Abstract
Glucocorticoid resistance (GR) is associated with exposure to chronic stress and an increased risk of metabolic and inflammatory disorders in both animal and human populations. Studies on ethnic disparities highlight the African-American (AA) population as having a high propensity to both GR and chronic stress exposure. Glucocorticoids and inflammation play a very important role in pregnancy outcome and fetal development. To date, however, the metabolites and metabolic pathways associated with GR during pregnancy have not been identified, obscuring the mechanisms by which adverse health consequences arise, and thus impeding targeted therapeutic intervention. The objective of this study was to perform untargeted high-resolution metabolomics (HRM) profiling on 273 pregnant AA women, to identify metabolites and metabolic pathways associated with GR during the first trimester of pregnancy and to evaluate their cross-sectional association with birth outcomes and psychosocial variables related to chronic stress exposure. For this study, GR was determined by the concentration of dexamethasone required for 50% inhibition (Dex IC50) of the cytokine tumor-necrosis factor alpha (TNF-alpha) release in vitro in response to a standard dose of lipopolysaccharide. The results for Metabolome-Wide Association Studies (MWAS) and pathway enrichment analysis for serum metabolic associations with Dex IC50, showed energy (nicotinamide and TCA cycle), amino acid, and glycosphingolipid metabolism as top altered pathways. Bioinformatic analysis showed that GR, as indicated by elevated Dex IC50 in the pregnant women, was associated with increased inflammatory metabolites, oxidative stress related metabolites, increased demand for functional amino acids to support growth and development, and disruption in energy-related metabolites. If confirmed in future studies, targeting these physiologically significant metabolites and metabolic pathways may lead to future assessment and intervention strategies to prevent inflammatory and metabolic complications observed in pregnant populations.
Keywords: Metabolomics, Glucocorticoid resistance, Health disparity, Pregnancy, Stress, Metabolites, Inflammation
Highlights
-
•
GR is associated with chronic stress and is a risk factor for adverse health outcomes, especially among African Americans.
-
•
Metabolites and metabolic pathways associated with GR relate to energy production, amino acid metabolism, and inflammation.
-
•
Findings provide a foundation for future studies investigating risk factors in this health disparity population.
1. Introduction
Glucocorticoids (GC) are steroid hormones under the influence of the hypothalamic-pituitary-adrenal (HPA) axis. GC mediate their actions by binding to glucocorticoid receptors, becoming a ligand dependent transcription factor that regulates key physiological events through target gene modulation in inflammation, glucose and fat metabolism, and cellular differentiation [1]. An important clinical pathological outcome observed is glucocorticoid resistance (GR). GR is identified in vitro by an increase in the dose of dexamethasone required to inhibit 50% (i.e., the IC50) of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), in the presence of the inflammatory agent bacterial lipopolysaccharide (LPS) [[2], [3], [4]]. Mechanisms for the manifestation of GR are still under investigation, however physiological stimuli such as chronic stress can activate the HPA axis thereby regulating plasma GC levels and GC action [5,6]. Genetic and epigenetic studies have shown that early exposure to altered glucocorticoid response (as early as beginning in utero) programs changes in neuroendocrine and immune mechanisms [7,8]. These altered glucocorticoid responses may further lead to increased vulnerability to adverse health outcomes later in life such as asthma [7]; insulin resistance [9]; inflammatory disease [10], metabolic syndrome [11], and potentially, adverse pregnancy outcomes [12,13]. Hence understanding what metabolites and metabolic pathways are associated with GR becomes necessary, in order ultimately to delineate mechanisms that lead to GR and identify therapeutic targets to prevent GR and its consequences.
Recent advances in metabolomics offer an opportunity to take this next step. Metabolomics includes multiple technologies that allow the identification of specific metabolites and complex metabolic pathways associated with health outcomes of interest [14,15]. A recent state-of the-science White Paper, emphasizes that an individual’s metabolic state, that is, the metabolites and metabolic pathways present, reflects the existing complex interactions between genome, proteome, diet, environmental exposures, and the gut microbiome [16]. These metabolites and pathways provide a real-time phenotype that can be screened to identify metabolic trends associated with adverse outcomes, potentially leading to early and targeted assessment and intervention strategies that influence those metabolites and pathways [15]. Metabolomics has been applied previously to identify biomarkers that associate with adverse pregnancy outcomes, including preterm birth, gestational diabetes mellitus, preeclampsia and small for gestational age (SGA) [[17], [18], [19], [20]], but has not been utilized to identify pathways related to GR or other measures of chronic stress exposure in pregnant women.
Importantly, ethnic disparities have been observed in glucocorticoid induced insulin resistance, wherein African Americans were more insulin resistant than Caucasian Americans under both placebo and dexamethasone treatments [9]. Compared to Caucasians, the National Academy of Medicine [21] and others (Kramer et al., 2011a; Mutambudzi et al., 2017; Parker and Douglas, 2010), have also identified stress as a key contributor to the health disparity in pregnancy outcomes experienced by AA women. Based on these ethnic disparities, and the future risk for GR-induced symptoms of metabolic syndrome in AA women, the present study was focused on utilizing metabolomics to identify metabolites and metabolic pathways associated with graded Dex IC50 response within the first trimester in AA women and to evaluate cross-sectional associations in light of personal factors such as age, body mass index (BMI), parity, and insurance status, and psychosocial measures of stress including adverse childhood experiences and exposure to discrimination. The potential to expose metabolites and metabolic pathways associated with GR and other measures of stress exposure during the first trimester of pregnancy, and to investigate their associations with pregnancy outcomes, will ultimately increase our ability to develop, deliver, and evaluate targeted clinical interventions that will improve maternal and fetal/infant health outcomes.
2. Materials and methods
Study population. Participants were drawn from women enrolled in the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort [22], for which pregnant US-born women who self-identify as African American (AA) are recruited from two hospitals in Atlanta, GA: a private hospital affiliated with Emory University that provides services for a socioeconomically and educationally diverse population and a county-supported hospital that provides services to the low-income and underserved. Both are staffed by Emory Obstetrics and Gynecology faculty. AA women presenting to the hospital-affiliated clinics for prenatal care between 8-14 weeks gestation, as determined by standard criteria based upon last menstrual period and/or first trimester ultrasound, were offered enrollment if they met the following inclusion criteria: 1) US-born black by self-report; 2) between 8-14 weeks gestation with a singleton pregnancy (verified by clinical record); 3) able to comprehend written and spoken English; 4) between 18-40 years of age; and 5) experiencing no chronic medical condition or taking prescribed chronic medications aside from prenatal vitamins (verified by clinical record). Women selected for inclusion in this study (N = 273) are a subsample of those enrolled into the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort between June, 2014, and January, 2017, for whom sufficient venous blood sample was available for determination of GR and metabolomics assays. The present study was approved by the Emory University Institutional Review Board; all participants provided written informed consent prior to enrollment and participation.
Sample collection and clinical data. At the 8-14 week enrollment encounter, participants completed questionnaires and provided biological samples as described in detail previously [22]. Briefly, women were informed of the study during their first prenatal visit by the prenatal clinic receptionist and those interested in learning more, then met with the study coordinator. After hearing details of the study and initial inclusion criteria, women who continued to express interest in participating provided informed consent after which they were accompanied to the clinical laboratory where a venous blood sample, in addition to that required for their initial prenatal visit, was collected for later analysis of in vitro WBC Dex IC50, an indicator of GR [3,23,24] and; (5) high-resolution serum metabolomics. After completing all biological sample collections, women returned to the waiting room where they next completed demographic and health questionnaires providing information on: (1) Maternal race, based on self-report; (2) Maternal age, parity, prenatal health insurance status (Medicaid, private) at the time of enrollment; (3) Psychosocial measures relevant to the analyses of this study including participants’ exposure to chronic stress and racial discrimination; (4) Fetal age at the time of the 1st trimester prenatal exam as determined by fetal ultrasound and/or last menstrual period; (5) Infant gestational age at delivery or the occurrence of a spontaneous abortion (e.g., miscarriage), defined as the loss of a fetus before the 20th week of pregnancy, and ascertained after delivery from the prenatal record by medical chart abstraction using a standardized chart abstraction tool; and (6) Body Mass Index (BMI) calculated from patients’ measured height at the first prenatal visit and patient-report of pre-pregnancy weight at the first visit.
In Vitro Dexamethasone Resistance Assay. Blood collected at the first prenatal visit (weeks 8–14 of gestation) was processed and analyzed to evaluate GR as described previously [25]. Briefly, whole blood was diluted 10:1 with sterile phosphate buffered saline, and 450 μL of this mixture was incubated in duplicate with LPS (from Escherichia Coli Serotype 055:B5, Millipore-Sigma; final concentration 30 ng/ml) for 24 h in the absence or presence of serial dilutions of dexamethasone (Millipore-Sigma, 10-9 to 10-6 M) in 24-well cell culture plates at 37 °C in 5% CO2. Culture well contents were centrifuged at 1000 X g for 10 min, and supernatants aliquoted and stored at −80 °C until assayed by ELISA (BD Biosciences) for TNF-α, a frequently used indicator cytokine, including in 50% of the GR-related studies identified in a recent meta-analysis [4]. The DEX concentration required to suppress 50% of the LPS-induced cytokine response was determined from supernatant concentrations of TNF-α using dose-response curves fitted by Prism (GraphPad Software, La Jolla, CA).
High-Resolution Metabolomics. High-resolution metabolomics (HRM) was completed using established methods [26,27]. A total of 50 μL of serum sample was treated with 100 μL of ice-cold LC-MS grade acetonitrile (Sigma Aldrich) containing 2.5 μL of internal standard solution with eight stable isotopic chemicals selected to cover a range of chemical properties. Following addition of acetonitrile, serum was equilibrated for 30 min on ice, and the precipitated proteins were removed by centrifugation (16.1 × g at 4 °C for 10 min). Resulting supernatant was maintained at 4 °C until analysis. Serum samples were analyzed in batches of 20; each batch included duplicate analysis of pooled human plasma (QStd) for quality control purposes.
Samples were analyzed in triplicate with 10 μL injections using hydrophilic interaction liquid chromatography (HILIC) column in positive electrospray ionization (ESI) mode (Thermo Scientific Dionex Ultimate 3000RSLCnano, Thermo Scientific Orbitrap Fusion Tribrid Mass Spectrometer). The high-resolution mass spectrometer was operated in full scan mode at 120,000 resolution and mass-to-charge ratio (m/z) range 85–1275. Raw data files were extracted and aligned using apLCMS [28] with modifications by xMSanalyzer [29]. Uniquely detected ions consisted of accurate mass m/z, retention time and ion abundance, referred to as m/z features. Prior to data analysis, m/z features were batch corrected using ComBat [30].
Metabolite Annotation. Metabolic features were annotated using with xMSannotator using HMDB database, wherein the confidence scores for annotation are derived from a multistage clustering algorithm [31]. Further identification of the selected metabolites by criteria of Schymanski [32] in combination with xMSannotator [31], included metabolite identification through matching to both m/z and retention time of a previously characterized authenticated chemical standard (level 1); annotated compounds matching m/z from databases wherein M+H is present and isotopes co-elute (level 2); annotated compounds matching m/z from databases wherein M+H is present and pathway metabolites present (level 3); m/z match from database within 5ppm tolerance with unique identifier (level 4). Database used for annotation include HMDB (Human Metabolome Database, http://www.hmdb.ca/) [33] and KEGG (Kyoto Encyclopedia of Genes and Genomes, http://www.genome.jp/kegg/) [34]. Additional manual search was done using METLIN (http://metlin.scripps.edu/index.php) at 5 ppm tolerance [35].
Bioinformatics analysis. A total of 16,481 m/z features were detected, which were further filtered to retain only features with non-zero values in >80% in all individuals and >50% in all technical replicates. The remaining 8830 m/z features were log2 transformed, quantile normalized and used for further regression based statistical analysis. A linear regression model adjusted for demographic covariates such as age, BMI and parity, was used to select metabolic features associated with increasing GR (Dex IC50) in a metabolome-wide-association study (MWAS). Threshold at P ≤ 0.05 without adjustment for multiple comparisons and for q ≤ 0.05 after correction for false discovery rate (FDR) was applied. FDR correction protects against type 1 error but not type 2 statistical error. Significant features (raw P < 0.05) were further studied by pathway enrichment analyses using mummichog 1.0.9 [36]. This approach protects against type 2 statistical error and protects against type 1 statistical error by permutation testing in pathway enrichment analysis by including all features at P < 0.05 [37]. Hierarchical cluster analysis (HCA) and partial least square discriminant analysis (PLS-DA) were used for comparison of the 1094 significant features obtained from lmreg model and plotted for quintile 1 (n = 55, Dex IC50 < 5348 pM) and quintile 5 (n = 55, Dex IC50 > 12898 pM) (raw P < 0.05) using Metaboanalyst [38]. Boxplots were plotted in R v.3.2.3.
Statistical Analyses. Descriptive analyses were conducted for the study population with median (range) and percentages (n) computed for demographic variables including age, BMI, parity and insurance status, each previously shown to impact pregnancy outcomes [[39], [40], [41], [42], [43]]. Spearman correlation was used to test for associations between Dex IC50 and demographic data, while crude odds ratio (OR) and adjusted OR were calculated using logistic regression modeling for pregnancy outcomes and psychosocial variables. Dex IC50 was used as a continuous variable in the linear regression analyses.
3. Results
3.1. Demographics and clinical parameters
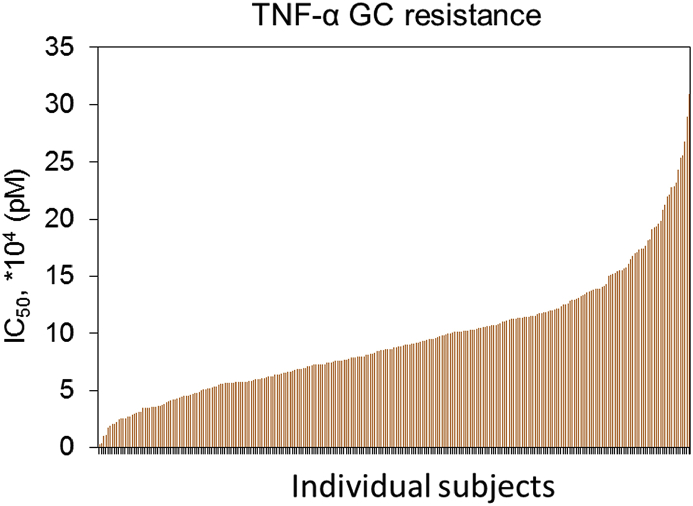
A total of 273 AA women at 8-14 weeks of gestation met criteria for inclusion in this study. Demographic and clinical characteristics for all 273 AA pregnant women, including serum Dex IC50 levels, maternal age in the first trimester, body mass index, parity, insurance status and education are summarized in Table 1. The concentration of dexamethasone required to suppress 50% of the LPS-induced TNF-α response (Dex IC50), a measure for GR, is depicted as a bar plot for each of the participating subjects (Fig. 1) and the range of values are presented as quintiles in Table 1. The range of Dex IC50 values for the study population was 125-fold and the median (range) was 9524 pM (246 - 30,888 pM). The median Dex IC50 for Quintile Q1, was 3593 pM and highest quintile, Q5 was 16,504 pM. Demographic survey results show that the median age for the study sample was 24 years; 17.9% of the subjects were greater than 30 years of age, and the majority were in the age range of 20–29 years (69.6%). The median BMI was 26.1 kg/m2 and ranged from 16.5 to 56.5 kg/m2; with 54.6% of the study population overweight. The study sample comprised of 48.7% subjects without previous birth history. A total of 77.3% of the study population had medical insurance from the government and 22.7% had private medical insurance. The majority of subjects either graduated high school (35.9%) or attended some college/technical school (86%). None of the demographic variables reported were correlated with serum Dex IC50 values.
Table 1.
Demographic and clinical characteristics of the study population. (Pregnant AA women, n = 273, ∗P < 0.05).
| Characteristics | Results | Quintile 1 | Quintile 5 |
|---|---|---|---|
| Number of subjects | 273 | 55 | 55 |
| DexIC50 (pM)a | 9524 (246–30,888) |
3593 (246–5348) |
16504 ∗ (12898–30888) |
| Age, ya | 24 (18–35) | 24 (19–35) | 23 (18–35) |
| <20 yb | 34 (12.5%) | 5 | 8 |
| 20–29 yb | 190 (69.6%) | 31 | 37 |
| ≥30 yb | 49 (17.9%) | 14 | 8 |
| BMI (kg/m2)a | 26.1 (16.5–56.5) | 26.6 (18.4–56.5) | 24.6 (16.5–45.6) |
| Parityb | |||
| 0 | 133 (48.7%) | 22 | 28 |
| 1 | 79 (28.9%) | 20 | 14 |
| 2 | 39 (14.3%) | 9 | 6 |
| 3 | 18 (6.6%) | 3 | 6 |
| 4 | 4 (1.5%) | 1 | 1 |
| Insurance Statusb | |||
| Government Insurance | 211 (77.3%) | 44 | 42 |
| Private Insurance | 62 (22.7%) | 11 | 13 |
median (range).
n (%).
Fig. 1.
Glucocorticoid resistance in pregnant African-American women. Dex IC50 in pM concentration required to suppress 50% of the LPS-induced cytokine response is plotted for each of the 273 pregnant African-American women. Each bar represents an individual, with the lowest Dex IC50 value recorded at 246 pM and highest at 30,888 pM. Details on the Dex IC50 values for each individual along with age, BMI and parity are provided in Supplemental Table 1.
3.2. Metabolome wide association study of glucocorticoid resistance
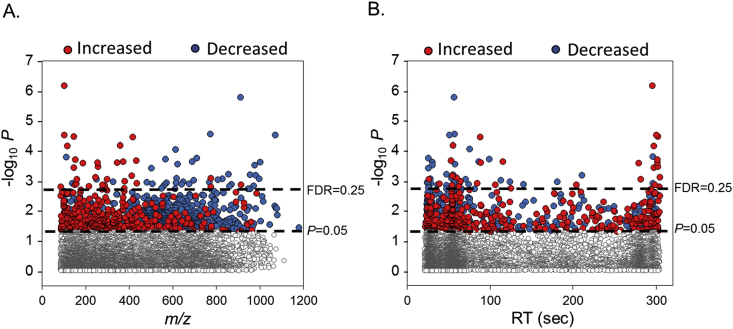
In order to investigate the global serum metabolic alterations associated with GR, we performed a metabolome wide association study of untargeted serum metabolic features identified by HRM. Using a linear regression model with Dex IC50 values as a continuous variable, a total of 75 out of 8830 were found as discriminatory features at a false discovery rate (FDR) of 0.25 and 1092 m/z features at a raw P value < 0.05. This selection was adjusted for demographic covariates such as age, BMI and parity. The Dex IC50 values, age, BMI and parity for each individual subject is provided in Supplemental Table 1. The type 1 Manhattan plot (-log10 P versus m/z) shows that these discriminatory features comprise a broad range of m/z (Fig. 2A), wherein red represents features that are positively and blue represents features negatively associated with Dex IC50. The top dotted horizontal line marks FDR 0.25 threshold and the bottom dotted line represents raw P value < 0.05 threshold (Fig. 2). Two thirds of all the 1092 discriminatory features were negatively associated with Dex IC50. Also 73% of features with an m/z less than 400 were positively associated with Dex IC50, while 84% of features greater than m/z 400 were negatively associated. The type 2 Manhattan plot (-log10 P versus chromatographic retention time) showed that 62% of the discriminatory features elute within 60 s (Fig. 2B). 78% of these early eluting features were negatively associated with Dex IC50, while the later eluting features comprised mostly of positively associated metabolic features, consistent with an amino acid elution pattern. The mass to charge, retention time and P-value for the 1092 discriminatory metabolites is provided in Supplemental Table 2.
Fig. 2.
Metabolome wide association study of chronic stress. (A) Type 1 Manhattan plot shows the negative log P (-log P) for each metabolite (m/z features) as a function of the mass to charge ratio (m/z). (B) Type 2 Manhattan plot shows the -log P for each metabolite as a function of retention time in seconds. Positively associated m/z features are represented as red dots and negative associations are represented as blue dots. The top dashed horizontal line represents an FDR cutoff of 25% using Benjamini-Hochberg correction and the lower dashed horizontal line represents a cutoff at raw P < 0.05. Details on the discriminatory metabolites are provided in Supplemental Table 2. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
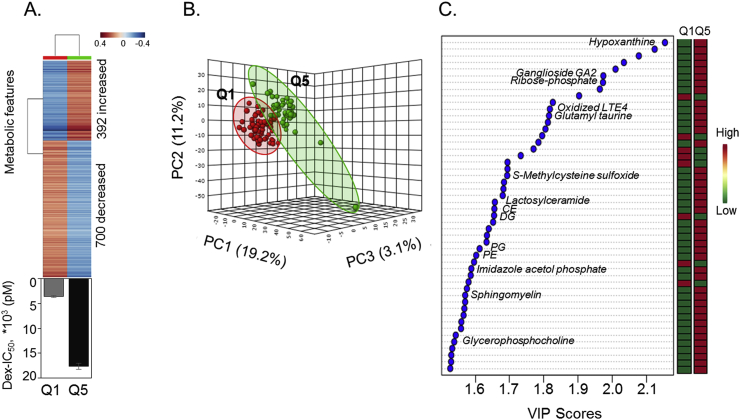
Hierarchal cluster analysis plot (HCA) of the 1092 features showed that a total of 392 m/z features increased while 700 m/z features decreased with an increase in Dex IC50 value as graphically represented by the highest Dex IC50 quintile (Q5, Dex IC50 mean ± SEM; 17,685 ± 590.1 pM, n = 55) compared to the lowest quintile (Q1, Dex IC50 mean ± SEM; 3552 ± 169.5 pM, n = 55) (Fig. 3A). These 1092 discriminatory metabolites selected by linear regression for all 273 AA women were further assessed by PLS-DA to identify the top discriminatory features that separated individuals in the highest Dex IC50 quintile, Q5, from the lowest quintile, Q1. In the 3-dimensional PCA score plot (Fig. 3B), principal component 1 accounted for 19.2% of the variance and principal component 2 accounted for 11.2%. The top 50 metabolites (VIP>1.5) responsible for the separation of Q1 and Q5 along principal component 1 are presented in Fig. 3C. These metabolites were annotated using xMSannotator with KEGG, Metlin and HMDB databases. These metabolites were comprised of increases in inflammatory markers such as glycosphingolipids –sphingomyelin, ceramides, ganglioside; and glycerophospholipids - PE (phosphotidyethanolamine), PG (phosphotidylglycerol), glycerophosphocholine and diglycerides (DG). The list also extended to other inflammatory markers such as oxidized LTE4 imidazole acetol phosphate and cholesterol ester (CE); oxidative stress related metabolite hypoxanthine and pentose phosphate pathway metabolite ribose phosphate, all of which increased with increases in Dex IC50 value.
Fig. 3.
Hierarchal cluster analysis and partial least square discriminant analysis dependent separation of Dex IC50 quintiles. (A) Supervised hierarchal clustering of the 1092 discriminatory metabolites from linear regression model is displayed for the lowest (Q1) and highest (Q5) Dex IC50 quintiles. A total of 392 metabolites increased and 700 metabolites decreased with increasing Dex IC50 values (Q1, Dex IC50 mean ± SEM; 3552 ± 169.5 pM, n = 55 and Q5, Dex IC50 mean ± SEM; 17,685 ± 590.1 pM, n = 55). Blue: metabolites that decreased and Red: metabolites that increased with increase in Dex IC50 values. (B) PLS-DA score plot for high-resolution metabolomics data resulting in separation of lowest (Q1) and highest (Q5), Dex IC50 quintiles along principal component 1 (PC1-19.2%), along principal component 2 (PC1-11.2%) and along principal component 3 (PC1-3.1%) is shown. (C) The top 50 discriminatory metabolic features contributing to the separation listed from top to bottom is shown with their VIP scores (variable importance in projection scores) and changes in intensity at Q1 and Q5 (VIP>1.5). The metabolite associated with the m/z features is putatively annotated as determined by KEGG, HMDB and Metlin ID within 5ppm tolerance. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Metabolic pathways and key metabolites associated with glucocorticoid resistance
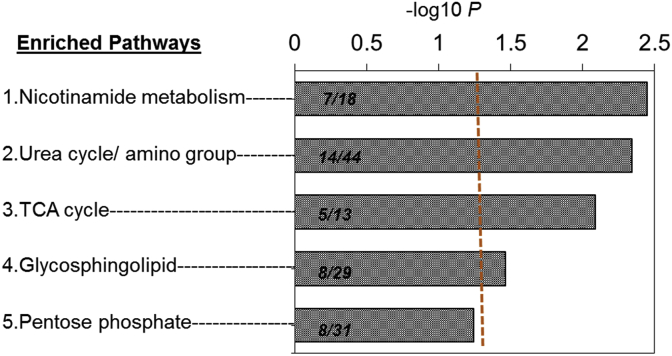
To investigate how these 1092 discriminatory m/z features obtained by the linear regression model were enriched in metabolic pathways, pathway enrichment analysis was performed using Mummichog [36]. Results showed the top 4 discriminatory pathways that were significantly altered by glucocorticoid resistance (P < 0.05). These included nicotinamide metabolism (P = 0.004); amino acid metabolism (P = 0.005); TCA cycle (P = 0.01) and glycosphingolipid metabolism (P = 0.04) (Fig. 4). Pentose phosphate pathway (P = 0.07) was not significant at the P-value cutoff.
Fig. 4.
Metabolic pathways associated with glucocorticoid resistance. The 1092 discriminatory metabolites from the regression model were used as Mummichog input for pathway enrichment analysis. Only pathways >4 overlapping metabolites are shown. The 4 metabolic pathways significantly affected by chronic stress (Dex IC50 measure) are shown by −log P and the number of matched features in each pathway are indicated in overlap/total size. The vertical line represents cut off at P < 0.05. The most significantly altered pathways were nicotinamide metabolism, amino acid metabolism, TCA cycle and glycosphingolipid metabolism (P < 0.05).
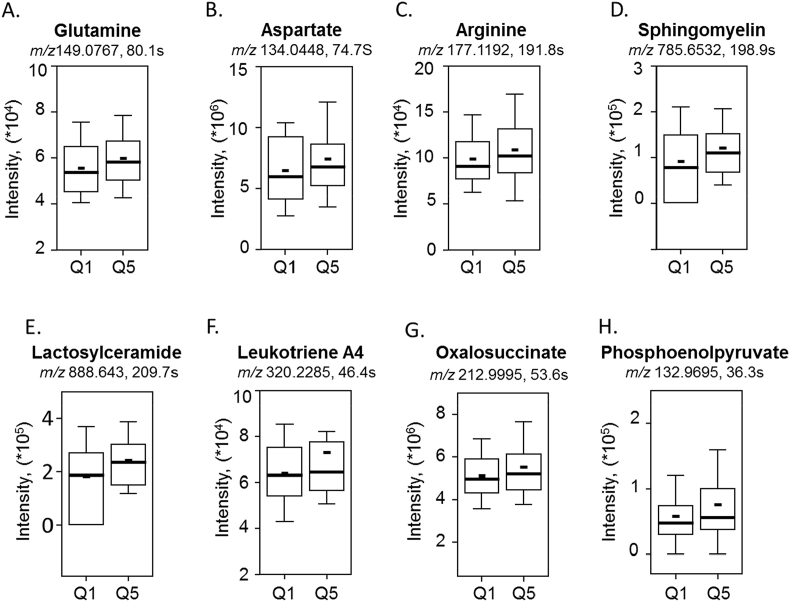
We further examined the key metabolites that were involved in the top discriminatory pathways associated with glucocorticoid resistance. The results are graphically depicted by raw intensity of each metabolite as a boxplot with10–90th percentile bars, at the lowest quintile (Q1) and highest quintile (Q5) for Dex IC50 (Fig. 5). The median and mean are represented as a solid long and short horizontal line respectively within each box. A number of key amino acids included in nicotinamide and amino acid metabolism increased with increase in Dex IC50 and comprised of metabolites such as glutamine (P = 0.0295), aspartate (P = 0.0385) and arginine (P = 0.0335) (Fig. 5A–C). Glycosphingolipids are an integral part of the cell membrane and play an important role in cell adhesion and as signaling molecules. In the glycosphingolipid metabolism, sphingomyelin hydrolysis is a major pathway for stress-induced ceramide generation [44]. Our results show that sphingomyelin (P = 0.0109) and multiple ceramide species including lactosylceramide (P = 0.0023) increased with increase in Dex IC50 values in the cohort, supporting the evidence for increased inflammation with increased Dex IC50 (Fig. 5D–E). Leukotrienes are eicosanoid lipid mediators of inflammation and the results show that leukotriene (P = 0.0090) also increased with increasing Dex IC50 (Fig. 5F). Energy metabolites such as oxalosuccinate (P = 0.0391) and phosphoenolpyruvate (P = 0.0475) also increased with increased Dex IC50, suggesting disruption in energy metabolism (Fig. 5G–H). Details on the identification of metabolites significantly associated with glucocorticoid resistance in the 273 pregnant AA women are provided in Supplemental Table 3.
Fig. 5.
Key discriminatory metabolites plotted from the significantly enriched metabolic pathways altered by glucocorticoid resistance. The raw intensity of the representative metabolites is plotted (A-H). Metabolites such as glutamine (A), aspartate (B), and arginine (C) from the amino acid and nicotinamide pathway; sphingomyelin (D), lactosylceramide (E), and leukotriene A4 (F) from glycosphingolipid metabolism; oxalosuccinate (G) and phosphoenolpyruvate (H) from energy metabolism. The raw intensity for each metabolite is presented as a boxplot with10–90th percentile bars. Q1 represents the lowest quintile and Q5 represents the highest quintile for Dex IC50 concentration. The median and mean are represented as a solid long and short horizontal line respectively within each box. (P < 0.05, n = 55).
3.4. Evaluation of pregnancy outcomes associated with glucocorticoid resistance
In order to determine the association of increased GR with adverse pregnancy outcomes including preterm birth and spontaneous abortion, odds ratio with and without adjustment for confounding factors was calculated (Supplemental Fig. 1). Results for adverse pregnancy outcomes showed that women with increased concentrations of Dex IC50 did not have increased odds of gestational age for birth <37 wk (OR = 1.88, 5%CI: 0.74, 95%CI: 4.78; P = 0.18), early term spontaneous birth (OR = 1.13, 5%CI: 0.45, 95%CI: 2.86; P = 0.80), preterm spontaneous birth (OR = 2.03, 5%CI: 0.6, 95%CI: 6.95; P = 0.26) or spontaneous abortion (OR = 2.26, 5%CI: 0.38, 95% CI: 13.51; P = 0.37). The results did not reach statistical significance even after adjustment for age, BMI and parity.
3.5. Evaluation of psychosocial variables with glucocorticoid resistance
Glucocorticoid resistance has been reported in populations exposed to chronic stress including, among others, women suffering from post-traumatic stress syndrome [45]. African American women in particular are more often exposed to chronic stress including perceived racism [46], neighborhood violence [47,48], and poverty [49] compared to Caucasian women and are also at a notably higher risk for nearly all types of adverse maternal, fetal, and infant outcomes [21,50,51]. In order to evaluate the strength of the association between GR and psychosocial factors in the current study population, odds ratio with and without adjustment for confounding factors was calculated (Supplemental Fig. 2). Results showed that there was no association between increased concentrations of Dex IC50 and psychosocial events such as adverse childhood experiences (ACE; OR = 0.73, 5%CI: 0.16, 95%CI: 3.46; P = 0.70), sexual life experience inventory (SLEI; OR = 1.05, 5%CI: 0.36, 95%CI: 3.06; P = 0.93), Krieger experiences of discrimination (KEOD; OR = 0.2, 5%CI: 0.03, 95%CI: 1.19; P = 0.08) and everyday discrimination scale (EDS; OR = 0.71, 5%CI: 0.26, 95% CI: 1.95; P = 0.51). The results did not reach statistical significance even after adjustment for age, BMI and parity. The results indicate that GR is not associated with psychosocial stress factors in this study cohort. The data thus suggest that GR could be attributed to a number of factors that may not be limited to standard chronic stress measures observed in ethnic communities, but could be an outcome of stressors such as environmental exposures [52], lifestyle choices [53] or genetic polymorphisms in glucocorticoid receptors [54].
4. Discussion
In this report, we describe the association between GR as reflected by increasing Dex IC50 during early pregnancy, and altered metabolic pathways in AA women, through serum metabolic phenotyping with high-resolution metabolomics. Glucocorticoids activate multiple biochemical processes that not only alter cell physiology at a systemic level for the mother and the fetus, but also facilitate long term adaption to post-uterine life [55]. Our findings, that GR in pregnant AA women, was associated with inflammatory metabolites, oxidative stress, disruption in energy metabolism and increased demand for functional amino acids essential for growth and development but not with preterm birth or other birth outcomes, may indicate that exposure to chronic stress has implications for fetal and child development rather than birth outcomes. Future study is essential.
Glutamine, aspartate and arginine are considered functional amino acids that alter metabolic processes that improve growth and development [56]. Previous amino acid profiling studies in pregnant women (>25 weeks gestational age) have shown that glutamine, aspartate and arginine are increased in plasma of women with gestational diabetes after adjusting for covariates such as gestational age and BMI [57]. Our results showed increases in these same three functional amino acids in association with increased Dex IC50. While indicating a relationship, given the cross-sectional nature of the study, directional effects of these associations cannot be determined. Moreover, although future studies to determine whether an increase in these amino acids is associated with disorders such as the development of gestational diabetes in subjects with GR are required, serum quantitation of amino acids along with routine laboratory tests, may someday play a role in pregnancy health monitoring to prevent future adverse metabolic outcomes in mother and the fetus.
Normally, circulating cortisol binds to glucocorticoid receptors in the WBC cytoplasm; once bound, the hormone-receptor complex translocates to the nucleus and inhibits further production of NF-κB and other transcription factors that regulate pro-inflammatory cytokine gene expression, limiting further production of pro-inflammatory cytokines [58]. In response to chronic stress, however, GR may develop, suppressing this feedback loop [12,45,59] and establishing a chronic inflammatory milieu. In both animal [60] and human [61,62] studies, biomarkers associated with oxidative stress and inflammation have been linked to adverse birth outcomes and inadequate energy availability early in pregnancy, disrupting fertility and fetal development [63,64]. Chronic inflammation is also associated with adverse health outcomes across the lifespan in non-pregnant populations [[65], [66], [67]], when present during pregnancy, it increases the risk of gestational diabetes and hypertension [[68], [69], [70]], and under some circumstance, maternal depressive symptoms [71] and autism spectrum disorder [72]. Sphingolipids have been implicated as mediators of cell-stress responses as they play an important role in intracellular signal transduction. Sphingomyelins and ceramides are mainly associated with low-density lipoproteins (LDL), thus alluding to their potential atherogenic properties [73]. Increased sphingomyelin and ceramides are associated with cardiovascular diseases [74], insulin resistance [75], type 2 diabetes and obesity [75]. They are also potent activators of the inflammatory cascade [76]. Our results showed that increased Dex IC50 was associated with increased serum sphingomyelin, leukotriene and multiple ceramide species, which indicate that GR within the first trimester is linked to increased inflammation in AA women. These findings may predispose the mother and/or the fetus to adverse outcomes, including maternal metabolic syndrome and insulin resistance, aa well as altered fetal development and/or future health risks for offspring [77,78]. Future studies that include assessment of offspring health outcomes are required to better understand the role of GR and early inflammatory metabolic alteration on long term offspring health and development.
Although mechanisms by which systemic and local inflammation associate with adverse health outcomes remain under investigation, inflammation increases oxidative stress [79,80], which could alter energy production and mitochondrial function [81]. Further evidence supports that glucocorticoid receptors (GRs) translocate into mitochondria and modulate mitochondrial gene expression, thereby altering mitochondrial function and impacting energy production [82]. The current study does indeed demonstrate that GR observed in pregnant AA women impacts the TCA cycle and suggests energy availability may be compromised with disruption in energy related precursor accumulation.
This study identified a wide range of Dex IC50 concentrations across the AA cohort. The level of Dex IC50 in this population was not associated with age, BMI, parity, pregnancy outcomes, psychosocial factors or standard indicators of SES variables including education or insurance [See Table 1, Supplementary Figs. S1 and S2, and Fig. 6]. While GR has been associated with chronic stress measures in other populations [3,23,59,83], inter-comparison of GR in AA women and to other ethnicities may highlight this association more significantly. Chronic stress across the lifetime of AA women was identified nearly thirty years ago by Geronimus [84] as “weathering”. Recent research by that team further clarifies this concept with results suggesting that, contrary to expectation, greater SES in AA women is associated with increased reports of both racial discrimination and symptoms of depression [85]. These results are consistent with our finding that Dex IC50 levels were not associated with SES indicators of education or insurance status. Other evidence also indicates that stressors experienced by AA women may not be improved with rising social status or income. For instance, research shows less improvement in infant birth weight in AA women with increasing SES compared to white women [86]. The latter observation suggests that this population may experience a biological vulnerability that could underlie a number of adverse health outcomes – including adverse pregnancy outcomes – disproportionately experienced by AA women. In the current study, we add to this important body of literature, by identifying several critical metabolites and metabolic pathways differentially expressed with rising Dex IC50 in pregnant AA women independent of typical SES indicators. Furthermore, our study shows that intra-comparison within the AA population indicates an association of GR as a function of Dex IC50, with chronic stress related perhaps to a mileu of environmental exposures, lifestyle choices, genetic polymorphisms and psychosocial variables, rather than a single factor. These findings also support the need for future studies to evaluate the contribution of these experiences to adverse birth outcomes comparing AA women to other ethnicities (Fig. 6).
Fig. 6.
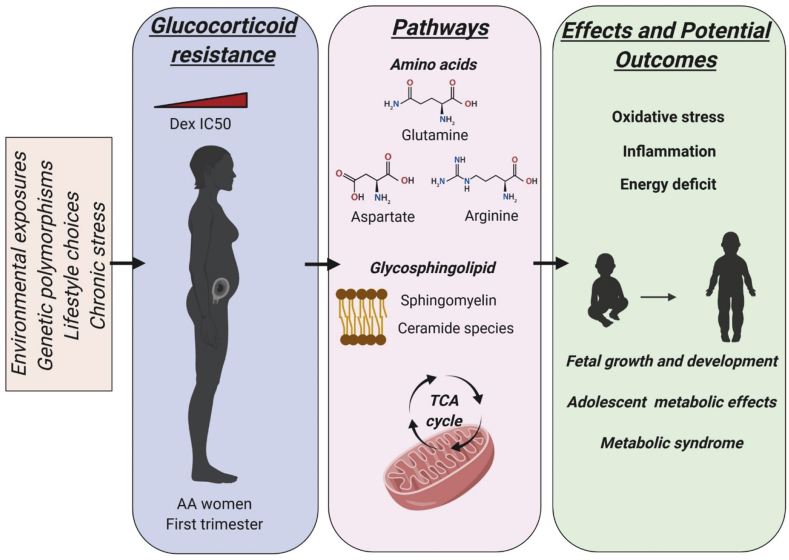
Proposed schematic for first trimester glucocorticoid resistance induced metabolic alterations leading to long term effects on maternal and offspring health in AA women. Glucocorticoid resistance observed in AA women within the first trimester could be an outcome of a combination of factors such as environmental exposures, genetic polymorphisms, lifestyle choices or standard chronic stress variables like psychosocial parameters. Increasing maternal DexIC50, as determined by in vitro testing within the first trimester is associated with disruption in amino acid metabolism, an increase in glutamine, aspartate and arginine, functional amino acids required to cope with growth and development; enrichment in glycosphingolipid metabolism with an increase in sphingomyelin and ceramide species that contribute to oxidative stress and inflammation; and disruption in mitochondrial TCA cycle with accumulation of energy metabolites. Thus glucocorticoid resistance induced alterations in maternal endogenous metabolic pathways within the first trimester, could lead to inflammation, oxidative stress and energy deficits that may influence the long term growth and development of the offspring, or to metabolic effects ultimately predisposing the mother and child to disease and metabolic syndrome.
As with most studies, this investigation has limitations. Although results suggest that GR is not associated with adverse birth outcomes in our study cohort, this may be related to small sample size in each group and requires further experimental validation with a larger cohort. With 273 AA women, and although the data show strong correlation of GR with inflammation, energy deficit and amino acid dysregulation, a larger sample size is essential to determine if lack of association between GR and standard chronic stress measures such as SES variables and psychosocial parameters still holds true. The study is also limited by its cross-sectional nature, which makes it impossible to know if the changes in metabolites are a consequence or a cause of GR. Additional information on GR changes across pregnancy and developmental parameters of the child at birth are likewise essential. Another potential limitation is the lack of a non-AA comparison group, which would further strengthen the associations between GR and reported chronic stress variables. However, a key tenant of health disparity research is that to understand the true underpinnings of health disparity, one must first look within the disparity group for group-defined risk and protective factors [87,88]. Our study also lacks the measure of serum cortisol within the group, which could further serve as a surrogate for in vitro Dex IC50 measures. However, based on metabolomics analysis, 18-hydroxycortisol and aldosterone sulfate, both downstream metabolites of cortisol increased and were significantly associated with Dex IC50, which further suggest that cortisol-related metabolites are upregulated and associated strongly with Dex IC50 measure in AA women within the first trimester (See Supplementary Table S3). And finally, our study did not include an assessment of offspring health outcomes, which could further inform the consequences of early pregnancy GR, observed in AA women.
In conclusion, this study of AA pregnant women during their first trimester utilized HRM to identify GR-associated dysregulation of maternal metabolic pathways with the potential to influence maternal health and/or offspring development. These pathways are linked to increases in inflammatory metabolites, oxidative stress related metabolites, increased demand for functional amino acids to cope with growth and development, and disruption in energy related metabolism. Given the high prevalence of adverse pregnancy outcomes among AA women of all SES levels compared to white women, future studies are essential to provide an additional foundation for interventions or monitoring strategies related to stress and coping that will eliminate this persistent health disparity and improve the maternal and childhood health of all members of our population.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was funded by grants from the National Institutes of Health R01NR4800; 3R01NR4800; and P50ES026071.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2020.100001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chriguer R.S., Elias L.L., da Silva I.M., Jr., Vieira J.G., Moreira A.C., de Castro M. Glucocorticoid sensitivity in young healthy individuals: in vitro and in vivo studies. J. Clin. Endocrinol. Metab. 2005;90(11):5978–5984. doi: 10.1210/jc.2005-0067. [DOI] [PubMed] [Google Scholar]
- 2.Leistner C., Menke A. How to measure glucocorticoid receptor’s sensitivity in patients with stress-related psychiatric disorders. Psychoneuroendocrinology. 2018;91:235–260. doi: 10.1016/j.psyneuen.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Pace T.W., Hu F., Miller A.H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrin A.J., Horowitz M.A., Roelofs J., Sunszain P.A., Pariante C.M. Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Front. Psychiatr. 2019;10:423. doi: 10.3389/fpsyt.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delbende C., Delarue C., Lefebvre H., Bunel D.T., Szafarczyk A., Mocaer E., Kamoun A., Jegou S., Vaudry H. Glucocorticoids, transmitters and stress. Br. J. Psychiatr. Suppl. 1992;(15):24–35. [PubMed] [Google Scholar]
- 6.Merkulov V.M., Merkulova T.I., Bondar N.P. Mechanisms of brain glucocorticoid resistance in stress-induced psychopathologies. Biochemistry (Mosc.) 2017;82(3):351–365. doi: 10.1134/S0006297917030142. [DOI] [PubMed] [Google Scholar]
- 7.Wright R.J. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr. Perinat. Epidemiol. 2007;21(Suppl 3):8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 8.Wright R.J. Stress and acquired glucocorticoid resistance: a relationship hanging in the balance. J. Allergy Clin. Immunol. 2009;123(4):831–832. doi: 10.1016/j.jaci.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frazier B., Hsiao C.W., Deuster P., Poth M. African Americans and Caucasian Americans: differences in glucocorticoid-induced insulin resistance. Horm. Metab. Res. 2010;42(12):887–891. doi: 10.1055/s-0030-1265131. [DOI] [PubMed] [Google Scholar]
- 10.Barnes P.J., Adcock I.M. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang M. The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr. Metab. 2005;2(1):3. doi: 10.1186/1743-7075-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corwin E.J., Guo Y., Pajer K., Lowe N., McCarthy D., Schmiege S., Weber M., Pace T., Stafford B. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology. 2013;38(9):1786–1796. doi: 10.1016/j.psyneuen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie S., Anderson C.M. Racial discrimination and leukocyte glucocorticoid sensitivity: implications for birth timing. Soc. Sci. Med. 2018;216:114–123. doi: 10.1016/j.socscimed.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng S., Shah S.H., Corwin E.J., Fiehn O., Fitzgerald R.L., Gerszten R.E., Illig T., Rhee E.P., Srinivas P.R., Wang T.J., Jain M., American Heart Association Council on Functional, G. Translational B., Council on C., Stroke N., Council on Clinical, C. Stroke C. Potential impact and study considerations of metabolomics in cardiovascular health and disease: a scientific statement from the American heart association. Circ. Cardiovasc. Genet. 2017;10(2) doi: 10.1161/HCG.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S., Dunlop A.L., Jones D.P., Corwin E.J. High-resolution metabolomics: review of the field and implications for nursing science and the study of preterm birth. Biol. Res. Nurs. 2016;18(1):12–22. doi: 10.1177/1099800415595463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beger R.D., Dunn W., Schmidt M.A., Gross S.S., Kirwan J.A., Cascante M., Brennan L., Wishart D.S., Oresic M., Hankemeier T., Broadhurst D.I., Lane A.N., Suhre K., Kastenmuller G., Sumner S.J., Thiele I., Fiehn O., Kaddurah-Daouk R., for "Precision M., Pharmacometabolomics Task Group"-Metabolomics Society, I. Metabolomics enables precision medicine: "A white paper, community perspective". Metabolomics. 2016;12(10):149. doi: 10.1007/s11306-016-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delplancke T.D.J., Wu Y., Han T.L., Joncer L.R., Qi H., Tong C., Baker P.N. Metabolomics of pregnancy complications: emerging application of maternal hair. BioMed Res. Int. 2018;2018:2815439. doi: 10.1155/2018/2815439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heazell A.E.P., Bernatavicius G., Warrander L., Brown M.C., Dunn W.B. A metabolomic approach identifies differences in maternal serum in third trimester pregnancies that end in poor perinatal outcome. Reprod. Sci. 2012;19(8):863–875. doi: 10.1177/1933719112438446. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Lu Y.P., Reichetzeder C., Kalk P., Kleuser B., Adamski J., Hocher B. Maternal PCaaC38:6 is associated with preterm birth - a risk factor for early and late adverse outcome of the offspring. Kidney Blood Press. Res. 2016;41(3):250–257. doi: 10.1159/000443428. [DOI] [PubMed] [Google Scholar]
- 20.Thomas M.M., Sulek K., McKenzie E.J., Jones B., Han T.L., Villas-Boas S.G., Kenny L.C., McCowan L.M.E., Baker P.N. Metabolite profile of cervicovaginal fluids from early pregnancy is not predictive of spontaneous preterm birth. Int. J. Mol. Sci. 2015;16(11):27741–27748. doi: 10.3390/ijms161126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behrman R.E., Butler A.S. National Academies Press; (US)Washington DC: 2007. Preterm Birth: Causes, Consequences, and Prevention. [PubMed] [Google Scholar]
- 22.Corwin E.J., Hogue C.J., Pearce B., Hill C.C., Read T.D., Mulle J., Dunlop A.L. Protocol for the Emory University African American Vaginal, oral, and gut microbiome in pregnancy cohort study. BMC Pregnancy Childbirth. 2017;17(1):161. doi: 10.1186/s12884-017-1357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller G.E., Cohen S., Ritchey A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 24.Bekhbat M., Rowson S.A., Neigh G.N. Checks and balances: the glucocorticoid receptor and NFkB in good times and bad. Front. Neuroendocrinol. 2017;46:15–31. doi: 10.1016/j.yfrne.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce B.D., Biron C.A., Miller A.H. Neuroendocrine-immune interactions during viral infections. Adv. Virus Res. 2001;56:469–513. doi: 10.1016/s0065-3527(01)56036-4. [DOI] [PubMed] [Google Scholar]
- 26.Go Y.M., Walker D.I., Liang Y., Uppal K., Soltow Q.A., Tran V., Strobel F., Quyyumi A.A., Ziegler T.R., Pennell K.D., Miller G.W., Jones D.P. Reference standardization for mass spectrometry and high-resolution metabolomics applications to exposome research. Toxicol. Sci. 2015;148(2):531–543. doi: 10.1093/toxsci/kfv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson J.M., Yu T., Strobel F.H., Jones D.P. A practical approach to detect unique metabolic patterns for personalized medicine. Analyst. 2010;135(11):2864–2870. doi: 10.1039/c0an00333f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu T., Park Y., Li S., Jones D.P. Hybrid feature detection and information accumulation using high-resolution LC-MS metabolomics data. J. Proteome Res. 2013;12(3):1419–1427. doi: 10.1021/pr301053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uppal K., Soltow Q.A., Strobel F.H., Pittard W.S., Gernert K.M., Yu T., Jones D.P. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinf. 2013;14:15. doi: 10.1186/1471-2105-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 31.Uppal K., Walker D.I., Jones D.P. xMSannotator: an R package for network-based annotation of high-resolution metabolomics data. Anal. Chem. 2017;89(2):1063–1067. doi: 10.1021/acs.analchem.6b01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schymanski E.L., Jeon J., Gulde R., Fenner K., Ruff M., Singer H.P., Hollender J. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ. Sci. Technol. 2014;48(4):2097–2098. doi: 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- 33.Wishart D.S., Jewison T., Guo A.C., Wilson M., Knox C., Liu Y., Djoumbou Y., Mandal R., Aziat F., Dong E., Bouatra S., Sinelnikov I., Arndt D., Xia J., Liu P., Yallou F., Bjorndahl T., Perez-Pineiro R., Eisner R., Allen F., Neveu V., Greiner R., Scalbert A. HMDB 3.0--the human metabolome database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith C.A., O’Maille G., Want E.J., Qin C., Trauger S.A., Brandon T.R., Custodio D.E., Abagyan R., Siuzdak G. METLIN: a metabolite mass spectral database. Ther. Drug Monit. 2005;27(6):747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- 36.Li S., Park Y., Duraisingham S., Strobel F.H., Khan N., Soltow Q.A., Jones D.P., Pulendran B. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013;9(7) doi: 10.1371/journal.pcbi.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uppal K., Walker D.I., Liu K., Li S., Go Y.M., Jones D.P. Computational metabolomics: a framework for the million metabolome. Chem. Res. Toxicol. 2016;29(12):1956–1975. doi: 10.1021/acs.chemrestox.6b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia J., Wishart D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinf. 2016;55:14 10 1–14 10 91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 39.Li N., Liu E.Q., Guo J., Pan L., Li B.J., Wang P., Liu J., Wang Y., Liu G.S., Baccarelli A.A., Hou L.F., Hu G. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0082310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Med I. America’s Uninsured Crisis: Consequences for Health and Health Care; 2009. America’s Uninsured Crisis: Consequences for Health and Health Care; pp. 1–214. [PubMed] [Google Scholar]
- 41.Merklinger-Gruchala A., Jasienska G., Kapiszewska M. Short interpregnancy interval and low birth weight: a role of parity. Am. J. Hum. Biol. 2015;27(5):660–666. doi: 10.1002/ajhb.22708. [DOI] [PubMed] [Google Scholar]
- 42.Schimmel M.S., Bromiker R., Hammerman C., Chertman L., Ioscovich A., Granovsky-Grisaru S., Samueloff A., Elstein D. The effects of maternal age and parity on maternal and neonatal outcome. Arch. Gynecol. Obstet. 2015;291(4):793–798. doi: 10.1007/s00404-014-3469-0. [DOI] [PubMed] [Google Scholar]
- 43.Sharifzadeh F., Kashanian M., Jouhari S., Sheikhansari N. Relationship between pre-pregnancy maternal BMI with spontaneous preterm delivery and birth weight. J. Obstet. Gynaecol. 2015;35(4):354–357. doi: 10.3109/01443615.2014.968101. [DOI] [PubMed] [Google Scholar]
- 44.Verheij M., Bose R., Lin X.H., Yao B., Jarvis W.D., Grant S., Birrer M.J., Szabo E., Zon L.I., Kyriakis J.M., Haimovitz-Friedman A., Fuks Z., Kolesnick R.N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380(6569):75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 45.Pace T.W., Wingenfeld K., Schmidt I., Meinlschmidt G., Hellhammer D.H., Heim C.M. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav. Immun. 2012;26(1):13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 46.Berger M., Sarnyai Z. "More than skin deep’’: stress neurobiology and mental health consequences of racial discrimination. Stress. 2015;18(1):1–10. doi: 10.3109/10253890.2014.989204. [DOI] [PubMed] [Google Scholar]
- 47.Beard J.H., Morrison C.N., Jacoby S.F., Dong B.D., Smith R., Sims C.A., Wiebe D.J. Quantifying disparities in urban firearm violence by race and place in Philadelphia, Pennsylvania: a cartographic study. Am. J. Publ. Health. 2017;107(3):371–373. doi: 10.2105/AJPH.2016.303620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galin J., Abrams B., Leonard S.A., Matthay E.C., Goin D.E., Ahern J. Living in violent neighbourhoods is associated with gestational weight gain outside the recommended range. Paediatr. Perinat. Epidemiol. 2017;31(1):37–46. doi: 10.1111/ppe.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mode N.A., Evans M.K., Zonderman A.B. Race, neighborhood economic status, income inequality and mortality. PloS One. 2016;11(5) doi: 10.1371/journal.pone.0154535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeFranco E.A., Hall E.S., Muglia L.J. Racial disparity in previable birth. Am. J. Obstet. Gynecol. 2016;214(3):394 e1–7. doi: 10.1016/j.ajog.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 51.Martin J.A., Hamilton B.E., Osterman M.J., Driscoll A.K., Mathews T.J. Births: final data for 2015. Natl. Vital Stat. Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 52.Rider C.F., Carlsten C. Air pollution and resistance to inhaled glucocorticoids: evidence, mechanisms and gaps to fill. Pharmacol. Ther. 2019;194:1–21. doi: 10.1016/j.pharmthera.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Bel E.H. Smoking: a neglected cause of glucocorticoid resistance in asthma. Am. J. Respir. Crit. Care Med. 2003;168(11):1265–1266. doi: 10.1164/rccm.2309009. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y., Albert D., McMahon R.J., Dodge K., Dick D., Conduct Problems Prevention Research G. Glucocorticoid receptor (NR3C1) gene polymorphism moderate intervention effects on the developmental trajectory of african-American adolescent alcohol abuse. Prev. Sci. 2018;19(1):79–89. doi: 10.1007/s11121-016-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fowden A.L., Li J., Forhead A.J. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc. Nutr. Soc. 1998;57(1):113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- 56.Wu G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010;1(1):31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahimi N., Razi F., Nasli-Esfahani E., Qorbani M., Shirzad N., Larijani B. Amino acid profiling in the gestational diabetes mellitus. J. Diabetes Metab. Disord. 2017;16:13. doi: 10.1186/s40200-016-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Besedovsky H., del Rey A., Sorkin E., Dinarello C.A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 59.Miller G.E., Chen E., Fok A.K., Walker H., Lim A., Nicholls E.F., Cole S., Kobor M.S. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U. S. A. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kauffman A.S., Bojkowska K., Rissman E.F. Critical periods of susceptibility to short-term energy challenge during pregnancy: impact on fertility and offspring development. Physiol. Behav. 2010;99(1):100–108. doi: 10.1016/j.physbeh.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan Y.K., Loh P.S. Handedness in man: the energy availability hypothesis. Med. Hypotheses. 2016;94:108–111. doi: 10.1016/j.mehy.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Kind K.L., Moore V.M., Davies M.J. Diet around conception and during pregnancy--effects on fetal and neonatal outcomes. Reprod. Biomed. Online. 2006;12(5):532–541. doi: 10.1016/s1472-6483(10)61178-9. [DOI] [PubMed] [Google Scholar]
- 63.Della Torre S., Benedusi V., Fontana R., Maggi A. Energy metabolism and fertility: a balance preserved for female health. Nat. Rev. Endocrinol. 2014;10(1):13–23. doi: 10.1038/nrendo.2013.203. [DOI] [PubMed] [Google Scholar]
- 64.King J.C. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J. Nutr. 2003;133(5 Suppl 2):1732S–1736S. doi: 10.1093/jn/133.5.1732S. [DOI] [PubMed] [Google Scholar]
- 65.Go Y.M., Jones D.P. Redox theory of aging: implications for health and disease. Clin. Sci. (Lond.) 2017;131(14):1669–1688. doi: 10.1042/CS20160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones D.P., True H.D., Patel J. Leukocyte trafficking in cardiovascular disease: insights from experimental models. Mediat. Inflamm. 2017;2017:9746169. doi: 10.1155/2017/9746169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez-Candales A., Hernandez Burgos P.M., Hernandez-Suarez D.F., Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J. Nat. Sci. 2017;3(4) [PMC free article] [PubMed] [Google Scholar]
- 68.Coussons-Read M.E., Lobel M., Carey J.C., Kreither M.O., D’Anna K., Argys L., Ross R.G., Brandt C., Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav. Immun. 2012;26(4):650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olson D.M., Severson E.M., Verstraeten B.S., Ng J.W., McCreary J.K., Metz G.A. Allostatic load and preterm birth. Int. J. Mol. Sci. 2015;16(12):29856–29874. doi: 10.3390/ijms161226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang S., Ding Z., Liu H., Chen Z., Wu J., Zhang Y., Yu Y. Association between mental stress and gestational hypertension/preeclampsia: a meta-analysis. Obstet. Gynecol. Surv. 2013;68(12):825–834. doi: 10.1097/OGX.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 71.Corwin E.J., Pajer K., Paul S., Lowe N., Weber M., McCarthy D.O. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav. Immun. 2015;49:86–93. doi: 10.1016/j.bbi.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ornoy A., Weinstein-Fudim L., Ergaz Z. Prenatal factors associated with autism spectrum disorder (ASD) Reprod. Toxicol. 2015;56:155–169. doi: 10.1016/j.reprotox.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 73.Deevska G.M., Sunkara M., Morris A.J., Nikolova-Karakashian M.N. Characterization of secretory sphingomyelinase activity, lipoprotein sphingolipid content and LDL aggregation in ldlr-/- mice fed on a high-fat diet. Biosci. Rep. 2012;32(5):479–490. doi: 10.1042/BSR20120036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang X.C., Paultre F., Pearson T.A., Reed R.G., Francis C.K., Lin M., Berglund L., Tall A.R. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2000;20(12):2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 75.Huang H., Kasumov T., Gatmaitan P., Heneghan H.M., Kashyap S.R., Schauer P.R., Brethauer S.A., Kirwan J.P. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity. 2011;19(11):2235–2240. doi: 10.1038/oby.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boon J., Hoy A.J., Stark R., Brown R.D., Meex R.C., Henstridge D.C., Schenk S., Meikle P.J., Horowitz J.F., Kingwell B.A., Bruce C.R., Watt M.J. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62(2):401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boyle A.K., Rinaldi S.F., Norman J.E., Stock S.J. Preterm birth: inflammation, fetal injury and treatment strategies. J. Reprod. Immunol. 2017;119:62–66. doi: 10.1016/j.jri.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 78.Kahraman S., Dirice E., De Jesus D.F., Hu J., Kulkarni R.N. Maternal insulin resistance and transient hyperglycemia impact the metabolic and endocrine phenotypes of offspring. Am. J. Physiol. Endocrinol. Metab. 2014;307(10):E906–E918. doi: 10.1152/ajpendo.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carta S., Castellani P., Delfino L., Tassi S., Vene R., Rubartelli A. DAMPs and inflammatory processes: the role of redox in the different outcomes. J. Leukoc. Biol. 2009;86(3):549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- 80.Jones D.P. Redefining oxidative stress. Antioxidants Redox Signal. 2006;8(9–10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 81.Pekala J., Patkowska-Sokola B., Bodkowski R., Jamroz D., Nowakowski P., Lochynski S., Librowski T. L-carnitine--metabolic functions and meaning in humans life. Curr. Drug Metabol. 2011;12(7):667–678. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- 82.Du J., McEwen B., Manji H.K. Glucocorticoid receptors modulate mitochondrial function: a novel mechanism for neuroprotection. Commun. Integr. Biol. 2009;2(4):350–352. doi: 10.4161/cib.2.4.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walsh C.P., Ewing L.J., Cleary J.L., Vaisleib A.D., Farrell C.H., Wright A.G.C., Gray K., Marsland A.L. Development of glucocorticoid resistance over one year among mothers of children newly diagnosed with cancer. Brain Behav. Immun. 2018;69:364–373. doi: 10.1016/j.bbi.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geronimus A.T. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn. Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 85.Hudson D.L., Neighbors H.W., Geronimus A.T., Jackson J.S. Racial discrimination, John Henryism, and depression among african Americans. J. Black Psychol. 2016;42(3):221–243. doi: 10.1177/0095798414567757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colen C.G., Geronimus A.T., Bound J., James S.A. Maternal upward socioeconomic mobility and black-white disparities in infant birthweight. Am. J. Publ. Health. 2006;96(11):2032–2039. doi: 10.2105/AJPH.2005.076547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Green N.S., Damus K., Simpson J.L., Iams J., Reece E.A., Hobel C.J., Merkatz I.R., Greene M.F., Schwarz R.H., March Of Dimes Scientific Advisory Committee On, P Research agenda for preterm birth: recommendations from the March of Dimes. Am. J. Obstet. Gynecol. 2005;193(3 Pt 1):626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 88.Hogan V.K., Richardson J.L., Ferre C.D., Durant T., Boisseau M. A public health framework for addressing black and white disparities in preterm delivery. J. Am. Med. Women’s Assoc. 2001;56(4):177–180. (1972) 205. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.