Abstract
Functional Near-Infrared Spectroscopy (fNIRS) assesses human brain activity by noninvasively measuring changes of cerebral hemoglobin concentrations caused by modulation of neuronal activity. Recent progress in signal processing and advances in system design, such as miniaturization, wearability and system sensitivity, have strengthened fNIRS as a viable and cost-effective complement to functional Magnetic Resonance Imaging (fMRI), expanding the repertoire of experimental studies that can be performed by the neuroscience community. The availability of fNIRS and Electroencephalography (EEG) for routine, increasingly unconstrained, and mobile brain imaging is leading towards a new domain that we term “Neuroscience of the Everyday World” (NEW). In this light, we review recent advances in hardware, study design and signal processing, and discuss challenges and future directions towards achieving NEW.
1. Introduction
Over the last three decades, powerful neuroimaging techniques such as functional Magnetic Resonance Imaging (fMRI) have provided great insights into the healthy functioning brain and led to numerous advances in characterizing, diagnosing and treating various brain disorders. To date, however, our understanding of how the brain functions is mostly based on single-snapshot experiments under constrained conditions. fMRI constrains subjects to the supine position in a highly artificial surrounding and the corresponding physical constraints prevent naturalistic movement, cognition, and interaction with the environment. The ecological validity of traditional laboratory-based experimentation, and the extent of the representativeness of such laboratory findings to their real world counterparts, have been a concern for a long time [1]. Neuroimaging under static and artificial lab settings can, in fact, only provide information on how the brain works under those select conditions, and not on how it responds to naturalistic stimuli under realistic, dynamic, complex, multisensory and often unpredictable real-world environments [2], [3]. Solving this complex problem will lead to a dramatic advancement in our understanding of human brain function. One path towards this goal is to greatly reduce the constraints of traditional lab-based studies and to enable researchers to gradually expand the complexity of their experiments towards naturalistic stimuli and environments, while always maintaining the desired degree of control and repeatability.
In recent years, there have been rapid technological advances in the functional Near Infrared Spectroscopy (fNIRS) field (also in combination with Electroencephalography (EEG)), that enable linking brain activity to human movement, cognition, and social interaction continuously, in real time, and in the Everyday World (Figure 1). fNIRS and EEG are two safe and non-invasive neuroimaging techniques that enable mobile brain imaging over long periods of time. fNIRS measures the cortical hemodynamic response function (i.e., deoxygenated, oxygenated, and total hemoglobin) that is strongly correlated with the fMRI blood oxygen-level dependent (BOLD) signal [4]. Due to its great potential ecological validity, it has been established as a viable alternative to fMRI for certain paradigms and populations [5]–[7], and has been employed in the study of various real world phenomena [8]. As the field continues to grow, we expect many new important applications to be identified (see Figure 1).
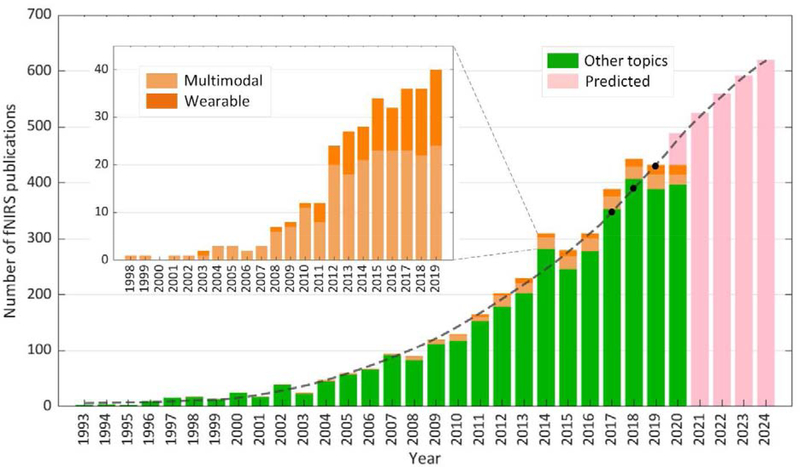
Figure 1.
The figure illustrates the growth of fNIRS publications from 1993 to 2019 as assessed on October 31st 2020, and our predictions for the period 2021 to 2024. The dashed black line represents the growing trend, and the black dots indicate the prediction (2017 to 2019) in our previous paper [6], showing that real publications in the last three years match well with the prediction. We expect the total number of fNIRS papers to continue to grow in the next several years as we illustrate with the magenta bars. This graph also highlights the growth of papers published utilizing multimodal measurements or wearable fNIRS since its first implementation in 1998. These statistics were obtained from a database created by a Web of Science search with the search terms ‘fNIRS’ and ‘brain’. ‘Multimodal’ refers to synchronous measurements of fNIRS and other neuroimaging modalities. Search keywords for multimodal fNIRS studies were a combination of ‘fNIRS’ and any one of the following words: ‘multimodal’, ‘bimodal’, ‘hybrid’ or ‘EEG’. Search keywords for wearable fNIRS studies were a combination of ‘fNIRS’ and any one of the following words: ‘wearable’, ‘wireless’, ‘fiberless’ or ‘modular’.
In this Opinion article, we briefly summarize the main advances in the fNIRS field over the last 5 years, and provide our perspective on what opportunities and challenges lie ahead, with a particular emphasis on the emergence of a new field of study that we term Neuroscience of the Everyday World (NEW). We structure this article into three sections to discuss Advances in fNIRS Instrumentation, Studies, and Signal Processing.
2. Advances in fNIRS instrumentation
2.1. Wearable fNIRS instrumentation and multimodality integration
Continuous Wave (CW) fNIRS systems have rapidly advanced towards lightweight, wearable, and fiberless designs [7], [9] that directly place light emitters and receivers on the scalp. The level of integration [10], channel count, and wearability of CW fNIRS instruments are continuously increasing as more High-Density Diffuse Optical Tomography (HD-DOT) systems emerge [11]. Other variations, such as time-domain fNIRS (TD-fNIRS), are also rapidly progressing towards mobile instruments [12], [13], and topics discussed in this paper apply to both domains. Among the main objectives in wearable fNIRS instrument design is the optimization of signal quality and sensitivity, and the improvement of usability and robustness to motion artifacts.
Novel system architectures profit from the steady progress in miniaturized, high-performance and low-cost (embedded) electronics and a broader availability of NIR source and detector components that is due to a general increase in NIR-based applications in the fNIRS domain and beyond (e.g. health-trackers, LiDAR technology). In wearable CW fNIRS, light emitting diodes (LEDs) have largely replaced lasers because they are cheaper, easier to work with, and just as powerful. Conventional avalanche photodiode (APDs) and regular silicon photodiodes (SiPDs), which are less sensitive, but more cost-efficient and simpler in their implementation and miniaturization, are still the dominant fNIRS detector types [9]. Recently, new sensors from the APD family - single-photon avalanche diode (SPAD) arrays and silicon photo-multipliers (SiPMs) - are now being tested in fNIRS systems because of their potential sensitivity advantages [14]–[16]. Aside from wearability, the overall miniaturization of fNIRS technology and integrated optodes also enables whole-scalp high-density measurements and, as opposed to using fibers, greatly reduces weight and motion artifacts.
Multimodal fNIRS data acquisition systems are becoming more common, as multimodal physiological data can greatly help to explain variance in the fNIRS signal. Simultaneous EEG and fNIRS promises complementary information about the neural state [17]–[19]. Non-neural auxiliary signals, such as blood pressure and acceleration, have proven to improve the filtering of physiological interference and motion artifacts (see section 4, Advances in Signal Processing). fNIRS instruments will increasingly have to support integration with these and other emerging modalities, such as eye-tracking, Augmented and Virtual Reality (AR/VR) devices.
2.2. Challenges and Future Directions
Advances in fibreless optode designs, NIR LED performance and detector sensitivity together with an overall increased understanding of fNIRS systems design have enabled more robust and sensitive measurements. To maintain this performance during real world mobility, new designs should consider constant and secure coupling to the scalp given the additional susceptibility to motion artifacts. Moreover, unexpected and ever-changing light conditions require additional considerations. For instance, a challenge for fNIRS in mobile environments or in combination with other devices that make use of near infrared (NIR) light sources (such as eye trackers, AR, VR and motion tracking set ups) lies in obtaining robust measurements despite NIR interference or strong ambient light sources such as the sun. Adaptation of dynamic range, rejection of modulated interference, established techniques like light baffling (e.g., covering the head with an opaque cap) or the use of optical filters are critical in these settings. HD-DOT setups are gaining prominence as they allow scalp regression during image reconstruction and more robustly measure brain activation with better spatial resolution and contrast to noise ratio [20]. HD-DOT measurements also provide data redundancy that can compensate for some low-quality channels due to hair interference. In addition to HD-DOT, whole head fNIRS is desirable in real-world settings due to the engagement of multiple cortical units —e.g., due to simultaneous presentation of multiple stimuli (e.g., visual/auditory/cognitive/motor during talking while walking) or a single complex stimulus activating multiple cortical regions. With the complexity of multisensory and motor inputs, reliable cross-modality synchronization and precise determination of stimulus timings will become even more crucial in analyzing multimodal data. One increasingly used tool for this purpose is the Lab Streaming Layer [21].
One should note that minimization of power consumption is even more critical with the trends towards multimodal, high-density, whole-head measurements with additional modalities providing physiological and contextual information, especially for recordings of long periods of time. While long-life, light and safe battery designs are critical, careful power management can also be employed to maximize the battery life. For example, firmware can be optimized to support a context-sensitive adaptation of the acquisition rate.
In summary, the continued evolution of wearable fNIRS systems with increasing channel density, improved brain sensitivity and coverage, and increased miniaturization of probes and a stronger integration with additional modalities, along with improvements in usability, will greatly accelerate the expansion of experimental studies that go beyond conventional lab-based settings.
3. Advances in Neuroscience Studies
3.1. Novel paradigms
More and more studies are appearing using wearable neuroimaging systems that provide reliable data from freely moving subjects, demonstrating the feasibility of brain imaging in real-world contexts, including: spatial cognition while walking [7]; allocation of attention across multiple sensory-cognitive processing demands during movement [22]; sports, social interactions, and neuroergonomics (e.g., during driving/flying [23], [24]). Studies of pathological brain function in more natural environments are now appearing for psychiatric or autism spectrum disorders [25]. The social neurosciences are now greatly benefiting from hyperscanning of socially interacting people [26]. Social perception and interaction studies in adults and children are being conducted to identify brain-behavior relations, such as imitation behavior [27], emotion perception [28], affective touch [29] and deceptive [30] and cooperative games [31]. Wearable systems also enable unconstrained studies in clinical settings, as needed for rehabilitation, neurological diseases [32], developmental neuroscience (typical, mental retardation [33], autism, attention deficit hyperactivity disorder, traumatic brain injury, neurodevelopment in rural areas [34]), psychiatric disorders (bipolar disorder, schizophrenia, and game addiction) [35], and intraoperative studies.
Virtual reality (VR) technology provides a platform to explore new theories and methods on how the brain functions in naturalistic situations (such as navigation, shopping) by simulating the real-world scenarios with more controlled settings and thus helps to prepare us as we move towards more chaotic, real life neuroimaging. It can be expected that the combination of VR and wearable brain imaging will greatly expand the possibilities for gait, neuroeconomic, and neuroergonomics studies, and for clinical studies requiring, for instance, neurofeedback for neuromodulation [36]. Although feasibility studies exist [37], [38], existing commercial VR devices with head-mounted displays can induce extra discomfort when combined with EEG and/or fNIRS devices. Therefore, effort is focused on the development of modified VR hardware that can be easily used in conjunction with brain imagers [39].
The field of brain computer interfaces (BCI) also requires wireless brain imaging technology and multimodality measurements. Specifically, the use of combined fNIRS-EEG measurements in BCI applications pushes the field to determine on how to spatially and temporally integrate the information coming from different modalities that target different aspects of the brain activity (e.g. neuronal activity versus hemodynamics) [19].
3.2. Challenges and Future Directions
Moving neuroimaging research out of the lab and into the real world requires novel experimental paradigms, recording procedures and data analysis methods. While early studies are mostly limited by the head coverage and preparation time, the development of modular wearable neuroimaging devices will enable studies with whole head coverage from larger groups, and thus enable the investigation of more complex interactions such as between teacher-student, child-parent, leader-team and patient-clinician. Moreover, the integration of neuroimaging with movement tracking in the Everyday World has promise to expand upon movement-based sensorimotor phenotypes and biomarkers, differentiate between restitution and compensation motor recovery mechanisms, and advance the fields of tele-rehabilitation and digital therapeutics [40].
As wearable neuroimaging devices increase in portability and affordability, they will become more pervasive in the market, opening up questions about the privacy and security of human data. Consumer markets such as BCIs for device control or self-monitoring, non-invasive neurostimulation, and neuromarketing applications produce large volumes of data in an unprotected and loosely regulated manner [41]. These data may involve personally identifiable information that could reveal sensitive data such as user’s health status, preferences, intentions, and moods, without the knowledge of the user. Therefore, proactive, cooperative and interdisciplinary efforts are needed from users, commercial industry and policymakers to increase the privacy and security of brain-related data.
4. Advances in Signal Processing
4.1. Artifact rejection and nuisance regression
With the novel studies using mobile fNIRS instruments in natural environments, the fNIRS signal will become more prone to motion-induced artifacts and systemic interference. Novel processing methods have to address complex characteristics of the signal [42], [43], such as 1) heterogeneity [44], non-instantaneous and non-constant coupling of brain and physiological nuisance signals in measurement channels, 2) correlated physiological noise, and 3) statistical dependencies of the underlying neuronal and systemic physiological processes.
Typical fNIRS analysis pipelines for stimulus-based paradigms involve a General Linear Model (GLM) [45]. Independent measurements of physiological signals (e.g., short-separation measurements [46]) or motion [accelerometer]) allow for a more accurate and statistically robust estimation of the evoked brain activity by simultaneously extracting hemodynamic response function (HRF) and the confounding signals [47]–[49]. Recent GLM expansions include pre-whitening/pre-coloring of the data [42] or make use of methods from machine learning to learn statistics of the multivariate data (i.e. by employing temporally embedded Canonical Correlation Analysis (tCCA)) to extract more optimal nuisance regressors for improved GLM-based brain activity estimation [50]. Feature-based analysis is an alternative approach that also utilizes GLM, albeit in a different way [51]. In this approach, one convolves each event/object captured with an HRF model, and calculates the temporal correlation between the modeled time-course and the measured brain signal.
4.2. Single trial analysis and multimodal processing
Recently, single-trial detection, real-time analysis, and classification of fNIRS signals using machine learning have also come into focus, often motivated by BCI applications (see [52] for a review and [24] for an example of real time working memory load estimation). A number of recent reviews of fNIRS or concurrent fNIRS-EEG approaches exist [53]. GLM-based concepts can be used to learn individual HRF regressors for single trial analysis [54].
4.3. Challenges and Future Directions
Improvements in the decomposition of fNIRS signals:
The use of fNIRS systems in more complex, dynamic and multisensory environments further increases the need for an improved understanding of systemic physiological confounds in the signal, and for robust approaches to separate the weak neural information from strong physiological contamination and other artifacts. HD-DOT helps by improving brain versus scalp discrimination from multi-distance measurements. Multimodal approaches can further help to identify and separate non-neuronal components from the fNIRS signal. Due to a current lack of standardization of fNIRS data analysis pipelines and parameter selection and reporting, the comparison and interpretation of reported results can be challenging. To address this, guidelines have recently been published and endorsed by the Society for fNIRS [55].
Linking brain activity with behavior:
In neuroscientific experiments, the timing of distinct behaviors, stimuli, and corresponding brain signals need to be precisely linked. In most common statistical models, this timing information is, then, used to generate regressors for brain activity estimation. In experiments under less restricted settings with continuous recordings in the Everyday World, a big challenge is the control, classification, and labeling of behavior and stimuli. While there are statistical techniques to estimate stimulus onsets from the time series alone [56], more complex scenarios will require the recording and analysis of additional contextual information about the environment, and the human perception of and interaction with it. These contexts can be generated from audio and video recordings, GPS position data, motion sensors and eye tracking. Eye-trackers are especially critical in providing information on the focus of attention in real world environments. Calibration without having a fixed point of reference, as well as eye fixations on elements beyond the range of calibration, are challenges waiting to be addressed [2]. With the current speed of innovation in the fields of artificial intelligence (e.g., object and voice recognition), we expect that context and label generation will increasingly be automated and will replace manual data annotation for context generation.
Real-time processing:
When offline-processing of brain-activity is not sufficient, for instance in BCI applications, single-trial analysis is required. Conventional regression approaches (e.g,. the offline GLM) are not computationally efficient for real-time analysis. Instead, the GLM must be recast as a state-space problem and solved using a Kalman filter for robust regression of fNIRS signals, especially for systems with dynamic statistical properties [49]. Future approaches can improve performance by using multimodal regressors [50] that are dynamically adapted, and also by dynamically incorporating information about the confidence in signal quality, contexts and labels.
5. Vision and Outlook: Neuroscience in the Everyday World
It is a straightforward conceptualization and engineering effort to combine these trends toward a wearable, high density, and multimodal fNIRS technology with state-of-the-art machine learning into a solution that synergistically time-locks brain activation patterns to behavioral data in increasingly unconstrained naturalistic environments which we term the Neuroscience in the Everyday World (NEW).
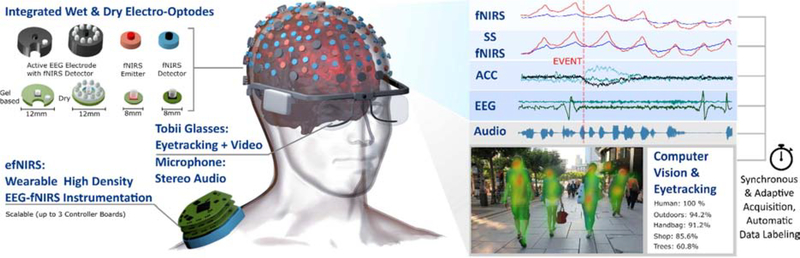
Our vision for NEW is a portable, miniaturized, lightweight, high-density, wearable combined fNIRS – EEG – Eye-tracking system that permits long duration continuous monitoring of normal / altered brain activity during movement, perception, and social interaction in real time and in the real world (Figure 2). We are currently developing such a system with a compact design that is extendable to 128 optodes, based on our previous work on scalable openfNIRS and wearable hybrid fNIRS-EEG instrumentation [18], [57].
Figure 2:
The authors’ ongoing approach to implement a version of the NEW Concept. Hybrid wearable high density fNIRS-EEG instrumentation (efNIRS) combined with eye tracking (here Tobii Glasses) and cloud-based computer vision for object recognition and automatic stimulus tracking / data labeling.
The Everyday World yields a plethora of multimodal sensory stimuli, often not well-defined. Signal cleaning, data annotation, and cue labeling face new challenges. Research in the NEW field, as we envision it, will holistically tackle these and other challenges outlined in this paper, i.e., by a combined high-density multimodal hardware and signal analysis effort. On the continuum from ‘lab-based’ to ‘free in the wild’, iterative progress will depend on the interaction between the improvement of robustness in brain-activity estimation and the resulting permissible level of experimental freedom, stimulus complexity and control. The analysis workflow for data collected has to accomplish (1) robust removal of nuisance signals from fNIRS/EEG signals, (2) automatic annotation of and adaptation to real world stimuli, and (3) joint analysis of multimodal fNIRS/EEG and behavioral data.
The field will likely tackle this challenge by using state-of-the-art multivariate Machine Learning methods. Experimental context will be automatically generated from the audio, video, and eye-tracking data using available cutting-edge computer vision / text to speech solutions; leading towards automatic, continuous and probabilistic data labeling that enables segmentation of the fNIRS-EEG signals using keyword / context search (Figure 2). Identified objects and scenes and their likelihood can be organized into predefined event-categories to construct HRF regressors. These regressors, along with the novel nuisance regressors from our multimodal tCCA-based approach, will then be employed in the extended GLM tCCA analysis of brain activity. This analysis approach allows subjects to perform tasks as they would in the Everyday World, while still supporting standard cognitive neuroscience data analysis methodology.
6. Conclusion
The fNIRS field is continuously growing and rapidly advancing towards more mobile, high-density, and multimodal devices, enabling studies outside of the laboratory. While there is increasing evidence for opportunities to further expand our understanding of the brain in situations that could not previously be investigated [7], [9], a vast number of challenges and limitations will have to be addressed to advance this NEW field beyond the current exploratory state [3], [58]. To enable routine neuroimaging in the Everyday World, improvements in usability, signal quality, and context-sensitivity are vital. Both instrumentation performance (affecting signal to noise ratio, SNR) and signal modeling and processing (affecting contrast to noise ratio, CNR) have to progress in concert. The usability, unobtrusiveness, and robustness of wearable fNIRS instrumentation will have to be further advanced together with probe density and system sensitivity to realize improvements in signal quality. The contrast between desired brain activation and other confounders in the acquired signals can then be elevated by incorporating novel analysis methods with independent measurements of confounding signals (such as systemic physiology or motion) as well as information from other modalities (e.g., EEG). Context-sensitivity, supported by eye tracking, movement monitoring, and artificial intelligence, will be invaluable for enabling automatic data labeling in wearable/naturalistic applications, elevating the need for human/computer for stimulus labeling or presentation. Great care will have to be taken to continuously counterbalance the newly-gained freedom from experimental constraints with a good control of environmental interaction and stimuli. This exciting new field offers a large number of highly interdisciplinary challenges and opportunities that will have to be jointly addressed with engineering, computer science and neuroscience efforts in the coming years.
7. Acknowledgment
This work was funded by NIH U01EB029856.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Neisser U, Cognition and Reality. W.H. Freeman & Co Ltd, 1976. [Google Scholar]
- [2].Ladouce S, Donaldson DI, Dudchenko PA, and Ietswaart M, “Understanding Minds in Real-World Environments: Toward a Mobile Cognition Approach,” Front. Hum. Neurosci, vol. 10, January. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ward JA, Pinti P, Amft O, and Van Laerhoven K, “Wearables and the Brain,” IEEE Pervasive Comput, vol. 18, no. 1, pp. 94–100, 2019. [Google Scholar]
- [4].Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, and Boas DA, “A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans,” Neuroimage, vol. 29, no. 2, pp. 368–382, January. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boas DA, Elwell CE, Ferrari M, and Taga G, “Twenty years of functional near-infrared spectroscopy: introduction for the special issue.,” Neuroimage, vol. 85 Pt 1, pp. 1–5, January. 2014. [DOI] [PubMed] [Google Scholar]
- [6].Yücel MA, Selb JJ, Huppert TJ, Franceschini MA, and Boas DA, “Functional Near Infrared Spectroscopy: Enabling Routine Functional Brain Imaging,” Curr. Opin. Biomed. Eng, vol. 4, pp. 78–86, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pinti P et al. , “A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments,” Jpn. Psychol. Res, vol. 60, no. 4, pp. 347–373, October. 2018.* A recent comprehensive review on wearable fNIRS imaging in naturalistic settings.
- [8].Butler LK, Kiran S, and Tager-Flusberg H, “Functional Near-Infrared Spectroscopy in the Study of Speech and Language Impairment Across the Life Span: A Systematic Review,” Am. J. Speech-Language Pathol, vol. 29, no. 3, pp. 1674–1701, August. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhao H and Cooper RJ, “Review of recent progress toward a fiberless, whole-scalp diffuse optical tomography system,” Neurophotonics, vol. 5, no. 01, p. 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Choi JK, Kim JM, Hwang G, Yang J, Choi MG, and Bae HM, “Time-Divided Spread-Spectrum Code-Based 400 fW-Detectable Multichannel fNIRS IC for Portable Functional Brain Imaging,” IEEE J. Solid-State Circuits, vol. 51, no. 2, pp. 484–495, February. 2016. [Google Scholar]
- [11].Chitnis D et al. , “Functional imaging of the human brain using a modular, fibre-less, high-density diffuse optical tomography system,” Biomed. Opt. Express, vol. 7, no. 10, pp. 4275–4288, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lacerenza M et al. , “Wearable and wireless time-domainnear-infrared spectroscopy system for brainand muscle hemodynamic monitoring,” Biomed. Opt. Express, vol. 11, no. 10, pp. 5934–5949, 2020.** This paper introduces a wearable time-domain near infrared spectroscopy that can be used in the Everyday World.
- [13].Kernel I, “Kernel Flow 50,” 2020. [Online]. Available: www.kernel.com. [Accessed: 26-Oct-2020].** This initiative introduces an affordable wearable-time-domain system.
- [14].Zimmermann R, Braun F, Achtnich T, Lambercy O, Gassert R, and Wolf M, “Silicon photomultipliers for improved detection of low light levels in miniature near-infrared spectroscopy instruments,” Biomed. Opt. Express, vol. 4, no. 5, p. 659, May 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wyser D, Lambercy O, Scholkmann F, Wolf M, and Gassert R, “Wearable and modular functional near-infrared spectroscopy instrument with multidistance measurements at four wavelengths,” Neurophotonics, vol. 4, no. 04, p. 1, August. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dalla Mora A et al. , “The SiPM revolution in time-domain diffuse optics,” Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip, vol. 978, no. July, p. 164411, 2020. [Google Scholar]
- [17].Kassab A et al. , “Multichannel wearable fNIRS-EEG system for long-term clinical monitoring,” Hum. Brain Mapp, vol. 39, no. 1, pp. 7–23, January. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].von Lühmann A, Wabnitz H, Sander T, and Müller K-R, “M3BA: A Mobile, Modular, Multimodal Biosignal Acquisition Architecture for Miniaturized EEG-NIRS-Based Hybrid BCI and Monitoring,” IEEE Trans. Biomed. Eng, vol. 64, no. 6, pp. 1199–1210, 2017.** Paper introduces a wearable fNIRS-EEG system with embedded motion sensor.
- [19].Ahn S and Jun SC, “Multi-Modal Integration of EEG-fNIRS for Brain-Computer Interfaces – Current Limitations and Future Directions,” Front. Hum. Neurosci, vol. 11, October. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wheelock MD, Culver JP, and Eggebrecht AT, “High-density diffuse optical tomography for imaging human brain function,” Rev. Sci. Instrum, vol. 90, no. 5, p. 051101, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boulay C, “Lab streaming layer (LSL) summary,” GitHub, 2018. . [Google Scholar]
- [22].Ladouce S, Donaldson DI, Dudchenko PA, and Ietswaart M, “Mobile EEG identifies the reallocation of attention during real-world activity,” Sci. Rep, vol. 9, no. 1, p. 15851, December. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Causse M, Chua Z, Peysakhovich V, Del Campo N, and Matton N, “Mental workload and neural efficiency quantified in the prefrontal cortex using fNIRS,” Sci. Rep, vol. 7, no. 1, p. 5222, December. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gateau T, Ayaz H, and Dehais F, “In silico vs. Over the Clouds: On-the-Fly Mental State Estimation of Aircraft Pilots, Using a Functional Near Infrared Spectroscopy Based Passive-BCI,” Front. Hum. Neurosci, vol. 12, May 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang F and Roeyers H, “Exploring brain functions in autism spectrum disorder: A systematic review on functional near-infrared spectroscopy (fNIRS) studies,” Int. J. Psychophysiol, vol. 137, pp. 41–53, 2019. [DOI] [PubMed] [Google Scholar]
- [26].Quaresima V and Ferrari M, “Functional Near-Infrared Spectroscopy (fNIRS) for Assessing Cerebral Cortex Function During Human Behavior in Natural/Social Situations: A Concise Review,” Organ. Res. Methods, vol. 22, no. 1, pp. 46–68, January. 2019. [Google Scholar]
- [27].Oliver D, Tachtsidis I, and de C AF. Hamilton, “The role of parietal cortex in overimitation: a study with fNIRS,” Soc. Neurosci, vol. 13, no. 2, pp. 214–225, March. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balconi M, Grippa E, and Vanutelli ME, “What hemodynamic (fNIRS), electrophysiological (EEG) and autonomic integrated measures can tell us about emotional processing,” Brain Cogn, vol. 95, pp. 67–76, April. 2015. [DOI] [PubMed] [Google Scholar]
- [29].Bennett RH, Bolling DZ, Anderson LC, Pelphrey KA, and Kaiser MD, “fNIRS detects temporal lobe response to affective touch,” Soc. Cogn. Affect. Neurosci, vol. 9, no. 4, pp. 470–476, April. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang M, Liu T, Pelowski M, and Yu D, “Gender difference in spontaneous deception: A hyperscanning study using functional near-infrared spectroscopy,” Sci. Rep, vol. 7, no. 1, p. 7508, December. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lu K, Xue H, Nozawa T, and Hao N, “Cooperation Makes a Group be More Creative,” Cereb. Cortex, vol. 29, no. 8, pp. 3457–3470, 2019.** This paper exemplifies how hyper scanning allows for studying the neuroscience of abstract concepts such as creativeness.
- [32].Bonilauri A, S. I. F, Pugnetti L, Baselli G, and Baglio F, “A Systematic Review of Cerebral Functional Near-Infrared Spectroscopy in Chronic Neurological Diseases-Actual Applications and Future Perspectives,” Diagnostics, vol. 10, no. 8, p. 581, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zennifa F, Ide J, Noguchi Y, and Iramina K, “Monitoring of cognitive state on mental retardation child using EEG, ECG and NIRS in four years study,” in 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2015, pp. 6610–6613. [DOI] [PubMed] [Google Scholar]
- [34].Lloyd-Fox S et al. , “Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa.,” Sci. Rep, vol. 4, no. 1, p. 4740, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chiarelli AM, Zappasodi F, Di Pompeo F, and Merla A, “Simultaneous functional near-infrared spectroscopy and electroencephalography for monitoring of human brain activity and oxygenation: a review,” Neurophotonics, vol. 4, no. 04, p. 1, August. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Teo W-P et al. , “Does a Combination of Virtual Reality, Neuromodulation and Neuroimaging Provide a Comprehensive Platform for Neurorehabilitation? – A Narrative Review of the Literature,” Front. Hum. Neurosci, vol. 10, June. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Park JL, Dudchenko PA, and Donaldson DI, “Navigation in Real-World Environments: New Opportunities Afforded by Advances in Mobile Brain Imaging,” Front. Hum. Neurosci, vol. 12, September. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McKendrick R et al. , “Into the Wild: Neuroergonomic Differentiation of Hand-Held and Augmented Reality Wearable Displays during Outdoor Navigation with Functional Near Infrared Spectroscopy,” Front. Hum. Neurosci, vol. 10, May 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Landowska A, Royle S, Eachus P, and Roberts D, “Testing the Potential of Combining Functional Near-Infrared Spectroscopy with Different Virtual Reality Displays—Oculus Rift and oCtAVE,” pp. 309–321, 2018. [Google Scholar]
- [40].Porciuncula F et al. , “Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances,” PM&R, vol. 10, pp. S220–S232, September. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ienca M, Haselager P, and Emanuel EJ, “Brain leaks and consumer neurotechnology,” Nat. Biotechnol, vol. 36, no. 9, pp. 805–810, 2018. [DOI] [PubMed] [Google Scholar]
- [42].Huppert TJ, “Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy,” Neurophotonics, vol. 3, no. 1, p. 10401, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].von Lühmann A, Boukouvalas Z, Müller K-R, and Adali T, “A new blind source separation framework for signal analysis and artifact rejection in functional Near-Infrared Spectroscopy,” Neuroimage, vol. (accepted), 2019. [DOI] [PubMed] [Google Scholar]
- [44].Wyser D, Mattille M, Wolf M, Lambercy O, Scholkmann F, and Gassert R, “Short-channel regression in functional near-infrared spectroscopy is more effective when considering heterogeneous scalp hemodynamics,” Neurophotonics, vol. 7, no. 03, September. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, and Frackowiak RSJ, “Statistical parametric maps in functional imaging: A general linear approach,” Hum. Brain Mapp, 1994. [Google Scholar]
- [46].Saager R and Berger A, “Measurement of layer-like hemodynamic trends in scalp and cortex: implications for physiological baseline suppression in functional near-infrared spectroscopy,” J. Biomed. Opt, vol. 13, no. 3, p. 034017, 2008. [DOI] [PubMed] [Google Scholar]
- [47].Yücel MA et al. , “Short separation regression improves statistical significance and better localizes the hemodynamic response obtained by near-infrared spectroscopy for tasks with differing autonomic responses,” Neurophotonics, vol. 2, no. 3, p. 035005, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kirilina E, Yu N, Jelzow A, Wabnitz H, Jacobs AM, and Tachtsidis I, “Identifying and quantifying main components of physiological noise in functional near infrared spectroscopy on the prefrontal cortex.,” Front. Hum. Neurosci, vol. 7, no. December, p. 864, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gagnon L, Perdue K, Greve DN, Goldenholz D, Kaskhedikar G, and Boas DA, “Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling,” Neuroimage, vol. 56, no. 3, pp. 1362–1371, June. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].von Lühmann A, Li X, Müller KR, Boas DA, and Yücel MA, “Improved physiological noise regression in fNIRS: A multimodal extension of the General Linear Model using temporally embedded Canonical Correlation Analysis,” Neuroimage, vol. 208, no. December 2019, p. 116472, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fishell AK, Burns-Yocum TM, Bergonzi KM, Eggebrecht AT, and Culver JP, “Mapping brain function during naturalistic viewing using high-density diffuse optical tomography,” Sci. Rep, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].He B, Yuan H, Meng J, and Gao S, “Brain-Computer Interface,” in Neural Engineering, Springer, 2020, pp. 131–183. [Google Scholar]
- [53].Hong K-S, Khan MJ, and Hong MJ, “Feature Extraction and Classification Methods for Hybrid fNIRS-EEG Brain-Computer Interfaces,” Front. Hum. Neurosci, vol. 12, June. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].von Lühmann A, Ortega-Martinez A, Boas DA, and Yücel MA, “Using the General Linear Model to Improve Performance in fNIRS Single Trial Analysis and Classification: A Perspective,” Front. Hum. Neurosci, vol. 14, February. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yücel MA et al. , “Best practices for fNIRS publications,” Neurophotonics, vol. 8, no. 01, January. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pinti P et al. , “A novel GLM-based method for the Automatic IDentification of functional Events (AIDE) in fNIRS data recorded in naturalistic environments,” Neuroimage, vol. 155, no. May, pp. 291–304, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zimmermann BB, Tamborini D, Selb J, Martinez AO, and Boas DA, “Development of a Wearable fNIRS System Using Modular Electronic Optodes for Scalability,” in Biophotonics Congress: Optics in the Life Sciences Congress 2019 (BODA,BRAIN,NTM,OMA,OMP), 2019, p. BW1A.3. [Google Scholar]
- [58].Matusz PJ, Dikker S, Huth AG, and Perrodin C, “Are We Ready for Real-world Neuroscience?,” J. Cogn. Neurosci, vol. 31, no. 3, pp. 327–338, March. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]