Abstract
Ubiquitin-specific peptidase 6 (USP6) is a hominoid-specific gene residing on chromosome 17p13 and serves as a deubiquitinating enzyme with a diverse set of functions including intracellular trafficking, inflammatory signaling, cell transformation and protein turnover. USP6 rearrangements were first identified in aneurysmal bone cysts, resulting in promoter swapping and over-expression of wild type USP6. Several morphologically overlapping fibroblastic/myofibroblastic tumors are known to harbor USP6 rearrangements, including nodular fasciitis, cellular fibroma of tendon sheath, myositis ossificans and fibro-osseous pseudotumor of digits. Over the past few years, fusions involving the USP6 gene and various partner genes have been described in these neoplasms. The current World Health Organization Classification of Tumors of Soft Tissue suggests that USP6-rearranged lesions are typically benign and usually self-limited in their growth. This review provides an updated overview of the clinical, histological and molecular genetic features of USP6-associated fibroblastic/myofibroblastic tumors and discusses how these lesions should be best classified.
Keywords: Nodular fasciitis, fibroma of tendon sheath, myositis ossificans, fibro-osseous pseudotumor of digits, USP6, review
Ubiquitin-specific peptidase 6 (USP6), located on chromosome 17p13, is a hominoid-specific gene primarily expressed in testicular tissue and encodes a deubiquitinating enzyme that plays critical roles in diverse cellular processes such as intracellular trafficking, inflammatory signaling, cell transformation and protein turnover (1,2). USP6 was initially cloned from transfected DNA of human Ewing sarcoma cells (3). USP6 rearrangements were first identified in a primary aneurysmal bone cyst (ABC) (4). Subsequently, various fusion partners for USP6 were discovered in ABC (5). These alternative gene fusions induce tumorigenesis by a promoter-swapping mechanism that drives transcriptional up-regulation of USP6. It was also shown that USP6 induces expression of matrix metalloproteinase through activation of the classical nuclear factor-kappaB pathway (6). Furthermore, recent studies demonstrated that USP6 promotes tumorigenesis through multiple pathways, including Wnt, Jak1-STAT3 and c-Jun (7-9).
The new fifth edition of the World Health Organization Classification of Tumors of Soft Tissue was published in early 2020. Several benign fibroblastic/myofibroblastic tumor subtypes are characterized by certain morphologies or distinctive anatomical distributions, including nodular fasciitis, cellular fibroma of tendon sheath, myositis ossificans and fibro-osseous pseudotumor of digits. Most notably, these benign tumors usually harbor USP6 rearrangements (10-13). In this review, we present an updated overview of the clinical, histological and molecular genetic features of USP6-associated fibroblastic/myofibroblastic tumors and discusses their relationships to one another. The corresponding clinico-pathological and molecular characteristics are summarized in Table I.
Table I. Clinicopathological and molecular characteristics of USP6-associated fibroblastic/myofibroblastic tumors.
USP6: Ubiquitin-specific peptidase 6; MYH9: myosin heavy chain 9; RRBP1: ribosome binding protein 1; CALU: calumenin; CTNNB1: catenin beta 1; MIR22HG: MIR22 host gene; SPARC: secreted protein acidic and cysteine rich; THBS2: thrombospondin 2; COL6A2: collagen type VI alpha 2 chain; SEC31A: SEC31 homolog A; COPII: coat complex component; EIF5A: eukaryotic translation initiation factor 5A; COL1A1: collagen type I alpha 1 chain; COL1A2: collagen type I alpha 2 chain; PAFAH1B1: platelet activating factor acetylhydrolase 1b regulatory subunit 1; SERPINH1: serpin family H member 1; COL3A1: collagen type III alpha 1 chain; PPP6R3: protein phosphatase 6 regulatory subunit 3; PKM: pyruvate kinase M1/2; RCC1: regulator of chromosome condensation 1; ASPN: asporin.
Nodular Fasciitis
Nodular fasciitis is a benign self-limited fibroblastic/myofibroblastic neoplasm of unknown etiology. It equally affects males and females and occurs in all age groups but more often in young adults (20 to 40 years of age). The upper extremities are most frequently affected, followed by trunk and head and neck. Most cases involve the subcutaneous tissue and underlying fascia. Nodular fasciitis typically grows rapidly and reaches its final size within a few weeks. Mild pain or tenderness may be present. The lesion usually measures 2 to 3 cm in diameter. Several subtypes of nodular fasciitis are recognized on the basis of the anatomical location, including intravascular fasciitis and cranial fasciitis (14).
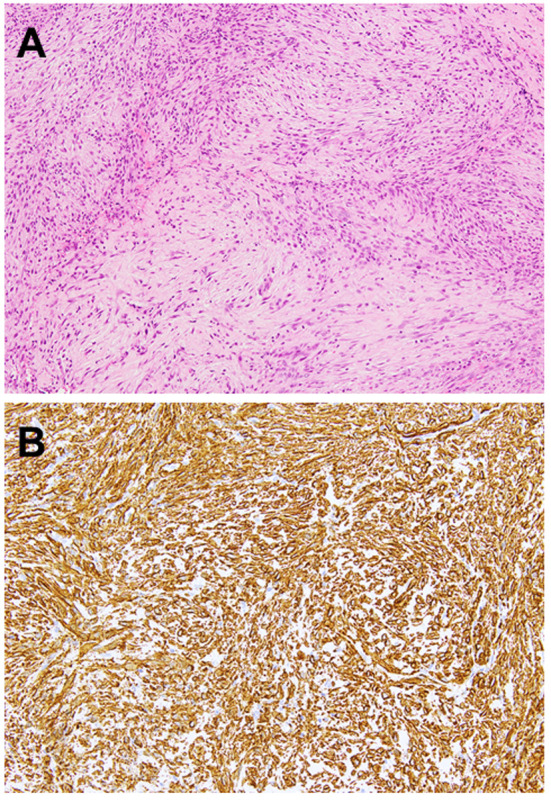
Histologically, nodular fasciitis consists of a proliferation of spindle-shaped cells in a myxoid or collagenous stroma. The neoplastic cells are typically slender and delicate, mimicking the appearance of fibroblasts in tissue culture and are arranged in whorls, short fascicles or haphazardly (Figure 1A). Mitotic activity is easily identified but atypical mitoses are not observed. Extravasated erythrocytes, lymphocytes and osteoclast-like giant cells are frequently identified (14). Occasionally, osseous metaplasia may be present as seen in myositis ossificans and fibro-osseous pseudotumor of digits (13). Immunohistochemically, the neoplastic cells are usually positive for smooth muscle actin (SMA) (Figure 1B) and muscle specific actin (MSA). Focal desmin expression is occasionally found.
Figure 1. Histological and immunohistochemical features of nodular fasciitis. A: Nodular fasciitis consists of plump spindle-shaped cells with a vague fascicular and storiform pattern. Extravasated erythrocytes and lymphocytes can be observed (hematoxylin and eosin staining, original magnification ×100). B: The neoplastic cells are diffusely positive for smooth muscle actin (original magnification ×200).
Clonal chromosomal aberrations were detected in five cases of nodular fasciitis (15-19). Rearrangements involving 3q21 and 15q22-q26 were identified in a small subset of nodular fasciitis.
In 2011, USP6 rearrangements were detected in 44 of 48 (92%) cases of nodular fasciitis (20). In that study, myosin heavy chain 9 (MYH9), located on chromosome 22q12.3, was identified as a novel fusion partner and it was shown that increased expression of USP6 could induce formation of a tumor clinically and histologically similar to human nodular fasciitis in xenograft models. Since then, various USP6 fusion partners were discovered in nodular fasciitis and its subtypes, including ribosome binding protein 1 (RRBP1), calumenin (CALU), catenin beta 1 (CTNNB1), MIR22 host gene (MIR22HG), secreted protein acidic and cysteine rich (SPARC), thrombospondin 2 (THBS2), collagen type VI alpha 2 chain (COL6A2), SEC31 homolog A, COPII coat complex component (SEC31A), eukaryotic translation initiation factor 5A (EIF5A), collagen type I alpha 1 chain (COL1A1), collagen type I alpha 2 chain (COL1A2), platelet activating factor acetylhydrolase 1b regulatory subunit 1 (PAFAH1B1), serpin family H member 1 (SERPINH1) and collagen type III alpha 1 chain (COL3A1) (21-27). Some fusion partners appear to be shared across neoplasms (5,24). These molecular studies indicate that the most frequent fusion partner of USP6 is MYH9 in nodular fasciitis. Wang et al. (24) suggested that the MYH9-USP6 fusion is not related bone formation. Furthermore, a protein phosphatase 6 regulatory subunit 3 (PPP6R3)-USP6 fusion with gene amplification was detected in two cases of nodular fasciitis with malignant behavior (28,29). It is suggested that this fusion and/or USP6 amplification may be associated with malignant condition. However, further studies with a large number of cases are needed to better understand the correlation between certain gene fusions and distinct biological behavior.
Cellular Fibroma of Tendon Sheath
Fibroma of tendon sheath is a benign fibroblastic/myofibroblastic neoplasm that is usually attached to a tendon (sheath) of the fingers. It may occur at any age but has a peak incidence in the third to fifth decades of life, with a male predominance. Fibroma of tendon sheath typically presents as a firm, small (usually less than 3 cm), slow-growing, painless mass (30). A cellular variant of this neoplasm has been described as having morphological overlap with nodular fasciitis (31).
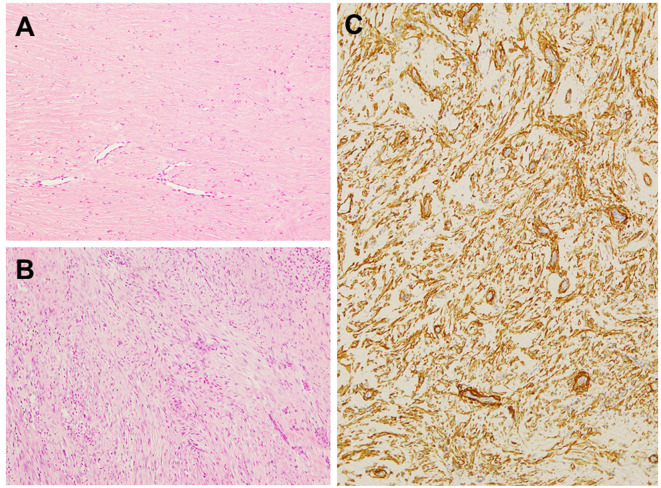
Histologically, fibroma of tendon sheath consists of bland spindle cells in a dense collagenous stroma (Figure 2A). Cytological atypia is not observed and mitotic activity is low. There are characteristic slit-like thin-walled vessels or clefts at the periphery of the lesion. Degenerative features such as myxoid change, osseous metaplasia and pleomorphism may be present (30). Cellular fibroma of tendon sheath is generally defined as a tumor having areas of increased cellularity and a nodular fasciitis-like appearance as well as identifiable histological features of classical fibroma of tendon sheath (Figure 2B). Immunohistochemically, the neoplastic cells are often positive for SMA (Figure 2C). Expression of desmin, S-100 protein and FOS-like antigen 1 is absent (32).
Figure 2. Histological and immunohistochemical features of classical and cellular variants of fibroma of tendon sheath. A: Classical fibroma of tendon sheath consists of bland spindle cells in a dense collagenous stroma. Slit-like vessels can be seen (hematoxylin and eosin staining, original magnification ×100). B: Cellular fibroma of tendon sheath contains areas of increased cellularity composed of spindled-to-stellae cells arranged in a vague fascicular pattern (hematoxylin and eosin staining, original magnification ×100). C: The tumor cells are positive for smooth muscle actin (original magnification ×100).
Clonal chromosomal alterations were detected in four cases of classical fibroma of tendon sheath (33-36). These cytogenetic studies suggest that 11q rearrangement may be characteristic of classical fibroma of tendon sheath. It is of interest that this chromosomal rearrangement has also been observed in desmoplastic fibroblastoma (32), which can show morphological overlap with classical fibroma of tendon sheath.
Cellular fibroma of tendon sheath appears to be genetically distinct from classical fibroma of tendon sheath. UPS6 rearrangements were detected in 6 of 9 (67%) cases of cellular fibroma of tendon sheath but not in classical fibroma of tendon sheath (37). In 2020, various USP6 fusion partners were discovered in a subset of cellular fibroma of tendon sheath, including pyruvate kinase M1/2 (PKM), regulator of chromosome condensation 1 (RCC1), asporin (ASPN), COL1A1, COL3A1 and MYH9 (24,38). In view of the similar morphological and molecular genetic features, a subset of cellular fibroma of tendon sheath may in fact be tenosynovial nodular fasciitis.
Myositis Ossificans and Fibro-Osseous Pseudotumor of Digits: Are they Related?
According to the current World Health Organization Classification of Tumors of Soft Tissue, myositis ossificans and fibro-osseous pseudotumor of digits belong to the same neoplastic spectrum, with definite bone-forming capacity (39).
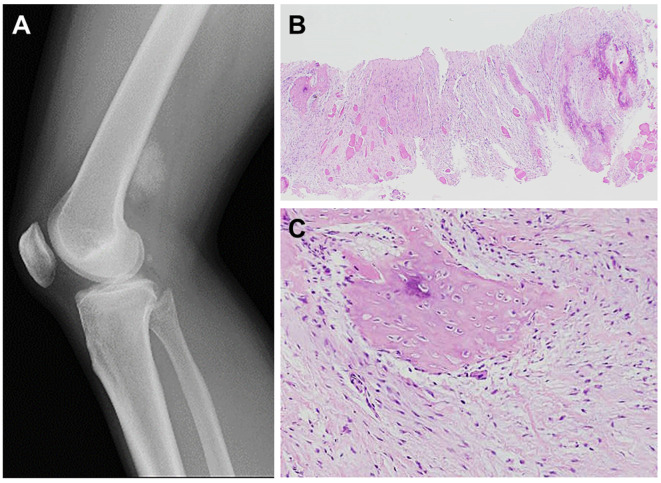
Myositis ossificans is a benign self-limited fibroblastic/myofibroblastic neoplasm typically occurring within the skeletal muscle. A history of trauma is sometimes absent, and the lesion may be an incidental finding. It equally affects males and females and usually occurs in physically active adolescents and young adults. Although myositis ossificans can occur anywhere in the body, the most common locations are the thigh, lower leg and buttock. Deep-seated lesions may involve both muscle and underlying periosteum. The clinical features depend on the phase; in the early phase (1-2 weeks), the involved area is swollen and painful. Eventually, it evolves into a firm, rapidly growing, painless mass (Figure 3A). The diameter ranges from 2 to 12.5 cm (median of 5 cm) (40).
Figure 3. Radiographical and histological features of myositis ossificans. A: Lateral radiograph shows a densely mineralized mass in the posterior aspect of the distal thigh. B: Myositis ossificans shows a distinct zonal pattern with a central spindle cell proliferation surrounded by a peripheral bone formation. Skeletal muscle is entrapped (hematoxylin and eosin staining, original magnification ×20). C: Myositis ossificans is composed of (myo)fibroblasts and foci of osteoid formation (hematoxylin and eosin staining, original magnification ×100).
Histologically, myositis ossificans is characterized by the presence of a distinct zonal pattern with a peripheral rim of mature bone and a central cellular area composed of (myo)fibroblasts and immature bone (Figure 3B and C). In the early phase, myositis ossificans demonstrates a close resemblance to nodular fasciitis, with a highly cellular proliferation of (myo)fibroblasts within a variably myxoid stroma. The constituent (myo)fibroblasts display a mild degree of cellular pleomorphism and rather prominent mitotic activity. In the late phase, on the other hand, myositis ossificans consists almost entirely of mature lamellar bone. Woven bone rimmed by uniform osteoblasts is present throughout the lesion. Entrapment of atrophic muscle fibers is often observed. Necrosis is usually absent. Immunohistochemically, the neoplastic cells may express SMA and MSA, suggesting myofibroblastic differentiation.
In 2008, UPS6 rearrangements were identified in 2 cases with radiological and histological features consistent with myositis ossificans (41). These cases were at that time considered to be the early phase of soft-tissue ABC rather than myositis ossificans. In 2018, Bekers et al. (42) confirmed the presence of USP6 rearrangements in 8 of 9 (89%) cases of myositis ossificans. Recently, COL1A1 was detected as a fusion partner of USP6 in a subset of myositis ossificans (24,43,44). It is of interest that this gene fusion has also been found in soft-tissue ABC (24,45,46). These findings suggest that myositis ossificans and soft-tissue ABC are closely related entities.
Fibro-osseous pseudotumor of digits is a rare benign self-limited neoplasm usually occurring in the subcutaneous tissue of the digits (Figure 4A). The etiology of this neoplasm remains unclear. It predominantly affects young to middle-aged adults, with a female predominance. The index finger appears to be a preferential location. Fibro-osseous pseudotumor of digits typically grows rapidly and the clinical features evolve over time. In the early phase, there is a variably painful fusiform swelling (47). Eventually, it becomes firm and well-demarcated. The diameter ranges from 0.2 to 5 cm (median of 1.5 cm) (48).
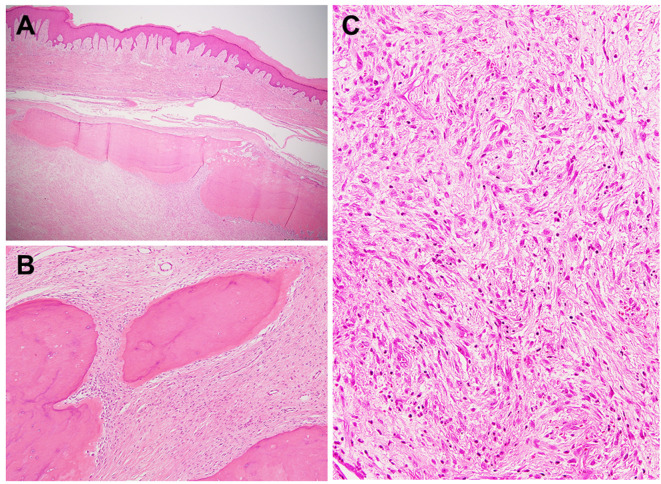
Figure 4. Histological features of fibro-osseous pseudotumor of digits. A: Fibro-osseous pseudotumor of digits involves the subcutaneous tissue. Mature bone is present at the periphery of the lesion (hematoxylin and eosin staining, original magnification ×20). B: Fibro-osseous pseudotumor of digits shows a (myo)fibroblastic proliferation and mature woven bone formation (hematoxylin and eosin staining, original magnification ×100). C: The central portion is composed of (myo)fibroblasts with a storiform pattern, resembling nodular fasciitis (hematoxylin and eosin staining, original magnification ×200).
The histological features of fibro-osseous pseudotumor of digits are very similar to those of myositis ossificans. Minor histological differences are believed to be related to the different location of involvement (40). Fibro-osseous pseudotumor of digits shows an irregular multinodular growth pattern and is histologically composed of a mixture of (myo)fibroblasts, osteoblasts and bony trabeculae with various stages of maturation (Figure 4B and C). Like myositis ossificans, the zonal pattern may be seen (48). Mitotic activity may be high but abnormal mitotic figures are not observed. Immunohistochemically, the neoplastic cells may express SMA. No immunoreactivity has been reported for desmin, S-100 protein and CD34 (49).
In 2018, UPS6 rearrangements were detected in 4 of 5 (80%) cases of fibro-osseous pseudotumor of digits (50). Recently, COL1A1 was detected as a fusion partner of USP6 in a significant number of fibro-osseous pseudotumor of digits (24,44). Intriguingly, almost all of bone-forming USP6-rearranged neoplasms adopt COL1A1 as the fusion partner, including myositis ossificans, fibro-osseous pseudotumor of digits and soft-tissue ABC. We suggest that these bone-forming soft tissue lesions belong to the same spectrum of USP6-induced neoplasms in light of their overlapping, occasionally indistinguishable morphology.
Molecular Diagnostics
In current practice, molecular genetic assays can serve as a useful diagnostic adjunct for soft tissue tumors (10). Notably, the development of next-generation sequencing has advanced our knowledge of molecular genetics in soft tissue tumors (22,51).
Because of its rapid growth, high cellularity and high mitotic activity, nodular fasciitis can be misdiagnosed as a malignant soft tissue tumor such as low-grade fibrosarcoma or low-grade myxofibrosarcoma, often leading to unnecessarily aggressive treatment. Similarly, myositis ossificans and fibro-osseous pseudotumor of digits can be easily confused with extraskeletal or parosteal osteosarcoma. Cellular fibroma of tendon sheath may be histologically confused with low-grade fibromyxoid sarcoma and low-grade myofibroblastic sarcoma. It is of interest that the most important differential diagnoses do not show USP6 rearrangements. Therefore, the detection of USP6 rearrangements and/or USP6 fusions would be useful for the diagnosis of these benign fibroblastic/myofibroblastic neoplasms, especially in small biopsy specimens.
Behavior and Treatment
In our experience, conservative treatment is a reasonable first-line approach for benign self-limited neoplasms such as nodular fasciitis and myositis ossificans. In bone-forming cases, repeated radiographical examinations should be obtained during the follow-up period to document the maturation of the lesion and the absence of destructive growth.
Nodular fasciitis is a benign self-limiting process. Spontaneous regression is well documented and simple excision without attention to margins is sufficient. Local recurrence is very uncommon and typically cured by simple re-excision. Although extremely rare, malignant clinical behavior has been described in the literature (28,29).
Simple excision is the treatment of choice for fibroma of tendon sheath but local recurrence is seen in 5-10% of cases (30). Recurrence is not aggressive and typically respond to simple re-excision. No cases of malignant transformation have been reported. There is no relationship between morphological variants and different clinical outcomes.
Undoubtedly, myositis ossificans and fibro-osseous pseudotumor of digits are benign self-limiting conditions similar to nodular fasciitis. Simple excision is the treatment of choice for these lesions and prognosis is excellent. Malignant transformation in myositis ossificans, usually into osteosarcoma, has been described (52) but it is exceptionally rare. On the other hand, there is no evidence of malignant transformation of fibro-osseous pseudotumor of digits.
Conclusion
Almost all of USP6-associated fibroblastic/myofibroblastic tumors have a benign clinical course. Over the past decade, a number of novel fusions involving the USP6 gene have been identified in nodular fasciitis, cellular fibroma of tendon sheath, myositis ossificans and fibro-osseous pseudotumor of digits. These fusion partners act as an ectopic promotor leading to transcriptional activation of USP6. A subset of cellular fibroma of tendon sheath are probably in fact tenosynovial variants of nodular fasciitis. Myositis ossificans and fibro-osseous pseudotumor of digits may represent a morphological spectrum of the same biological entity, related to and sometimes indistinguishable from soft-tissue ABC. Further studies are required to determine whether different fusion partners are associated with distinct morphological features and biological behavior of USP6-induced neoplasms.
Conflicts of Interest
The Authors declare no conflicts of interest associated with this article.
Authors’ Contributions
SN researched the literature and drafted the article. JN collected the data and was a major contributor to writing the article. MA, KK and KN performed the histological evaluations. TY reviewed the article. All Authors read and approved the final article.
Acknowledgements
The Authors are grateful to Dr. Hiroshi Iwasaki (Emeritus Professor, Department of Pathology, Faculty of Medicine, Fukuoka University) for his expert opinion and valuable comments on the histological diagnosis.
References
- 1.Paulding CA, Ruvolo M, Haber DA. The Tre2 (USP6) oncogene is a hominoid-specific gene. Proc Natl Acad Sci USA. 2003;100:2507–2511. doi: 10.1073/pnas.0437015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira AM, Chou MM. The TRE17/USP6 oncogene: a riddle wrapped in a mystery inside an enigma. Front Biosci (Schol Ed) 2012;4:321–334. doi: 10.2741/271. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Hillova J, Mariage-Samson R, Onno M, Huebner K, Cannizzaro LA, Boghosian-Sell L, Croce CM, Hill M. A novel transcriptional unit of the Tre oncogene widely expressed in human cancer cells. Oncogene. 1992;7:733–741. [PubMed] [Google Scholar]
- 4.Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, Bridge JA, Perez-Atayde AR, Fletcher JA. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64:1920–1923. doi: 10.1158/0008-5472.can-03-2827. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira AM, Perez-Atayde AR, Dal Cin P, Gebhardt MC, Chen CJ, Neff JR, Demetri GD, Rosenberg AE, Bridge JA, Fletcher JA. Aneurysmal bone cyst variant translocations upregulate USP6 transcription by promoter swapping with the ZNF9, COL1A1, TRAP150 and OMD genes. Oncogene. 2005;24:3419–3426. doi: 10.1038/sj.onc.1208506. [DOI] [PubMed] [Google Scholar]
- 6.Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H, Jiang T, Lev D, Welman A, Blobel GA, Oliveira AM, Chou MM. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-ĸB. Oncogene. 2010;29:3619–3629. doi: 10.1038/onc.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madan B, Walker MP, Young R, Quick L, Orgel KA, Ryan M, Gupta P, Henrich IC, Ferrer M, Marine S, Roberts BS, Arthur WT, Berndt JD, Oliveira AM, Moon RT, Virshup DM, Chou MM, Major MB. USP6 oncogene promotes Wnt signaling by deubiquitylating Frizzleds. Proc Natl Acad Sci USA. 2016;113:E2945–E2954. doi: 10.1073/pnas.1605691113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quick L, Young R, Henrich IC, Wang X, Asmann YW, Oliveira AM, Chou MM. JAK1-STAT3 signals are essential effectors of the USP6/TRE17 oncogene in tumorigenesis. Cancer Res. 2016;76:5337–5347. doi: 10.1158/0008-5472.CAN-15-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Yang H, He Y, Li T, Feng J, Chen W, Ao L, Shi X, Lin Y, Liu H, Zheng E, Lin Q, Bu J, Zeng Y, Zheng M, Xu Y, Liao Z, Lin J, Lin D. Ubiquitin-specific protease USP6 regulates the stability of the c-JUN protein. Mol Cell Biol. 2018;38:e00320–17. doi: 10.1128/MCB.00320-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12–18. doi: 10.3892/ol.2012.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira AM, Chou MM. USP6-induced neoplasms: the biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol. 2014;45:1–11. doi: 10.1016/j.humpath.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Erber R, Agaimy A. Misses and near misses in diagnosing nodular fasciitis and morphologically related reactive myofibroblastic proliferations: experience of a referral center with emphasis on frequency of USP6 gene rearrangements. Virchows Arch. 2018;473:351–360. doi: 10.1007/s00428-018-2350-0. [DOI] [PubMed] [Google Scholar]
- 13.Hiemcke-Jiwa LS, van Gorp JM, Fisher C, Creytens D, van Diest PJ, Flucke U. USP6-associated neoplasms: a rapidly expanding family of lesions. Int J Surg Pathol. 2020;28:816–825. doi: 10.1177/1066896920938878. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira AM, Wang J, Wang WL. Lyon, IARC Press. 2020. Nodular fasciitis. In: World Health Organization Classification of Tumours of Soft Tissue and Bone; pp. pp. 49–50. [Google Scholar]
- 15.Sawyer JR, Sammartino G, Baker GF, Bell JM. Clonal chromosome aberrations in a case of nodular fasciitis. Cancer Genet Cytogenet. 1994;76:154–156. doi: 10.1016/0165-4608(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 16.Birdsall SH, Shipley JM, Summersgill BM, Black AJ, Jackson P, Kissin MW, Gusterson BA. Cytogenetic findings in a case of nodular fasciitis of the breast. Cancer Genet Cytogenet. 1995;81:166–168. doi: 10.1016/0165-4608(94)00229-5. [DOI] [PubMed] [Google Scholar]
- 17.Weibolt VM, Buresh CJ, Roberts CA, Suijkerbuijk RF, Pickering DL, Neff JR, Bridge JA. Involvement of 3q21 in nodular fasciitis. Cancer Genet Cytogenet. 1998;106:177–179. doi: 10.1016/s0165-4608(98)00066-1. [DOI] [PubMed] [Google Scholar]
- 18.Donner LR, Silva T, Dobin SM. Clonal rearrangement of 15p11.2, 16p11.2, and 16p13.3 in a case of nodular fasciitis: additional evidence favoring nodular fasciitis as a benign neoplasm and not a reactive tumefaction. Cancer Genet Cytogenet. 2002;139:138–140. doi: 10.1016/s0165-4608(02)00613-1. [DOI] [PubMed] [Google Scholar]
- 19.Velagaleti GVN, Tapper JK, Panova NE, Miettinen M, Gatalica Z. Cytogenetic findings in a case of nodular fasciitis of subclavicular region. Cancer Genet Cytogenet. 2003;141:160–163. doi: 10.1016/s0165-4608(02)00725-2. [DOI] [PubMed] [Google Scholar]
- 20.Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasm induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427–1433. doi: 10.1038/labinvest.2011.118. [DOI] [PubMed] [Google Scholar]
- 21.Patel NR, Chrisinger JSA, Demicco EG, Sarabia SF, Reuther J, Kumar E, Oliveira AM, Billings SD, Bovée JVMG, Roy A, Lazar AJ, Lopez-Terrada DH, Wang WL. USP6 activation in nodular fasciitis by promotor-swapping gene fusions. Mod Pathol. 2017;30:1577–1588. doi: 10.1038/modpathol.2017.78. [DOI] [PubMed] [Google Scholar]
- 22.Lam SW, Cleton-Jansen AM, Cleven AHG, Ruano D, van Wezel T, Szuhai K, Bovée JVMG. Molecular analysis of gene fusions in bone and soft tissue tumors by anchored multiplex PCR-based targeted next-generation sequencing. J Mol Diagn. 2018;20:653–663. doi: 10.1016/j.jmoldx.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Lenz J, Michal M, Svajdler M, Ptakova N, Lenz D, Konecna P, Kavka M. Novel EIF5A-USP6 gene fusion in nodular fasciitis associated with unusual pathologic features: a report of a case and review of the literature. Am J Dermatopathol. 2020;42:539–543. doi: 10.1097/DAD.0000000000001602. [DOI] [PubMed] [Google Scholar]
- 24.Wang JC, Li WS, Kao YC, Lee JC, Lee PH, Huang SC, Tsai JW, Chen CC, Chang CD, Yu SC, Huang HY. Clinicopathological and molecular characterization of USP6-rearranged soft tissue neoplasms: the evidence of genetic relatedness indicates an expanding family with variable bone-forming capacity. Histopathology. 2020 doi: 10.1111/his.14268. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y, Peng R, Chen H, Zhuang H, He X, Zhang H. Atypical nodular fasciitis with a novel PAFAH1B1-USP6 fusion in a 22-month-old boy. Virchows Arch. 2020 doi: 10.1007/s00428-020-02961-y. [DOI] [PubMed] [Google Scholar]
- 26.Paulson VA, Stojanov IA, Wasman JK, Restrepo T, Cano S, Plunkitt J, Duraisamy S, Harris MH, Chute DJ, Al-Ibraheemi A, Church AJ. Recurrent and novel USP6 fusions in cranial fasciitis identified by targeted RNA sequencing. Mod Pathol. 2020;33:775–780. doi: 10.1038/s41379-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, He X, Qiu Y, Chen H, Zhuang H, Yao J, Zhang H. Novel CTNNB1-USP6 fusion in intravascular fasciitis of the large vein identified by next-generation sequencing. Virchows Arch. 2020;477:455–459. doi: 10.1007/s00428-020-02792-x. [DOI] [PubMed] [Google Scholar]
- 28.Guo R, Wang X, Chou MM, Asmann Y, Wenger DE, Al-Ibraheemi A, Molavi DW, Aboulafia A, Jin L, Fritchie K, Oliveira JL, Jenkins RB, Westendorf JJ, Dong J, Oliveira AM. PPP6R3-USP6 amplification: novel oncogenic mechanism in malignant nodular fasciitis. Genes Chromosomes Cancer. 2016;55:640–649. doi: 10.1002/gcc.22366. [DOI] [PubMed] [Google Scholar]
- 29.Teramura Y, Yamazaki Y, Tanaka M, Sugiura Y, Takazawa Y, Takeuchi K, Nakayama T, Kaneko T, Musha Y, Funauchi Y, Ae K, Matsumoto S, Nakamura T. Case of mesenchymal tumor with the PPP6R3-USP6 fusion, possible nodular fasciitis with malignant transformation. Pathol Int. 2019;69:706–709. doi: 10.1111/pin.12851. [DOI] [PubMed] [Google Scholar]
- 30.Sciot R, Cunha IW. Lyon, IARC Press. 2020. Fibroma of tendon sheath. In: World Health Organization Classification of Tumours of Soft Tissue and Bone. pp. pp. 67–68. [Google Scholar]
- 31.Chung EB, Enzinger FM. Fibroma of tendon sheath. Cancer. 1979;44:1945–1954. doi: 10.1002/1097-0142(197911)44:5<1945::aid-cncr2820440558>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama S, Nishio J, Aoki M, Nabeshima K, Yamamoto T. An update on clinicopathological, imaging and genetic features of desmoplastic fibroblastoma (collagenous fibroma) In Vivo. 2021;35:69–73. doi: 10.21873/invivo.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dal Cin P, Sciot R, De Smet L, Van den Berghe H. Translocation 2;11 in a fibroma of tendon sheath. Histopathology. 1998;32:433–435. doi: 10.1046/j.1365-2559.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 34.Nishio J, Iwasaki H, Nagatomo M, Naito M. Fibroma of tendon sheath with 11q rearrangements. Anticancer Res. 2014;34:5159–5162. [PubMed] [Google Scholar]
- 35.Suzuki K, Yasuda T, Suzawa S, Watanabe K, Kanamori M, Kimura T. Fibroma of tendon sheath around large joints: clinical characteristics and literature review. BMC Musculoskelet Disord. 2017;18:376. doi: 10.1186/s12891-017-1736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinstein A, Fitzhugh V, Ahmed I, Vosbikian M. A case of 14-year-old male with fibroma of tendon sheath of the hand with novel chromosomal translocation 4;10. Case Rep Orthop. 2019;2019:3514013. doi: 10.1155/2019/3514013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter JM, Wang X, Dong J, Westendorf J, Chou MM, Oliveira AM. USP6 genetic rearrangements in cellular fibroma of tendon sheath. Mod Pathol. 2016;29:865–869. doi: 10.1038/modpathol.2016.83. [DOI] [PubMed] [Google Scholar]
- 38.Mantilla JG, Gross JM, Liu YJ, Hoch BL, Ricciotti RW. Characterization of novel USP6 gene rearrangements in a subset of so-called cellular fibroma of tendon sheath. Mod Pathol. 2021;34:13–19. doi: 10.1038/s41379-020-0621-1. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira AM, Rosenberg AE. Lyon, IARC Press. 2020. Myositis ossificans and fibro-osseous pseudotumour of digits. In World Health Organization Classification of Tumours of Soft Tissue and Bone. pp. pp. 53–54. [Google Scholar]
- 40.de Silva MV, Reid R. Myositis ossificans and fibroosseous pseudotumor of digits: a clinicopathological review of 64 cases with emphasis on diagnostic pitfalls. Int J Surg Pathol. 2003;11:187–195. doi: 10.1177/106689690301100305. [DOI] [PubMed] [Google Scholar]
- 41.Sukov WR, Franco MF, Erickson-Johnson M, Chou MM, Unni KK, Wenger DE, Wang X, Oliveira AM. Frequency of USP6 rearrangements in myositis ossificans, brown tumor, and cherubism: molecular cytogenetic evidence that a subset of “myositis ossificans-like lesions” are the early phases in the formation of soft-tissue aneurysmal bone cyst. Skeletal Radiol. 2008;37:321–327. doi: 10.1007/s00256-007-0442-z. [DOI] [PubMed] [Google Scholar]
- 42.Bekers EM, Eijkelenboom A, Grünberg K, Roverts RC, de Rooy JWJ, van der Geest ICM, van Gorp JM, Creytens D, Flucke U. Myositis ossificans – another condition with USP6 rearrangement, providing evidence of a relationship with nodular fasciitis and aneurysmal bone cyst. Ann Diagn Pathol. 2018;34:56–59. doi: 10.1016/j.anndiagpath.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Flucke U, Bekers EM, Creytens D, van Gorp JM. COL1A1 is a fusion partner of USP6 in myositis ossificans - FISH analysis of six cases. Ann Diagn Pathol. 2018;36:61–62. doi: 10.1016/j.anndiagpath.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Švajdler M, Michal M, Martínek P, Ptáková N, Kinkor Z, Szépe P, Švajdler P, Mezencev R, Michal M. Fibro-osseous pseudotumor of digits and myositis ossificans show consistent COL1A1-USP6 rearrangement: a clinicopathological and genetic study of 27 cases. Hum Pathol. 2019;88:39–47. doi: 10.1016/j.humpath.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Song W, Suurmeijer AJH, Bollen SM, Cleton-Jansen AM, Bovée JVMG, Kroon HM. Soft tissue aneurysmal bone cyst: six new cases with imaging details, molecular pathology, and review of the literature. Skeletal Radiol. 2019;48:1059–1067. doi: 10.1007/s00256-018-3135-x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Hwang S, Benayed R, Zhu GG, Mullaney KA, Rios KM, Sukhadia PY, Agaram N, Zhang Y, Bridge JA, Healey JH, Athanasian EA, Hameed M. Myositis ossificans-like soft tissue aneurysmal bone cyst: a clinical, radiological, and pathological study of seven cases with COL1A1-USP6 fusion and a novel ANGPTL2-USP6 fusion. Mod Pathol. 2020;33:1492–1504. doi: 10.1038/s41379-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishio J, Iwasaki H, Soejima O, Naito M, Kikuchi M. Rapidly growing fibro-osseous pseudotumor of the digits mimicking extraskeletal osteosarcoma. J Orthop Sci. 2002;7:410–413. doi: 10.1007/s007760200070. [DOI] [PubMed] [Google Scholar]
- 48.Moosavi CA, Al-Nahar LA, Murphey MD, Fanburg-Smith JC. Fibroosseous pseudotumor of the digit: a clinicopathologic study of 43 new cases. Ann Diagn Pathol. 2008;12:21–28. doi: 10.1016/j.anndiagpath.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhry IH, Kazakov DV, Michal M, Mentzel T, Luzar B, Calonje E. Fibro-osseous pseudotumor of the digit: a clinicopathological study of 17 cases. J Cutan Pathol. 2010;37:323–329. doi: 10.1111/j.1600-0560.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 50.Flucke U, Shepard SJ, Bekers EM, Tirabosco R, van Diest PJ, Creytens D, van Gorp JM. Fibro-osseous pseudotumor of digits - expanding the spectrum of clonal transient neoplasms harboring USP6 rearrangement. Ann Diagn Pathol. 2018;35:53–55. doi: 10.1016/j.anndiagpath.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Dermawan JK, Cheng YW, Tu ZJ, Meyer A, Habeeb O, Zou Y, Goldblum JR, Billings SD, Kilpatrick SE, Reith JD, Shurtleff SA, Farkas DH, Rubin BP, Azzato EM. Diagnostic utility of a custom 34-gene anchored multiplex PCR-based nextgeneration sequencing fusion panel for the diagnosis of bone and soft tissue neoplasms with identification of novel USP6 fusion partners in aneurysmal bone cysts. Arch Pathol Lab Med, 2020 doi: 10.5858/arpa.2020-0336-OA. [DOI] [PubMed] [Google Scholar]
- 52.Konishi E, Kusuzaki K, Murata H, Tsuchihashi Y, Beabout JW, Unni KK. Extraskeletal osteosarcoma arising myositis ossificans. Skeletal Radiol. 2001;30:39–43. doi: 10.1007/s002560000298. [DOI] [PubMed] [Google Scholar]