SUMMARY
In mammals with binocular vision, integration of the left and right visual scene relies on information in the center visual field, which are relayed from each retina in parallel and merge in the primary visual cortex (V1) through the convergence of ipsi- and contralateral geniculocortical inputs as well as transcallosal projections between two visual cortices. The developmental assembly of this binocular circuit, especially the transcal-losal pathway, remains incompletely understood. Using genetic methods in mice, we found that several days before eye-opening, retinal and callosal activities drive massive apoptosis of GABAergic chandelier cells (ChCs) in the binocular region of V1. Blockade of ChC elimination resulted in a contralateral eye-domi-nated V1 and deficient binocular vision. As pre-vision retinal activities convey the left-right organization of the visual field, their regulation of ChC density through the transcallosal pathway may prime a nascent binocular territory for subsequent experience-driven tuning during the post-vision critical period.
In Brief
Wang et al. demonstrate that during the development of primary visual cortex, spontaneous retinal activities before vision onset coordinate with callosal inputs to eliminate a large fraction of GABAergic chandelier interneuron in the binocular zone (BZ). This may prime a nascent BZ for subsequent visual experience-dependent tuning of binocular vison.
INTRODUCTION
In mammals with more front-oriented eyes, the seamless integration of the left and right visual scene into binocular vision is crucial for many visually guided behaviors. Information about the central visual field is relayed from the left and right temporal retinas separately to the lateral geniculate nuclei (LGN) and converges in the lateral regions of V1, where neurons respond to inputs from both eyes, defining the binocular zone (BZ) (Seabrook et al., 2017). In the rodent visual system, over 95% of the retinal fibers cross at the optic chiasm (Cowey and Perry, 1979; Lund, 1965; Sefton and Dreher, 1995), and direct ipsilateral eye input to V1 is very limited. Surprisingly, however, most cortical neurons in the lateral segment of rodent V1, which maps the central part of the visual field, are highly binocular (Gordon and Stryker, 1996). This is because in addition to the geniculo-cortical pathway, a set of V1 pyramidal neurons (PyNs) projects to the contralateral BZ through a transcallosal pathway, which also contributes to binocular vision. Up to 40%–50% of ipsilateral eye V1 responses in rodents derive from the transcallosal pathway (Cerri et al., 2010; Lee et al., 2019; Pietrasanta et al., 2012; Restani et al., 2009; Zhao et al., 2013), indicating that callosal inputs play a significant role in binocular vision. The developmental assembly of functional binocular circuits from the retina to visual cortex—especially the role of the transcallosal pathway—is not well understood.
Seminal studies demonstrated that visual experience-dependent tuning of V1 binocular properties takes place during a postnatal critical period in juvenile life (Wiesel and Hubel, 1965), a principle of neural plasticity that manifests across mammalian species (Hensch, 2005). Even before vision onset, however, the spatial and topographic organizations of retinal inputs convey key features of the visual world, including the vertical meridian bridging the left and right visual field. This information is relayed along the visual pathway to V1 as spatiotemporal patterns of spontaneous activity within and between the two eyes (Ackman et al., 2012; Thompson et al., 2017). How this early retinal activity coordinates with the developing geniculo-cortical and transcallosal pathways to shape the binocular properties in V1 before vision onset is unknown. In particular, the role of cortical GABAergic interneurons in this process has not been explored.
Among diverse cortical interneurons, chandelier cells (ChCs) are one of the most distinctive types that selectively innervate pyramidal neurons (PyNs) at axon initial segments, the spike initiation site (Somogyi et al., 1982). As a single ChC innervates hundreds of PyNs, ChCs may exert powerful control over the firing of PyN ensembles (Lu et al., 2017; Tamás and Szabadics, 2004; Woodruff et al., 2010). As such, the proper integration of ChCs into V1 neural circuits may significantly shape visual response properties. However, the role of ChCs in visual cortex processing and development has not been studied due to the lack of specific experimental access. Using genetic labeling and manipulation (Lu et al., 2017; Taniguchi et al., 2013), we have characterized the distribution and circuit integration of ChCs in the developing mouse visual cortex. Surprisingly, we observed a ~50% reduction of ChC density at the BZ compared with the surrounding visual areas during the second postnatal week, which resulted from a massive ChC apoptosis. This ChC elimination is triggered by transcallosal input as well as retinal activity during the several days prior to eye opening. Notably, blocking ChC elimination in the BZ resulted in selective weakening of the ipsilateral eye responsiveness, leading to a contralateral-dominated V1 and profound deficit in binocular vision. Our results suggest that patterned spontaneous retinal activities, which reflect the left-center-right organization of the visual world, regulate the developmental integration of a specific cortical inhibitory interneuron type through coordination with the transcallosal pathway, thereby priming a V1 territory for subsequent experience-dependent tuning of binocular visual function.
RESULTS
Massive ChC Elimination at the Binocular Zone during Second Postnatal Week before Vision
We labeled ChCs through targeting their embryonic progenitors by tamoxifen administration to pregnant Nkx2.1-CreER;Ai14 mice (Taniguchi et al., 2013; Wang et al., 2019). This method allows tracking the entire developmental trajectory of ChCs from their birth in embryonic medial ganglionic eminence (MGE) to their migration and circuit integration in mature cortex, including examining their distribution across primary (V1) and lateral secondary visual areas (V2L) (Figures 1A, 1B, and S1). To demarcate the subregion of BZ that receives callosal input, we injected AAV-GFP into the contralateral hemisphere and visualized callosal axons that innervate the border between V1 and V2L, which was additionally identified by the expression pattern of type 2 muscarinic acetylcholine receptors (Wang et al., 2011) (Figures 1A, 1B, and S1; STAR Methods). We define this callosal recipient subregion of the BZ as the callosal binocular zone (cBZ), which is essentially the V1/V2L border region. We quantified the density of layer 2 (L2) ChCs along the anterior-posterior axis of mature visual cortex (>P28) and found that the density in the cBZ was only half that of neighboring V1 or V2L (Figures 1K–1M). Notably, there was no areal difference in ChC density between the medial monocular zone and medial secondary visual areas (V2M), indicating that the differential distribution of ChCs is a unique property at the cBZ of V1 (Figures S2D and S2E). Importantly, the decrease in ChCs at the cBZ is not evident in other interneuron types such as parvalbumin (PV) or calretinin (CR) interneurons (Figures S2A–S2C). Although we observed a decrease in the number of both PV and CR expressing cells in V2L, which is consistent with previous reports (Kim et al., 2017), their density at cBZ is similar to the rest of V1. Furthermore, reduced ChC density was not evident in other cortical regions receiving callosal projections, such as the S1–S2 (Figures S2F–S2H) and M1–S1 borders (Figures S2I–S2K).
Figure 1. Massive ChC Elimination at the Binocular Zone during Second Postnatal Week.
(A) Schematic of experimental design. AAV-EGFP was injected into visual cortex at P0 and ChC density in contralateral cortex analyzed at indicated time points.
(B) Example AAV-EGFP-labeled callosal axon projection into contralateral V1/V2L border region where ChCs express RFP. The region that received callosal input is labeled as callosal binocular zone (cBZ).
(C, E, G, I, and K) Left: example coronal image of ChC distribution at P7 (C), P10 (E), P12 (G), P14 (I), and >P28 (K). White line marks the cBZ. Right: example ChC distribution across the visual cortical areas at P7, P10, P12, P14, and P28. The coronal sections were aligned at the medial most edge of V1.
(D, F, H, J, and L) Mean ChC density in V1, cBZ, and V2L across development: P7 (D) (n = 13 animals), P10 (F) (n = 12 animals), P12 (H) (n = 4 animals), P14 (J) (n = 13 animals), and >P28 (L) (n = 16 animals).
(M) Normalized ChC density to V1 across development. At P7, ChC density at the cBZ is initially higher than V1; ChCs are progressively eliminated between P7–P14, reaching adult-like distribution by P14. P7 and P10 data were not normally distributed; therefore Wilcoxon matched-pairs signed-rank test was used. *p < 0.05, **p < 0.01, ***p < 0.001. All statistics performed with paired t test unless stated otherwise. Scale bar, 200 μm.
See also Figures S1, S2, and S3.
To explore the developmental basis of ChC density reduction in cBZ, we first characterized the developmental profile of ChC distribution across the visual cortex at multiple postnatal ages. Following their generation from the MGE (Sultan et al., 2018; Taniguchi et al., 2013) and migration into the cortex (Taniguchi et al., 2013), a large subset of young ChCs settled in L2 by the end of the first postnatal week and was densely distributed across cortical areas including V1 and V2L (Figure 1C) (Taniguchi et al., 2013). Interestingly, ChC density at P7 was initially significantly higher in cBZ than in V1 (Figures 1C, 1D, and 1M). Thereafter, ChC density progressively decreased in the second postnatal week in the cBZ, attaining a similar density as V1 by P10 (Figures 1E, 1F, and 1M) and further reduced to only half that of neighboring V1 and V2L by P14 (Figures 1G–1J and 1M); a density similar to that of P28 (Figures 1K–1M).
In addition to the reduction of ChC density in cBZ relative to V2L, we also observed an overall reduction of ChC density across the visual cortex (Figures 1C–1L and S3). This is consistent with previous findings of the developmental elimination of GABAergic neurons in early postnatal period (Southwell et al., 2012; Wong et al., 2018), suggesting that ChC reduction is a component of this more general process. To quantify this broad developmental reduction of ChCs across visual cortex, we compared ChC density separately within V1, cBZ, and V2L across development among littermates to control for the inherent variability of tamoxifen induction that labeled ChCs across different litters. We found a substantial reduction of ChC density between P7 and P10 across all three visual areas. Interestingly, whereas ChC elimination in V1 and V2L effectively ceased by P10, ChC elimination in the cBZ continued between P10–P14 (Figure S3). Therefore, the massive reduction of ChC density in cBZ relative to V1 in the second postnatal week was superimposed upon a more general and likely earlier epoch of developmental reduction of ChCs across the visual cortex.
Developmental ChC Elimination Is Mediated through Apoptosis
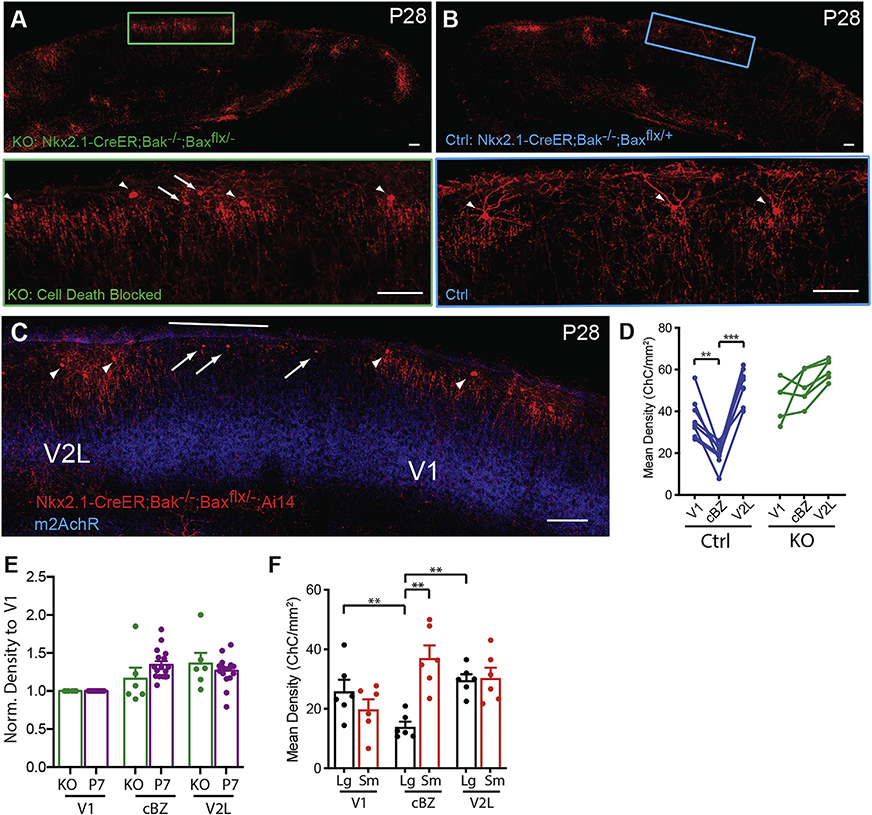
There is evidence that cortical GABAergic neurons undergo apoptosis during early postnatal life (Southwell et al., 2012; Wong et al., 2018), but the mechanism and function of this process is not fully understood. To investigate the cellular mechanism of ChC elimination, we blocked apoptosis in ChCs by crossing Nkx2.1-CreER;A14 mice to Bak−/−Baxf/− conditional knockout mice (Wong et al., 2018). At P28, the ChC density in the cBZ of these mice was comparable to that of nearby V1 and V2L (Figure 2A–D) and closely resembled the distribution pattern of wild-type (WT) P7 mice (before ChC elimination; normalized density shown in Figure 2E). Interestingly, we observed two readily distinguishable populations of ChCs in apoptosis-blocked mutants: one with normal morphology and another with a dramatically smaller cell body and short neurites that were concentrated at the cBZ (Figure 2F). The atrophic ChCs likely represent those that would have been eliminated during normal development, but remained due to apoptosis blockade. By analyzing the two ChC populations separately, we found that significantly more atrophic ChCs were located at the cBZ than the neighboring areas, whereas the distribution of ChCs with normal morphology closely resembled that of the visual cortex in WT mice (Figure 2F). These results indicate that the developmental elimination of ChCs in visual cortex is mediated by apoptosis, and this process is specifically enhanced in the cBZ relative to neighboring visual areas.
Figure 2. Developmental Chandelier Cell Elimination Is Mediated through Apoptosis.
(A and B) Blockade of apoptosis in visual cortex of Nkx2.1-CreER;Bak−/−;Baxf/− mouse resulted in more ChCs (A) than controls (B).
(C) More ChCs remain at cBZ in apoptosis blocked mice.
(D) More ChCs remain at cBZ in knockout animals (n = 6 animals) than the controls (n = 8 animals).
(E) The distribution pattern of ChCs in knockout adult animals closely resembles the pattern in wild-type P7 mice before ChC elimination.
(F) Most of the remaining RFP+ cells at the cBZ are small atrophic ChCs that are destined to die but are blocked from apoptosis. Arrows point to small atrophic ChCs (sm). Arrowheads point to normal ChCs (Lg). *p < 0.05, **p < 0.01, ***p < 0.001. All statistics performed with paired t test unless stated otherwise. Scale bar, 100 μm. Data are represented as mean ± SEM.
trans-Callosal Axons and Activity Regulate ChC Elimination at the cBZ
To explore the role of neural activity in ChC apoptosis, we considered the major excitatory input sources to developing L2 ChCs. L2 ChC2s extend their prominent apical dendrites in layer 1 and likely receive local glutamatergic inputs from nearby pyramidal neurons and long-range inputs from other ipsi-lateral cortical areas, thalamus, and contralateral cortex. Among these, the contralateral callosal input specifically targets the BZ and is thus uniquely poised to influence ChCs in the BZ, compared with those in neighboring visual areas. Furthermore, the innervation and elaboration of callosal axons in the upper layers of V1 (Mizuno et al., 2007; Wang et al., 2007) parallels the time course of L2 ChC apoptosis. In contrast, the thalamic inputs invade V1 as early as P0 (Kageyama and Robertson, 1993), which precedes ChC apoptosis; and the inputs from higher visual areas do not innervate V1 until around P14, after the completion of most ChC apoptosis (Dong et al., 2004). We therefore investigated whether there was a link between callosal input and ChC apoptosis in the cBZ. We used in utero electroporation at embryonic day 15.5 (E15.5) to label L2/3 callosal PyNs with EGFP (Figure 3A). Callosal axons extended to the contralateral hemisphere and arborized densely in a restricted border region between V1 and V2L (Figures 3B and 3C). Co-electroporation of a potassium channel Kir2.1, which reduces neuronal activity, dramatically reduced the growth and invasion of callosal axons into contralateral cortex (Figures 3D and 3E), consistent with previous findings (Mizuno et al., 2007; Wang et al., 2007). Importantly, reduced callosal axon innervation resulted in an increase in ChC density at the cBZ (Figures 3D–3F), suggesting that callosal axon invasion promotes ChC elimination.
Figure 3. Transcallosal Axons and Activity Regulate ChC Elimination at the cBZ.
(A) Experimental design with CAG-Kir 2.1-EGFP or CAG-EGFP electroporation at E15.5 targeting layer 2/3 callosal PyNs in visual cortex and tamoxifen labeling of ChCs at E17.5.
(B and C) In a control animal, EGFP-labeled V1 L2/3 PyNs sent callosal axons innervating the contralateral cBZ (C1) where few ChCs were present (C2).
(D) In Kir2.1 in utero electroporated animals, there was a reduction in the callosal axonal projection to cBZ (D1) that prevented ChC elimination at cBZ (D2).
(E) Cumulative distribution of callosal axon intensity at the contralateral cBZ showing lack of projections in Kir2.1-expressing animals (p < 0.001, Kolmogorov-Smirnov [K-S] test), but normal projection intensity in GFP-expressing animals.
(F) Mean ChC density across V1 and V2L showing rescue of ChCs at the cBZ in Kir2.1-expressing animals (n = 8 animals) compared to GFP controls (n = 4 animals).
(G) Schematic of suppressing callosal activity with H4MDi-Citrine electroporation at E15.5 and daily CNO intraperitoneal (i.p.) injections between P8–P14.
(H and I) Silencing callosal neuron activity between P8–P14 did not affect callosal projection into contralateral cortex (H1, I1) but prevented ChC elimination at the cBZ (H2, I2).
(J) Cumulative distribution of callosal axon intensity at the contralateral cBZ showing normal projection intensity in H4MDi electroporated animals treated with saline or CNO between P8-P14 (K-S test).
(K) Mean ChC density across V1 and V2L showing rescue of ChCs at the cBZ in H4MDi-expressing animals treated with CNO (n = 7 animals) compared with saline controls (n = 5 animals). *p < 0.05, **p < 0.01, ***p < 0.001. All statistics performed with paired t test unless stated otherwise. Scale bar, 200 μm.
See also Figures S4, S5, S6, and S7.
To examine the role of callosal activity in regulating ChC survival, we expressed Hm4Di (inhibitory DREADD receptor) (Zhu and Roth, 2014) in L2/3 PyNs via electroporation at E15.5 (Figures 3G–3K) or via cortical injection of an AAV vector at P0–P1 (Figure S4). Application of clozapine-N-oxide (CNO) suppressed firing of V1 PyNs in vitro (Figures S5A–S5E) and decreased wide-field spontaneous neuronal activity in vivo (Figures S5F–S5I). In addition, the chronic experiments revealed that ~19 h after the last CNO dose, both V1 cortices were active but had a significant decrease in spontaneous activation frequency in the inhibitory DREADD-treated hemisphere (Figure S6; V1 right). Indeed, chronic suppression of PyN activity via CNO led to reduced inter-hemispheric activity correlations without affecting within-hemisphere correlations (2-way ANOVA between treatment and visual subregion; F = 4.44, p = 0.02) (Figure S6). Importantly, daily application of CNO from P8–P14 prevented ChC death at ~30, leading to significantly higher ChC density in the cBZ compared to saline controls (Figures 3H, 3I, and 3K), without affecting callosal projection patterns (Figure 3J). To assay whether CNO itself affects ChC elimination, we injected AAV-GFP into the visual cortex at P0 with CNO application from P8–P14 and found that CNO did not influence callosal projections or ChC elimination in the absence of Hm4Di (Figures S7A–S7E). Together these results suggest that ChC elimination at the cBZ is regulated by callosal PyN activity, likely through coordinating callosal neuron activity across the two hemispheres.
The precision of callosal projections to the contralateral V1/V2L border region is shaped by retinal inputs during a perinatal critical period (Olavarria et al., 1987). Previous studies in the rat have shown that perinatal monocular enucleation (ME) induces broadened and ectopic callosal projections into V1 (Olavarria et al., 1987). We therefore investigated the role of developing retinal inputs on callosal axon projection to V1 in mice and the impact on ChCs at cBZ. We found that P0 ME in mice also resulted in broadened callosal terminations in the contralateral visual hemisphere that expanded into the lateral portion of V1 (Figures 4A, 4B, 4D, and 4F). Importantly, this led to a correspondingly expanded reduction in ChC density (Figures 4C, 4E, and 4G), indicating that invading callosal axons were sufficient to eliminate ChCs, even in ectopic locations. Consistent with this notion, we found that ChC density in the lateral V1, which was innervated by the expanded callosal projections, was lower than the callosal-free medial V1 (Figure 4G). These results suggest that the impact of callosal axons and activity on ChC density at the cBZ is powerfully regulated by the early ascending inputs from the retina, and these perinatal retinal inputs not only play a crucial role in shaping transcallosal axon projections to the cBZ but also regulate ChC survival at the cBZ.
Figure 4. Ectopic Callosal Axon Projections Promotes ChC Elimination within V1.
(A) Schematic of experimental design with monocular inoculation at P0–P1 and AAV-EGFP cortical injection at ~30.
(B and C) Control animal with normal callosal projection to cBZ (B) and lack of ChCs at cBZ (C).
(D and E) ME animal with broad and ectopic callosal projections into V1 (D) and a reduction in ChC density within V1 (E).
(F) Widening of callosal projection axon innervations into V1 in ME animals at P0 (p < 0.001, K-S test).
(G) Reduction in ChC density within lateral portion of V1 in ME animals due to widening of callosal recipient zone (control: n = 7 animals; ME: n = 8 animals). *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar, 100 μm. All statistics performed with paired t test unless stated otherwise. Data are represented as mean ± SEM.
Retinal Activity during Second Postnatal Week Promotes ChC Elimination
Prior to eye opening, developing retinal ganglion cells generate spontaneous and correlated waves of activity that sweep across the retina (Arroyo and Feller, 2016; Kirkby et al., 2013; Rossi et al., 2001; Thompson et al., 2017) and are enhanced by light just prior to eye opening (Tiriac et al., 2018). Retinal waves preferentially initiate in the ventral-temporal retina, which relays information about the binocular visual field to the BZ region of cortex where transcallosal axons terminate (Ackman et al., 2012). To examine how retinal activity might influence the bilateral coordination of cortical activity and ChC survival, we enucleated one eye at P6, prior to ChC elimination in the cBZ (Figure 5A). Whereas callosal projections were not affected (Figure 5B), consistent with a previous study (Olavarria et al., 1987), ChCs were spared from apoptosis at the cBZ (Figures 5C and 5D). To further explore the role of retinal activity in this process, we unilaterally blocked retinal activity between P7–P14 through intravitreal injection of the sodium channel blocker, tetrodotoxin (TTX) (Figure 5E). Although callosal projections were not affected by this manipulation (Figures 5F, 5H, and 5J), ChC elimination at the cBZ was compromised (Figures 5G, 5I, and 5K). Notably, bilateral blockade of retinal activity between P7–P14 also reduced ChC elimination at the cBZ (Figures 5L–5O), suggesting that the overall level and coordination of retinal activity promotes ChC elimination in the cBZ.
Figure 5. Retinal Activity during Second Postnatal Week Promotes ChC Elimination at the cBZ.
(A) Time course of tamoxifen induction at E17.5, and schematic of monocular enucleation (ME) at P6 and AAV-EGFP at ~30.
(B and C) Example image of callosal projection (B) and ChC distribution (C) at the cBZ between V1 and V2L in ME animals (n = 6 animals).
(D) Mean ChC density across V1and V2L showing ME at P6 prevents ChC elimination at the cBZ.
(E) Schematic of manipulating retinal activity with intravitreal TTX injection.
(F and G) In H2O intravitreal-injected control animal, EGFP-labeled callosal projection to the contralateral cBZ (F) with few ChCs (G).
(H and I) Example of TTX-injected animals with EGFP-labeled callosal axons (H) and ChCs remaining at the cBZ (I).
(J) Similar callosal projection intensity at cBZ between TTX- and H2O-treated controls (p = 0.32, K-S test).
(K) Mean ChC density across V1, cBZ, and V2L showing rescue of ChCs at the cBZ after TTX injection (control: n = 6 animals; TTX: n = 12 animals). These data were not normally distributed; therefore Wilcoxon matched-pairs signed-rank test was used.
(L) Time course of tamoxifen induction at E17.5, and schematic of daily binocular TTX retinal injection between P7–P14.
(M and N) Example image of ChC distribution at the cBZ between V1 and V2L in H2O control (M) and TTX-injected (N) animals.
(O) Decrease in ChC density at the cBZ in H2O control (n = 3 animals), but not binocular TTX-treated animals (n = 6 animals).
(P) Time course of tamoxifen induction at E17.5, dark rearing between P7–P14 and AAV-EGFP at ~30.
(Q and R) Example image of callosal projection (Q) and ChC distribution (R) at the cBZ between V1 and V2L in ME animals (n = 6 animals).
(S) Mean ChC density across V1 and V2L showing dark rearing between P7–P14 (n = 5 animals) does not affect ChC elimination at the cBZ. *p < 0.05, **p < 0.01, ***p < 0.001. All statistics performed with paired t test unless stated otherwise. Scale bar, 200 μm.
A recent study showed that even before eye opening, light stimuli play a role in promoting spontaneous activity in the retina (Tiriac et al., 2018). To dissociate the role of inherent and light-regulated retinal activity on ChCs in the cBZ, we eliminated light stimuli before eye opening, by placing the animals in complete darkness between P7–P14, and then assayed the distribution of ChCs at ~45 (Figures 5P–5S). We found that dark-reared animals had a similar ChC density as normal reared animals, suggesting that the regulation of ChC density at the cBZ is independent of light stimulation before eye opening. Together, these results suggest that inherent spontaneous activity of the developing retina before eye opening, which propagates along the visual pathway, plays a key role in regulating ChC density in the cBZ by coordinating callosal activity across the two hemispheres.
Excess ChCs at the Binocular Zone Alters Normal Binocularity and Visually Guided Behavior
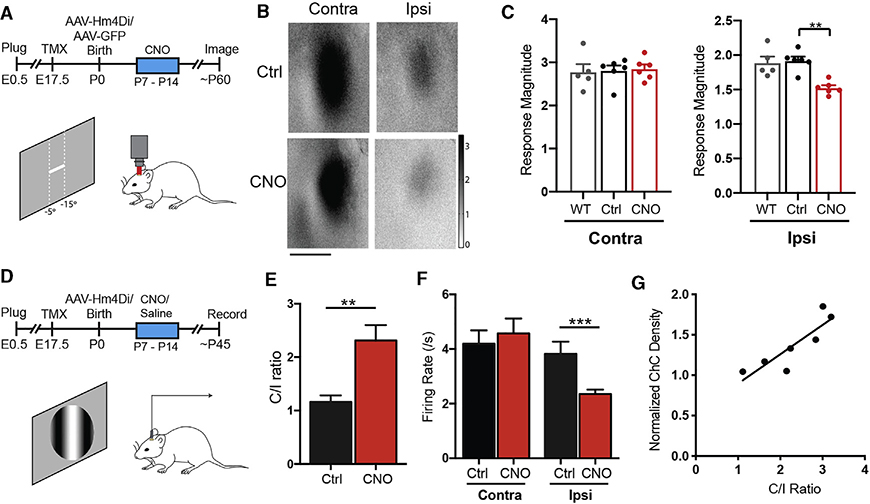
To investigate the functional impact of a developmental reduction of ChC density at the BZ, we first examined neuronal response properties in V1 binocular neurons in animals with excess ChCs following suppression of callosal activity with inhibitory DREADD during the second postnatal week (Figures 3K and S4). We used intrinsic signal optical imaging to measure visual responses in the visual cortex and analyzed the mean response magnitude in the BZ after separate stimulation of each eye to examine potential changes in contra- and/or ipsilateral responses (Figure 6A). We observed a consistent reduction of ipsilateral but not contralateral responses in mice with excess ChCs (Figures 6B and 6C). We substantiated this result by using in vivo single unit extracellular recordings of binocularly responsive neurons in the BZ (Figure 6D). Consistent with the imaging result, we recorded significantly higher contralateral/ipsilateral response ratios (C/I ratio) in mice with excess ChCs than in control mice (Figure 6E), and a reduction of ipsilateral but not contralateral responses in mice with excess ChCs (Figure 6F). Notably, the number of ChCs at the cBZ positively correlated with the shift in ocular dominance (Figure 6G). Monocular response properties, such as orientation selectivity, direction selectivity, spatial frequency preference, and binocular matching of orientation preference were normal in ChC-excess animals (Figures S8A–S8E). Therefore, the persistence of excess ChCs at the cBZ specifically disrupts the binocularity of V1 neurons by suppressing their responses to ipsilateral eye input.
Figure 6. Excess ChCs at the Binocular Zone Alters Normal Binocularity.
(A) Experimental designing for assaying response properties in ChC-excess V1 using intrinsic imaging in lightly anesthetized mice.
(B) Response magnitude maps of binocular visual cortex of adult mice with or without inhibitory DREADD manipulation between P7–P14.
(C) Mean response magnitude expressed as fractional change in light reflectance elicited by stimulation of the contralateral (left) or ipsilateral (right) eye of mice. Inhibitory DREADD between P7–P14 reduces ipsilateral eye responsiveness but does not affect contralateral eye (Mann-Whitney test). WT, no manipulation; Ctrl, AAV-GFP injection at P0 with CNO injections between P7–P14; CNO, AAV-Hm4Di injection at P0 with CNO injections between P7–P14.
(D) Experimental designing for assaying response properties in ChC-excess V1 using extracellular recording in lightly anesthetized mice.
(E) Contralateral/ipsilateral response ratio (C/I ratio) showing a shift in ocular dominance in CNO-treated animals (p < 0.01) (Ctrl, n = 102 cells, 5 animals; CNO-treated, n = 128 cells, 7 animals).
(F) Weakened ipsilateral eye responsiveness in CNO-treated animals (n = 128 cells, 7 animals) than controls (n = 102 cells, 5 animals).
(G) ChCs density at the cBZ is positively correlated with C/I ratio in CNO-treated animals. *p < 0.05, **p < 0.01, ***p < 0.001. All statistics performed with unpaired t test unless stated otherwise. Data are represented as mean ± SEM.
See also Figure S8.
To examine whether changes in binocular properties due to surplus ChCs might impact visual function and visually guided behavior, we first tested these mice using both a virtual optokinetic task (Figure 7A) and forced two-choice visual water maze task (the visual water task; Figure 7D). In the optokinetic test, which is primarily mediated by subcortical visual centers (Douglas et al., 2005), ChC-excess mice exhibited normal spatial visual thresholds, visual acuity (Figure 7B), and contrast sensitivity (Figure 7C). These results suggest that the function of the visual system, as measured via the optokinetic system, was not disrupted in these animals. We next tested whether visual acuity and contrast sensitivity was affected at the cortical level using the visual water task, thresholds measured in which depend on the visual cortex (Prusky and Douglas, 2004). Mice were trained to perform a swimming test of visual discrimination between one monitor displaying a sinusoidal grating and the other of uniform gray (Figure 7D). During the training period, both ChC-excess and control mice learned to perform the task above 70% accuracy after 7–10 sessions (Figure S8F). Spatial acuity was determined by gradually increasing the spatial frequency of the testing grating until the success rate is below 70% (see STAR Methods). ChC-excess and control mice had similar spatial acuity (Figure 7E). We also measured contrast threshold in these mice at a spatial frequency that elicits the highest sensitivity; 0.20 cpd (Prusky and Douglas, 2004). ChC-excess and control mice had similar contrast sensitivity (Figure 7F). Together with in vivo physiology assessment (Figures S8A–S8E), the results show that ChC-excess mice do not have impaired spatial vision in 2D.
Figure 7. Excess ChCs at the Binocular Zone Perturbs Binocularly Guided Visual Behavior.
(A) Schematic of virtual optomotor system for testing visual acuity and contrast sensitivity.
(B) CNO-treated animals showed similar maximum spatial frequency that could elicit head tracking to control animals (control, N = 7; CNO, N = 8).
(C) Contrast sensitivity at six different spatial frequencies were measured with virtual optokinetic stimuli. CNO-treated animals showed similar contrast sensitivity to control animals (control, N = 7; CNO, N = 8).
(D) Schematic of forced two-choice visual water maze task for testing visual acuity and contrast sensitivity.
(E and F) Based on visual water maze task, CNO-treated animals have normal visual acuity (E) (control, N = 5; CNO, N = 5, p = 0.96) and contrast sensitivity at 0.20 cpd spatial frequency (F) (control, N = 5; CNO, N = 5, p = 0.47).
(G) Schematic of visual cliff test.
(H and I) Example trial movement tracking of control (H) and CNO-treated (I) animals.
(J) CNO-treated animals spend more time over at the deep side (31.8% ± 2.3%; n = 22) than the control (17.5% ± 1.3%; n = 10).
(K) Both CNO-treated and control animals make similar numbers of approaches to cross the “cliff” (control, 20.5 ± 1.9; CNO, 25.3 ± 1.9; p = 0.13).
(L) CNO-treated animals cross the “visual cliff” more often than control (total: control, 44.0% ± 6.8%; CNO, 74.0% ± 3.6% of approaches; middle: control,30.7% ± 8.0%; CNO, 69.6% ± 4.0%).
(M) ChCs density at the cBZ is highly correlated with the percentage of crossing in CNO-treated animals. *p < 0.05, **p < 0.01, ***p < 0.001. All statistics performed with unpaired t test unless stated otherwise. Data are represented as mean ± SEM.
See also Figures S7 and S8.
To specifically probe visual function dependent on binocularity, we used a visual cliff test in which a high-contrast checkerboard was placed at two different heights below an open field arena covered by a transparent glass floor. This behavioral paradigm creates the visual illusion of a cliff (Figure 7G), and mice with normal binocular vision tend to avoid crossing the visual cliff and remain in the apparent shallow side (Baroncelli et al., 2013; Castaño-Castaño et al., 2019). In saline-treated controls, mice usually approached the virtual cliff, paused to inspect the cliff, and then frequently retreated to the shallow side of the arena as expected from previous studies (Figure 7H; Video S1). In striking contrast, ChC-excess mice approached the visual cliff at a similar frequency but often without pause, and crossed the cliff without hesitation (Figures 7I–7L; Video S2). Accordingly, ChC-excess crossed the visual cliff significantly more frequently (Figure 7L) spent double the time on the cliff side than controls (Figure 7J). It is possible that mice might use the arena walls to explore and cross the visual cliff independent of visual cue. We therefore analyzed crosses at the sidewalls and center separately. We found that in the center region away from arena walls, ChC-excess mice still crossed the visual cliff significantly more than controls, suggesting a visual deficit (Figure 7L). Notably, the density of ChCs at the cBZ was tightly correlated with the percentage of center crossing in ChC-excess animals (Figure 7M). Furthermore, we performed visual cliff test in the same set of ChC-excess animals that appeared normal visually in both optokinetic test and visual water task and found the these animals crossed visual cliff more often and spent more time in the deep end of the arena (Figures S8G–S8J). As an additional control, CNO application by itself between P8–P14 without inhibitory DREADD, which does not alter ChC density at cBZ (Figures S7A–S7E), did not affect animals’ performance in this task either; it was similar to saline-treated controls (Figures S7F–S7I). Together, these results suggest that ChC-excess mice have a major and specific deficit in binocular vision, possibly including depth perception.
DISCUSSION
The assembly of eye-to-brain connections for binocular vision involves sequential steps from developmental programs guiding axon growth and their topographic organization (Feldheim et al., 1998; Herrera et al., 2003; Petros et al., 2008, 2010), to activity and experience dependent refinement of synaptic connections (Seabrook et al., 2017). In this study, we discovered a crucial step bridging these events. We demonstrate that retinal activity before eye opening, which conveys information about the basic organization of left/right monocular and central binocular visual field, influences the development of the transcallosal pathway in visual cortex, which in turn regulates the density of a powerful inhibitory interneuron type in the binocular region of V1; this is a necessary step for subsequent appropriate development of binocular neural response properties and binocular vision (Figure 8). These findings suggest that an early retinal initiated and neural activity-mediated developmental program, likely common to species with front-oriented eyes, primes the nascent V1 for subsequent experience-dependent plasticity in building cortical circuits for binocular vision. The reduction of ChC-mediated inhibition in the cBZ might also facilitate fast and efficient transcallosal communication (Engel et al., 1991; Kiper et al., 1999; Nowak et al., 1995), which is thought to link left and right V1 neurons with similar retinotopic and receptive field properties for achieving seamless integration of visual scenes, stereopsis, and motion tracking (Gardner and Cynader, 1987; Lee et al., 2019; Peiker et al., 2013; Rochefort et al., 2009; Schmidt et al., 1997).
Figure 8. A Model for Chandelier Cell Elimination and Visual System Development.
(A) In rodents, information in the central visual field is first projected separately to the temporal region of the left and right retina, relayed largely in parallel through the lateral geniculate nuclei (LGN), and converge at the lateral region of the primary visual cortex (V1), defining the binocular zone (BZ). In addition to this convergence of ipsi- and contralateral LGN-V1 inputs, pyramidal neurons at BZ project to the contralateral BZ, forming a transcallosal pathway that also contributes to binocular response properties (defined as callosal binocular zone, cBZ, shown in green).
(B) Chandelier cell elimination takes place between P7 to P14 before eye opening. The timing coincides with axonal projections of transcallosal neurons onto the contralateral hemisphere as well as spontaneous retinal activity before eye opening (Huberman et al., 2008).
(C) At P7, abundance of ChCs at the cBZ may form promiscuous connectivity between ChCs and callosal projecting neurons. As GABAergic transmission may be depolarizing at early postnatal ages, ChCs innervating callosal PyNs might promote their firing, forming a transcallosal loop, which is further driven by coordinated bilateral retinal inputs. Such a transient over-excited network may drive the elimination of “mis-wired” ChCs through apoptosis, resulting a reduction in ChCs by P14.
The developmental enhancement of cortical GABAergic inhibition has been implicated in ocular dominance (OD) plasticity and experience dependent modification of binocular vision during a postnatal critical period after eye opening (Fagiolini and Hensch, 2000; Hensch, 2005; Hensch et al., 1998; Kuhlman et al., 2013). In particular, the maturation of PV-expressing and fast-spiking basket interneurons is crucial in regulating the onset and closure of critical period plasticity between the third and fifth postnatal week in mice (Hanover et al., 1999; Huang et al., 1999). Here, we show that well before the onset of OD plasticity and even before vision onset, the proper circuit integration of ChCs in the cBZ of V1 is regulated by spontaneous retinal activity during the second postnatal week, setting the stage for subsequent experience dependent tuning of binocular vision. Therefore different types of interneurons appear to play distinct roles at different steps during the progressive assembly of cortical circuits for binocular vision. The mechanisms through which ChC elimination in the cBZ promotes the development of binocular responses remain to be elucidated. As the contralateral eye input initially dominates cortical responses at eye opening in rodents (Faguet et al., 2009; Trachtenberg, 2015), a reduction of cortical inhibition through ChC elimination might enable the strengthening of the initially weak ipsilateral responses through either thalamo-cortical and/or transcallosal connections (Cerri et al., 2010; Restani et al., 2009), thereby priming the nascent V1 for subsequent experience-driven OD plasticity during the post-vision critical period (Hensch, 2005). Importantly, disruption of this ChC elimination process resulted in profound deficits in the visual cliff test that requires binocular vision and likely stereopsis (Baroncelli et al., 2013; Castaño-Castaño et al., 2019). Whether such deficit in visually guided behavior is due to a reduced binocularity and/or impairment in binocular integration (i.e., disparity tuning) (Scholl et al., 2013) remains to be investigated.
Recent studies have demonstrated that, during the first postnatal week, the survival of MGE clade PV and SST interneurons depends on the level of excitatory inputs they receive from local PyNs, an activity dependent mechanism through suppressing PTEN-mediated apoptosis for adjusting the appropriate ratio of excitatory and inhibitory neurons that may manifest across cortical areas (Wong et al., 2018). Consistent with this finding, we observe a reduction of ChC density across the developing early postnatal visual cortex (Figure S3) (and across other cortical areas [data not shown]), which might be driven by the same mechanism as that of PV and SST interneurons, such as the level of excitatory inputs from local PyNs. Superimposed upon this broad reduction of GABAergic neurons across the cortex, we have uncovered an additional and distinct process of activity regulated apoptosis of cortical interneurons, which is restrict to ChCs at the cBZ during the second postnatal week. In particular, a substantial set of ChCs are further eliminated by long-range callosal inputs, presumably in parallel and following the more general interneuron apoptosis process, as a crucial step for the proper development of binocular visual circuits.
The mechanisms by which retinal and callosal activity regulate ChC apoptosis at V1 cBZ remains to be determined. It is possible that the young ChCs at cBZ initially innervate both non-callosal and callosal PyNs, while the latter establish bilateral reciprocal excitatory connections. As GABAergic transmission might be depolarizing at this postnatal period (Dehorter et al., 2012; Pan-Vazquez et al., 2020; Rinetti-Vargas et al., 2017; Sauer and Bartos, 2011), ChCs innervating callosal PyNs could promote their firing, forming a transcallosal loop that is further driven by coordinated bilateral retinal inputs. Such a transient over-excited network might drive the elimination of mis-wired ChCs through apoptosis, thereby shaping a fast transcallosal network by preventing ChC-mediated inhibition at later stages. Although early PV and SST cells may also depolarize callosal PyNs, their targeted synapses at the soma and dendrite might be less effective in promoting PyN firing. In addition to the developing visual system, other sensory organs also generate early spontaneous activities prior to sensory input that propagate into the CNS and influence the development of the corresponding sensory cortex (Kirkby et al., 2013; Li and Crair, 2011). Young ChCs in other sensory cortical areas receiving callosal inputs may also be wired into a similar excitatory loop, but the bilateral correlation of peripheral inputs to other areas (e.g., barrel and auditory cortex) (Rock and Apicella, 2015; Shuler et al., 2001) may not be as synchronous and coordinated as in the visual pathway to produce the level of over-excitation that eliminates ChCs in V1. In addition to L2/3, a significant population of ChCs also resides in L5/6. In fact, callosal projection neurons also extend their axon terminals in L5/6 and my play a role in regulating deep layer ChC density and the binocular properties L5/6 pyramidal cells. It is possible that, in addition to the reduction of ChC density, other cellular processes regulated by the transcallosal axons might also contribute to the development of functional binocular circuitry.
Our results begin to reveal how developmental genetic programs and activity regulated mechanisms coordinate in the wiring of a distinct cortical neuron type toward shaping a specific circuit function. Previous studies suggest that the ChC identity is largely specified through MGE lineage progression and birth timing (Sultan et al., 2018; Taniguchi et al., 2013), and young ChCs are endowed with cell intrinsic mechanism for their laminar settlement and synaptic innervation (Taniguchi et al., 2013). Here, we demonstrate that, upon reaching their cortical destination, the circuit integration of ChCs is regulated by neural activity-mediated mechanisms in the developing visual cortex. This is a crucial step through which the organization of the peripheral sense organ, which reflects basic organizational features of the physical world, can prime the cortical territory for experience-dependent tuning of sensory perception.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Z. Josh Huang (huangj@cshl.edu; josh.huang@duke.edu).
Materials Availability
Materials generated in this study are available on request to the Lead Contact.
Data and Code Availability
The datasets and code generated during this study are available upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Adult Nkx2.1 -CreER mice (The Jackson Laboratory stock 014552) were crossed with adult Rosa26-lox-stop-lox-TdTomato (Ai14) reporter (The Jackson Laboratory stock 007914) to produce Nkx2.1 –CreER;Ai14 (het/homo) males, which were then crossed to Swiss Webster or C57B6 (Taconic) females. The pregnant females were induced with tamoxifen (TM) at E17.5 (see Method Details below), and then TX induced Nkx2.1 –CreER;Ai14 (het/homo) offsprings were used for experiments. Neonatal offspring of Emx1-Cre mice (The Jackson Laboratory stock 005628) crossed with Ai95-floxed mice (The Jackson laboratory stock 024105) were used for in vivo calcium imaging experiments. All the male and female offspring age between 1–8 weeks were used in this study. Animals were allocated to experimental groups at random both within litter and across multiple litters.
Mouse Husbandry Procedures
All mouse colonies at Cold Spring Harbor Laboratory (CSHL) were maintained in accordance with husbandry protocols approved by the IACUC (Institutional Animal Care and Use Committee reference number 16–13-09–8) or Yale IACUC, U. S. Department of Health and Human Services and Institution guidelines (for in-vivo calcium imaging experiments using Emx1-cre/Ai95 mice, IACUC, reference number 2017–11141). Mice were housed by gender in groups of 2–4 with access to food and water ad libitum and 12-hour light-dark cycle, except when placed in a breeder pair.
METHOD DETAILS
Viral constructs
AAV-CB7-EGFP was purchased from Penn Vector Core, Philadelphia, PA. AAV-Hsyn-HA-HM4D(Gi)-IRES-mCitrine was purchased from UNC vector core, Chapel Hill, North Carolina. AAV-hSyn1-HA-hM4D(Gi)-IRES-mCitrine-WPRE-hGHp(A) from ETH Zurich stock # V 92–8. pAAV-hSyn-DIO-hM4D(Gi)-mCherry was a gift from Bryan Roth (Addgene viral prep # 44362-AAV8; http://addgene.org/44362; RRID:Addgene_44362).
DNA plasmids
CAG-Kir2.1 plasmid was a gift from Dr. Yu-Qiang Ding. pCAGGS-IRES-EGFP plasmid was a gift from Dr. Linda Van Aelst. pAAV-hSyn-HA-hM4D(Gi)-IRES-mCitrine was a gift from Dr. Bryan Roth (Addgene viral prep # 50464-AAV8; http://addgene.org/50464; RRID:Addgene_50464). pAAV-CAGGS-HA-hM4D(Gi)-IRES-mCitrine plasmid cassettes were assembled and cloned using standard molecular cloning protocols with restriction enzymes from New England Biolabs. CMV-CAAGS promoter was subcloned into pAAV-Hsyn-HA-hM4D(Gi)-IRES_mCitrine replacing Hsyn promoter. All constructs were sequenced to ensure their fidelity and proper reversed orientation of given inserts
Tamoxifen Induction
In order to properly identify embryonic day 17.5 (E17.5) for tamoxifen (TM) inductions, Swiss Webster or C57B6 (Taconic) females were housed with Nkx2.1-CreER;Ai14 (het/homo) males overnight and females were checked for vaginal plug by 8–9 am the following morning. Tamoxifen (TM) was dissolved in corn oil (20 mg/ml) overnight, at room temperature under constant stirring. Stocks were stored as individual aliquots at 4°C degrees for no more than one month. Following light isoflurane anesthesia pregnant females were given oral gavage administration of TM (dose: 3 mg / 30 g of body weight) at gestational day E17.5 (embryonic day 17.5) for dense revealing of ChCs. In rare instances, TM induction lead to dystocia in pregnant females and emergency caesarian sections were performed. Pups retrieved following caesarians were housed with Swiss Webster foster mothers until weaning age.
In utero electroporation
Timed pregnant female mice (crossed to were Nkx2.1-CreER;Ai14) were used for in utero electroporation. The animal was anesthetized, the abdominal cavity was cut open, and the uterine horns were exposed. Approximately 1–2 mg DNA was injected into the lateral ventricle at E15–E16 by using a pulled glass micropipette. The angle of electrical paddles was adjusted to target visual cortex. Square electric pulses (45 V, 50 ms) were passed five times at 1 s intervals. Embryos were put back into the cavity as soon as the electroporation was done. Embryos were allowed to develop normally to birth.
Surgical procedures of stereotaxic injection: adult
Adult animals were anesthetized by inhalation of isoflurane (2%). Mice were mounted in a stereotaxic headframe (Kopf Instruments Model 940 series). Coordinates were identified for primary visual cortex (Adults: 3.0–3.3 mm lateral from the midline and 0.5–0.8 mm anterior from the lambda suture). An incision was made over the scalp, a small burr hole was made into the skull and brain surface was exposed. A pulled glass pipette tip of 20–30 mm containing virus was lowered into the brain. Pulses were delivered using a Picospritzer (General Valve Corp) at a rate of 30 nl/minute; the pipette was left in the brain for 5–10 minutes to prevent backflow. Following the injection, the pipette was withdrawn, the incision was closed with tissue glue, and animals recovered.
Surgical procedures of stereotaxic injection: P0-P1 pups
P0–1 mice underwent hypothermia anesthesia in preparation for surgery. They were mounted and stabilized in a neonatal injection apparatus developed in-house. Neonates have closed ear canals and earbars cannot be used for head stabilization and alignment. A small scalp incision was made and V1 was targeted with a MP285 micromanipulator (Sutter Instruments). The targeted coordinates are 1.5mm lateral from the midline and 0.5mm anterior from the lambda suture. A pulled glass capillary (3.5” #3–000-203-G/X capillary, Drummond Scientific Co, or G150TF-4 capillary, Warner Instruments; P-97 pipette puller, Sutter Instruments) mounted on a Nanjoject III (Drummond Scientific Co) or a Picospritzer (General Valve Corp) was filled with virus and slowly lowered until piercing the surface of the skull. The capillary was raised to the desired depth, followed by a waiting period of 30–60 s, and 200–250nl was injected at rate of 1–2nl/s. Once the injection was completed, there was a 30–60 s waiting period followed by capillary withdrawal and closing of the incision with tissue glue (Vetbond). Pups recovered on a heating pad before returning to the home cage.
Surgical procedures for in vivo imaging
P9-P14 mice were deeply anesthetized with 2% isoflurane, received local analgesia (0.5% lidocaine) and maintained on a heated water pad (HTP-1500, Adroit Medical Systems). Scalp and connective tissue were removed to expose the skull, headbars placed over the occipital and nasal bones, and cyanoacrylate glue applied to head fix the animal and covers the skull. Imaging began after 3h+ of recovery from anesthesia and the animal was periodically hydrated with subcutaneous saline. After 1h+ of spontaneous activity recordings, control animals received a single CNO (10mg/Kg) i.p. injection to assess acute effects.
Histology
For histology, animals were perfused with 4% PFA in PBS. Brains were removed and post fixed overnight in same fixative. Coronal brain slices were sectioned at a 75 mm thickness via vibratome. Sections were blocked with 10% normal goat serum in 0.5% Triton in PBS and then incubated overnight with combinations of the following primary antibodies diluted in block solution: rabbit polyclonal RFP (1:1000, Rockland) and chicken polyclonal anti-GFP (1:1000, Aves) for fluorophore preservation of tdTomato (from Ai14) and GFP virus expression, mouse monoclonal anti-parvalbumin (1:1000 Sigma), rabbit polyclonal Calretinin (1:250, Immunostar), rat monoclonal anti-muscarinic acetylcholine receptor m2 (m2AchR) (1:500, Millipore Sigma), and rabbit 5-hydroxytryptamine (5-HT) (1:1000 Immunostar). Sections were incubated with appropriate Alexa fluor dye-conjugated IgG secondary antibodies (1:500, Molecular Probes). Sections were washed and mounted with Fluoromount-G (Southern Biotech).
Intravitreous injections
Every 24 hr from P7 to P14, mice were anesthetized using isoflurane (2%), and 1 mL of 10 uM tetrodotoxin (Sigma, MO) in sterile H2O, or sterile H2O alone, was injected intravitreously into one or both eyes. Eye injections at P7 the eyelids were cut open. A 34G needle attached to a Hamilton (Reno, NV) microsyringe was used to inject the solution at the rate of about 1 ml/min into the vitreous humor at the ora serrata. The needle is withdrawn after holding it in place for 30 s to 1 min. The animals were allowed to survive to P40–P60 before analysis.
Monocular enucleation and dark-rearing
For monocular enucleation in the first few days postnatally (P0-P1), the pups were placed under anesthesia through cooling; otherwise, for monocular enucleation at P6, the animal were anesthetized using isoflurane (2%). The eyelids were gently pried open (the eyes open at day 13) to expose the globe. Curved forceps were used to gain access to the perimeter and back of the globe. The globe was removed whole after the posterior bundle containing the optic nerve and arteries were pinched and transected. The eyelids were then repositioned and sealed using ophthalmic surgical adhesive. After recovery from anesthesia, the animals were returned to their mothers.
For short term dark rearing, pups along with their mother were placed in complete darkness from P7 to P14, then the animals were placed back to regular dark/light cycle until ~45.
Chemical-genetic manipulation
For the chemical-genetic manipulation, Nkx2.1-CreER;Ai14 mice that received injections of either the AAV/plasmid form of hM4DimCitrine or the EGFP (control) into visual cortex were intraperitoneally (i.p.) injected with CNO (10 mg/kg) or saline.
Widefield calcium imaging and assessment of mCherry-expression
Widefield calcium imaging was performed using a Zeiss Axiozoom V.16 with PlanNeoFluar Z 1C, 0.25 NA objective and equipped with an ET-EGFP filter (Chroma, 49002). Epifluorescence excitation was delivered with a 460nm LED source (X-cite XLED1, Excelitas Technologies). Emissions were collected with a sCMOS camera (pco.edge 4.2) with 512×500 resolution after 4×4 pixel binning, and frames acquired using Camware software (pco). Each recording lasted 10 minutes at 10 frames/second (100ms exposure time), and at least 60 minutes of data were collected per animal. AAV8-hSyn-DIO-hM4D(Gi)- mCherry was confirmed using the same setup for widefield calcium imaging, a 565nm LED source (X-cite XLED1, Excelitas Technologies) and an ET-AlexFluor568 filter set (Chroma 49031).
Intrinsic signal optical imaging
Prior to imaging, mice were implanted with a head bar and underwent skull thinning a according to established protocols (Silasi et al., 2013). The ocular dominance of these mice was determined 7–14 days later after the preparation by optical imaging of intrinsic signal (Zhang et al., 2015) under isofluorane anesthesia (0.5%–0.75% in O2) in combination with Chlorprothixene (2mg/kg, i.m.). The animal’s temperature was maintained at 37.5°C. The eyes were protected with a thin layer of silicon oil. These experiments were run blind to the experimental identity of the mice. The visual stimulus was a thin bar (2° in height and 20° in width) drifting continuously and periodically upward or downward. It was shown between −5 to 15° azimuth (the vertical meridian was defined as 0° with negative values for ipsilateral visual field) and full-screen elevations. The spatial frequency of the drifting bar was one cycle/100°, and the temporal frequency one cycle/8 s. Images were acquired under 625 nm red light illumination using a CMOS camera (Photonfocus, MV1-D1312–40-G2–12) with a long-pass filter (T660lpxrxt, Chroma) and the Fourier component of the reflectance changes was extracted at the temporal frequency of the stimulus as described previously (Kalatsky and Stryker, 2003).
In vitro electrophysiology
Slice preparation
We used wild-type mice electroporated with pAA-CAGGS-HA-hM4D(Gi)-IRES-mCitrine at E15.5 to confirm suppression of neuronal activity by chemical genetic method. Mice (~P7) were anesthetized with isoflurane before decapitation.
The dissected brain was rapidly immersed in ice-cold, oxygenated, artificial cerebrospinal fluid (section ACSF: 110 mM choline-Cl, 2.5 mM KCl, 4mM MgSO4, 1mM CaCl2, 1.25 mM NaH2PO4, 26mM NaHCO3, 11mM D-glucose, 10 mM Na ascorbate, 3.1 Na pyruvate, pH 7.35, 300 mOsm) for 1 min. Coronal prefrontal cortical slices were sectioned at 300 mm thickness using a vibratome (HM 650 V; Microm) at 1–2°C and incubated with oxygenated ACSF (working ACSF; 124mM NaCl, 2.5 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, 11 mM D-glucose, pH 7.35, 300mOsm) at 34°C for 30 min, and then transferred to ACSF at room temperature (25°C) for > 30 min before use. Whole cell patch recordings were directed electroporated hemisphere, the morphology of subcortical whiter matter and corpus callosum as primary landmarks according to the atlas (Paxinos and Watson Mouse Brain in Stereotaxic Coordinates, 3rd edition).
Patch pipettes were pulled from borosilicate glass capillaries with filament (1.2 mm outer diameter and 0.69 inner diameter; Warner Instruments) with a resistance of 3–6 MW. The pipette recording solution consisted of 130 mM potassium gluconate, 15 mM KCl, 10 mM sodium phosphocreatine, 10 mM HEPES, 4 mM ATP·Mg, 0.3 mM GTP, and 0.3 mM EGTA (pH 7.3 adjusted with KOH, 300 mOsm). Whole cell recordings from GFP labeled cells in layer 2/3 were made with Axopatch 700B amplifiers (Molecular Devices, Union City, CA) using an upright microscope (Olympus, Bx51) equipped with infrared-differential interference contrast optics (IR-DIC) and fluorescence excitation source. GFP negative cells were blindly selected within 100 um distance to GFP positive cells as controls. Both IR-DIC and fluorescence images were captured with a digital camera (Microfire, Optronics, CA). All recordings were performed at 33–34°C with the chamber perfused with oxygenated working ACSF. Recordings were made with two MultiClamp 700B amplifiers (Molecular Devices). The membrane potential was maintained at −75mV in the voltage clamping mode and zero holding current in the current clamping mode, without the correction of junction potential. Signals were recorded and low-pass filtered, digitalized at 20 kHz (DIGIDATA 1322A, Molecular Devices) and further analyzed using the pClamp 10.3 software (Molecular Devices) for intrinsic properties and synaptic features.
In vivo electrophysiology
Nkx2.1-CreER;Ai14 mice (TM induction at E17.5) age between 2–4 months were placed in a stereotaxic apparatus on a heating pad. The animal’s temperature was monitored and maintained at 37°C through a feed-back heater control module (Frederick Haer Company, Bowdoinham, ME). Silicon oil was applied to both eyes to prevent them from drying. The animals were lightly anesthetized under isoflurane anesthesia (0.5%–0.75% in O2) in combination with Chlorprothixene (2mg/kg, i.m.). A small craniotomy (2 mm2) was performed at the left hemisphere to expose the cortex for recording. Visual stimuli was generated with MATLAB programs developed by Dr. Xu An using the Psychophysics Toolbox extensions (An et al., 2014). An Asus monitor (120 Hz refresh rate) was placed at 25 cm in front of the animal to display the stimuli, with its midline aligned with the animal. Stimuli were presented to either eye separately with the other eye occluded. A 32-Channel electrode array consisting of 8 tetrodes (A4×2-tet-5mm-150–200-121-A32, Neuronexus) was penetrated perpendicular to the pial surface in the binocular zone of V1 (3.0–3.3 mm lateral from the midline and 0.5–0.8 mm anterior from the lambda suture). Only cells in upper layer 2–3 were recorded and analyzed. Action potentials were recorded extracellularly (sampled at 32 kHz) with Cheetah32 system (Neuralynx, Inc.). The animals were euthanized at the end of the recording.
Visual cliff test
Mice were placed in a 61 cm X 61 cm box with a clear acrylic (ShopPoPdisplays) base. A high-contrast grating was attached to the underside of one half of the box. The box placed such that the clear half of the base protruded from a table, revealing a drop to the floor of approximately 100 cm. Mice were placed in the center of the box and their behavior monitored for 10 min via a digital video camera mounted above the floor of the box. The data were analyzed, blind to genotype, from video recordings. An approach was defined as moving from the patterned region toward the “cliff” with the nose of the animal within 5 cm of the midline. Crossing was defined as the animal completely crossing the midline from the patterned to the clear half of the box. Retreating was defined as moving such that the head was within 5 cm of the midline and retreating without the entire body crossing the border (Leamey et al., 2007). Subsequent approaches were not scored until the mouse had moved outside the 5-cm range. The analysis was further divided into middle crossing and side crossing, where the middle crossing was scored when the animal was more than 5cm away from the sidewalls while the side crossing was scored when the animal crossed the cliff within 5 cm from the sidewalls.
Virtual optokinetic task
Spatial frequency and contrast thresholds for optokinetic tracking of sine-wave gratings were measured using a virtual optokinetic system (OptoMotry, CerebralMechanics, Medicine Hat, Alberta, Canada) (Douglas et al., 2005; Prusky et al., 2004) Vertical sine-wave gratings projected as a virtual cylinder and drifting at 12°/s, or gray of the same mean luminance, were displayed on four computer monitors arranged in a square around a testing platform positioned at the epicenter. At a testing session, a mouse was placed on the platform and allowed to freely move, and the center of the cylinder was fixed between its eyes to maintain the spatial frequency of the grating when the mouse changed its position. Gray was projected when the mouse was ambulating, and a grating was projected when it was stationary, which when visible to the animal, elicited tracking movement of the head and neck. The presence of tracking under each stimulus condition was appraised via live video with a yes/no criterion by an observer blind to the group identity of the mice, and a threshold for tracking was established using a method of limits procedure. A spatial frequency threshold (i.e., acuity) - the highest spatial frequency to elicit tracking of a grating at maximal contrast) through each eye (Douglas et al., 2005) - was obtained in a testing session in a few minutes. In some sessions, spatial frequency thresholds (highest spatial frequency to elicit tracking), and contrast thresholds (lowest contrast to elicit tracking) at six spatial frequencies (0.031, 0.064, 0.092, 0.103, 0.192, 0.272 c/d to generate a contrast sensitivity function (CSF)) through each eye separately were measured (14 thresholds), in ~30 min. Michelson contrast sensitivity was calculated from the contrast thresholds using the average screen luminance (max-min)/(max+min). Experimental animals and their controls were assessed on the same day. Thresholds for all animals were obtained under photopic lighting conditions (screen luminance = 54 lux), which selectively measures cone-based visual function (Alam et al., 2015).
Forced Two-Choice Visual Water Maze Task
Mice were trained to perform a forced two-choice test of visual discrimination in a trapezoidal shaped pool (Figure 7K) following previously published protocol (Prusky and Douglas, 2004; Prusky et al., 2000). In short, two monitors were placed at the wide end of the pool, in which one monitor displayed a stationary vertical sinusoidal grating, while the other displayed uniform gray of the same mean luminance. The pool was filled with water monitored at 22°C. A moveable platform was submerged below the water surface directly under the monitor with the testing grating. A midline divider was placed between the monitors to partially bisect the tank from the monitors to the middle of the tank where it creates a choice line. The animals were released from the short end of the tank and swam toward the monitors. The animal was considered to have made a choice once the whole body crosses the choice line. Once the animals made the correct choice and reaches the hidden platform, it was quickly removed from the water. If the animal chose incorrectly, it is forced to swim longer. During the training phase, the animals were required to achieve at least 80% accuracy in identifying low spatial frequency gratings in three consecutive sessions before moving to testing phase. The testing of visual acuity and contrast sensitivity followed closely to the published protocol (Prusky and Douglas, 2004; Prusky et al., 2000). These tests were run blind to the experimental identity of the mice. Briefly, during visual acuity test, the spatial frequency of the testing grating was increased, and the acuity was determined as the spatial frequency in which the success rate fell below 70%. Similarly, the animal’s contrast sensitivity at the spatial frequency of 0.20 cpd was determined by gradually decreasing the testing contrast until the success rate fell below 70%.
QUANTIFICATION AND STATISTICAL ANALYSIS
In-vivo electrophysiology and calcium imaging summary data values were obtained with MATLAB 2017a (MathWorks). All data were tested and plotted using GraphPad Prism 8.0.2 for Windows (GraphPad Software) or MATLAB 2017a (MathWorks). Shapiro-Wilk test was used to test for normality for all the test groups. Based on the normality results, either KS test or Wilcoxon matched-pairs signed rank test were used for datasets with non-normal distribution, and unpaired or paired Student’s t tests were used for datasets with normal distribution. Data are presented as mean ± s.e.m or mean ± SD (for in-vivo calcium imaging data). For in-vivo calcium imaging data, statistical tests were 2-way ANOVAS followed by Sidak’s multiple comparisons tests. All the experimental data were replicated in multiple animals, and were stated in the figure legends of the corresponding experiments. No method was used to predetermine sample sizes. If not specifically indicated, and a p value < 0.05 was considered significant. The significance was marked as *, p < 0.05; **, p < 0.01 and ***, p < 0.001.
Image acquisition and analysis
Images were acquired by confocal microscopy (Zeiss LSM 780). All images were processed using ImageJ software. To determine the callosal projecting region, Z stack images (10mm optical sections for 75 mm sections) were acquired with 10X objective and stitched using Bioformat plugin in ImageJ software (p. 42). Maximum intensity projections of the Z stacks were obtained. Region of callosal axon projections at V1/V2L border was selected and threshold using ImageJ IsoData method to determine the area of callosal projection zone (Figure S1A). The selected region was then outlined using the Analyze Particle function in ImageJ software. The L2 ChCs located within the outlined region are defined as the ChCs in the cBZ. In the Kir2.1 treated animals, where the callosal projections were disrupted, we used the expression of m2AchR to determine the callosal projection zone. First, we identified the lateral most callosal projection zone at the cBZ in control animals and correlate that point with the m2AchR expression profile (Figures S1C and S1D). On average, the m2AchR expression reduces to 93% of the peak intensity at the lateral edge of callosal projection zone (Figure S1E). The average width of callosal projection zone is 272mm ± 5mm. Then, in Kir2.1 treated animals, we determined the region where m2AchR expression reduces to 93% of the peak intensity as the lateral edge of callosal projection zone and used 272mm ± 5mm as the width of the zone. For estimating callosal projection zone at P7 when callosal axons have not reached contralateral cortex, we used the expression of 5-hydroxytryptamine (5-HT) to identify V1 (Chou et al., 2013) and estimate the lateral most 200mm as the cBZ that receive callosal input. By P10, we can reliably label callosal axons in the contralateral cortex, in which the average width is 210mm ± 3mm (Figure S1B).
For ChC density quantification, ChC were visually identified and their cell bodies were manually counted in a double-blinded manner throughout the different cortical regions for all the sections with cBZ labeled by callosal axons. We only restrict our analysis to L2 ChCs with cell bodies located at L1-L2 border (within 100um from the pia surface). For whole cortex ChC representations, the imaged sequential brain slices were manually aligned using TrakEM2 function in Fiji software. The ChCs were manually counted and corresponding ChC locations were recorded from the cell counter function in Fiji software to generate 2D representation of ChC locations.
To control for the tamoxifen induction variability for ChC labeling, all the comparisons made across brain regions (i.e., V1, V2L, cBZ) were made within the same animal. To quantify the developmental changes of ChC density in V1, V2, and cBZ across development, animals of different ages were normalized to their littermate controls with the same tamoxifen induction.
Calcium signal detection and analysis
Image processing and calcium signal detection was performed using the freely available wholeBrainDX software developed by James Ackman (available at https://github.com/ackman678/wholeBrainDX) and written for MATLAB (MathWorks) (Ackman et al., 2014) (Gaussian filter(s) = 3px, dF/F threshold = 2 SD). Visual Cortex ROIs were individually defined using functional data (domain activation frequency and/or duration maps), hand-drawn on ImageJ software and divided into lateral and medial regions according to V1’s apex and base. Activations were assigned to ROIs based on their centroid, and activation frequency was calculated as the mean number of individual activations per minute, averaged across recordings.
We performed a variety of seed-based correlations (available at https://github.com/CrairLab/Yixiang_OOP_pipeline) to quantitatively measure the spatial extent of similar V1 activity within- and across- hemispheres. Seed-based correlation analyses reveal regions with similar activity patterns, where tight correlations are interpreted as representing similar functional roles. Individual seed-based correlation maps were computed for seeds from a grid covering lateral V1 of the DREADD-injected hemisphere (range = 29–51 seeds, mean = 36.52, median = 35, Figure S6) Maps from individual recordings were averaged to obtain a mean correlation map per seed. For each mean map, we calculated the proportion of each V1 region tightly correlated with the seed (pearson’s correlation coefficient 3 0.7), then averaged these values across maps, and normalized them relative to the seed region (V1 lateral of the DREADD-injected hemisphere). All averaging and normalizations were done within animal.
Intrinsic signal optical imaging: ocular dominance Analysis
The response magnitude map acquired by ipsilateral eye stimulation was first smoothed by a circular averaging filter (25 μm in radius) and then thresholded at 30% of the peak response amplitude, to determine the binocular zone for ocular dominance analysis. Average magnitude values were presented as a measure of response strength separately for each eye. The response magnitude values from multiple sessions were averaged to determine representative values for each animal. All the response magnitudes were expressed after multiplication by 104. The Mann–Whitney test was used to test for statistical significance between two groups in optical imaging experiments. Statistical analyses and graphs were done with Prism (GraphPad Software). *p < 0.05 and **p < 0.01.
In vivo electrophysiology: data analysis
All data analysis was carried out using built-in and custom-built software in MATLAB (Mathworks). Spikes were manually sorted into clusters (presumptive neurons) offline based on peak amplitude and waveform energy using the MClust software (A.D. Redish). Cluster quality was quantified using isolation distance and L-ratio. Putative cells with isolation distance < 20 or L-ratio > 0.1 were excluded.
Sinusoidal grating drifting perpendicular to their orientation were used to determine V1 neuron’s orientation selectivity and spatial tuning. The drifting direction and spatial frequency of the gratings (full contrast and temporal frequency of 2 Hz) were varied between 0° – 330 (12 steps at 30° spacing) and 0.01–0.08 cycle/degree (in 4 logarithmic steps) in a pseudorandom order. The mean firing rate during the period of stimulus presentation was used to generate the direction tuning curves. The preferred direction was determined as the one that gave maximum response, averaged across all spatial frequencies. The preferred spatial frequency was the one that gave peak response at this direction. A vector summation method was used to quantitatively characterize the direction tuning curves:
Where θk and rK are the direction of motion and mean firing rate, respectively. The complex phase and amplitude of the resultant S represent the preferred direction and direction selective index (DSI), respectively. DSI varies between 0, for a cell that responds equally to all directions, and 1, for cells that only responds to a single direction. Preferred orientation (pref_O) and orientation selective index (OSI) to sine-wave grating were calculated by substituting 2θk for θk, while π was added to the resultant phase before it was halved as the moving directions of grating were always perpendicular to the orientations. The difference in preferred orientation between the two eyes was calculated by subtracting ipsilateral pref_O from contralateral pref_O along the 180° cycle (−90° to 90°). The absolute values of these differences (ΔO) were used in all quantifications for binocular matching of orientation preference. The ODI for each cell was calculated as (C − I)/(C + I), where C and I represent the maximum response magnitude for the contralateral and ipsilateral eyes, respectively. The ODI ranges from −1 to 1, where positive values indicate contralateral bias and negative values indicate ipsilateral bias.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-RFP | Rockland | Cat#600–402-379; RRID:AB_828390 |
| Chicken Polyclonal anti-GFP | Aves | Cat#GFP-1010; RRID: AB_2307313 |
| mouse monoclonal anti-parvalbumin | Sigma | Cat# P3088, RRID:AB_477329 |
| rabbit polyclonal anti-Calretinin | Immunostar | Cat# 24445, RRID:AB_572223 |
| rat monoclonal anti-muscarinic acetylcholine receptor m2 | Millipore Sigma | Cat# MAB367, RRID:AB_94952 |
| rabbit anti-5-hydroxytryptamine | Immunostar | Cat# 20080, RRID:AB_572263 |
| Virus Strains | ||
| pENN.AAV.CB7.CI.eGFP.WPRE.rBG | Penn Vector core | Cat# AV-8-PV1963 |
| AAV-Hsyn-HA-HM4D(Gi)-IRES-mCitrine | UNC Vector core | N/A |
| AAV-hSyn1-HA-hM4D(Gi)-IRES-mCitrine-WPRE-hGHp(A) | ETH Zurich | Cat# V 92–8 |
| AAV8-hSyn-DIO-hM4D(Gi)- mCherry | (Krashes et al., 2011) | Addgene Cat# 44362-AAV8 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| clozapine-N-oxide | Millipore Sigma | Cat# C0832–5MG |
| tamoxifen | Millipore Sigma | Cat# T5648–5G |
| tetrodotoxin | Millipore Sigma | Cat# 554412 |
| Critical Commercial Assays | ||
| OptoMotry | CerebralMechanics Inc. | http://www.cerebralmechanics.com |
| Experimental Models: Organisms/Strains | ||
| Nkx2–1tm1.1(cre/ERT2)Zjh Mus musculus | The Jackson Laboratory | RRID:IMSR_JAX:014552 |
| B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J Mus musculus | The Jackson Laboratory | RRID:IMSR_JAX:007914 |
| B6.129S2-Emx1tm1(cre)Krj/J Mus musculus | The Jackson Laboratory | RRID:IMSR_JAX:005628 |
| B6J.Cg-Gt(ROSA)26Sortm95.1(CAG-GCaMP6f) Hze/MwarJ Mus musculus | The Jackson Laboratory | RRID:IMSR_JAX:028865 |
| Recombinant DNA | ||
| CAG-Kir2.1 | Dr. Yu-Qiang Ding | N/A |
| pCAGGS-IRES-EGFP | Dr. Linda Van Aelst | N/A |
| pAAV-hSyn-HA-hM4D(Gi)-IRES-mCitrine | Dr. Bryan Roth | Addgene Cat #50464-AAV8 |
| Software and Algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij |
| GraphPad Prism 7 | GraphPad software | N/A |
| MATLAB | Mathworks | https://www.mathworks.com/; RRID:SCR_001622 |
| Psychophysics Toolbox extensions | Psychtoolbox | http://psychtoolbox.org |
| wholeBrainDX | Dr. James Ackman | https://github.com/ackman678/wholeBrainDX |
| Yixiang_OOP_pipeline | Crair Lab | https://github.com/CrairLab |
| Cheetah32 | Neuralynx | https://neuralynx.com/hardware/retired/cheetah32 |
| MClust | A.D. Redish | http://redishlab.neuroscience.umn.edu/mclust/MClust.html |
| pClamp 10.3 | Molecular Devices | https://mdc.custhelp.com/app/answers/detail/a_id/18779//axonpclamp-10electrophysiology-data-acquisition-%26-analysissoftware-download |
Highlights.
Massive chandelier cell (ChC) apoptosis in the binocular zone prior to vision onset
Callosal input from contralateral visual cortex drives ChC elimination
Spontaneous retinal activities prior to vision regulate ChC elimination
Preventing ChCs elimination results in deficient binocularity and visual behavior
ACKNOWLEDGMENTS
We thank Yueyi Zhang for her assistance with colony maintenance. This work was supported in part by NIH (R01 MH094705-06 and 1S10OD021759-01 to Z.J.H., 2P01AG001751-33A1 to G.T.P., and R01 EY015788 to M.C.C.), CSHL Robertson Neuroscience Fund (to Z.J.H.), and NRSA Postdoctoral Fellowship (NIH5F32NS096877-03 to B.-S.W.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.neuron.2020.11.004.
REFERENCES
- Ackman JB, Burbridge TJ, and Crair MC (2012). Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackman JB, Zeng H, and Crair MC (2014). Structured dynamics of neural activity across developing neocortex. bioRxiv. 10.1101/012237. [DOI] [Google Scholar]
- Alam NM, Altimus CM, Douglas RM, Hattar S, and Prusky GT (2015). Photoreceptor regulation of spatial visual behavior. Invest. Ophthalmol. Vis. Sci 56, 1842–1849. [DOI] [PubMed] [Google Scholar]
- An X, Gong H, Yin J, Wang X, Pan Y, Zhang X, Lu Y, Yang Y, Toth Z, Schiessl I, et al. (2014). Orientation-cue invariant population responses to contrast-modulated and phase-reversed contour stimuli in macaque V1 and V2. PLoS ONE 9, e106753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo DA, and Feller MB (2016). Spatiotemporal Features of Retinal Waves Instruct the Wiring of the Visual Circuitry. Front. Neural Circuits 10, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Braschi C, and Maffei L (2013). Visual depth perception in normal and deprived rats: effects of environmental enrichment. Neuroscience 236, 313–319. [DOI] [PubMed] [Google Scholar]
- Castaño-Castaño S, Feijoo-Cuaresma M, Paredes-Pacheco J, Morales-Navas M, Ruiz-Guijarro JA, Sanchez-Santed F, and Nieto-Escámez F (2019). tDCS recovers depth perception in adult amblyopic rats and reorganizes visual cortex activity. Behav. Brain Res 370, 111941. [DOI] [PubMed] [Google Scholar]
- Cerri C, Restani L, and Caleo M (2010). Callosal contribution to ocular dominance in rat primary visual cortex. Eur. J. Neurosci 32, 1163–1169. [DOI] [PubMed] [Google Scholar]
- Chou S-J, Babot Z, Leingärtner A, Studer M, Nakagawa Y, and O’Leary DDM (2013). Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science 340, 1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, and Perry VH (1979). The projection of the temporal retina in rats, studied by retrograde transport of horseradish peroxidase. Exp. Brain Res 35, 457–464. [DOI] [PubMed] [Google Scholar]
- Dehorter N, Vinay L, Hammond C, and Ben-Ari Y (2012). Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur. J. Neurosci 35, 1846–1856. [DOI] [PubMed] [Google Scholar]
- Dong H, Wang Q, Valkova K, Gonchar Y, and Burkhalter A (2004). Experience-dependent development of feedforward and feedback circuits between lower and higher areas of mouse visual cortex. Vision Res 44, 3389–3400. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, and Prusky GT (2005). Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis. Neurosci. 22, 677–684. [DOI] [PubMed] [Google Scholar]
- Engel AK, König P, Kreiter AK, and Singer W (1991). Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science 252, 1177–1179. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, and Hensch TK (2000). Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404, 183–186. [DOI] [PubMed] [Google Scholar]
- Faguet J, Maranhao B, Smith SL, and Trachtenberg JT (2009). Ipsilateral eye cortical maps are uniquely sensitive to binocular plasticity. J. Neurophysiol 101, 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sánchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, and Rico B (2017). Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron 95, 639–655. [DOI] [PubMed] [Google Scholar]
- Favuzzi E, Deogracias R, Marques-Smith A, Maeso P, Jezequel J, Exposito-Alonso D, Balia M, Kroon T, Hinojosa AJ, F Maraver E, and Rico B (2019). Distinct molecular programs regulate synapse specificity in cortical inhibitory circuits. Science 363, 413–417. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisén J, Lu Q, Barbacid M, and Flanagan JG (1998). Topographic guidance labels in a sensory projection to the forebrain. Neuron 21, 1303–1313. [DOI] [PubMed] [Google Scholar]
- Gardner JC, and Cynader MS (1987). Mechanisms for binocular depth sensitivity along the vertical meridian of the visual field. Brain Res 413, 60–74. [DOI] [PubMed] [Google Scholar]
- Gordon JA, and Stryker MP (1996). Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci 16, 3274–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, and Stryker MP (1999). Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci 19, RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, and Kash SF (1998). Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, and Mason CA (2003). Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell 114, 545–557. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, and Tonegawa S (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755. [DOI] [PubMed] [Google Scholar]
- Ishino Y, Yetman MJ, Sossi SM, Steinecke A, Hayano Y, and Taniguchi H (2017). Regional Cellular Environment Shapes Phenotypic Variations of Hippocampal and Neocortical Chandelier Cells. J. Neurosci 37, 9901–9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama GH, and Robertson RT (1993). Development of geniculocortical projections to visual cortex in rat: evidence early ingrowth and synaptogenesis. J. Comp. Neurol 335, 123–148. [DOI] [PubMed] [Google Scholar]
- Kalatsky VA, and Stryker MP (2003). New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron 38, 529–545. [DOI] [PubMed] [Google Scholar]
- Kim Y, Yang GR, Pradhan K, Venkataraju KU, Bota M, García Del Molino LC, Fitzgerald G, Ram K, He M, Levine JM, et al. (2017). Brain-wide Maps Reveal Stereotyped Cell-Type-Based Cortical Architecture and Subcortical Sexual Dimorphism. Cell 171, 456–469.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiper DC, Knyazeva MG, Tettoni L, and Innocenti GM (1999). Visual stimulus-dependent changes in interhemispheric EEG coherence in ferrets. J. Neurophysiol 82, 3082–3094. [DOI] [PubMed] [Google Scholar]
- Kirkby LA, Sack GS, Firl A, and Feller MB (2013). A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, and Trachtenberg JT (2013). A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamey CA, Merlin S, Lattouf P, Sawatari A, Zhou X, Demel N, Glendining KA, Oohashi T, Sur M, and Fässler R (2007). Ten_m3 regulates eye-specific patterning in the mammalian visual pathway and is required for binocular vision. PLoS Biol 5, e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Vandemark K, Mezey D, Shultz N, and Fitzpatrick D (2019). Functional Synaptic Architecture of Callosal Inputs in Mouse Primary Visual Cortex. Neuron 101, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, and Crair MC (2011). How do barrels form in somatosensory cortex? Ann. N Y Acad. Sci 1225, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Tucciarone J, Padilla-Coreano N, He M, Gordon JA, and Huang ZJ (2017). Selective inhibitory control of pyramidal neuron ensembles and cortical subnetworks by chandelier cells. Nat. Neurosci 20, 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD (1965). Uncrossed Visual Pathways of Hooded and Albino Rats. Science 149, 1506–1507. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, and Tagawa Y (2007). Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J. Neurosci 27, 6760–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Munk MH, Nelson JI, James AC, and Bullier J (1995). Structural basis of cortical synchronization. I. Three types of interhemispheric coupling. J. Neurophysiol 74, 2379–2400. [DOI] [PubMed] [Google Scholar]
- Olavarria J, Malach R, and Van Sluyters RC (1987). Development of visual callosal connections in neonatally enucleated rats. J. Comp. Neurol 260, 321–348. [DOI] [PubMed] [Google Scholar]
- Pan-Vazquez A, Wefelmeyer W, Gonzalez Sabater V, Neves G, and Burrone J (2020). Activity-Dependent Plasticity of Axo-axonic Synapses at the Axon Initial Segment. Neuron 106, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiker C, Wunderle T, Eriksson D, Schmidt A, and Schmidt KE (2013). An updated midline rule: visual callosal connections anticipate shape and motion in ongoing activity across the hemispheres. J. Neurosci 33, 18036–18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, and Mason CA (2008). Retinal axon growth at the optic chiasm: to cross or not to cross. Annu. Rev. Neurosci 31, 295–315. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Bryson JB, and Mason C (2010). Ephrin-B2 elicits differential growth cone collapse and axon retraction in retinal ganglion cells from distinct retinal regions. Dev. Neurobiol 70, 781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrasanta M, Restani L, and Caleo M (2012). The corpus callosum and the visual cortex: plasticity is a game for two. Neural Plast. 2012, 838672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, and Douglas RM (2004). Characterization of mouse cortical spatial vision. Vision Res 44, 3411–3418. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, and Douglas RM (2000). Behavioral assessment of visual acuity in mice and rats. Vision Res 40, 2201–2209. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, and Douglas RM (2004). Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci 45, 4611–4616. [DOI] [PubMed] [Google Scholar]
- Restani L, Cerri C, Pietrasanta M, Gianfranceschi L, Maffei L, and Caleo M (2009). Functional masking of deprived eye responses by callosal input during ocular dominance plasticity. Neuron 64, 707–718. [DOI] [PubMed] [Google Scholar]
- Rinetti-Vargas G, Phamluong K, Ron D, and Bender KJ (2017). Periadolescent Maturation of GABAergic Hyperpolarization at the Axon Initial Segment. Cell Rep 20, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Buzás P, Quenech’du N, Koza A, Eysel UT, Milleret C, and Kisvárday ZF (2009). Functional selectivity of interhemispheric connections in cat visual cortex. Cereb. Cortex 19, 2451–2465. [DOI] [PubMed] [Google Scholar]
- Rock C, and Apicella AJ (2015). Callosal projections drive neuronal-specific responses in the mouse auditory cortex. J. Neurosci 35, 6703–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, and Changeux JP (2001). Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc. Natl. Acad. Sci. USA 98, 6453–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JF, and Bartos M (2011). Postnatal differentiation of cortical interneuron signalling. Eur. J. Neurosci. 34, 1687–1696. [DOI] [PubMed] [Google Scholar]
- Schmidt KE, Kim DS, Singer W, Bonhoeffer T, and Löwel S (1997). Functional specificity of long-range intrinsic and interhemispheric connections in the visual cortex of strabismic cats. J. Neurosci 17, 5480–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Burge J, and Priebe NJ (2013). Binocular integration and disparity selectivity in mouse primary visual cortex. J. Neurophysiol 109, 3013–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Burbridge TJ, Crair MC, and Huberman AD (2017). Architecture, Function, and Assembly of the Mouse Visual System. Annu. Rev. Neurosci 40, 499–538. [DOI] [PubMed] [Google Scholar]
- Sefton AJ, and Dreher B (1995). Visual System. The Rat Nervous System (Academic Press; ), pp. 833–898. [Google Scholar]
- Shuler MG, Krupa DJ, and Nicolelis MA (2001). Bilateral integration of whisker information in the primary somatosensory cortex of rats. J. Neurosci 21, 5251–5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silasi G, Boyd JD, Ledue J, and Murphy TH (2013). Improved methods for chronic light-based motor mapping in mice: automated movement tracking with accelerometers, and chronic EEG recording in a bilateral thin-skull preparation. Front. Neural Circuits 7, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Freund TF, and Cowey A (1982). The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience 7, 2577–2607. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, et al. (2012). Intrinsically determined cell death of developing cortical interneurons. Nature 491, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinecke A, Hozhabri E, Tapanes S, Ishino Y, Zeng H, Kamasawa N, and Taniguchi H (2017). Neocortical Chandelier Cells Developmentally Shape Axonal Arbors through Reorganization but Establish Subcellular Synapse Specificity without Refinement. eNeuro 4, ENEURO.0057–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan KT, Liu WA, Li ZL, Shen Z, Li Z, Zhang XJ, Dean O, Ma J, and Shi SH (2018). Progressive divisions of multipotent neural progenitors generate late-born chandelier cells in the neocortex. Nat. Commun 9, 4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Y, Gallo NB, Wang M, Yu J-R, and Van Aelst L (2019). Axo-axonic Innervation of Neocortical Pyramidal Neurons by GABAergic Chandelier Cells Requires AnkyrinG-Associated L1CAM. Neuron 102, 358–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás G, and Szabadics J (2004). Summation of unitary IPSPs elicited by identified axo-axonic interneurons. Cereb. Cortex 14, 823–826. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Lu J, and Huang ZJ (2013). The spatial and temporal origin of chandelier cells in mouse neocortex. Science 339, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Gribizis A, Chen C, and Crair MC (2017). Activity-dependent development of visual receptive fields. Curr. Opin. Neurobiol 42, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Smith BE, and Feller MB (2018). Light Prior to Eye Opening Promotes Retinal Waves and Eye-Specific Segregation. Neuron 100, 1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT (2015). Competition, inhibition, and critical periods of cortical plasticity. Curr. Opin. Neurobiol 35, 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-L, Zhang L, Zhou Y, Zhou J, Yang X-J, Duan SM, Xiong Z-Q, and Ding Y-Q (2007). Activity-dependent development of callosal projections in the somatosensory cortex. J. Neurosci 27, 11334–11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Gao E, and Burkhalter A (2011). Gateways of ventral and dorsal streams in mouse visual cortex. J. Neurosci 31, 1905–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tucciarone J, Jiang S, Yin F, Wang BS, Wang D, Jia Y, Jia X, Li Y, Yang T, et al. (2019). Genetic Single Neuron Anatomy Reveals Fine Granularity of Cortical Axo-Axonic Cells. Cell Rep 26, 3145–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, and Hubel DH (1965). Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol 28, 1060–1072. [DOI] [PubMed] [Google Scholar]
- Wong FK, Bercsenyi K, Sreenivasan V, Portalás A, Fernández-Otero M, and Marín O (2018). Pyramidal cell regulation of interneuron survival sculpts cortical networks. Nature 557, 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Anderson SA, and Yuste R (2010). The enigmatic function of chandelier cells. Front. Neurosci 4, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, An X, Liu H, Peng J, Cai S, Wang W, Lin DT, and Yang Y (2015). The topographical arrangement of cutoff spatial frequencies across lower and upper visual fields in mouse V1. Sci. Rep 5, 7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu M, and Cang J (2013). Sublinear binocular integration preserves orientation selectivity in mouse visual cortex. Nat. Commun 4, 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, and Roth BL (2014). DREADD: a chemogenetic GPCR signaling platform. Int. J. Neuropsychopharmacol 18, pyu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and code generated during this study are available upon request.