Abstract
Purpose of review
The burden of heart failure (HF) is a significant national and global public health problem, with prevalence rates on the rise. Given the significant morbidity, mortality, and healthcare costs attributable to HF, it is of utmost importance to utilize preventive strategies to prevent the development of HF. Therefore, we sought to address how a multi-modal risk assessment approach can be used to stratify patients for HF risk and guide implementation of therapeutic strategies to prevent HF.
Recent findings
New externally validated, multivariate prediction models for incident HF can be applied in the general population and may be used to aide clinicians in assessing individualized HF risk and screening for HF. Recent clinical trial data suggest a natriuretic peptide biomarker-based screening approach coupled with team-based cardiovascular care to focus on optimization of guideline-directed medical therapy may help prevent new-onset HF. However, widespread implementation of clinical risk scores and/or biomarkers is needed.
Summary
In addition to promoting a heart healthy lifestyle, prevention and management of modifiable risk factors, including intensive blood pressure lowering and use of sodium-glucose cotransporter-2 inhibitors, can prevent incident HF.
Keywords: Preention, Heart failure, Hypertension, Diabetes
Introduction
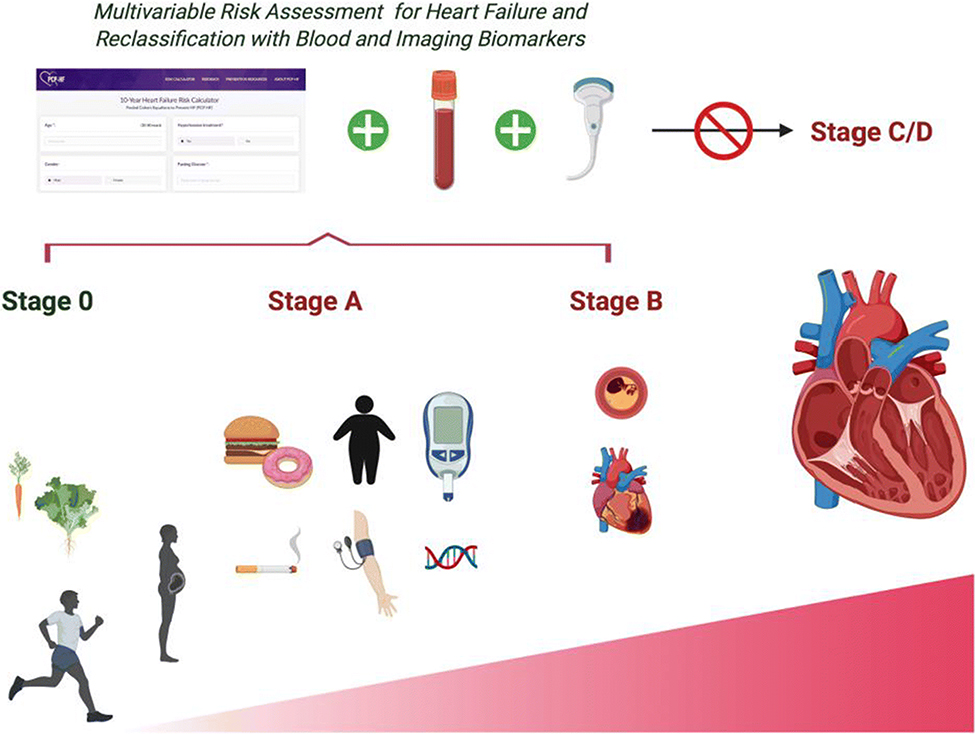
An estimated 6 million Americans have heart failure (HF), with prevalence rates on the rise [1]. By 2030, projections estimate the prevalence of HF will increase by 46%, resulting in greater than 8 million adults with HF [2]. HF is a global public health problem as well, with an estimated 26 million cases of HF worldwide [3]. The overall lifetime risk for heart failure is estimated to range between 20 and 45% [1]. The economic impact of HF in the United States (US) is significant, with an estimated total cost for HF of $30 billion and is projected to increase to an estimated $70 billion by 2030 [1]. As outlined by national practice guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA), screening is essential for early identification of those with HF or at increased risk for HF, as evidence supports that the onset of HF can be delayed or prevented by targeting modifiable risk factors [4, 5]. However several barriers to screening exist, including poor understanding of HF among the general population and lack of widespread adoption of a HF risk assessment and screening protocol [5]. In contrast with coronary artery disease, incorporation of a risk-based assessment for heart failure at an early stage is not part of routine clinical practice. Externally validated, multivariate prediction models for incident heart failure that can be applied in the general population may be used to aide clinicians in screening for HF and assessing individualized HF risk. Furthermore there are emerging strategies for the primary prevention of heart failure, including natriuretic peptide screening protocols, evidence-based intensive blood pressure lowering, and utilization of novel therapies, such as sodium-glucose cotransporter-2 (SGLT2) inhibitors. This review will discuss how a multi-modal risk assessment approach can be used to stratify patients for HF risk to then guide implementation of therapeutic strategies to prevent HF (Fig. 1).
Fig 1.
Multi-modal risk assessment approach to estimate and reclassify heart failure risk in the general population based on known risk factors within the context of the American College of Cardiology and American Heart Association heart failure staging construct.
Risk assessment of and screening for heart failure
Assessment for modifiable heart failure risk factors
HF is largely attributable to cumulative exposure to modifiable risk factors over the life course. The ACC/AHA guidelines define 4 stages as a progression of those at risk for HF (Stage A and B), which may result in an asymptomatic period that may last years to decades. Progression to symptomatic HF is labeled Stage C and advanced end-stage HF, D [4]. Given increases in mortality with transition from Stage A to Stage B and Stage B to Stage C, intervention to prevent progression to symptomatic heart failure is critical.
The AHA defines ideals cardiovascular health (CVH) as both ideal health behaviors (nonsmoking, body mass index < 25 kg/m2, physical activity at goal levels, and pursuit of a diet consistent with current guideline recommendations) and ideal health factors (untreated blood pressure < 120/< 80 mmHg, fasting blood glucose < 100 mg/dL, and untreated total cholesterol < 200 mg/dL). However, the prevelance of ideal cardiovascular in the US is very low [6, 7]. Traditional risk factors for HF are common, with up to one-third of the US adult population having at least one HF risk factor, including hypertension, elevated body mass index (BMI), physical inactivity, diabetes mellitus (DM), tobacco use, and coronary artery disease (CAD) [8]. When approaching HF risk assessment, it is essential to characterize CVH and assess for these modifiable HF risk factors.
Hypertension is highly prevalent and one of the most important modifiable risk factors for HF [9]. The Framingham Heart Study (FHS) showed that hypertension antedated development of HF in 91% of patients. The risk of developing HF in hypertensive patients in comparison with normotensive individuals was almost 2-fold greater in men and 3-fold greater in women [10]. Neurohormonal blockade with anti-hypertensive agents that block the renin-angiotensin-aldosterone system (RAAS) in particular may be an appealing strategy for risk reduction of HF compared with other blood pressure lowering agents. However, robust data supports the greatest impact on prevention results from the magnitude of BP lowering, independent of anti-hypertensive class. Similarly, DM increases the relative risk of HF by almost 2-fold in men and 4-fold in women [11,12,13,14]. Furthermore, poor glycemic control independently increases the risk of HF; with each 1% increase in hemoglobin A1c (HbA1c), there is an 8–36% increased risk of incident HF [15,16,17,18].
The relationship both of elevated BMI and physical inactivity with an increased risk of HF is also well-established [19,20,21,22,23]. Those with an elevated BMI have an earlier onset of HF than those with a normal BMI [19,20,21]. In the FHS, each increment of 1 kg/m2 in BMI was associated with an increased risk of HF by 5% for men and 7% for women [23]. Furthermore, increasing BMI has been shown to have a dose-response linear relationship with HF with preserved ejection fraction (EF) [19]. Analogously, physical inactivity has an inverse dose-response relationship with HF risk [22].
Cigarette smoking increases the risk of incident HF, independent of coronary artery disease (CAD) status [24, 25]. Cigarette smoking has been linked with oxidative stress and deleterious inflammatory effects on the cardiovascular system [26, 27]. Additionally, CAD is a well-described risk factor for HF and shares a pathophysiologic relationship with the aforementioned risk factors in addition to dyslipidemia [28].
Heart failure risk assessment models
Several multi-variable models exist to estimate future risk for HF. Ideally these models allow for individualized HF risk assessments in patients at risk for HF (stage A) and to better identify those with asymptomatic structural heart disease (stage B) to implement targeted prevention strategies. These tools also play an essential role in investigational strategies for HF prevention.
The Health, Aging and Body Composition (Health ABC) Heart Failure Risk score is an externally validated risk prediction model for incident risk of HF in the elderly. This 9-variable model was developed in a cohort of 2935 people aged 70–79 years participating in the Health ABC Study in the US. The scoring system classifies patients into four groups of 5-year HF risk (5%, 5–10%, 10–20%, 20%) using the following variables: age, history of CAD, history of smoking, systolic blood pressure, heart rate, serum glucose, serum creatinine, serum albumin, and electrocardiographic left ventricular hypertrophy (LVH). While this model had good calibration (χ2 6.24, p = 0.621) and discrimination performance (C index of 0.73) across sex and race overall, the risk was underestimated in white men [29, 30].
The Heart “OMics” in AGEing (HOMAGE) study developed a 5 year incident HF risk prediction model in the elderly using a meta-analysis of 10,236 people from three cohorts (Health ABC Study, Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiaco (PREDICTOR), and Prospective Study of Pravastatin in the Elderly at Risk (PROSPER)). The PREDICTOR trial included a population-based registry of 2001 people over the age of 65 years in four Italian cities. The PROSPER trial cohort included 5804 people over age 70 years with a history of vascular disease or high risk of developing vascular disease in Scotland, Ireland, and The Netherlands. The HOMAGE risk prediction model was externally validated using the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), including a cohort of 13,121 high-risk hypertensive patients aged 40 to 80 years, in Scandinavian countries, the United Kingdom, and Ireland [31,32,33]. This study validated 10 variables to predict a 5-year risk of incident heart failure hospitalization (including age, sex, BMI, smoking, diabetes mellitus, history of CAD, antihypertensive medication use, systolic blood pressure, heart rate, and serum creatinine), and showed good discrimination (C index 0.71) and calibration (χ2 7.9, p = 0.54) [33].
Limitations of the Health ABC and HOMAGE models include non-generalizability to middle-aged and young adults. While several other multi-variable models exist to estimate future risk for heart failure (Table 1), incorporation of these models into clinical practice has not been adopted given these prior models are derived from non-contemporary cohorts with limited ethnic diversity and lack of extensive external validation in a population without prevalent cardiovascular disease [34, 35•].
Table 1.
Characteristics, Risk Factors, and Endpoints of HF Risk Scores
| Risk score information | Risk factors/covariates/endpoint | ||||||||||||
| Risk equation | Cohorts and countries | Participants’ race/ethnic Background | Participants’ Hx of CVD | Participant age range | Data collection years | Risk window (years) | Age | Sex | Race | Hx of CHD | SBP | BP Tx | |
| PCP-HF | FOF, CHS, ARIC, CARDIA, MESA | European-American, African-American | Free of CHD and stroke at baseline | 30–80 years | 1985–2002 | 10 | X | X | X | X | X | ||
| WATCH-DM | ACCORD, ALLHAT USA | European-American, Hispanic, African-American, | Included participants with hx of CHD | 40–79 years | 2001, 2003–2005 | 5 | X | X | X | ||||
| TIMI - HF | SAVOR-TIMI 53, DECLARE-TIMI 58 | European, European-American, African-American, Hispanic | Included participants with hx of CHD | Over age 40 years | 2010–2011 | 2.1 | x | ||||||
| Risk score information | Risk factors/covariates/endpoint | ||||||||||||
| Risk equation | Total Chol | LDL Chol | HDL Chol | DM | Fasting glucose | DM Tx | Smoking | BMI | Heart Rate | ECG | Biomarkers (e.g., albumin, BNP) | Endpoint | |
| PCP-HF | X | X | X | X | X | X | X (QRS) | HF | |||||
| WATCH-DM | X | X | X | X (QRS) | HF | ||||||||
| TIMI - HF | |||||||||||||
The Pooled Cohort equations to Prevent Heart Failure (PCP-HF) is a race- and sex-specific 10-year risk equation for developing incident HF. This contemporary analysis utilized 33,010 people, aged 30 to 79 years from pooled individual-level data from 7 population-based cohorts with long-term follow-up. These included 5 large, racially and geographically diverse, National Heart, Lung, and Blood Institute–sponsored cohort studies for derivation, including the ARIC (Atherosclerosis Risk In Communities) study, CARDIA (Coronary Artery Risk Development in Young Adults) study, CHS (Cardiovascular Health Study), FOF (Framingham Heart Study Offspring Cohort), and MESA (Multi-Ethnic Study of Atherosclerosis). The study design for these cohorts has been previously described [36,37,38,39,40,41,42]. Age, BMI, systolic blood pressure, fasting blood glucose levels, cholesterol levels, smoking status, and QRS duration were identified as predictors of the risk of developing HF over 10 years. A web-based tool exists of this prediction model (available at pcphf.org). This model was further externally validated in the Prevention of Renal and Vascular End-stage Disease cohort and Jackson Heart Study where it demonstrated good-to-excellent discrimation (C index ranging from 0.71 to 0.88 in white and black men and women), and strong calibration (χ2 < 20 for all sex-race groups). Overall, the PCP-HF tool has excellent discrimination and calibration among a diverse group of US and European adults enhancing its broad generalizability and may be a valuable tool in risk assessment of HF on both an individual and population-based level [35•].
To focus on participants with diabetes who are at heightened risk, the WATCH-DM risk score (Weight [BMI], Age, hyperTension, Creatinine, HDL-C, Diabetes control [fasting plasma glucose], QRS Duration, MI, and CABG) leveraged machine learning to develop a ten clinical variable score to predict risk of incident HF hospitalization using clinical trial data. This risk score was developed using a cohort 8756 participants from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, and was externally validated in a cohort of individuals with diabetes mellitus in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). The study design for these trials has been previously described [43, 44]. This risk score utilizes ten variables routinely obtained for patients with DM including: age, BMI, systolic and diastolic blood pressure, fasting plasma glucose, high-density lipoprotein level, serum creatinine, QRS duration, and history of prior myocardial infarction or CABG. The WATCH-DM Risk score provides a 5-year risk assessment for incident heart failure in patients with diabetes and also provides an integer-based risk score (very low, low, average, high, or very high risk) for ease of integration in clinical use, with good discrimination (C index 0.72) and calibration (χ2 10.58, p = 0.23) [45•].
The TIMI Risk Score for Heart Failure in Diabetes (TRS-HF DM) is an externally validated risk score to predict hospitalization for heart failure (HHF) in patients with DM. This tool was derived using 8212 patients from the placebo arm of the SAVOR-TIMI 53 trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) and externally validated in the placebo-treated patients in the DECLARE-TIMI 58 trial (Dapagliflozin Effect on Cardiovascular Events– Thrombolysis in Myocardial Infarction 58). The study design of these trials has been previously described [46, 47]. Five clinical variables were indentified to predict the risk of HHF, including prior heart failure, history of atrial fibrillation, coronary artery disease, estimated glomerular filtration rate, and urine albumin-to-creatinine ratio. The TRS-HF DM score provides an integer-based for HHF (low, intermediate, high, and very high risk) with good discrimination (C index 0.78 to 0.81) and calibration (χ2 4.64, p = 0.20) [47].
Biomarker screening
The current ACC/AHA HF Guidelines list a class IIa recommendation to obtain a screening natriuretic peptide in those at risk for HF [4]. This recommendation was based on evidence from the St Vincent’s Screening to Prevent Heart Failure (STOP-HF) and PONTIAC trials, which showed natriuretic peptide screening paired with early intervention prevents the development of HF. The STOP-HF Trial was a single-center, unblinded study of 1374 people at risk for HF in Ireland (with hypertension, diabetes mellitus, or known vascular disease) without know structural heart disease or symptoms of HF who were randomized to B-type natriuretic peptide (BNP) screening or standard of care. Groups in the intervention arm with a BNP level > 50 pg/ml underwent echocardiography and referral to a team-based specialist cardiovascular service. All patients received coaching by a specialist nurse who emphasized individual risk status and the importance of adherence to medication and healthy lifestyle behaviors. The BNP-based screening group had reduction in the primary endpoint of asymptomatic LV dysfunction with or without newly diagnosed heart failure [48•]. In PONTIAC, 300 patients with diabetes mellitus type 2 without a history of cardiac disease and a N-terminal pro b-type natriuretic peptide (NTproBNP) > 125 pg/ml were randomized to individualized visits for the up-titration of renin-angiotensin system (RAS) antagonists and beta-blockers versus usual care. The treatment group had a reduction in the composite endpoint of hospitalization or death due to cardiac disease [49].
Electrocardiography (ECG) screening
Abnormal baseline ECG abnormalities have been shown to be predictive of future HF events. In a middle-aged adult cohort without cardiovascular disease (MESA), a QRS duration greater than 100 ms was significantly associated with incident HF suggesting that ECG abnormalities may identify left ventricular structural changes in asymptomatic patients [50]. An analysis of 2915 older adults participating in the Health ABC study showed baseline and new ECG abnormalities are independently associated with increased risk of incident HF after adjusting for clinical HF risk factors. Minor abnormalities minor were ST segment or T-wave abnormalities. Criteria for major ECG abnormalities included Q-QS wave abnormalities, major ST-T abnormalities, LVH, atrial fibrillation or atrial flutter, Wolff-Parkinson-White, complete bundle-branch block, or intraventricular block. The risk of heart failure increased significantly with the increasing severity of ECG abnormalities [51]. While increased QRS duration has been incorporated into contemporary pooled-cohort heart failure risk calculators, identification of more severe ECG abnormalities may prompt consideration of assessment with an echocardiogram to evaluate for asymptomatic structural heart disease.
Echocardiogram screening
An abnormal ECG or elevated natriuretic peptide level should prompt consideration for an echocardiogram to evaluate for asymptomatic left ventricular dysfunction. While evidence to support community-wide echocardiographic screening for asymptomatic left ventricular dysfunction is inadequate [52, 53], some evidence suggests a screening echocardiogram may benefit individuals at increased risk for HF.
In a community-based screening study in 438 asymptomatic adults over age 65 with at least one HF risk factor used clinical characteristics and functional capacity, followed by ECG abnormalities to define low- and high-risk groups for new HF. Those identified to be high risk for HF were more likely to have an abnormal echocardiographic findings, and therefore may potentially benefit from cardioprotective medications [54]. Furthermore, a screening echocardiogram protocol in 5227 adults over age 60 recruited from primary care clinics found increased detection of both Stage B and Stage C HF, with subsequent increase in use of cardioprotective medications (including ace-inhibitors, beta-blockers) in these groups [55]. However, data supporting that screening echocardiographic assessments improve HF and cardiovascular outcomes is lacking.
Assessment for heart failure risk enhancers
One limitation of relying wholly on multi-variable HF risk assessment tools is lack of inclusion of some known risk enhancers for HF in these tools. It is important to consider these risk-enhancing comorbidities to personalize HF risk screening assessment, as these risk prediction tools may underestimate risk. In addition to traditional risk factors, conditions to consider include adverse pregnancy complications, systemic inflammatory conditions, atrial fibrillation, history of treatment for cancer, chronic kidney disease, liver disease, and sleep disorders.
Complications of pregnancy including pre-eclampsia, gestational hypertension, gestational diabetes, and premature delivery portend an increased risk of future heart failure [56, 57]. Pre-eclampsia has been linked with a four-fold increased risk of incident heart failure [58•]. Systemic inflammatory conditions including connective tissue disorders and human immunodeficiency virus (HIV) are also associated with an increased risk for HF patients with rheumatoid arthritis (RA) are estimated to be at twice the increased risk of incident HF [59, 60]. RA enhances the risk of HF from both ischemic and non-ischemic causes, with associations between increased disease activity and incident non-ischemic HF [61]. Patients with lupus are at an increased risk of HF, attributed to accelerated atherosclerosis and higher cardiovascular risk factor disease burden (i.e., hypertension) in comparison with those without lupus [60, 62, 63]. HIV is an independently associated risk factor for heart failure [64]. In comparing people living with HIV to non-HIV infected controls, the reported relative risk of heart failure and diastolic dysfunction in those with HIV was 1.7 and 3.0, respectively [65]. Atrial fibrillation (AF) is a known risk factor for heart failure, with some reports of a 3-to-4 times higher incidence of heart failure in those who have AF in comparison with those without AF [66,67,68,69]. Furthermore, permanent AF has been shown to portend higher risk of incident HF in comparison with paroxysmal AF [70]. The inter-relationship between AF and HF is complex, particularly given these conditions often co-exist with shared risk factors and pathophysiology.
Cancer treatments including anthracyclines, human epidermal growth factor receptor 2 antagonists and chest radiation portended a risk for both early-onset and late-onset HF [71,72,73,74]. Other disease states linked with increased incidence of HF include chronic kidney disease [75,76,77], non-alcoholic fatty liver disease [78], and obstructive sleep apnea [79]. An important component of a HF risk assessment is family history. Obtaining a 3-generation family history of HF is recommended [80]. If the family history is concerning for a genetic cardiomyopathy, the patient’s individual HF risk should not be assessed only using pooled cohort data. Instead, consider referral to genetic counseling, genetic testing, and further cardiac testing [80, 81].
Treatments aimed at heart failure prevention
Once a HF risk assessment is complete, risk stratification should help drive the management for that patient. In all patients, including those without HF risk factors, the cornerstone of the discussion should be counseling on the importance of a heart healthy lifestyle to optimize cardiovascular health to reduce the lifetime risk of incident HF. In those who are at increased risk for HF, additional preventive strategies should be targeted at optimizing modifiable risk factors.
Lifestyle
The AHA has defined ideal cardiovascular health based on seven metrics that compose Life’s Simple 7, which include not smoking, maintaining an optimal BMI, physical activity at goal levels, healthy diet, optimize cholesterol, reduce blood glucose levels, and control blood pressure [73]. Greater adherence to Life’s Simple 7 has been associated with a lower lifetime risk of heart failure and greater preservation of cardiac structure and function [82, 83].
Evidence suggests adherence to a Mediterranean or DASH-type (Dietary Approaches to Stop Hypertension) diet may protect against new onset of HF [84, 85•]. Data from a large, diverse, and contemporary US population without known CAD found adherence to a plant-based dietary pattern was associated with a 41% lower risk of incident HF hospitalization; in contrast, a southern-based diet was associated with a 72% higher risk of HF hospitalization [86].
Increased physical activity is associated with lower incidence of HF. Data from the Physicians Healthy Study [87] and the Women’s Health initiative [88], both large-cohort community-based studies demonstrated relative high levels of physical activity are associated with a lower risk of HF [89]. A prospective study of 137,303 women aged 50 to 79 years showed that higher levels of self-reported total recreational activity and higher levels of walking are associated with a lower risk of developing HF [90]. Furthermore, data from the California Men’s Health Study, a cohort of 82,695 men over age 45 years without HF followed for 10 years showed increased physical activity and lower sedentary time were associated with lower HF risk [91].
Obesity prevention and management
In patients with obesity, weight loss has been shown to reduce the incidence of HF. Clinicians should integrate weight loss treatment in patient’s care plans, including lifestyle, pharmacologic, and surgical weight loss strategies. In a study of nearly 40,000 obese people without heart failure in a Scandinavian registry, gastric bypass surgery was associated with approximately half the incidence of HF in comparison with intensive lifestyle treatment. These effects were largely mediated by weight loss, with a 22.6 kg more weight loss in the surgery group at 2 years, and appeared to be partially mediated by effects of treating atrial fibrillation, diabetes mellitus, and hypertension [92].
While overweight and obesity are associated with greater incidence of HF, the findings of an “obesity parodox” in patients with HF, whereby the association of obesity is associated with longer survival, has raised concern about intentional weight loss following disease onset. It is important to clarify the other potential drivers of lower BMI that may occur due to frailty, increased catabolic state, or lack of metabolic reserve resulting in weight loss. Therefore, it is not that obesity is protective, but the severity of illness may contribute to the so-called “obesity paradox” [21, 93,94,95]. Ultimately, focus on maintenance of a normal body weight would allow for promotion of CVH and promotion of a healthier, longer life free of HF.
Hypertension management
Controlling hypertension is associated with a lower risk of incident HF [8]. Current ACC/AHA guidelines recommend targeting a blood pressure of < 130/80 mmHg in patients at increased risk of HF (stage A) [10]. Secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated targeting a systolic blood pressure of less than 120 mmHg is associated with reduced incidence of HF. In this population of patients with hypertension at increased risk of cardiovascular disease but without diabetes, intensive blood pressure lowering by targeting a systolic blood pressure of < 120 mmHg in comparison with targeting < 140 mmHg was associated with a significant reduction in new HF. However the rates of adverse events (AF) were higher in the intensive blood pressure group, including hypotension, syncope, electrolyte abnormalities and acute kidney injury [96, 97, 98•]. One approach to better inform who would benefit from more intensive blood pressure lowering would be the use of risk stratification using multivariate risk modeling. A recent post hoc analysis using SPRINT data applied the PCP-HF prediction model to stratify the SPRINT cohort into three tertiles of 10-year incident HF risk (low, intermediate, or high). In the high predicted HF risk group, there was a significant reduction in incident HF in the intensive BP lowering arm. However, this benefit of an intensive blood pressure target was not observed within low and intermediate heart failure risk group. In the intensive versus standard BP treatment arms, serious adverse events occurred in 40.9 vs. 38.7% in the high predicted HF risk group, respectively. This data suggests intensive BP lowering for HF prevention is most beneficial to individuals at high predicted risk of HF and minimizes unnecessary AE in those individuals at lower risk of HF [99].
Glucose-lowering therapy
Currently, limited data are available to guide targeted hemoglobin A1c goals in patients with type-2 diabetes mellitus to reduce the incidence of HF [100]. Sodium-glucose cotransporter-2 (SGLT2) inhibitors are the first class of glucose-lowering therapies that have been shown to reduce the risk of HF hospitalization in patients with DM [47, 100,101,102]. SGLT2 inhibitors have been shown to have a similar relative risk reduction of hospitalization for heart failure (HHF) across a wide spectrum of baseline cardiovascular risk [48, 103]. Applying the Health ABC HF Risk Score to stratify patients with DM and without HF by baseline 5-year HF risk demonstrated the use of a SGLT2 inhibitor reduced cardiovascular mortality and HF hospitalizations in patients at both high and lower risk of HF [104]. Futhermore, utilizing the TRS-HF DM to assess baseline risk for HHF in patients with DM, those with the highest baseline risk for HHF derive the greatest absolute risk reduction of HHF from SGLT2 inhibitors [48•].
Notably, the majority of patients in cardiovascular outcome trials for SLGT2 inhibitors did not have HF at baseline. Therefore, the reductions in heart failure events provide compelling evidence for the benefit of SLGT2 inhibitors in the primary prevention of incident HF among patients with diabetes, irrespective of glycemic control [103]. The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) Trial randomized patients with NYHA Class II-IV HF with an EF < 40% HFrEF to dapaglifozin or placebo, in those with and without T2DM. The risk of worsening HF or death from cardiovascular causes was lower in those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes [105]. These data suggest SGLT2 inhibitors may have a therapeutic role to prevent incident HF in both those with and without diabetes.
Conclusion
Externally validated, multivariate risk assessment of HF may be incorporated into routine clinical practice for personalized risk assessment and targeted prevention strategies. Using a step-wise approach, once a patient is identified at increased risk, practitioners may consider a biomarker assessment (i.e., natriuretic peptide) or cardiac testing (i.e., echocardiogram). Furthermore, data suggests a natriuretic peptide biomarker-based screening coupled with optimization of guideline-directed medical therapy can help prevent new-onset HF. Utilization of these tools may improve identification of those (a) with stage B HF to implement guideline-directed medical therapy or (b) moderate-to-high HF risk individuals who would benefit from targeted primary prevention strategies (i.e., lower blood pressure targets or, initiation of SGLT2 inhibitors, or neurohormonal blockade). Further studies are need to develop a cost-effective strategy for further cardiac testing in those identified at increased risk of HF. Despite the promising accumulating data on risk prediction for HF, categorizing HF as a single entity is an important limitation that must be acknowledged. HF is a heterogenous syndrome with different subtypes that may require consideration of different predictors and further personalization with improved phenotyping of HF risk.
In the future, more widespread utilization of genetic testing may be incorporated for a more precision medicine-focused approach to HF risk assessment. Advances in knowledge about the genetic impact on HF risk are growing rapidly [106•]. Increased accessibility to and reduced cost of an individual’s genomics data may lead to identification of new risk-enhancing genes in asymptomatic patients. For example, truncating variants in the Titin gene (TTNtvs), a known pathologic variant in familial and sporadic dilated cardiomyopathies [107], have been recently associated with risk for several other non-ischemic cardiomyopathies, including peripartum cardiomyopathy [108], alcohol-induced cardiomyoatphy [109], and cancer therapy–induced cardiomyopathy [110]. In the general population, in comparsion with noncarriers, asymptomic carriers of TTNtvs are at a substantially increased relative risk of having a subnormal ejection fraction and developing HF in the future [111]. However, not all carriers of TTNtvs go on to develop HF; therefore, further data is needed understand the determinants of disease expression to guide HF preventation strategies in asymptomatic TTNtvs carriers [111, 112]. In addition to genomics, other rapidly expanding HF “omics” data (i.e., metabolomics, pharmacogenomics, epigenomics, proteomics, microbiomics) are promising to enhance understanding of HF pathophysiology, characterization of HF sub-phenotypes, and development novel HF therapeutics, but the additional clinical predictive value of this data is not yet clear [113]. These data will need to be combined with current models for a more personalized risk assessment and consideration of therapeutic strategies.
Furthermore, novel systems of care approach for HF risk assessment and screening are needed to allow more widespread delivery of effective, efficient strategies focused on HF prevention. Get With the Guidelines – Heart Failure (GWG-HF) is an voluntary, hospital-based quality initiative to improve adherence to guideline driven in-hospital treatment of heart failure. Data has shown since implementation of GWG-HF, hospitals participating in the GWG-HF program have had improvements in their processes of care for patients with HF [114]. Further development of systems of care–based programs targeted at heart failure prevention are urgently needed to improve implementation of HF prevention in clinical practice.
Ultimately, the most important way to prevent HF is to promote a heart healthy lifestyle from birth to older adulthood. A comprehensive patient-centered lifecourse approach that accounts for maintenance of optimal CVH (including healthy diet, 150 min of moderate to vigorous physical activity, avoidance of tobacco exposure, maintenance of a normal BMI) is needed to reduce individual-level and population-level morbidity and mortality related to HF [115].
Acknowledgments
Funding Information
This work is supported by grants from the American Heart Association (no. 19TPA34890060) and the National Institutes of Health/National Heart, Lung, and Blood Institute (KL2TR001424) to Dr. Khan.
Footnotes
Conflict of Interest
Lua A. Jafari declares that she has no conflict of interest. Rachel M. Suen declares that she has no conflict of interest. Sadiya S. Khan declares that she has no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sponsor did not contribute to design and conduct of the study, collection, management, analysis, or interpretation of the data or preparation, review, or approval of the manuscript. The authors take responsibility for decision to submit the manuscript for publication.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–19. 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123–33. 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira JP, Kraus S, Mitchell S, Perel P, Pineiro D, Chioncel O, et al. World Heart Federation Roadmap for Heart Failure. Glob Heart. 2019;14(3):197–214. 10.1016/j.gheart.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Li C, Zhao G, Pearson WS, Capewell S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation. 2009;120(13):1181–8. 10.1161/CIRCULATIONAHA.108.835728. [DOI] [PubMed] [Google Scholar]
- 8.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic followup study. Arch Intern Med. 2001;161(7):996–1002. 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287(8):1003–10. 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–62. [PubMed] [Google Scholar]
- 11.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, et al. Predictors of heart failure among women with coronary disease. Circulation. 2004;110(11):1424–30. 10.1161/01.CIR.0000141726.01302.83. [DOI] [PubMed] [Google Scholar]
- 12.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 13.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–9. 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–8. 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 15.Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103(22):2668–73. 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 16.Pazin-Filho A, Kottgen A, Bertoni AG, Russell SD, Selvin E, Rosamond WD, et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2008;51(12):2197–204. 10.1007/s00125-008-1164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care. 2010;33(9):2084–9. 10.2337/dc10-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol. 2017;69(9):1129–42. 10.1016/j.jacc.2016.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen K, Mariosa D, Adami HO, Held C, Ingelsson E, Lagerros YT, et al. Dose-response relationship of total and leisure time physical activity to risk of heart failure: a prospective cohort study. Circ Heart Fail. 2014;7(5):701–8. 10.1161/CIRCHEARTFAILURE.113.001010. [DOI] [PubMed] [Google Scholar]
- 21.Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018;3(4):280–7. 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, et al. Dose-Response Relationship Between Physical Activity and Risk of Heart Failure: A Meta-Analysis. Circulation. 2015;132(19):1786–94. 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 23.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–13. 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura D, Cain LR, Mentz RJ, White WB, Blaha MJ, DeFilippis AP, et al. Cigarette Smoking and Incident Heart Failure: Insights From the Jackson Heart Study. Circulation. 2018;137(24):2572–82. 10.1161/CIRCULATIONAHA.117.031912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson M, Dardari Z, Kianoush S, Hall ME, DeFilippis AP, Keith RJ, et al. Relation Between Cigarette Smoking and Heart Failure (from the Multiethnic Study of Atherosclerosis). Am J Cardiol. 2019;123(12):1972–7. 10.1016/j.amjcard.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J Am Coll Cardiol. 2017;70(2):230–51. 10.1016/j.jacc.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, et al. Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes: Clinical Perspectives From the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J Am Coll Cardiol. 2015;66(12):1378–91. 10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Ziaeian B, Fonarow GC. Epidemiology and etiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–78. 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1(2):125–33. 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, et al. Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circ Heart Fail. 2010;3(4):495–502. 10.1161/CIRCHEARTFAILURE.109.904300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mureddu GF, Tarantini L, Agabiti N, Faggiano P, Masson S, Latini R, et al. Evaluation of different strategies for identifying asymptomatic left ventricular dysfunction and pre-clinical (stage B) heart failure in the elderly. Results from ‘PREDICTOR’, a population based-study in central Italy. Eur J Heart Fail. 2013;15(10):1102–12. 10.1093/eurjhf/hft098. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomized controlled trial. Lancet. 2002;360(9346):1623–30. 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang WY, Zhang ZY, et al. Risk for Incident Heart Failure: A Subject-Level Meta-Analysis From the Heart “OMics” in Aging (HOMAGE) Study. J Am Heart Assoc. 2017;6(5). 10.1161/JAHA.116.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Echouffo-Tcheugui JB, Greene SJ, Papadimitriou L, Zannad F, Yancy CW, Gheorghiade M, et al. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail. 2015;8(3):438–47. 10.1161/CIRCHEARTFAILURE.114.001896. [DOI] [PubMed] [Google Scholar]
- 35.Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, et al. 10-Year Risk Equations for Incident Heart Failure in the General Population. J Am Coll Cardiol. 2019;73(19):2388–97. 10.1016/j.jacc.2019.02.057• This externally validated, pooled cohort equation is a tool to aide clinicians to estimate an individual’s 10-year risk for heart failure and helps identify those at highest risk who may benefit from more intensive prevention strategies.
- 36.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 37.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 38.Diercks GF, Janssen WM, van Boven AJ, Bak AA, de Jong PE, Crijns HJ, et al. Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular ENdstage Disease Intervention Trial [PREVEND IT]). Am J Cardiol. 2000;86(6):635–8. 10.1016/s0002-9149(00)01042-0. [DOI] [PubMed] [Google Scholar]
- 39.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. [DOI] [PubMed] [Google Scholar]
- 40.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–16. 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 41.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 42.Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–4-17. [PubMed] [Google Scholar]
- 43.Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, et al. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118(22):2259–67. 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segar MW, Vaduganathan M, Patel KV, McGuire DK, Butler J, Fonarow GC, et al. Machine Learning to Predict the Risk of Incident Heart Failure Hospitalization Among Patients With Diabetes: The WATCH-DM Risk Score. Diabetes Care. 2019. 10.2337/dc19-0587•This study utilized machine learning to develop the WATCH-DM Risk score, which is a tool that provides a 5-year risk assessment for incident heart failure in patients with diabetes.
- 46.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 47.Berg DD, Wiviott SD, Scirica BM, Gurmu Y, Mosenzon O, Murphy SA, et al. Heart Failure Risk Stratification and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Type 2 Diabetes Mellitus. Circulation. 2019. 10.1161/CIRCULATIONAHA.119.042685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, et al. Natriuretic peptide based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66–74. 10.1001/jama.2013.7588• This study created a baseline risk assessment tool for hospitalization for heart failure (HHF) among patients with DM. It demonstrated that patients derive benefit from SGLT2-inhibitors to prevent HHF regardless of their baseline risk, and that those with the highest baseline risk derived the greatest absolute reduction of HHF.
- 49.Huelsmann M, Neuhold S, Resl M, Strunk G, Brath H, Francesconi C, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365–72. 10.1016/j.jacc.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 50.Ilkhanoff L, Liu K, Ning H, Nazarian S, Bluemke DA, Soliman EZ, et al. Association of QRS duration with left ventricular structure and function and risk of heart failure in middle-aged and older adults: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur J Heart Fail. 2012;14(11):1285–92. 10.1093/eurjhf/hfs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gencer B, Butler J, Bauer DC, Auer R, Kalogeropoulos A, Marques-Vidal P, et al. Association of electrocardiogram abnormalities and incident heart failure events. Am Heart J. 2014;167(6):869–75 e3. 10.1016/j.ahj.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138(11):907–16. 10.7326/0003-4819-138-11-200,306,030-00012. [DOI] [PubMed] [Google Scholar]
- 53.Lindekleiv H, Lochen ML, Mathiesen EB, Njolstad I, Wilsgaard T, Schirmer H. Echocardiographic screening of the general population and long-term survival: a randomized clinical study. JAMA Intern Med. 2013;173(17):1592–8. 10.1001/jamainternmed.2013.8412. [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Wang Y, Nolan M, Negishi K, Okin PM, Marwick TH. Community Screening for Nonischemic Cardiomyopathy in Asymptomatic Subjects 9/=65 Years With Stage B Heart Failure. Am J Cardiol. 2016;117(12):1959–65. 10.1016/j.amjcard.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 55.Ghany R, Palacio A, Chen G, Dawkins E, Ghany A, Forbes E, et al. A screening echocardiogram to identify diastolic dysfunction leads to better outcomes. Echocardiography. 2017;34(8):1152–8. 10.1111/echo.13615. [DOI] [PubMed] [Google Scholar]
- 56.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–80. 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daubert MA, Douglas PS. Primary Prevention of Heart Failure in Women. JACC Heart Fail. 2019;7(3):181–91. 10.1016/j.jchf.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). 10.1161/CIRCOUTCOMES.116.003497• This review discusses sex-specific risk factors for heart failure in women and strategies to prevent heart failure in women.
- 59.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–20. 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 60.Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: a focus on heart failure. Heart Fail Clin. 2014;10(2):339–52. 10.1016/j.hfc.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mantel A, Holmqvist M, Andersson DC, Lund LH, Askling J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J Am Coll Cardiol. 2017;69(10):1275–85. 10.1016/j.jacc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 62.Kim CH, Al-Kindi SG, Jandali B, Askari AD, Zacharias M, Oliveira GH. Incidence and risk of heart failure in systemic lupus erythematosus. Heart. 2017;103(3):227–33. 10.1136/heartjnl-2016-309,561. [DOI] [PubMed] [Google Scholar]
- 63.Dhakal BP, Kim CH, Al-Kindi SG, Oliveira GH. Heart failure in systemic lupus erythematosus. Trends Cardiovasc Med. 2018;28(3):187–97. 10.1016/j.tcm.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 64.Yen YF, Ko MC, Yen MY, Hu BS, Wang TH, Chuang PH, et al. Human Immunodeficiency Virus Increases the Risk of Incident Heart Failure. J Acquir Immune Defic Syndr. 2019;80(3):255–63. 10.1097/QAI.0000000000001917. [DOI] [PubMed] [Google Scholar]
- 65.Erqou S, Lodebo BT, Masri A, Altibi AM, Echouffo-Tcheugui JB, Dzudie A, et al. Cardiac Dysfunction Among People Living With HIV: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2019;7(2):98–108. 10.1016/j.jchf.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, et al. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J. 2006;27(8):936–41. 10.1093/eurheartj/ehi694. [DOI] [PubMed] [Google Scholar]
- 67.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133(5):484–92. 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D, et al. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15(8):843–9. 10.1093/eurjhf/hft041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5. 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 70.Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, et al. Predictors and Prognostic Implications of Incident Heart Failure in Patients With Prevalent Atrial Fibrillation. JACC Heart Fail. 2017;5(1):44–52. 10.1016/j.jchf.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 71.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34(15):1102–11. 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 72.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95(7):1592–600. 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 73.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60(24):2504–12. 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 74.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61(23):2319–28. 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 75.He J, Shlipak M, Anderson A, Roy JA, Feldman HI, Kallem RR, et al. Risk Factors for Heart Failure in Patients With Chronic Kidney Disease: The CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc. 2017;6(5). 10.1161/JAHA.116.005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dhingra R, Gaziano JM, Djousse L. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail. 2011;4(2):138–44. 10.1161/CIRCHEARTFAILURE.109.899070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waheed S, Matsushita K, Sang Y, Hoogeveen R, Ballantyne C, Coresh J, et al. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2012;60(2):207–16. 10.1053/j.ajkd.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15(7):425–39. 10.1038/s41575-018-0010-0. [DOI] [PubMed] [Google Scholar]
- 79.Holt A, Bjerre J, Zareini B, Koch H, Tonnesen P, Gislason GH, et al. Sleep Apnea, the Risk of Developing Heart Failure, and Potential Benefits of Continuous Positive Airway Pressure (CPAP) Therapy. J Am Heart Assoc. 2018;7(13). 10.1161/JAHA.118.008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52. 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 81.Hershberger RE, Cowan J, Morales A, Siegfried JD. Progress with genetic cardiomyopathies: screening, counseling, and testing in dilated, hypertrophic, and arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Heart Fail. 2009;2(3):253–61. 10.1161/CIRCHEARTFAILURE.108.817346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, et al. American Heart Association’s Life’s Simple 7: Avoiding Heart Failure and Preserving Cardiac Structure and Function. Am J Med. 2015;128(9):970–6 e2. 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uijl A, Koudstaal S, Vaartjes I, Boer JMA, Verschuren WMM, van der Schouw YT, et al. Risk for Heart Failure: The Opportunity for Prevention With the American Heart Association’s Life’s Simple 7. JACC Heart Fail. 2019;7(8):637–47. 10.1016/j.jchf.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Aggarwal M, Bozkurt B, Panjrath G, Aggarwal B, Ostfeld RJ, Barnard ND, et al. Lifestyle Modifications for Preventing and Treating Heart Failure. J Am Coll Cardiol. 2018;72(19):2391–405. 10.1016/j.jacc.2018.08.2160. [DOI] [PubMed] [Google Scholar]
- 85.Sanches Machado d’Almeida K, Ronchi Spillere S, Zuchinali P, Correa Souza G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients. 2018;10(1). 10.3390/nu10010058• This review highlights the evidence for lifestyle modifications to prevent heart failure including diet, physical activity, and weight management.
- 86.Lara KM, Levitan EB, Gutierrez OM, Shikany JM, Safford MM, Judd SE, et al. Dietary Patterns and Incident Heart Failure in U.S. Adults Without Known Coronary Disease. J Am Coll Cardiol. 2019;73(16):2036–45. 10.1016/j.jacc.2019.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302(4):394–400. 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Agha G, Loucks EB, Tinker LF, Waring ME, Michaud DS, Foraker RE, et al. Healthy lifestyle and decreasing risk of heart failure in women: the Women’s Health Initiative observational study. J Am Coll Cardiol. 2014;64(17):1777–85. 10.1016/j.jacc.2014.07.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nayor M, Vasan RS. Preventing heart failure: the role of physical activity. Curr Opin Cardiol. 2015;30(5):543–50. 10.1097/HCO.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.LaMonte MJ, Manson JE, Chomistek AK, Larson JC, Lewis CE, Bea JW, et al. Physical Activity and Incidence of Heart Failure in Postmenopausal Women. JACC Heart Fail. 2018;6(12):983–95. 10.1016/j.jchf.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Young DR, Reynolds K, Sidell M, Brar S, Ghai NR, Sternfeld B, et al. Effects of physical activity and sedentary time on the risk of heart failure. Circ Heart Fail. 2014;7(1):21–7. 10.1161/CIRCHEARTFAILURE.113.000529. [DOI] [PubMed] [Google Scholar]
- 92.Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M. Weight Loss and Heart Failure: A Nationwide Study of Gastric Bypass Surgery Versus Intensive Lifestyle Treatment. Circulation. 2017;135(17):1577–85. 10.1161/CIRCULATIONAHA.116.025629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsujimoto T, Kajio H. Abdominal Obesity Is Associated With an Increased Risk of All-Cause Mortality in Patients With HFpEF. J Am Coll Cardiol. 2017;70(22):2739–49. 10.1016/j.jacc.2017.09.1111. [DOI] [PubMed] [Google Scholar]
- 94.Piepoli MF, Corra U, Veglia F, Bonomi A, Salvioni E, Cattadori G, et al. Exercise tolerance can explain the obesity paradox in patients with systolic heart failure: data from the MECKI Score Research Group. Eur J Heart Fail. 2016;18(5):545–53. 10.1002/ejhf.534. [DOI] [PubMed] [Google Scholar]
- 95.Khan SS, Carnethon MR, Lloyd-Jones DM. The Obesity Paradigm and Lifetime Risk of Cardiovascular Disease-Reply. JAMA Cardiol. 2018;3(9):896–7. 10.1001/jamacardio.2018.1840. [DOI] [PubMed] [Google Scholar]
- 96.Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Upadhya B, Rocco M, Lewis CE, Oparil S, Lovato LC, Cushman WC, et al. Effect of Intensive Blood Pressure Treatment on Heart Failure Events in the Systolic Blood Pressure Reduction Intervention Trial. Circ Heart Fail. 2017;10(4). 10.1161/CIRCHEARTFAILURE.116.003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Upadhya B, Stacey RB, Kitzman DW. Preventing Heart Failure by Treating Systolic Hypertension: What Does the SPRINT Add? Curr Hypertens Rep. 2019;21(1):9. 10.1007/s11906-019-0913-3• A secondary analysis of the SPRINT trial demonstrated that targeting a systolic blood pressure of less than 120 mmHg is associated with reduced incidence of HF.
- 99.Molsberry RJ, Lloyd-Jones DM, Ning H, Lewis CE, Yancy CW, Shah SJ, et al. Would Risk-Stratified Intensive Blood Pressure Lowering Prevent Heart Failure More Effectively? J Card Fail. 2019;25(8):S95–S6. 10.1016/j.cardfail.2019.07.273. [DOI] [Google Scholar]
- 100.Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 Diabetes Mellitus and Heart Failure, A Scientific Statement From the American Heart Association and Heart Failure Society of America. J Card Fail. 2019;25(8):584–619. 10.1016/j.cardfail.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–28. 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 102.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–57. 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 103.Greene SJ, Butler J. Primary Prevention of Heart Failure in Patients With Type 2 Diabetes Mellitus. Circulation. 2019;139(2):152–4. 10.1161/CIRCULATIONAHA.118.037599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation. 2019;139(11):1384–95. 10.1161/CIRCULATIONAHA.118.037778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019. 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 106.Mital S, Musunuru K, Garg V, Russell MW, Lanfear DE, Gupta RM, et al. Enhancing Literacy in Cardiovascular Genetics: A Scientific Statement From the American Heart Association. Circ Cardiovasc Genet. 2016;9(5):448–67. 10.1161/HCG.0000000000000031• This prospective, randomized control trial of patients with systolic heart failure showed the use of an SGLT2 inhibitor reduced cardiovascular death and heart failure events in patients both with and without diabetes. This landmark trial was the first to demonstrate the benefit of SGLT2 inhibitors for cardiovascular outcomes in patients without diabetes.
- 107.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619–28. 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N Engl J Med. 2016;374(3):233–41. 10.1056/NEJMoa1505517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ware JS, Amor-Salamanca A, Tayal U, Govind R, Serrano I, Salazar-Mendiguchia J, et al. Genetic Etiology for Alcohol-Induced Cardiac Toxicity. J Am Coll Cardiol. 2018;71(20):2293–302. 10.1016/j.jacc.2018.03.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, et al. Genetic Variants Associated With Cancer Therapy-Induced Cardiomyopathy. Circulation. 2019;140(1):31–41. 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pirruccello JP, Bick A, Chaffin M, Aragam KG, Choi SH, Lubitz SA, et al. Titin Truncating Variants in Adults Without Known Congestive Heart Failure. J Am Coll Cardiol. 2020;75(10):1239–41. 10.1016/j.jacc.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ware JS, Cook SA. Role of titin in cardiomyopathy: from DNA variants to patient stratification. Nat Rev Cardiol. 2018;15(4):241–52. 10.1038/nrcardio.2017.190. [DOI] [PubMed] [Google Scholar]
- 113.Cresci S, Pereira NL, Ahmad F, Byku M, de Las FL, Lanfear DE, et al. Heart Failure in the Era of Precision Medicine: A Scientific Statement From the American Heart Association. Circ Genom Precis Med. 2019;12(10):458–85. 10.1161/HCG.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 114.Heidenreich PA, Hernandez AF, Yancy CW, Liang L, Peterson ED, Fonarow GC. Get With The Guidelines program participation, process of care, and outcome for Medicare patients hospitalized with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5(1):37–43. 10.1161/CIRCOUTCOMES.110.959122. [DOI] [PubMed] [Google Scholar]
- 115.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–414. 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]