Abstract
Although lesion-deficit case studies are foundational in cognitive neuroscience, published papers presenting single lesion cases are declining. In this review, we argue that there is a valuable place for single-case lesion-deficit research, especially when combined with functional neuroimaging methods, such as functional magnetic resonance imaging (fMRI). To support this, we present a summary of notable findings from single-case combined lesion-deficit and fMRI studies published in recent years (2017–2020). These studies show the unique value that this combined approach brings to the understanding of complex functions, brain-level connectivity, and plasticity and recovery. We encourage researchers to consider combining lesion-deficit and functional imaging methods in the analysis of single cases, as this approach affords unique opportunities to address challenging unanswered questions about brain-behavior relationships.
Keywords: case studies, lesion method, fmri
Introduction
Single-case lesion-deficit studies form the foundations of cognitive neuroscience research. Famous cases such as Mr. Leborgne and S.M. are fundamental to our understanding of language and emotional processing, respectively, and are widely taught in introductory neuroscience textbooks [1,2]. Similarly, the stories of Phineas Gage and patient E.V.R. demonstrated the link between personality and brain tissue [3,4]. Patient H.M. provided researchers the ability to discern the role of the hippocampus in episodic memory, resulting in a landmark paper that is among the most cited in neuroscience [5,6]. In each of these examples, substantial impact on the understanding of brain-behavior relationships was made by groundbreaking single-case lesion-deficit studies.
Lesion-deficit case studies are essentially serendipitous in nature, as scientists lack experimental control over whether any participant will have an interesting focal brain lesion and associated deficits [7,8]. As such, single-case lesion-deficit studies have often featured participants with unique or paradoxical behavioral presentations. While lesion-deficit studies with multiple participants are well-suited for testing predictions of theoretical models, at times the patterns of behavior observed in a single case report are unexpected and cannot be explained using current models. Such singular cases may prompt entirely new formulations of brain-behavior relationships [8]. The power of compelling case studies to reshape models and theories is one of the valuable features of single-case lesion studies. For example, the report of a patient who had lost the ability to name verbs but not nouns helped establish the non-intuitive dissociation between these two lexical processes [9].
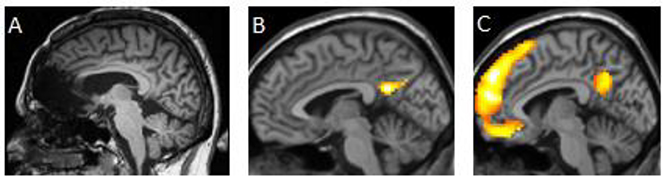
Despite their potential to make impactful contributions, single-case studies seem to have fallen out of favor in neuroscience. Journals are increasingly unwilling to publish single-case studies [8,10] or limit publication to occurrences that are “strikingly unusual” [11]. Journals that do accept single-case studies report that the number of manuscript submissions on single-case studies is decreasing [7]. The advent of neuroimaging techniques has largely been blamed for this decline [12]. However, researchers could take this as an opportunity to “join forces” – viz., to use the classic lesion-deficit approach in single-case studies and combine this with neuroimaging methods, particularly functional magnetic resonance imaging (fMRI). Figure 1 illustrates an example of this approach.
Fig 1.
Sample images indicating how a combined single-case lesion deficit and fMRI approach may be used to understand emotional processing. (A) MR image (mid-sagittal view of right hemisphere) depicting a lesion to the ventromedial prefrontal cortex in one participant. (B) Brain regions activated (p<.01) in this participant during an emotional processing task, plotted on a template brain (same mid-sagittal perspective as in A). (C) Brain regions activated in a matched, neurotypical comparison cohort engaging in the same task (same mid-sagittal perspective as in A and B). The activation depicted in (B) suggests that the patient is using a preserved remnant of an emotion processing circuit to achieve some degree of correct performance on the task.
Combining lesion-deficit and fMRI methods can give insight into a variety of neural processes, such as the roles of circumscribed brain regions within larger network activation, recovery processes following brain injury, and the impact of rehabilitation therapies on brain function. In this review, we present a summary of recent (2017–2020) contributions this combined approach has made to the field of neuroscience. We argue that studies combining single-case lesion-deficit with fMRI methods can make significant and meaningful contributions to our understanding of brain-behavior relationships, particularly in the areas of connectivity and plasticity.
Connectivity
Functional imaging methods afford researchers a window into patterns of connectivity within the brain. The entire brain can be surveyed and areas whose activation patterns oscillate in tandem can be interpreted as functionally connected. The lesion-deficit method may be used in conjunction with fMRI methods to enhance our understanding of network function and organization [13]. By comparing the network activation patterns of patients with unique lesions to those of an appropriately-matched comparison group, valuable insights can be gleaned regarding the role that a particular brain region may play (or not) within the network.
Recent work has used this approach to further clarify vision pathways. Canonical understandings of visual processing describe a flow of information from retina to thalamus to primary visual cortex, at which point information is distributed to various cortical areas responsible for processing motion, color, objects, faces, and location in space. However, recent papers report on individuals who retained some level of visual processing despite destruction of this pathway. Two studies report on participants with damage to the occipital lobe; the participants have no static (form) vision yet can perceive motion. Motion tasks conducted in the scanner elicited activation in middle temporal cortex [14*,15]. An additional case-report describes a 7-year-old who maintained conscious perception of visual stimuli and residual fMRI activation during visual tasks despite destruction of the white matter tracts that carry information to visual cortex [16]. Taken together, these studies indicate the existence of additional visual pathways, possibly subcortical in nature, that bypass primary visual cortex. A recent report of a patient with prosopagnosia has also challenged the assumption that face processing is linear and hierarchical. When presented with images of unfamiliar faces, the patient activated anterior portions of a face-processing network, despite having damage to key posterior regions crucial for face processing [17*]. This study suggests that face recognition may emerge from multiple parallel pathways that relay information from the visual cortex to various face-processing regions throughout cortex.
Language networks have also been further elucidated using a combined lesion-deficit and fMRI approach. Compared to vision, the precise pathways by which language is processed are less firmly established. Experiments in a surgical patient have supported the role of a recently-identified frontal fiber pathway in connecting brain regions important for planning to those important for lexical processing [18]. A peculiar case of a bilingual man whose stroke-induced aphasia was worse in his non-dominant language also helped to elucidate brain regions active in language processing, particularly in bilingual individuals. Functional neuroimaging showed that brain areas associated with two language processing networks exhibited decreased connectivity when compared to a matched comparison, yet connectivity between brain areas involved in the language control network were not found to be similarly affected [19]. It was concluded that the disconnection between the language control network and language processing networks led to the participant being unable to inhibit his dominant language, leading to more severe aphasia symptoms in his non-dominant language. In a third report, resting state fMRI in a 43-year-old woman who exhibited non-sequential spelling following a cerebellar lesion demonstrated an impairment of cerebellar connectivity to areas known to be involved in handwritten spelling [20].
Other notable recent works have shown that damage to anterior cingulate cortex is associated with diminution of the body’s “chill” response to pleasant and unpleasant sounds. Activation in areas involved in auditory recognition and working memory, as well as the subjective experience of “chills,” was preserved [21]. Another study indicated that lesions to periaqueductal gray and superior colliculus may be implicated in a psychotic behavior phenotype due to connectivity between these brainstem structures and the amygdala [22].
Plasticity
Single-case studies utilizing fMRI approaches are particularly well-suited for the investigation of plasticity and reorganization following brain injury. Plasticity outcomes following brain lesions can be variable and are influenced by individual factors [23,24], and repeated measurements of groups may be potentially insensitive to highly different patterns of changes across participants. Thus, following one participant over time may yield valuable results.
FMRI methods can be used to elucidate changes in brain network structure after injury [25]. One such example is a patient who developed severe amnesia following a thalamic lesion. Upon further study of this patient, a prior lesion to the hippocampus was discovered. The study authors suggest that this prior damage may have caused the participant to rely on right-hemisphere-based memory circuits, which were then disrupted following the new thalamic lesion [26*]. This reorganization of function can happen quickly; Gould and colleagues report the case of a patient whose motor cortex became compressed under subdural fluid following brain surgery. The motor regions were shown to reorganize and shift to adjacent brain regions in the same hemisphere in a matter of months, then shift again over the course of 4 months once the pressure subsided [27]. Functional connectivity has also been proposed to serve as a valuable tool for understanding plasticity following childhood lesions [28]. For example, reorganization of language has recently been reported in a case of penetrating brain injury experienced in infancy [29]. The unusual nature of these reports highlights the importance of publishing single-case lesion-deficit studies that include fMRI measures.
Current research on the underlying mechanisms suggests that reorganization includes recruitment of additional brain regions and return of neural activation back to baseline [30**], but the physiological underpinnings of global connectivity changes are not yet understood [31]. The ability to study one participant over time and acquire multiple fMRI scans at various stages of recovery affords a unique opportunity to capture plastic changes that may be taking place. For example, a recent report described a woman who presented with severe deficits in lexical processing following a stroke and was assessed over the course of one year. She recovered her abilities to read and spell, and improvement of these functions was associated with increased activation in areas involved in orthographic lexical processing. Notably, these areas were distinct from the area affected by the stroke [32]. This finding indicates that strokes can disrupt connectivity in distant areas, and that successful recovery may include reestablishing these connections. Similarly, in a patient who developed hemispatial neglect due to stroke, and then recovered, resting state fMRI scans acquired 4 days and 6 months after the stroke showed that improvement in symptoms was associated with additional multisensory integration [33].
In addition to improving our understanding of plasticity, combining lesion-deficit and fMRI methods may yield valuable insights into the mechanisms and effectiveness of rehabilitation. For example, after a 14-week period of rehabilitation, a stroke patient with spatial neglect demonstrated improvement in their attention, along with increased activity in bilateral frontal areas [34]. This suggests that rehabilitation can induce sustained increases in brain activity, and that frontal involvement may be a key part of recovery from neglect. Similarly, rehabilitation after stroke can lead to changes in network organization. One study showed that a virtual reality rehabilitation protocol led not only to improvements in function, but also increased network metrics in the ipsilesional hemisphere [35]. This supports the possibility that an increase in functional connectivity is an important component of recovery.
Changes in patterns of activation after rehabilitation can also be used to assess the efficacy of novel treatments [36]. This has been particularly true in reports of novel treatments for aphasia. How the brain may reorganize in response to treatment for aphasia remains unclear, and the reorganization that takes place may depend on various factors, including the specific deficit, language process assessed, lesion site, treatment type, and intensity of treatment [37**]. Due to heterogeneity across patients, a single-case study approach with robust functional imaging measures prior to and following intervention may be valuable for assessing treatment efficacy. FMRI measures have been shown to be correlated with success in language therapy [38]. In a patient with conduction aphasia, investigators described no improvement in language abilities after two months of speech language therapy. However, after repetitive transcranial magnetic stimulation (rTMS) to left Broca’s area, language ability significantly improved. Application of rTMS was also associated with a shift from loose and extensive activation patterns to more focused activation of areas associated with the left language network [39]. Additional research has shown that a novel rehabilitation technique for treating aphasia is associated with changes in areas involved with sensorimotor interactions, attention, motor planning, and internal models for speech [40]. In addition to their scientific contributions, these studies highlight the importance of individualized treatment options for patients.
Taken together, the above works underscore the importance of single-case studies in elucidating the neural reorganization that takes place following focal injury. Single-case studies provide detail and nuance that is not available in studies where multiple individuals are averaged together, and as such, single-case studies are ideal for investigating heterogeneous recovery trajectories. Moreover, single-case studies afford researchers the opportunity to identify underlying patterns of plasticity that may be common between individuals. Additionally, single-case studies may provide empirical evidence to support proposed mechanisms of reorganization.
Discussion
Single-case studies combining lesion-deficit and fMRI methods have a place in modern neuroscience. In recent years, research utilizing this combined approach has provided valuable insights regarding network organization in the brain, particularly regarding vision, language, and attention processing networks. It has also broadened our understanding of plasticity after focal injury, including giving further insight into mechanisms that may be at play and the impact of rehabilitation techniques on brain reorganization.
Contemporary cognitive neuroscience has become enamored of “big data” approaches in fMRI studies, which include large cohorts of many hundreds of participants. While these studies have yielded valuable knowledge, they are not without drawbacks. FMRI studies are low-powered [41] and require large sample sizes [42]. While fMRI studies can infer relationships, they lack the causal impact of lesion-deficit methods, and thus depend heavily on existing cognitive theories to draw meaningful conclusions about brain-behavior relationships [8]. Additionally, due to large sample sizes and standardized data collection methods, it is difficult to gather the types of nuanced data that a thorough study of a single participant can provide. Finally, big data approaches are not ideal for understanding the nuanced reorganization that occurs following an injury or rehabilitation intervention.
Combined single-case lesion-deficit and fMRI approaches capitalize on the strengths of both methods and offer unique opportunities that large-scale fMRI studies or lesion-deficit studies alone cannot. Unlike fMRI, lesion-deficit studies can identify key brain regions necessary for function, and such studies are typically highly powered due to extremely large effect sizes. However, while lesion-deficit methods can determine the extent to which a participant may have lost and later regained a function, they cannot be used to discern where or how the neuronal basis for that function has reorganized. By combining lesion-deficit and fMRI approaches, researchers can leverage an approach that provides strong evidence of localization of function while also describing whole-brain changes in patterns of activation. Due to the compelling nature of this combined approach, a study with a sample size of one can lead to meaningful scientific discovery.
Of course, there are important limitations of single-case studies, particularly regarding lesion-deficit and fMRI methods. Due to the “serendipitous” nature of single-case lesion-deficit studies, researchers lack rigorous experimental control over potential confounds, such as comorbidities or socioeconomic factors, that may influence both neuroimaging results and the neuropsychological profile. For this reason, appropriately matched individuals should be included as comparison participants. Statistical methods specifically formulated for comparing single-cases to a matched group should also be employed[43,44]. These statistical methods can also be used when assessing functional imaging results[45,46]. Additionally, fMRI analyses require statistical modeling to account for potential confounds when data are not being averaged across individuals [47]. Collecting longer bouts of imaging data for the participant may help mitigate this concern [41,48].
In this review, we have underscored the value of combined single-case lesion-deficit and fMRI studies. We appreciate that the debate regarding the value of single-case studies is longstanding [49,50], and the inclusion of recent neuroimaging techniques presents exciting new opportunities to revisit this debate. Researchers wishing to report on unique and interesting single-cases with focal lesions could consider including fMRI measures of connectivity or plasticity in their data collection to broaden what questions might be asked or what conclusions might be drawn. Similarly, journals currently eschewing case-studies could reconsider the potential value of multimodal single-case study approaches.
Conclusion
In conclusion, a combined single-case lesion-deficit and fMRI approach provides researchers with a compelling, unique opportunity to study neural organization, plasticity, and recovery.
Highlights.
The number of published neuroscience papers on single-case studies is declining
Combining lesion-deficit case studies and functional imaging yields valuable insights
These insights go beyond those obtained by lesion-deficit or functional imaging alone
Acknowledgements
This research was supported in part by the National Institute of Mental Health (MH094258), the National Institutes of Health (NS103780), and the Kiwanis Neuroscience Research Foundation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broca PP: Remarques sur le siège de la faculté du langage articulé suivies d’une observation d’aphemie. Bull Soc Anthr 1861, 6:330–357. [Google Scholar]
- 2.Hyman BT, Tranel D: Neuropsychological Correlates of Bilateral Amygdala Damage. Arch Neurol 1990, 47:349–355. [DOI] [PubMed] [Google Scholar]
- 3.Harlow JM: Passage of an Iron Rod through the Head. Bost Med Surg J 1848, 39:389–393. [PMC free article] [PubMed] [Google Scholar]
- 4.Eslinger PJ, Damasio AR: Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology 1985, 35:1731–1741. [DOI] [PubMed] [Google Scholar]
- 5.Scoville WB, Milner B: Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957, 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squire LR: The Legacy of Patient H.M. for Neuroscience. Neuron 2009, 61:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubelli R, Della Sala S: Looking back to go forward: Promoting single case studies. Cortex 2017, 97:A1–A3. [DOI] [PubMed] [Google Scholar]
- 8.Medina J, Fischer-Baum S: Single-case cognitive neuropsychology in the age of big data. Cogn Neuropsychol 2017, 34:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damasio AR, Tranel D: Nouns and verbs are retrieved with differently distributed neural systems. In Proceedings of the National Academy of Sciences of the United States of America.. National Academy of Sciences; 1993:4957–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee A: A madness to the methods in cognitive neuroscience? J Cogn Neurosci 2005, 17:847–849. [DOI] [PubMed] [Google Scholar]
- 11.When once is enough. Nat Neurosci 2004, 7:93. [DOI] [PubMed] [Google Scholar]
- 12.Mazzi C, Savazzi S: The Glamor of Old-Style Single-Case Studies in the Neuroimaging Era: Insights From a Patient With Hemianopia. Front Psychol 2019, 10:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaidya AR, Pujara MS, Petrides M, Murray EA, Fellows LK: Lesion Studies in Contemporary Neuroscience. Trends Cogn Sci 2019, 23:653–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran A, MacLean MW, Hadid V, Lazzouni L, Nguyen DK, Tremblay J, Dehaes M, Lepore F: Neuronal mechanisms of motion detection underlying blindsight assessed by functional magnetic resonance imaging (fMRI). Neuropsychologia 2019, 128:187–197.* This paper describes a patient with blindsight abilites despite lesion to primary visual cortex, V1. Using an event-related motion detection design, the authors identified significant fMRI signal in contralateral superior colliculi and frontal areas, and in ipsilateral middle temporal cortex. This finding has important implications for a dorsal visual processing pathway that bypasses V1 and allows for blindsight.
- 15.Arcaro MJ, Thaler L, Quinlan DJ, Monaco S, Khan S, Valyear KF, Goebel R, Dutton GN, Goodale MA, Kastner S, et al. : Psychophysical and neuroimaging responses to moving stimuli in a patient with the Riddoch phenomenon due to bilateral visual cortex lesions. Neuropsychologia 2019, 128:150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikellidou K, Arrighi R, Aghakhanyan G, Tinelli F, Frijia F, Crespi S, De Masi F, Montanaro D, Morrone MC: Plasticity of the human visual brain after an early cortical lesion. Neuropsychologia 2019, 128:166–177. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Vuong QC, Rossion B: The cortical face network of the prosopagnosic patient PS with fast periodic stimulation in fMRI. Cortex 2019, 119:528–542.*This paper reports on patient P.S., a long-studied patient with prosopagnosia. In face-localizer and face-discrimination tasks, the authors found a face-selective network of regions activated in patient P.S. that is similar to those of a comparison group, apart from a lack of activation in the right fusiform face area. This study supports the existence of multiple streams of processing for face information and also reiterates the role of the right fusiform face area in the conscious recognition of faces.
- 18.Chernoff BL, Teghipco A, Garcea FE, Sims MH, Paul DA, Tivarus ME, Smith SO, Pilcher WH, Mahon BZ: A role for the frontal aslant tract in speech planning: A neurosurgical case study. J Cogn Neurosci 2018, 30:752–769. [DOI] [PubMed] [Google Scholar]
- 19.Van der Linden L, Laurence D, De Letter M, Wouter D, de Partz MP, Adrian I, Szmalec A: A case study about the interplay between language control and cognitive abilities in bilingual differential aphasia: Behavioral and brain correlates. J Neurolinguistics 2018, 46:37–68. [Google Scholar]
- 20.Lupo M, Siciliano L, Olivito G, Masciullo M, Bozzali M, Molinari M, Cercignani M, Silveri MC, Leggio M: Non-linear spelling in writing after a pure cerebellar lesion. Neuropsychologia 2019, 132:107143. [DOI] [PubMed] [Google Scholar]
- 21.Grunkina V, Holtz K, Klepzig K, Neubert J, Horn U, Domin M, Hamm AO, Lotze M: The Role of Left Hemispheric Structures for Emotional Processing as a Monitor of Bodily Reaction and Felt Chill – a Case-Control Functional Imaging Study. Front Hum Neurosci 2017, 10:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koropouli E, Melanitis N, Dimitriou VI, Grigoriou A, Karavasilis E, Nikita KS, Tzavellas E, Paparrigopoulos T: New-Onset Psychosis Associated With a Lesion Localized in the Rostral Tectum: Insights Into Pathway-Specific Connectivity Disrupted in Psychosis. Schizophr Bull 2020, 46:1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew S, Zavaliangos-Petropulu A, Jahanshad N, Lang CE, Hayward KS, Lohse KR, Juliano JM, Assogna F, Baugh LA, Bhattacharya AK, et al. : The ENIGMA Stroke Recovery Working Group: Big data neuroimaging to study brain–behavior relationships after stroke. Hum Brain Mapp 2020, doi: 10.1002/hbm.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernhardt J, Borschmann K, Boyd LA, Carmichael ST, Corbett D, Cramer SC, Hoffmann T, Kwakkel G, Savitz SI, Saposnik G, et al. : Moving rehabilitation research forward: Developing consensus statements for rehabilitation and recovery research. Int J Stroke 2016, 11:454–458. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Polimeni JR, Price CM, Edlow BL, McNab JA: Characterizing Signals Within Lesions and Mapping Brain Network Connectivity After Traumatic Axonal Injury: A 7 Tesla Resting-State FMRI Study. Brain Connect 2018, 8:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oedekoven CSH, Keidel JL, Anderson S, Nisbet A, Bird CM: Effects of amnesia on processing in the hippocampus and default mode network during a naturalistic memory task: A case study. Neuropsychologia 2019, 132:107104.* In this study, a participant is described who developed amnesia after a right thalamic stroke. There were no differences in the brain regions that responded to naturalistic 40-second narrative videos between the participant and a comparison group, but the participant did display decreased connectivity between the posterior midline cortex and the hippocampus. In the comparison group, the strength in connectivity while watching video between these regions correlated positively with the recall outside the scanner. This suggests that the interaction between these two regions may be important for encoding and retrieving of naturalistic events.
- 27.Gould L, Kelly ME, Mickleborough MJS, Ekstrand C, Brymer K, Ellchuk T, Borowsky R: Reorganized neural activation in motor cortex following subdural fluid collection: an fMRI and DTI study. Neurocase 2017, 23:292–303. [DOI] [PubMed] [Google Scholar]
- 28.Okerstrom-Jezewski K, Graft A, Denburg NL, Bruss J, Deifelt Streese C, Gratton C, Tranel D: How early damage to dorsomedial prefrontal hub in human brain networks affects long term cognitive, behavioral, and neuroanatomical outcomes. Psychol Neurosci [date unknown], [Google Scholar]
- 29.Burns TG, Semmel ES, Reisner A: A longitudinal evaluation of a penetrating traumatic brain injury: Theories of plasticity and vulnerability. Appl Neuropsychol 2020, doi: 10.1080/23279095.2020.1780239. [DOI] [PubMed] [Google Scholar]
- 30.Crofts A, Kelly ME, Gibson CL: Imaging Functional Recovery Following Ischemic Stroke: Clinical and Preclinical fMRI Studies. J Neuroimaging 2020, 30:5–14.** This review provides a succinct yet thorough description of the functional imaging changes that occur after stroke in humans. It then describes similar findings in animal models, and argues for the inclusion of resting state fMRI in stroke recovery research using animal models to better reflect the clinical findings seen in human studies.
- 31.Puig J, Blasco G, Alberich-Bayarri A, Schlaug G, Deco G, Biarnes C, Navas-Martí M, Rivero M, Gich J, Figueras J, et al. : Resting-state functional connectivity magnetic resonance imaging and outcome after acute stroke. Stroke 2018, 49:2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purcell J, Sebastian R, Leigh R, Jarso S, Davis C, Posner J, Wright A, Hillis AE: Recovery of orthographic processing after stroke: A longitudinal fMRI study. Cortex 2017, 92:103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrad J, Boegle R, Ertl M, Brandt T, Dieterich M: Recovery from Spatial Neglect with Intra- and Transhemispheric Functional Connectivity Changes in Vestibular and Visual Cortex Areas—A Case Study. Front Neurol 2018, 9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basagni B, Errante A, Pinardi C, De Gaetano K, Crisi G, De Tanti A, Fogassi L: Rehabilitation of unilateral spatial neglect: A combined behavioral and fMRI single-case study. Neuropsychology 2019, 33:343–357. [DOI] [PubMed] [Google Scholar]
- 35.Feitosa JA, Stefano Filho CA, Casseb RF, Camargo A, Martins BSG, Ballester BR, Omedas P, Verschure P, Oberg TD, Min LL, et al. : Complex network changes during a virtual reality rehabilitation protocol following stroke: A case study. In International IEEE/EMBS Conference on Neural Engineering, NER.. IEEE Computer Society; 2019:891–894. [Google Scholar]
- 36.Vourvopoulos A, Jorge C, Abreu R, Figueiredo P, Fernandes JC, Bermúdez i Badia S: Efficacy and brain imaging correlates of an immersive motor imagery BCI-driven VR system for upper limb motor rehabilitation: A clinical case report. Front Hum Neurosci 2019, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartwigsen G, Saur D: Neuroimaging of stroke recovery from aphasia – Insights into plasticity of the human language network. Neuroimage 2019, 190:14–31.** This comprehensive review addresses recovery from stroke-induced aphasia. It describes current scholarship regarding the timecourse and reorganization associated with recovery, use of neuroimaging in predicting recovery outcomes, and mechanisms underlying improvements after rehabilitation interventions. This review is notable for its in-depth and thorough treatment of the topic.
- 38.Iorga M, Higgins J, Caplan D, General M, Zinbarg HR, Thompson CK, Rapp B, Parrish TB: Predicting Language Recovery in Post-Stroke Aphasia using Behavior and Functional MRI. [date unknown], doi: 10.21203/rs.3.rs-75485/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Chen Y, Hu R, Yang L, Wang M, Zhang J, Lu H, Wu Y, Du X: RTMS treatments combined with speech training for a conduction aphasia patient. Med (United States) 2017, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haldin C, Acher A, Kauffmann L, Hueber T, Cousin E, Badin P, Perrier P, Fabre D, Perennou D, Detante O, et al. : Speech recovery and language plasticity can be facilitated by Sensori-Motor Fusion training in chronic non-fluent aphasia. A case report study. Clin Linguist Phonetics 2018, 32:595–621. [DOI] [PubMed] [Google Scholar]
- 41.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline JB, Vul E, Yarkoni T: Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017, 18:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner BO, Paul EJ, Miller MB, Barbey AK: Small sample sizes reduce the replicability of task-based fMRI studies. Commun Biol 2018, 1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford JR, Howell DC: Comparing an individual’s test score against norms derived from small samples. Clin Neuropsychol 1998, 12:482–486. [Google Scholar]
- 44.Hulleman J, Humphreys GW: Maximizing the power of comparing single cases against a control sample: An argument, a program for making comparisons, and a worked example from the Pyramids and Palm Trees test. Cogn Neuropsychol 2007, 24:279–291. [DOI] [PubMed] [Google Scholar]
- 45.Little DM, Thulborn KR, Szlyk JP: An fMRI study of saccadic and smooth-pursuit eye movement control in patients with age-related macular degeneration. Investig Ophthalmol Vis Sci 2008, 49:1728–1735. [DOI] [PubMed] [Google Scholar]
- 46.Price CJ, Crinion J, Friston KJ: Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging 2006, 23:816–826. [DOI] [PubMed] [Google Scholar]
- 47.Bollmann S, Puckett AM, Cunnington R, Barth M: Serial correlations in single-subject fMRI with sub-second TR. Neuroimage 2018, 166:152–166. [DOI] [PubMed] [Google Scholar]
- 48.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE: Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caramazza A: On drawing inferences about the structure of normal cognitive systems from the analysis of patterns of impaired performance: The case for single-patient studies. Brain Cogn 1986, 5:41–66. [DOI] [PubMed] [Google Scholar]
- 50.Zurif EB, Gardner H, Brownell HH: The case against the case against group studies. Brain Cogn 1989, 10:237–255. [DOI] [PubMed] [Google Scholar]