Abstract
Background.
Social impairments are important features of a substance use disorder diagnosis; and recent models suggest early impairments in socio-cognitive and -affective processes may predict future use. However, no systematic reviews are available on this topic.
Methods:
We conducted a systematic review and meta-analyses exploring the association between social- cognitive and -affective processes (empathy, callous-unemotional (CU) traits, theory of mind, and social cognition) and substance use frequency (alcohol, cannabis, general drug use). We examined moderating effects of study design, gender, age, and weather conduct problems were controlled for. We also review brain studies related to social cognition and substance use disorder (SUD) risk.
Results:
Systematic review suggested a negative association for positively valenced constructs with substance use but mixed results on the negatively valenced construct CU traits. Meta-analyses revealed moderate positive association between CU traits with alcohol and general drug use but no significance with cannabis use. Moderate effect sizes were found for CU traits in youth predicting severity of substance use by late adolescence and significantly accounted for variance independently of conduct problems. Significant moderators included gender proportions, sample type, and age. Neuroimaging meta-analysis indicated 10 coordinates that were different in youth at a high risk/with SUD compared to controls. Three of these coordinates associate with theory of mind and social cognition.
Conclusion:
Socio-cognitive and -affective constructs demonstrate an association with current and future substance use, and neural differences are present when performing social cognitive tasks in regions with strongest associations with theory of mind and social cognition.
Keywords: adolescents, substance use, risk, empathy, callous-unemotional traits, theory of mind, social cognition
Substance use commonly increases during early adolescence, around 13 years old, and peaks in late adolescence, around 18-19 years of age (Dennis & Scott, 2007; Rutherford et al., 2010). These normative age patterns of use see a steady decline into adulthood; however, a subset persist in their use, with some developing a substance use disorder (SUD). There are many preadolescent phenotypes known to increase risk of SUD such as reward sensitivity, poor impulse control, and behavioral disorders (Argyriou et al., 2018; Ryan et al., 2013; Verdejo-Garcia et al., 2008; Volkow et al., 2010). Identification of such risk factors help in prediction of SUD. However, there is one commonly overlooked phenotype – social functioning and its underlying factors.
Problems of social functioning and bonding have been well described in youth with persistent substance use and these problems are traditionally considered to result from substance use. For example, social impairment is one of four diagnostic domains of SUD involving strained and problematic interpersonal relationships as well as giving up major roles and social activities in order to use substances (American Psychiatric Association, 2013). However, recent models and preliminary evidence suggest factors underlying impaired social functioning and bonding (e.g. social cognition, empathy, theory of mind) may predispose to persistent substance use and SUD. Massey et al. (2017) propose that impairments in empathy (i.e. the ability to understand others thoughts and share their feelings) are an early SUD risk factor; in this model, those with social impairments are more likely to persist in substance use despite substance related social consequences. Additionally, prevention programs target social competence/skills as a means to reduce substance use (Das et al., 2016), further suggesting that social domains may lead into substance use and improving social skills may reduce future substance use risk.
Though potentially important to our understanding of addiction, behavioral and neural models of socio-cognitive and -affective processes are not well defined in models on substance use and addiction. For example, Volkow and colleagues (Volkow et al., 2016) emphasize social skills development and assessing social rewards for individuals SUD prevention and recovery. However, Volkow and colleagues’ biological model does not include socio-cognitive and - affective processes. Identifying whether reliable evidence exists on these associations can improve existing models by either including or excluding relevant socio- cognitive or -affective factors. It is plausible that excessive substance use disrupts factors underlying social bonding, and that impairments in factors underlying social bonding increases potential for substance use and later SUD (Young et al., 2011) – highlighting the need to elucidate social processes underlying social impairments both behaviorally and neurally as a phenotypic risk factor.
1.2. Socio-Cognitive and -Affective Factors Examined in this Review
1.2.1. Empathy
Empathy is generally agreed in the literature to involve both socio-cognitive and - affective components (Decety, 2010; Shamay-Tsoory et al., 2009). Cognitive empathy involves the ability to take on and understand others perspective, whereas Affective empathy involves sharing another’s emotional experience (Decety, 2011; Decety & Cowell, 2015). Neuroimaging meta-analyses distinguish these processes neurally, suggesting that cognitive processes involve the medial prefrontal cortex, temporoparietal junction, and the posterior cingulate cortex (constituting the default mode network). On the other hand, affective processes involve the anterior insula and anterior cingulate cortex (constituting the salience network) (Decety & Lamm, 2007; Eres et al., 2015; Fan et al., 2011; Lamm et al., 2011).
1.2.2. Callous-Unemotional Traits
Callous-Unemotional (CU) traits involve affective impairments including a lack empathy, remorse, and guilt (Frick & White, 2008) that are broader than, but include primary impairments of socio- affective processes (Jones et al., 2010; Schwenck et al., 2012; Winter et al., 2017). Behaviorally, those with CU traits are characterized by antisocial behavior such as persistent disregard for others, uncaring behavior, and the use of others for their own gain (Blair et al., 2014). Those with higher CU traits show less neural activity and aberrant connectivity patterns in default mode and salience networks (Pu et al., 2017; Umbach et al., 2017; Yoder et al., 2016).
1.2.3. Theory of Mind
Theory of mind is a socio-cognitive process referring to the ability to infer and attribute mental states to others and reason about the thoughts, beliefs, and emotions of others (Frith & Frith, 2005; Mitchell, 2005). Like other socio-cognitive processes, neural underpinnings of theory of mind primarily involve the default mode network (Li et al., 2014).
1.2.4. Social Cognition
Social cognition involves both social-cognitive and -affective processes that broadly refer to processes that interact with the social environment, including responding to social stimuli and making social decisions (Frith, 2008). This broad construct could subsume theory of mind and empathy with impairments at least partially involved in CU traits. Neural underpinnings of social cognition include the default mode and salience networks (Adolphs, 2009; Frith, 2007).
1.3. Neural Underpinnings of Addiction
Volkow et al. (2016) demonstrate those with addiction have neuroimaging findings that differ from typically developing brains overlapping brain regions underlying socio-cognitive and -affective processes. This includes the default mode, which in addiction is involved in preoccupation and anticipation of substance use, as well as the salience network which, in addiction, is involved in withdrawal and negative affect (Volkow et al., 2016). Beyond neural regions underlying social processes, the ventral striatum and global pallidus show aberrant functioning, which are involved in addictive processes of reward in response to substances during binging and intoxication. Even though brain differences beyond social processes are demonstrated, it is unknown if the overlapping brain regions between addiction and both socio-cognitive and -affective processes account for any predisposed vulnerability to persistent substance use.
1.4. Current Study
The present study examines the association between socio-cognitive and -affective processes with substance use and future substance use severity. It is plausible that deficits in socio-affective processes may attenuate the response to substance use-related consequences (Massey et al., 2017) and deficits in socio-cognitive processes may diminish perception of social connectedness (Galinsky et al., 2005; McWhirter et al., 2002; Wang et al., 2014) and/or engagement in prosocial behaviors (Fett et al., 2014; Imuta et al., 2016; Tamnes et al., 2018), leading individuals to prefer substances over social connection.
Here we aim to qualitatively synthesize and quantitatively examine available evidence on the association between socio-cognitive and -affective functioning in adolescents in relation to substance use and predictions of future use. Because social processes and substance use show gender-related differences (Brady & Randall, 1999; Christov-Moore et al., 2014; Geary, 2002; Russell et al., 2007; Stickle et al., 2012) that may also vary by sample type (clinical versus community; Colins et al., 2020; Cotter et al., 2018; Decety & Moriguchi, 2007; Herpers et al., 2016; Loney et al., 2003), we explore gender and sample effects. Age is also explored as a moderator because normative use patterns change with age (Dennis & Scott, 2007; Rutherford et al., 2010). In addition to the behavioral evidence we review relevant neural evidence of SUD risk.
We hypothesize that both socio-cognitive and -affective constructs will associate with concurrent substance use (e.g. cannabis and alcohol consumption) and predict severity of substance use (i.e. higher frequency) longitudinally. Specifically, positively valenced constructs (empathy, theory of mind, social cognition) will negatively associate, whereas negatively valenced construct (CU traits) will positively associate, with substance use, respectively. For moderation, we expected forensic and clinical samples to have lower ratings on socio-cognitive and -affective processes and higher rates of substance use in comparison to community samples. Because substance use and impairments in socio-cognitive and -affective processes underlie internalizing and externalizing behavioral issues (Bornstein et al., 2010; Gambin & Sharp, 2016) that are commonly present in those with a SUD (Colder et al., 2018; Currie et al., 2005; Jun et al., 2015), we expect studies controlling for conduct problems to have a weaker effect than those that do not account for this variance in the analysis. Additionally, we examine the effects of age and gender, but, because of the sparse literature, we made no directional hypotheses on these as moderators. The present study addresses a critical need by examining constructs underlying both cognitive and affective social processes in relation to substance use severity, as well as the underlying neural mechanisms, associated with concurrent use and prediction of future use severity.
2. Methods
Prior to the beginning of the study, the study protocol was registered (PROSPERO 2019 CRD42019124948). A four-step process then was implemented to select the studies to be included in the review in accordance with PRISMA guidelines (Moher et al., 2009). First, studies relevant to this review were identified through literature searches using PubMed, PsychINFO, and Web of Science. Searches consisted of keyword combinations of the terms (1) socio-cognitive and -affective processes (i.e. empathy, theory of mind, social cognition, and CU traits) and (2) substance use including specific substances (Supplemental Table 1). PubMed’s medical subject headings search and both PsychINFO and Web of Science term search guided search term selection. A forward and backward search using the identified articles was completed to locate additional articles.
2.1. Inclusion and Exclusion Criteria
We included studies if they (1) were empirical studies reporting on the association between socio- cognitive or -affective processes associating with substance use or SUD risk; (2) had substance use, addiction, SUD, or SUD risk as an outcome (via self-report, laboratory, or neurobiological measures); (3) used a sample that had a mean age ≤ 18, (4) used quantitative methods; and (5) published in English. Studies using measures of psychopathic traits in adolescents had to report on a subscale or measure assessing CU traits to be included. Additionally, we excluded studies with measures of emotional intelligence because the broadness of the construct is outside the aims of the present study.
2.2. Bias Assessment
Four reviewers independently assessed each included article using a modified version of the Cochrane bias assessment scale (Higgins et al., 2011). Reviewers discussed applicability of the article to the research question and came to consensus about inclusion and bias ratings. Because the current review of studies were all observational, a risk of bias was assessed as high, low, or unclear for the six domains: selection, measurement (e.g. reliability coefficients >.70), detection bias (appropriate measurement for construct studied), differential attrition, incomplete data, and selective reporting. Our qualitative synthesis focused on low-risk studies, defined as studies with low risk on most (4 out of 6) domains assessed. Medium risk studies were those that had high risk of bias on one domain or unclear risk of bias on most (4 out of 6). Studies deemed to be a high risk were those agreed to have a high risk of bias on more than one domain.
2.3. Data Extraction and Synthesis
All four authors extracted data and methods from the included studies using a custommade table (Table 1 [behavioral], Table 2 [imaging]). Data abstraction included study samples (e.g. sample type, size, groupings, diagnostic groups studied), key methods used (study design and type, measures used, analysis methods), and summary of the results (direction, strength, and significance of the relationship between empathy and substance use and risk outcomes). The strengths of association were assessed looking at the direction and magnitude of the association as well as the statistical significance.
Table 1.
Summary of Research on Social Cognitive and Affective Constructs Relationship with Substance Use in Adolescence (k=28).
| Study | Sample | Key methods | Summary of key results | Association | Social Construct |
Results |
|---|---|---|---|---|---|---|
| Empathy | ||||||
| Anh et al., (2011) | N=498 African American adolescents. Age= 11-15. 43% male. | Correlational study. Self-report empathy, drug refusal efficacy, drug use frequency (alcohol, marijuana, and cigarette use). Structural equation modeling. | General empathy has an indirect negative effect on drug use via drug refusal efficacy. | Concurrent | General (does not distinguish) |
Moderation model Empathy a Drug refusal β= .11* Drug refusal a use β= −.29* Indirect effect of empathy on drug refusal on drug use .11 *(−.29) = −.032* |
| Ferreira, Simoes et al. (2012) | N= 3,436 community sample adolescent students. Mean age 15. 47% male. | Correlational study. Self-report empathy (social and emotional competence) and frequency of substance use (tobacco use, drunkenness, and illicit drugs use). ANOVA and moderation. | Authors found a significant mean difference on empathy in youth who used either tobacco or illegal substances. Specifically, those who used had lower self-reported empathy when compared to youth who abstained. | Concurrent | General (does not distinguish) |
Empathy on use Tobacco: F= 7.69** Alcohol: ns Drugs: F= 32.20*** |
| Laghi et al. (2019) | N= 188 community samples of Italian high school students. Age= 15-19. 71.8% male. | Correlational study. Self-report of cognitive and affective empathy, number of binge drinking episodes in past 2 weeks, and self-efficacy in resisting peer pressure to drink. Moderation and hierarchical regression analysis. | Binge drinking was negatively correlated with empathetic concern. Perspective taking positively associates with an adolescent’s resistance to peer pressure. Direct findings did not hold in regression, but cognitive empathy moderated the association between resistance to peer pressure and drinking. | Concurrent | Cognitive & affective |
Binge drinking associating with Affective: r= −0.16* Cognitive: r= −0.06 Resistance to peer pressure associating with Affective: r= .12 Cognitive: r= .18* Drinking regressed on Affective: β = −.06 Cognitive: β = −.02 Resistance X cognitive β= .28* |
| Luengo et al. (1994) | N= 1,144 youth involved in the juvenile justice system (n= 103) and community sample (n= 1,041). Age= 14-18. 100% male. | Group compare study. Self-report empathy, impulsivity, sensation-seeking scale, and drug consumption. Pearson correlation and stepwise multiple regression. | Pearson correlations: cognitive and affective empathy had a significant negative association with drug use. (most associated with sensation seeking). Multiple regression: in controls, drug taking was predicted by affective empathy, self-esteem, and sensation seeking with affective empathy accounting for the most variance. In incarcerated samples it was predicted by sensation-seeking and impulsivity. |
Concurrent | Cognitive & affective |
Drug-use associating with Correlation whole sample Cognitive: r= −.10** Affective: r= −.09*** Regression with controls Cognitive: ns Affective: β= −.423** Regression with incarcerated people Sensation-seeking: .382* Impulsivity: .425* |
| Winters et al. (2020) | N=826 adolescents in outpatient substance use treatment. Age= 12-18. 18.68% male. | Cohort study (4 time points). Self-reported empathy and substance use over prior 3 months (alcohol, marijuana, cocaine, or a hallucinogen). Growth curve modeling. | Growth curve modeling: affective empathy consistently predicted reductions in substance use and cognitive empathy did not. | Prospective | Cognitive & affective |
Predicting substance use Affective: β= −.04** Cognitive: ns |
| Callous-Unemotional (CU) Traits | ||||||
| Andershed et al. (2018)(2) | N= 996 community sample of 12-year-old adolescents; 48% male. | Cohort study (3 time points). Self-report CU traits and substance use frequency. Correlation, multiple & logistic regression. | Baseline CU traits significantly correlated with substance use at 1st, 2nd, and 3rd year follow up for boys and year 3 for girls. In logistic regression neither conduct or CU added prediction to the stability of substance use. | Prospective | Affective deficits |
CU correlation with use Boys (girls) Y1: r= .14**(ns) Y2: r= .11*(ns) Y3: r= .10*(.09*) CP correlation with use = ns Stability of use over time CP only = ns CU only = ns |
| Anderson et al. (2018)(2) | N= 753 children (n= 367 high-risk for behavior problems, n= 386 controls). Assessed from grade 7 to | Cohort study (8 time points). Self-report CU traits and parent-report CD symptoms. Self-report | CU traits associated with mean increases in substance and cigarette use over the 8 time points but not the change in slope. | Prospective | Affective deficits |
CU with substance use: Intercept = 1.15** Slope = ns |
| Muratori et al. (2016)(2) | N= 59 youth with disruptive behavior disorders. Age = 9 at start of study. 95% male. | Cohort study (from age 9 to 15; 4 time points) parent-report CU traits and self-report of substance use frequency (alcohol, tobacco, marijuana, and overall substances) in the past month. Latent growth curve analysis. | Youth with a high and relatively consistent CU trait level from childhood to adolescence were at an increased risk for behavioral problems and substance use in adolescence. Also, no baseline environmental and clinical factors associated with levels of CU traits. | Prospective | Affective deficits |
CU correlating with use T1-T4: r= .17*-.66* CU growth curve Substance Use: Slope β= 1.04*** Intercept β= .48*** |
| Pechorro et al. (2016) a2 | N= 1,003, Portuguese public-school sample. Age= 12-20, (mean= 15.87). 52.6% male. | Group compare study. Self-report psychopathic traits, CU traits, empathy, alcohol abuse, and drug use. Correlations. | Psychopathic traits, notably CU traits, had a positive correlation with alcohol and cannabis use. | Concurrent | Affective deficits |
Youth Psychopathic Trait Inventory short version CU Alcohol (total sample): 0.15*** Cannabis (total sample): 0.11** |
| Pechorro et al. (2017) a2 (1) | N= 1,003, Portuguese public-school sample. Age= 12-20 (mean= 15.87). 52.6% male. | Group compare study. Self-report psychopathic traits, CU traits, empathy, alcohol abuse, and drug use. Correlations. | Psychopathic traits, notably CU traits, had a positive correlation with alcohol and cannabis use. | Concurrent | Affective deficits |
Youth Psychopathic Trait Inventory short version CU Alcohol (total sample): 0.18*** Cannabis (total sample): 0.13*** |
| Pechorro et al. (2017)a3 (1) | N= 377 forensic females (n= 274) and controls (n= 103). Age= 14-19. 0% male. | Group compare study. Self-report CU traits, empathy, and substance use frequency (alcohol, cannabis, and cocaine/heroin use). Pearson correlation. | Callousness was significantly negatively correlated with empathy (stronger negative relationship with affective than cognitive empathy) and was positively correlated with use of alcohol, cannabis, cocaine, and heroin. | Concurrent | Affective deficits |
CU total score associating with Alcohol r= .45*** Cannabis: r= .46*** Cocaine/heroin: r= .40*** |
| Pechorro et al. (2017)a3 | N= 377 forensic females (n= 274) and controls (n= 103). Age= 14-19. 0% male. | Group compare study. Self-report CU traits, empathy, and substance use frequency (alcohol, cannabis, and cocaine/heroin use). Pearson correlation. | Callousness was significantly negatively correlated with empathy (stronger negative relationship with affective than cognitive empathy) and was positively correlated with use of alcohol cannabis, cocaine, and heroin. | Concurrent | Affective deficits |
CU total score associating with Alcohol: r= .31*** Cannabis: r= .34*** Cocaine/heroin: r= .31*** |
| Pechorro et al. (2016) (1) | N= 221 forensic males. Age 13-20. 100% male. | Within subject study. Self-report CU traits. Frequency of substance use. Pearson correlation | CU traits positively associated with substance use (alcohol, cannabis, and cocaine/heroin). | Concurrent | Affective deficits |
CU total score associating with Alcohol: r= .26*** Cannabis: r= .32*** Cocaine/heroin: r= .17*** |
| Ray et al. (2016)(1) | N= 1,216 adolescents involved with the juvenile justice system (from Crossroads Study). Age= 13-17. 100% male. | Correlational study. Self-report CU traits, impulse control, and frequency of substance use across lifetime. Latent class analysis and logistic regression. | CU traits added to the predictive probability between substance use groups. | Concurrent | Affective deficits |
Abstainer & soft drug users: CU: OR= 1.03 Abstainer & hard drug users: CU: OR = 1.06*** Soft vs hard drug users: CU: OR = 1.03* |
| Thogersen et al. (2020) (1) | N=160 Norwegian adolescents with behavioral problems. Age= 11-19. 53.7% male. | Correlational study. Self-, parent-, teacher-report of CU traits and self-report frequency of problematic alcohol use (AUDIT). Logistic regression. | Self- and teacher- reported CU traits were significantly associated with problematic alcohol use. Parent-reported CU traits of adolescents failed to show a significant association with problematic alcohol use. | Concurrent | Affective deficits |
Reported CU traits and problematic alcohol use Self-reported OR= 1.09** Teacher-reported OR= 1.11** Parent-reported OR = 1.04, p= 0.173 |
| Thornton et al. (2019) | N= 1,216 male adolescents from juvenile justice system. Age= 13-17. 100% male. | Cohort study (4 time points). Self-report of CU traits and variety of substance use (13 substances surveyed at 2 time points by SU/AI). Zero-order correlations. | CU traits associated with substance use. Additionally, CU traits had an indirect effect on risky sexual behavior through adolescent’s substance use (regardless of the type of substance used). | Prospective | Affective deficits |
CU traits with substance use r= 0.29*** |
| Waller et al. (2018)a1 (1) | N= 1170 male forensic youth. Age= 14-18. 100% male. | Cohort and case control study (9 time points). Self-report CU traits, substance use (alcohol, and substance dependence) in the past 6 months. MANCOVA. | Youth with high and stable CU traits were associated with higher substance use, as well as harsh parenting, and contextual risk of violence exposure. Effect sizes (eta2) reported, and all are nominal to small (<= .03). | Prospective | Affective deficits |
CU trait groups on drug F= 11.67*** High > moderate*** High > low*** CU trait groups on alcohol F= 4.57* High > moderate*** High < low*** |
| Wymbs et al. (2012) (2) | N= 521 mixed sample community youth in 6th grade followed up at 9th grade. Age = 11-13.6 at start of study. 43% male. | Cohort study (2 time points). Self-report CU traits and frequency of alcohol and marijuana use in past 6 months. Hierarchal and logistic regression. | Self-reported CU traits at 6th grade predicted onset, reoccurrence, and impairment of substance use by 9th grade. Females with higher CD and CU had higher odds of endorsing alcohol or marijuana. | Prospective | Affective deficits |
Onset of impairment Self-report (parent report) CU: ns (.20*) CD:.51**(ns) CU&CD: −.11*(ns) Gender&CU: ns (ns) Gender&CD: −.33**(ns) Genders&CU&CD: ns(ns) Recurrent impaired use CU: ns(ns) CD: .19**(ns) CU&CD: −.06**(ns) Gender&CU: ns(ns) Gender&CD: −.15**(ns) Genders&CU&CD: .08*(ns) |
| Theory of Mind (ToM) | ||||||
| Lannoy et al. (2020) | N=202 French secondary school adolescents. Age= 13-20. 37.6% male. | Correlational study. Self-report empathy, depressive/anxiety symptoms, and alcohol consumption (AUD1T-C, assessing frequency and intensity of drinking) and binge drinking score. ToM assessed with Yoni’s task. Correlational analysis and hierarchical linear models. |
Binge drinking and alcohol abuse is negatively associated with second-order affective ToM. Empathy did not associate statistically. | Concurrent | Cognitive |
Alcohol Cognitive: ns Affective: ns ToM cognitive 1st: ns ToM affective 1st: ns ToM cognitive 2nd: ns ToM affective 2nd:−.19* Binge drinking Cognitive: ns Affective: ns ToM cognitive 1st: ns ToM affective 1st: ns ToM cognitive 2nd: ns ToM affective 2nd: −.22* |
| Social Cognition | ||||||
| Fluharty et al. (2018) | N= 3,058 youth age 8 (prospective) and N= 3,613 adolescents age 18 (retrospective). | Cohort and case control study (2 time points). Parent report social communication disorders (age 8). Self-report frequency, age at first use, and variety of use for alcohol, cigarette, and cannabis use (age 15 & 18). Self-report social reciprocity (age 18). Logistic regression. | Temporally going forward: age 8 poor non-verbal communication decreased odds of substance use in adolescence. Retrospectively after substance use: Poor social communication and reciprocity increased the odds of adolescent substance use. Sex-stratified analysis indicated no differences. |
Prospective & retrospective | General (does not distinguish) |
Social communication Prospective Alcohol: ns Tobacco: OR= 1.95*** Cannabis: ns Social reciprocity Retroactive Alcohol: OR= 1.44** Tobacco: OR= 1.92*** Cannabis: OR= 1.44** |
| Kirisci et al. (2004) | N= 215 youth, starting at ages= 10-12 and followed until 19. 100% Male. | Cohort study (4 time points). Self-report social cognition, beliefs about substance use, frequency of use (alcohol and marijuana consumption) in month prior to study, and risk of lifetime SUD. Social cognitive distortions report. Path analysis. | Inaccurate social cognition, significantly predicted by childhood SUD risk (measured by neurobehavioral disinhibition), predicted marijuana use at age 16 that led to prodromal SUD at age 19. | Prospective | Cognitive |
SUD risk (neurobehavioral disinhibiden) 10yo on social cognitive distortions 12yo: β= −31** (negative means more) Social cognitive 12-14yo distortions on marijuana use 16yo: β= −.13** (negative means more) Prodromal marijuana use on SUD 19yo: β= .31* |
CU= callous-unemotional, CD= conduct disorder, CP= conduct problem, ToM= theory of mind, ANOVA= analysis of variance.
#= studies using the same sample (same number indicates which studies use the same sample), + = elevated, □ = moderate, − = low
= reported on the model without executive functioning
= included in cross-sectional meta-analysis
= included in longitudinal meta-analysis.
ns = not significant
= p<.05
= p<.01
= p<.001.
Table 2.
Summary of Neuroimaging Research on Social Cognitive and Affective Constructs Relationship with Substance Use Disorder Risk in Adolescence (k = 4).
| Study | Sample | Key methods | Summary of key results | Association | Social Construct |
Results |
|---|---|---|---|---|---|---|
| Imaging Studies | ||||||
| Callous-Unemotional (CU) Traits | ||||||
| Sakai, Dalwania (1) | N= 66 male adolescents in a treatment program with conduct problems. Age=15-18. 100% male. | Group compare study. Task-based fMRI, Prosocial giving game “altruistic antisocial game” (AlAn’s), self-report of CU traits, empathy, as well as substance abuse or dependence across several substances. | The high CU traits group had higher prevalence of substance use. During the prosocial game, the LPE group (that has higher substance use) had a negative activation in the R-AI in comparison to both CD and controls. Severity of CU associated negatively with the L-IPL and positively with the L-PCC. | Concurrent | Affective deficits |
Brain region cluster Differences in insula Cluster (R-AI, IFG, and STG) between groups (high CU, conduct only, controls) F= 9.5; p< 0.001 LPE was negative With severity ot CU traits (whole brain): L-IPL t= −3.61 L- PCC t= −3.31 |
| Social Cognition | ||||||
| Cservenka, Fair (2) | N=36 adolescents with a FHP (n=19 high SUD risk) and FHN (controls n=17, 51% male). Age= 12-16. 49% male. | Group compare study. Seed-based resting state fMRI and task-based fMRI with emotional go-no-go task. | Emotional go-no-go task: FHP group had less activity to happy faces in the L. STG, I. insula, L. postcentral gyrus. (Implicated in affective social processing during a social processing task). Resting state: reduced bilateral amygdala connectivity in FHP. Reduced connectivity b/t superior frontal gyrus and amygdala associated with poor response inhibition during emotional task. | Concurrent | Cognitive & affective |
HR vs. LR on facial task L-STG: t= −3.20* L-STG, I.-AI, L-POG: t= −3.05* HR vs. LR connectivity L. amygdala L-SFG: t= −3.98* L-precuneus: t= −3.44* L-SFG: t= −3.44* L-cerebellum: t= −3.11* R-cerebellum: t= −3.57* R-MFG: t= −3.81* R-precentral gyrl: t= −4.04* R-amygdala R-cerebellum: t= −3.27* R-MFG: t= −3.65* R-MTG; t= −3.37* |
| Hulvershorn, Finn (3) | N= 37 non-substance using early adolescents at high risk for SUD or controls (19 HR, 18 controls). Age= 12-14. 67% male. | Group compare study. Task-based fMRI using an emotional face matching task. | HR youth had increase mPFC, precuneus, and occipital cortex activation relative to controls during the face matching condition relative to the control shape condition. | Concurrent | Cognitive & affective |
Face vs. Shape contrast High risk = higher activation R-mPFC: t= 3.88*** L-Precuneus: t= 4.02*** L-MOG: t= 4.34*** R-MOG: t= 4.73*** |
| O'Brien and Hill (4) | N= 78 third generation offspring of alcoholic families at high risk for SUD (n= 40) and controls (n= 38). Mean age= 11.78. 54% males. | Group comparison fMRI study. Clinical interviews for substance use and clinical conditions. Structural MRI and cox survival analysis. | The ratio of OFC to amygdala volume significantly predicted SUD survival time across the sample; reduction in survival time was seen in those with smaller ratios for both high-risk and low-risk groups. Morphology of prefrontal relative to limbic regions in adolescence prospectively predicts age of onset for substance use disorders. | Concurrent | Cognitive & affective |
Survival analysis for onset of substance use disorder OFC/amygdala: OR= .389* Family risk: OR= .418* Age: OR= .786** Sex: ns |
CU = callous-unemotional, CD = conduct disorder, FHP = family history positive for substance use disorder, HR = high risk, LR = low risk, LPE = low prosocial emotions.
AI = anterior insula, PCC = posterior cingulate cortex, mPFC = medial prefrontal cortex, MFG = medial frontal gyrus, STG = superior temporal gyrus, MTG = middle temporal gyrus, IFG = inferior frontal gyrus, SFG = superior frontal gyrus, OFC = orbital frontal cortex, MOG = mid occipital gyrus, PCG = postcentral gyrus.
R- = right, L- = left, fMRI = functional magnetic resonance imaging.
ns = not significant
= p < .05
= p < .01
= <.001.
2.4. Selection of Studies for Meta-Analysis
Studies included in the systematic review were screened by all authors for appropriateness for meta-analysis. Any disagreements about appropriateness for meta-analysis were resolved by first and second authors. Considerations included comparison of measures, study design, and number of studies available. For behavioral meta-analysis, we were only able to analyze CU traits studies due to the limited number of studies and lack of comparable outcome variables for other measures of social cognition. Inclusion for behavioral meta-analysis included a self-report measure for CU traits and substance use frequency measure. If male and female were reported separately, we treated each as a point estimate for an independent population for boys and girls. For the neuroimaging meta-analysis, we included studies if they performed a social task that compared blood oxygen level dependent (BOLD) responses between groups of youth who were deemed as a high risk for or current SUD with typically developing controls.
2.5. Behavioral Meta-Analytic Strategy
Effect sizes for analysis were converted to Hedge’s g for comparison using the “esc” package and, where necessary, we calculated standard error using the “dmeter” package (Harrer et al., 2019; Lüdecke, 2019). We conducted all analyses using the “metaphor” package for R version 4.0.2 (R Core Team, 2020; Viechtbauer, 2010). A priori, we expected studies were unlikely to be identical. We tested heterogeneity of the studies and if the test was insignificant, we compared random and fixed effect model results for comparison. If identical we reported the random-effects model. For cross-sectional studies we conducted separate meta-analyses by substance use outcome (i.e., alcohol, cannabis, general drugs). When included studies for an analysis included both between- and within- subject design, we accounted for potential differences by including study design as a moderator.
For longitudinal studies, we ran analyses on a general drug use frequency measure. An important consideration for this meta-analysis was controlling for effects of conduct problems. Where available, we calculated effect sizes for longitudinal analyses that controlled for conduct problems. We accounted for studies that did not control for conduct disorder in mediation. Most articles examined a baseline measure of CU traits predicting later substance use; thus we calculated effect sizes that assessed a constant CU trait over time predicting substance use when growth factors may have also been available (i.e. intercept betas in growth models used for effect sizes).
To statistically assess publication bias, we used a multifaceted approach including 1) a visual inspection of a funnel plot, 2) examined weighted regressions of funnel plot asymmetry using standard error, and 3) compared a trim-and-fill model using a L0 estimator. Using this approach, we would determine there is systematic bias if 1) there is considerable asymmetry in the funnel plot, 2) the regression required a significant statistic, and/or 3) trim-and-fill models substantively different than the model tested. If we determined there was bias and the trim-and-fill model corrected for this bias, then we report on the trim-and-fill model. We confirmed effect sizes by simulating 5,000 studies using the metaphor package simulate function. Simulated effect sizes within five-hundredths of a point from the original are considered confirmed.
After assessing the properties of the unconditional models, we explored moderation of the association between CU traits and substance use. Where relevant for all studies, we tested moderation for age, proportion of males in the sample, design (within or between subject), sample type (community or forensic/clinical), and whether they controlled for conduct disorder. For longitudinal studies, additional moderators were tested for time points, lag in time between each time point, and the interaction between them. To decrease Type I error inflation, we used Knapp and Hartung’s adjustment for the moderators significance (Viechtbauer, 2010).
2.6. Neuroimaging Studies Meta-Analytic Strategy
Neuroimaging meta-analyses were conducted on studies that provided coordinates with a social task comparing higher risk versus control participants. We conducted the neuroimaging meta-analysis using the activation likelihood estimation technique implemented in GingerALE (Turkeltaub et al., 2002; www.brainmap.org/ale/) using MNI152 coordinates. The likelihood of neural differences between groups who were categorized as a higher risk (externalizing symptoms and family history or higher instances of use and CU traits) and controls were estimated on contrasts reported in primary studies. Clusters forming thresholds included a false discovery rate (FDR) corrected p value at < 0.01 and a minimum volume of 500mm. We calculated the centroid of clusters identified in the analysis and compared with independent meta-analytic locations from the Neurosynth database (Yarkoni et al., 2009; www.neurosynth.org). From Neurosynth we examined associations of the coordinates identified ALE maps with social constructs (i.e. empathy, theory of mind, social cognition) as well as addiction to indicate if identified regions associated with similar findings across studies.
3. Results
3.1. Study characteristics
We identified 32 articles (Supplemental Figure 1) that were included in the systematic review (29 [88%] behavioral and four [12%] neuroimaging; Figure 1). Social constructs represented included: five empathy studies (15%), 21 CU trait studies (63%), two theory of mind studies (6%), and six social cognition studies (18%). Twelve (36%) were longitudinal studies and 22 (66%) were cross sectional studies. Out of the 21 CU trait studies, the meta-analysis consisted of six) cross-sectional studies (29% of CU studies) and seven for longitudinal studies (33% of CU studies) with a total of 21 relevant effect sizes. Three neuroimaging studies (75% of neuroimaging studies) were included in the neuroimaging meta-analysis. Behavioral study samples ranged from 63 to 3,436 participants, whereas neuroimaging study samples ranged from 16 to 78 participants. The mean age across studies was 14.92 (range= 11.5-18). The average proportion of males across studies is 65% (range 0-100%).
Figure 1.
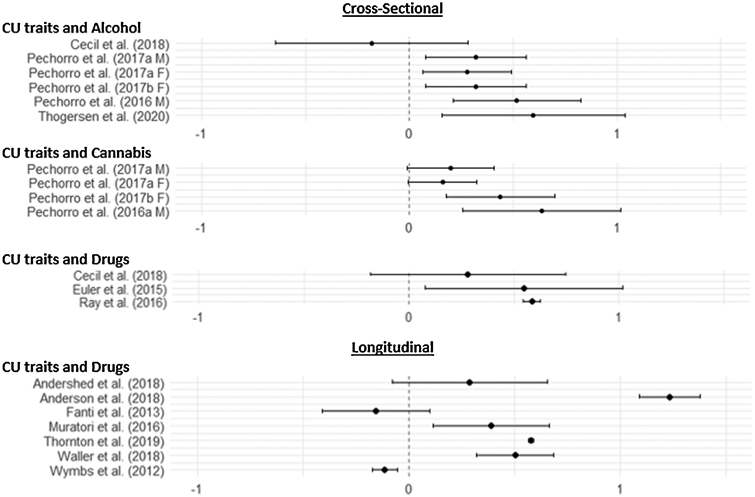
Figure Depicting Effect Size Results of Behavioral Meta-Analysis.
3.2. Risk of Bias Assessment
Of the 32 articles included, all authors agreed that 24 had a low risk of bias (73%), seven (22%) with medium risk, and two (6%) with high risk (Supplemental Table 2; Supplemental Figure 2). Amongst the medium risk studies, two had high risk for incomplete outcome data, one for selection bias, and one for measurement bias while the rest had more unclear ratings than low. High risk studies had high bias in more than one category including outcome data, detection, selective reporting, and measurement bias. The majority of unclear ratings occurred for selection (12) and incomplete data (11) bias. Overall, most studies were rated favorably with a low risk of bias indicating high quality evidence. Of the studies meeting inclusion criteria in the meta-analysis, except one medium bias study, all were rated a low bias.
3.3. Systematic review synthesis
3.3.1. Empathy
Five studies were found on the association between empathy and substance use including one longitudinal study (20%) and four cross-sectional designs (80%). Of these studies, two focused on general empathy (40%) whereas three differentiated between cognitive and affective processes (60%) (Table 1). The quality of evidence suggested only one study had a high risk of bias due to measurement and selective reporting bias whereas the other four studies had low risk of bias (Figure 2). Findings in this section did not appear to be due to individual study bias.
Figure 2.
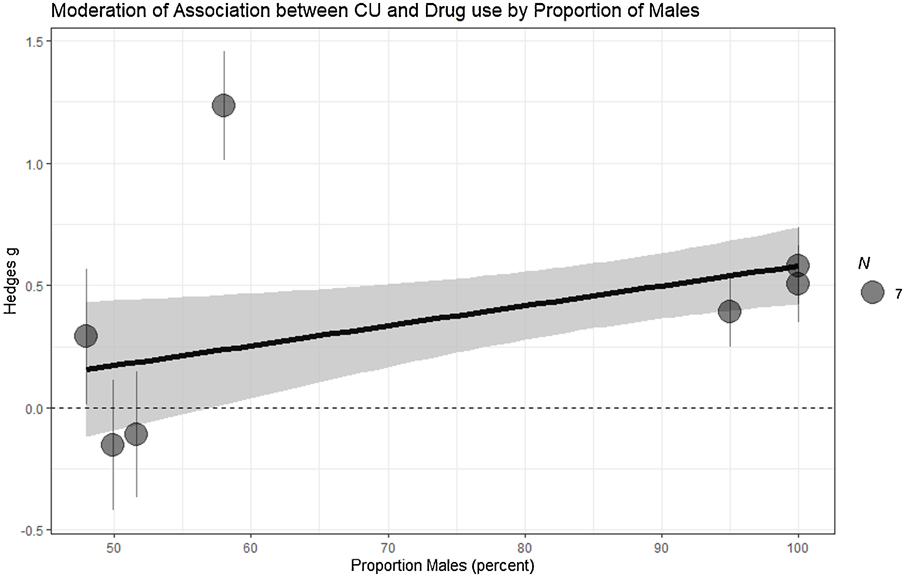
Figure Depicting the Moderating Effect of Proportion of Males on Effect Sizes in Longitudinal Studies
Associations between empathy and substance use.
Overall, cross-sectional findings suggest that general, as well as both cognitive and affective empathy, negatively associate with concurrent substance use (Anh et al., 2011; Ferreira et al., 2012; Laghi et al., 2019; Luengo et al., 1994) (Table 1). Of importance, two studies suggested an indirect effect via drug refusal efficacy – one study on general empathy and another on cognitive empathy (Anh et al., 2011; Laghi et al., 2019). Although all correlations showed negative associations, regressions of cross-sectional data controlling for covariates showed mixed results for the association between affective empathy and substance use. However, longitudinal findings found that increases in affective empathy over time predicted decreases in substance use (Winters et al., 2020).
Influences on relationship between empathy and substance use.
There was little evidence for other influences on the above associations. Other than one study finding general empathy did not moderate the association between gender and substance use (Anh et al., 2011), gender differences were not well examined in the available literature. We were unable to examine differences in community and clinical samples, whether behavioral issues accounted for differences, or effects of age from the available literature. Additionally, no neural studies were available in the present search. It is unclear if biological processes beyond subjective report may account for cognitive and affective empathy when associating with substance use. Moreover, future longitudinal studies could help untangle socio-cognitive processes relationship to substance by testing the moderating effect of drug refusal-efficacy.
3.3.2. Callous-Unemotional Traits
We included 21 studies focusing on CU traits. Ten of these studies were cross-sectional (50%), nine were longitudinal (45%), and one was retrospective (5%). Eight of these studies were of community samples and 12 sampled from forensic or clinical samples (Table 1). Risk of bias consensus suggested five articles (25%) had a medium risk of bias with the greatest bias in measurement and incomplete outcome data bias. Fifteen articles (75%) had low risk of bias (Figure 2). Studies risk of bias (between medium and low risk) did not change the pattern of findings across studies.
Associations between CU traits and substance use.
Overall findings on this association were mixed. Five cross-sectional studies (Pedro Pechorro et al., 2016; Pechorro, da Silva, et al., 2017; Pechorro, Gonçalves, et al., 2017; Pechorro, Hawes, et al., 2017; P. Pechorro et al., 2016) and three longitudinal studies (Andershed et al., 2018; Thornton et al., 2019; Wymbs et al., 2012) showed positive correlations (no controls) with substance use, which is supported in group comparison studies (Chabrol et al., 2012; Ray et al., 2016). Regarding conduct problems, two cross-sectional studies (Cecil et al., 2017; Euler et al., 2015) and two longitudinal studies (Andershed et al., 2018; Wymbs et al., 2012) found conduct with CU traits accounted for more variance in substance use. Two studies found CU traits associated with slight decreases in substance use over time (Fanti, 2013; Wymbs et al., 2012). However, four longitudinal studies (Anderson et al., 2018; Baskin-Sommers et al., 2015; Muratori et al., 2016; Waller et al., 2018) found that after accounting for conduct problems (either continuously or group separation) that consistency and/or growth in CU traits accounted for unique variance when predicting increases in substance use over time. Moreover, one study found CU traits moderated the relationship between conduct problems and substance use (Wymbs et al., 2012), whereas another found CU traits in addition to preexisting impulsivity accounted for increases in future substance use (Dubas et al., 2017).
Gender influence on CU traits and substance use.
Gender related findings were also mixed. Five cross-sectional studies (Pedro Pechorro et al., 2016; Pechorro, da Silva, et al., 2017; Pechorro, Gonçalves, et al., 2017; Pechorro, Hawes, et al., 2017; P. Pechorro et al., 2016) and two longitudinal studies (Andershed et al., 2018; Thornton et al., 2019) demonstrated positive associations for both males and females. However, one longitudinal study found within females there was no significant association between baseline CU traits and substance use, and CU traits protected females from later substance use (Wymbs et al., 2012).
Sample influence on CU traits and substance use.
The pattern of findings did not appear to be different in samples of community versus forensic samples.
Neuroimaging findings on CU traits and substance use.
Neuroimaging findings consisted of one study (Sakai et al., 2017) that concurrently compared adolescent controls with those with conduct issues and those with conduct issues with high CU traits on a prosocial giving game that was played in an fMRI machine (Table 2). Those who were high in CU traits had more substance use or dependence and less cognitive and affective empathy than conduct problems alone or controls. Sakai and colleagues found a negative mean activation in a right insula cluster (right insula, inferior frontal gyrus, and superior temporal gyrus) in those with CU traits that was not present in the other two groups. CU traits positively associated with the left inferior parietal lobule and left posterior cingulate cortex.
3.3.3. Theory of Mind and Social Cognition
We found only one study examining theory of mind and substance use (Table 1). That study had a medium risk of bias (Figure 2). We found 5 studies of social cognition: two behavioral longitudinal studies (40%) and three neuroimaging studies (60%) (Table 1). One study had a high level of bias for detection and incomplete outcome bias, and the other four studies had low bias risk (Figure 2). The high risk study on one parameter appeared to diverge from the consistency of the other results, which is discussed below.
Theory of mind associating with substance use.
When examining both cognitive and affective theory of mind, the only significant finding was the second order affective theory of mind (i.e. the ability to predict what one person thinks a different person thinks and feels). Interestingly, cognitive, and affective empathy did not have a significant association, yet the theory of mind finding held.
Social cognition associating with substance use.
Longitudinal studies agreed that social cognition (social reciprocity, level of social cognitive distortions) temporally associated with substance use in adolescents. One study found that higher risk for SUD predicted social cognitive distortions at 12-14 predicted marijuana use prodromal to SUD at 19 (Kirisci et al., 2004). Fluharty and colleagues (Fluharty et al., 2018) report similar findings from retrospective reports but not in prospective longitudinally collected data, though this study was rated as a high risk of bias. In addition, the social cognition variable assessed by Fluharty and colleagues (poor non-verbal communication at age 8 as a measure of social cognition) may better map on to developmental delays and not the socio-cognitive processes of interest here. We could not determine the effects of gender, sample, or conduct issues on these relationships. The findings generally support that social cognition is negatively associated with substance use.
Neuroimaging findings on social cognition and SUD risk.
Neuroimaging findings converge on the frontal cortex and amygdala across studies (Table 2). During facial recognition tasks, adolescents identified as a higher risk for SUD (family history of substance use and externalizing symptoms) showed higher activity in medial frontal and orbital regions both when looking at angry (Hulvershorn et al., 2013) and happy faces (Cservenka et al., 2014). During resting state, functional connectivity of the amygdala with the rest of the cortex was different in youth at high SUD risk when compared to controls (Cservenka et al., 2014). A brain volume study supported that a lower ratio of amygdala to orbital frontal cortex volume in adolescence predicted onset of SUD by late adolescence/early adulthood (O'Brien & Hill, 2017). Together these studies suggest patterns of differences in the medial and orbital frontal regions as well as subcortical amygdala are present in youth at higher risk for SUD.
3.3.4. Summary of Systematic Review
The positively-valenced constructs examined here appear to be negatively associated with substance use. When considering empathy, the cognitive components appear to indirectly associate with greater efficacy in refusing substances, whereas affective components have a direct negative association with substance use. Neither theory of mind nor social cognition studies corroborated the substance refusal finding; however, social cognitive errors longitudinally predicted substance use severity leading to SUD. Social reciprocity also retrospectively predicted odds of use. Gender and sample type (community vs. clinical) were not investigated in these studies. Both cognitive and affective components of social cognition appear to predict substance use but there is a clear need to further examine socio- cognitive processes in relation to drug refusal efficacy.
Amongst studies on the negatively valenced CU trait construct, the findings were mixed. A few studies showed a positive association, while others suggested that controlling for conduct disorder accounted for this association. Analyses examining the effects of sample type and gender were also mixed. Several studies showed an association between CU traits and both concurrent and future substance use. However, this systematic review could not determine if conduct disorder accounted for this association, or the magnitude of potential effects of sample type or gender. These inconsistencies are addressed by meta-analysis (below).
Systematic review of the available neuroimaging studies suggested that youth who at a higher risk for SUDshowed differences in medial frontal cortical regions (medial prefrontal cortex, orbital frontal cortex, medial orbital gyri, superior temporal gyri) and subcortical regions associated with emotional arousal (anterior insula and amygdala) when performing a social cognitive task. When performing a prosocial giving task, CU traits are associated with activation patterns in an insular cluster (right anterior insula, inferior frontal gyrus, and superior temporal gyrus) such that those with CU traits have a negative activation pattern whereas the other groups (conduct issues with low CU and controls) have a positive activation. Overall, it appears the insula, amygdala, and both medial and orbital frontal regions are important in socio-cognitive and -affective tasks in relation to risk for SUD.
3.4. Meta-analysis
3.4.1. Cross-Sectional Model of Alcohol
There were six separate effects included in the random effects model with concurrent alcohol use as the outcome (Table 3, Figure 1). The association between CU traits and alcohol use was moderate and positive (g= 0.32, p= 0.04, simulated g= 0.32). The Q-statistic suggested these studies were homogeneous (Q= 1.67, p= 0.89); however, examination of the fixed effects model revealed the same effect size as the random effect model. Visual inspection of the funnel plot did not reveal an obvious asymmetry, and this was supported by an insignificant regression test (t= −0.19, p= 0.85). A trim-and fill model detected one absent effect that raised the effect size slightly (g= 0.38, p= 0.01). Given the other two bias checks did not suggest any issues, and interpretations were not changed by trim-and-fill model results, we decided to report on the original random effects model.
Table 3.
Summary of findings from meta-analysis findings.
| Outcome | N | k | Hedge’s g | CI95 | Simulated Hedge’s g |
|---|---|---|---|---|---|
| Cross-Sectional | |||||
| Alcohol1 | 1,612 | 6 | 0.32 | 0.01, 0.63 | 0.32 |
| Cannabis | 1,380 | 4 | 0.31 | −0.03, 0.64 | 0.31 |
| Drugs1 | 1,415 | 3 | 0.58 | 0.34, 0.82 | 0.58 |
| Longitudinal | |||||
| Drugs | 3,736 | 7 | 0.47 | 0.34, 0.60 | 0.47 |
| Longitudinal: Gender | |||||
| Drugs | 1,619 | 6 | 0.53 | 0.26,0.80 | 0.53 |
= trim and fill model reported.
3.4.2. Cross-Sectional Model of Cannabis
There were four separate effects included in the random effects model for cross-sectional cannabis use (Table 3, Figure 1). The association between CU traits and cannabis use was moderate and positive but not significant (g = 0.31, p= 0.08, simulated g = 0.31). The Q-statistic suggested a homogenous sample (Q= 1.06, p= 0.79); however, examination of the fixed effects model revealed the same effect size as the random effect model. Visual inspection of the funnel plot did not reveal an obvious asymmetry, and this was supported by insignificant regression for asymmetry (t= 1.00, p= 0.32). A trim-and-fill model identified one absent effect that reduced the overall effect but did not change interpretation of results (g= 0.25, p= 0.12). Given the previous two checks were acceptable and there was no difference in interpretation, we report on the random effect model.
3.4.3. Cross-Sectional Model of Drugs
There were three separate effects included in the random effects model for cross-sectional drug use (Table 3, Figure 1). The positive association between CU traits and drug use was moderate (g= 0.56, p < 0.0001). The Q-statistic suggested a homogeneous sample (Q= 0.36, p= 0.84); however, examination of the fixed effects model revealed the same effect size as the random effect model. Visual inspection of the funnel plot revealed a potential asymmetry; however, there was an insignificant regression for asymmetry (t= −0.46, p= 0.65). A trim-and-fill model identified two absent effects (g= .58, p < 0.0001, simulated g= 0.58). Given we visually detected asymmetry and the trim-and-fill model detected two missing effects we report on the trim-and fill model – this did not change interpretation of the effect.
3.4.4. Longitudinal Model of Drugs
There were seven separate effects included in the random effects model for longitudinal drug use (Table 3, Figure 1). The association between CU trats and longitudinal drug use was moderate (g= 0.48, p < 0.001, simulated g= 0.48). Supporting the use of a random effects model, the Q-statistic was significant (Q= 24.69, p< 0.001). Visual inspection of the funnel plot did not reveal an obvious asymmetry, and this was supported by insignificant regression for asymmetry (t= 0.14, p= 0.88). A trim-and-fill model detected no missing effects.
3.5. Meta-Analysis Moderation Models
Despite four exceptions, moderation tests revealed little evidence of moderation (Table 4). Cross-sectional studies shown no moderating effects. Longitudinally, the association between CU traits and drug use was moderated by the proportion of males, with studies with a higher proportion of males showing a higher positive effect (Figure 2). The type of sample also moderated this association with forensic/clinical samples having a stronger positive effect (Figure 3). Age also moderated this association where higher longitudinal mean age collections shown stronger associations (Figure 4). In addition, we separately examined studies that did not control and did control for conduct problems. The studies that did not control for conduct problems had a stronger effect, but both study types had significant effects (Figure 3).
Table 4.
Estimates from models exploring moderation of the association between CU traits and substance use by study design and gender.
| Moderator | b | t | p |
|---|---|---|---|
| Cross-Sectional | |||
| Alcohol | |||
| Proportion: Male | 0.00 | 1.27 | 0.42 |
| Age | −0.08 | −0.95 | 0.51 |
| Sample: Forensic/Clinical | 0.13 | 1.43 | 0.52 |
| No Control: Conduct Problems | 0.13 | 0.54 | 0.61 |
| Design: Within-Subject | 0.33 | 1.50 | 0.37 |
| Cannabis | |||
| Proportion: Male | 0.00 | 1.28 | 0.42 |
| Age | 0.95 | 0.97 | 0.63 |
| Sample: Forensic/Clinical | 0.35 | 4.42 | 0.14 |
| Drugs | |||
| Proportion: Male | 0.00 | 2.27 | 0.26 |
| Age | −0.11 | −0.60 | 0.55 |
| Sample: Forensic/Clinical | 0.30 | 0.59 | 0.55 |
| No Control: Conduct Problems | 0.17 | 0.46 | 0.65 |
| Longitudinal | |||
| Drugs | |||
| Proportion: Male | 0.00 | 2.55 | 0.00 |
| Age | 0.17 | 3.51 | 0.00 |
| Sample: Forensic/Clinical | 0.34 | 2.24 | 0.02 |
| No Control: Conduct Problems | 0.33 | 2.26 | 0.02 |
| Design: Time Lag | −0.36 | −0.86 | 0.38 |
| Design: Timepoints | −0.08 | 0.40 | 0.68 |
| Design: Time Lag x Timepoints | 0.00 | 0.03 | 0.97 |
Figure 3.
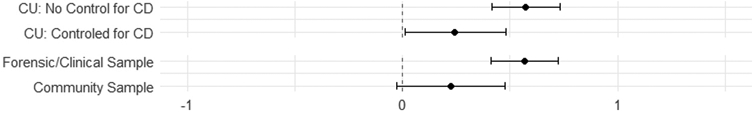
Figure Depicting Moderating Effects of Controlling for conduct problems and Sample type
Figure 4.
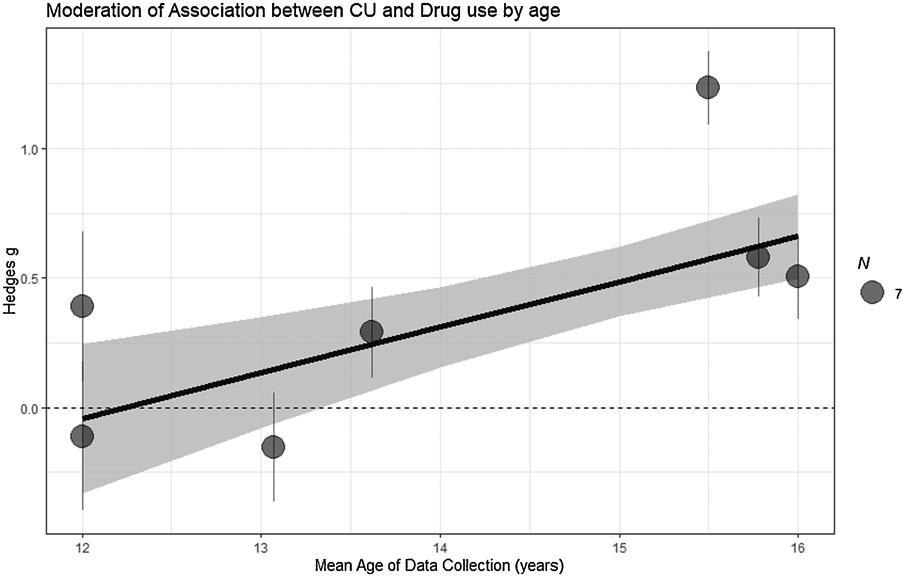
Figure Depicting Moderating Effect of Age on Effect Sizes in Longitudinal Studies
3.6. Neuroimaging Meta-Analysis
Analysis of neuroimaging data revealed statistically significant differences in 10 coordinates when comparing typically developing youth to those identified at a high risk (Table 5; Supplemental Figure 3). Consistent with the systematic review of neuroimaging studies, we see patterns of medial frontal regions, but little subcortical differences. We compared the coordinates identified in this meta-analysis with findings from the general literature, by searching those coordinates in Neurosynth. Three of the ten coordinates had significant associations with socio-cognitive and -affective processes (Figure 5); all other coordinates did not have significant findings with any terms on Neurosynth. The three coordinates that did associate had a low association with addiction and empathy and the highest associations (positive or negative) with social cognition and theory of mind. The angular gyrus and superior frontal medial gyrus had positive associations whereas the superior occipital lobe had negative associations with social cognition. The superior frontal medial gyrus had the strongest association.
Table 5.
Meta-Analysis of Neural Difference Between Youth at High Risk and Controls During Social Task and associations with neural maps.
|
MNI Coordinates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Hemisphere | Label | BA | ALE | Neural Map association (r) | |||
| 51 | 22 | −8 | Right | Orbital inferior frontal gyrus | 47 | 0.0090 | Addict | Emp | SC | ToM |
| −11 | −63 | 1 | Left | Lingual Gyrus | 19 | 0.0097 | – | – | – | – |
| 21 | −18 | −37 | Right | Parahippocampal gyrus | 35 | 0.0102 | – | – | – | – |
| 3 | 4 | 11 | Right | Caudate | – | 0.0102 | – | – | – | – |
| 2 | 26 | 53 | Right | Superior Frontal medial gyrus | 8 | 0.0098 | – | – | – | – |
| −21 | −96 | 12 | Left | Middle occipital gyrus | 18 | 0.0094 | – | – | – | – |
| −62 | −26 | 13 | Left | Superior temporal gyrus | 41 | 0.0094 | – | – | – | – |
| 38 | −76 | 19 | Right | Middle occipital gyrus | 19 | 0.0094 | – | – | – | – |
| 10 | 61 | 26 | Right | Superior Frontal medial gyrus | 9 | 0.0094 | 0.01 | 0.08 | 0.39 | 0.43 |
| −9 | −82 | 46 | Left | Superior occipital lobe | 7 | 0.0094 | 0.02 | −0.03 | 0.10 | 0.18 |
| −39 | 24 | 45 | Left | Frontal medial gyrus | 8 | 0.0098 | – | – | – | – |
| 49 | −72 | 34 | Right | Angular gyrus | 39 | 0.0098 | −0.01 | −0.06 | −0.23 | −0.23 |
| −58 | −35 | 47 | Left | Inferior parietal lobule | 40 | 0.0094 | – | – | – | – |
| −58 | 13 | −16 | Left | Superior temporal pole | 38 | 0.0102 | – | – | – | – |
All coordinate anatomical likelihood estimations have an FDR corrected p value of < .001. – = no association.
Addict = addiction map; Emp = Empathy map; SC = Social cognition map; ToM = Theory of mind map.
Figure 5.
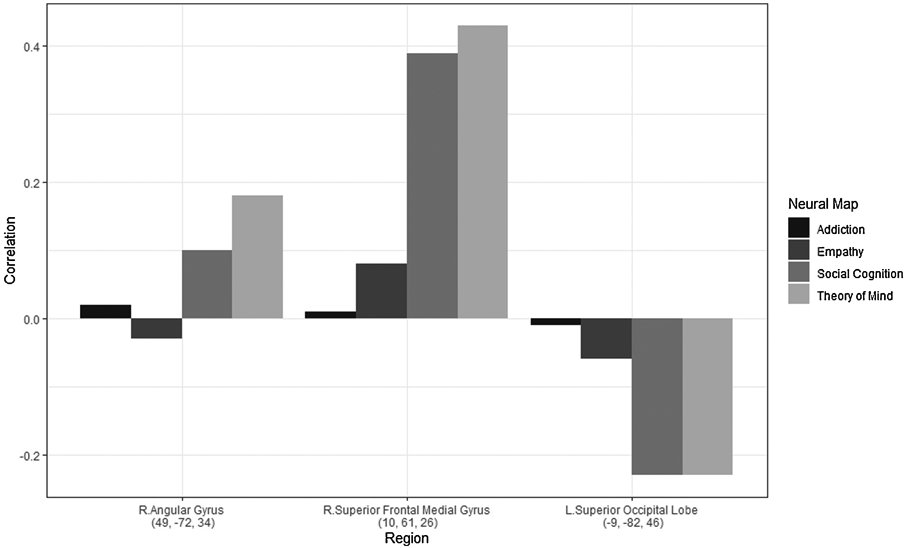
Figure Depicting Correlations of Neural Regions Meta-Analytic Neural Maps in Neurosynth
4. Discussion
The current review and meta-analysis represent a timely analysis of the literature on the association between socio-cognitive and -affective constructs with substance use. The literature for empathy and social cognition generally converge on a negative association with substance use. These studies did not capture gender differences, associations with age, or clinical samples. On the other hand, studies of CU traits produced several mixed findings on gender, impact of conduct problems, and sample differences in systematic review.
In the behavioral meta-analysis (on CU traits and substance use), we found that, although CU traits did not significantly associate with concurrent cannabis use, they did positively associate with concurrent use of alcohol and general drug use in adolescents. Longitudinal studies suggested CU traits positively associated with drug use over time. In addition, although this relationship with future substance use was weaker when conduct problems were accounted for, CU traits still contributed a significant effect independent of conduct problems. This finding, that CU traits predict future substance use independent of conduct problems, is of particular importance and is consistent with theory suggesting the impairment of social processes increase risk for SUD (Massey et al., 2017). Together with the systematic review, these findings call for further examination of positively valenced constructs (i.e. social cognition, theory of mind, empathy) to untangle specific socio-cognitive and -affective processes underlying adolescent substance use. Meta-analyses of these phenotypes will require more consistent measurement of social cognition and substance use outcomes across studies.
4.1. Gender Moderating Substance Use and CU Traits
Gender differences were not found for concurrent substance use in cross-sectional samples, but in longitudinal studies males have a stronger positive effect between CU traits and substance use compared to females. Males have been reported to have higher rates of CU traits than females, and CU traits as currently described may better capture male-centric aspects of this construct. Alternatively, it may be that males are, in part, at greater risk for increased substance use due to higher baseline levels of CU traits. A single study suggested that CU traits were protective of substance use within females (Wymbs et al., 2012), Based on the available data, we can neither substantiate nor counter this claim in the present analysis, but some prior studies had a low proportion of males and those studies showed mixed results, with both significant and insignificant effects. The mediating effects of factors, such as emotional arousal or social perceptions, may be different for males and females. Additional studies are required to help us understand gender differences in relation to CU traits and future severity of substance use.
4.2. Study Sample Type Moderating CU Traits Association with Substance Use
Samples that were forensic/clinical compared to community samples had larger positive effects in longitudinal studies. This finding substantiated our hypothesis that forensic/clinical samples would have higher levels of socio- affective impairments and this would predict increased substance use over time. Importantly, the effect with the community sample was of a moderate size but not statistically significant – it is difficult to arrive at firm conclusions regarding the CU traits to substance use relationship in community samples. Further testing is needed to parse apart the multitude of processes (e.g. low affective arousal, less spontaneous perspective taking, impairments in sharing other’s emotions) that may be specific to forensic and clinical samples.
4.3. Age Moderating CU Traits Relationship with Substance Use
Mean age of the sample did not moderate cross-sectional studies, but in longitudinal samples greater mean age was associated with higher effect sizes. This finding is within the curve of normative substance use that peaks during in late adolescents (Dennis & Scott, 2007; Rutherford et al., 2010). Although a valuable finding, it is plausible that this reflects a normative pattern of use that may decline as expected even in the presence of CU traits. Future studies that capture age ranges beyond the adolescent years will be important for determining whether CU traits may contribute to persistence in substance use beyond normative substance use patterns in adolescents.
4.4. Conduct Problem Controls Moderating CU Traits Relationship with Substance Use
In cross-sectional studies, controlling for conduct issues did not moderate the association between CU traits and substance use. Longitudinally, we demonstrated a significant moderation such that studies that did not control for conduct issues had a higher effect. However, it is important to note that studies that controlled for conduct issues still had a significant positive moderate effect. This suggests that CU traits account for some unique variance in predicting substance use longitudinally, independent of conduct problems. Massey and colleague’s model (Massey et al., 2017), supports the importance of including moderators of response to social consequences and perception of use in future studies. Additionally, investigating the role that comorbid mental health symptoms or associated traits, such as anxiety and impulsivity, play in these relationships would similarly be important.
4.5. Neuroimaging Meta-Analysis
Neural differences were observed in adolescents identified at a high risk for SUD (or already having SUD) while engage in tasks related to social cognition. Some neural coordinates identified in our meta-analysis also are known to undergird social cognition and theory of mind (e.g., from search of the Neurosynth database). Notably, these regions did not have a strong association with maps of addiction. It may be that youth at this age do not yet show full neural patterns of addictions and these will later emerge. However, the neural differences in regions associating with social processes may indicate early warning signs for later substance use severity. This analysis did not find differences in subcortical regions such as the amygdala in the literature review. However, the studies included involved social tasks that are more likely to probe task-positive regions that are more cortical. Future studies could target subcortical regions to clarify this association with substance use. We found no longitudinal studies for the neural meta-analysis that examining tasks of social cognition and future risk of substance use. Such studies would be critical to develop models of neural development of social cognition in relation to substance use.
4.6. Limitations
The present findings must be interpreted in light of some limitations. First, in some analyses we consider single substance use (cannabis and alcohol) and in other analyses we considered general drug use. These choices were based on the available literature but also may limit the generalizability of our findings. Because this is a relatively new area of investigation, we had to examine multiple constructs that, although are overlapping, have some conceptual and measurement differences. However, the consistent patterns of findings of an association across these constructs with substance use both cross-sectional and longitudinally suggest the robustness of these relationships. Additionally, the current review only identified a small number of studies for meta-analysis and our behavioral meta-analyses could only focus on CU traits.
5. Conclusion
The current systematic review suggests an association between social cognitive and affective constructs with both concurrent and future substance use. The relationship between positively valenced constructs and substance use phenotypes was consistently seen across gender and study sample type. Specifically, affective constructs appeared to negatively associate directly with substance use whereas cognitive constructs appeared to have an indirect effect through drug refusal efficacy. Thus, positively valenced socio-cognitive and -affective constructs appear to be multidimensional with important contributions to adolescent substance use. Differences were also shown in the brains of youth identified at a high risk versus controls. However, the systematic review of CU traits did not demonstrate consistent results relating to substance use.
The meta-analytic review was able to clarify some of these inconsistencies, showing moderate effect sizes between CU traits and both frequency of substance use concurrently and future substance use. We found differences in the presence of gender, sample type, controlling for conduct issues, and age. Of particular importance CU traits accounted for variance independently of conduct problems. Thus, these findings suggest socio-cognitive and -affective processes that are involved in substance use behavior in adolescents are multidimensional.
The present literature still leaves several questions to build on. Future studies could further examine the positively valenced constructs in relation to gender and sample type as well as test the indirect effects of socio-cognitive processes on drug refusal efficacy. Additionally, future studies should examine gender differences in the relationship between CU traits and future substance use. The meta-analysis revealed that samples with larger proportions of females had lower effect sizes (some studies were even insignificant), whereas all male studies had significant effects. This likely reflects some of the inconsistencies in the available literature. More studies are needed to parse why females may show differences in social processes in relation to substance use. Overall, the present work provides evidence that socio-cognitive and - affective processes do predict substance use in adolescents. More work is needed to assess if causal effects exist.
Supplementary Material
Highlights.
Social processes negatively associate with adolescent substance use
Callous-unemotional traits have moderate effects on substance use
Callous-unemotional traits effect is independent of conduct issues
Age, gender, and sample type moderate callous-unemotional traits effect
Brain differences of youth at high risk associate with social processes
Acknowledgments
Drew E Winters, PhD. was supported by a training grant from National Institutes of Mental Health, T32MH015442
Footnotes
Conflict of interest
Authors have no conflicts of interest to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R (2009). The social brain: neural basis of social knowledge. Annu Rev Psychol 60, 693–716. 10.1146/annurev.psych.60.110707.163514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Publishing. https://books.google.com/books?id=EIbMlwEACAAJ [Google Scholar]
- Andershed H, Colins OF, Salekin RT, Lordos A, Kyranides MN, & Fanti KA (2018). Callous-unemotional traits only versus the multidimensional psychopathy construct as predictors of various antisocial outcomes during early adolescence. Journal of Psychopathology and Behavioral Assessment, 40(1), 16–25. 10.1007/s10862-018-9659-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SL, Zheng Y, & McMahon RJ (2018). Do callous–unemotional traits and conduct disorder symptoms predict the onset and development of adolescent substance use? Child Psychiatry and Human Development. 10.1007/s10578-018-0789-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anh BN, Clark TT, & Faye ZB (2011). Empathy and drug use behaviors among African- American adolescents. Journal of Drug Education, 41(3), 289–308. 10.2190/DE.41.3.d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou E, Um M, Carron C, & Cyders MA (2018). Age and impulsive behavior in drug addiction: A review of past research and future directions. Pharmacology, biochemistry, and behavior, 164, 106–117. 10.1016/j.pbb.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Waller R, Fish AM, & Hyde LW (2015). Callous-unemotional traits trajectories interact with earlier conduct problems and exec-utive control to predict violence and substance use among high risk male adolescents. J Abnorm Child Psychol, 43(8), 1529–1541. 10.1007/s10802-015-0041-8 [DOI] [PubMed] [Google Scholar]
- Blair RJ, Leibenluft E, & Pine DS (2014). Conduct disorder and callous-unemotional traits in youth. N Engl J Med, 371(23), 2207–2216. 10.1056/NEJMra1315612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomstein MH, Hahn C-S, & Haynes OM (2010). Social competence, externalizing, and internalizing behavioral adjustment from early childhood through early adolescence: Developmental cascades. Development and psychopathology, 22(4), 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, & Randall CL (1999). Gender differences in substance use disorders. Psychiatric Clinics of North America, 22(2), 241–252. 10.1016/S0193-953X(05)70074-5 [DOI] [PubMed] [Google Scholar]
- Cecil CAM, McCrory EJ, Barker ED, Guiney J, & Viding E (2017). Characterising youth with callous–unemotional traits and concurrent anxiety: Evidence for a high-risk clinical group. European Child & Adolescent Psychiatry. 10.1007/s00787-017-1086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol H, Chauchard E, Goutaudier N, & van Leeuwen N (2012). Exploratory study of the psychopathological profiles of adolescent cannabis users. Addictive Behaviors, 37(10), 1109–1113. 10.1016/j.addbeh.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Christov-Moore L, Simpson EA, Coudé G, Grigaityte K, Iacoboni M, & Ferrari PF (2014). Empathy: Gender effects in brain and behavior. Neuroscience & Biobehavioral Reviews, 46(Part 4), 604–627. 10.1016/j.neubiorev.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, Frndak S, Lengua LJ, Read JP, Hawk LW, & Wieczorek WF (2018). Internalizing and externalizing problem behavior: A test of a latent variable interaction predicting a two-part growth model of adolescent substance use. Journal of Abnormal Child Psychology, 46(2), 319–330. 10.1007/s10802-017-0277-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colins OF, Fanti KA, & Andershed H (2020). The DSM-5 Limited Prosocial Emotions Specifier for Conduct Disorder: Comorbid Problems, Prognosis, and Antecedents. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2020.09.022 [DOI] [PubMed] [Google Scholar]
- Cotter J, Granger K, Backx R, Hobbs M, Looi CY, & Barnett JH (2018). Social cognitive dysfunction as a clinical marker: a systematic review of meta-analyses across 30 clinical conditions. Neuroscience & Biobehavioral Reviews, 84, 92–99. 10.1016/j.neubiorev.2017.11.014 [DOI] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, & Nagel BJ (2014). Emotional processing and brain activity in youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research, 38(7), 1912–1923. 10.1111/acer.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Patten SB, Williams JV, Wang J, Beck CA, El-Guebaly N, & Maxwell C (2005). Comorbidity of major depression with substance use disorders. The Canadian Journal of Psychiatry, 50(10), 660–666. 10.1177/070674370505001013 [DOI] [PubMed] [Google Scholar]
- Das JK, Salam RA, Arshad A, Finkelstein Y, & Bhutta ZA (2016). Interventions for Adolescent Substance Abuse: An Overview of Systematic Reviews. Journal of Adolescent Health, 59(4), S61–S75. 10.1016/j.jadohealth.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J (2010). The neurodevelopment of empathy in humans. Dev Neurosci, 32(4), 257–267. 10.1159/000317771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J (2011). Dissecting the neural mechanisms mediating empathy. Emotion Review, 3(1), 92–108. 10.1177/1754073910374662 [DOI] [Google Scholar]
- Decety J, & Cowell JM (2015). Empathy, justice, and moral behavior. AJOB neuroscience, 6(3), 3–14. 10.1080/21507740.2015.1047055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Lamm C (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist, 13(6), 580–593. 10.1177/1073858407304654 [DOI] [PubMed] [Google Scholar]
- Decety J, & Moriguchi Y (2007). The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. Biopsychosocial Medicine, 1, 22–22. 10.1186/1751-0759-1-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, & Scott CK (2007). Managing addiction as a chronic condition. Addiction Science & Clinical Practice, 4(1), 45–55. 10.1151/ascp074145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubas JS, Baams L, Doornwaard SM, & van Aken MAG (2017). Dark personality traits and impulsivity among adolescents: Differential links to problem behaviors and family relations. Journal of Abnormal Psychology, 126(7), 877–889. 10.1037/abn0000290 (Dark Personality Traits: Challenges and Innovations) [DOI] [PubMed] [Google Scholar]
- Eres R, Decety J, Louis WR, & Molenberghs P (2015). Individual differences in local gray matter density are associated with differences in affective and cognitive empathy. Neuroimage, 117, 305–310. 10.1016/j.neuroimage.2015.05.038 [DOI] [PubMed] [Google Scholar]
- Euler F, Jenkel N, Stadler C, Schmeck K, Fegert JM, Kölch M, & Schmid M (2015). Variants of girls and boys with conduct disorder: Anxiety symptoms and callous-unemotional traits. Journal of Abnormal Child Psychology, 43(4), 773–785. 10.1007/s10802-014-9946-x [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, & Northoff G (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews, 35(3), 903–911. 10.1016/j.neubiorev.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Fanti KA (2013). Individual, social, and behavioral factors associated with co-occurring conduct problems and callous-unemotional traits. Journal of Abnormal Child Psychology, 41(5), 811–824. 10.1007/s10802-013-9726-z [DOI] [PubMed] [Google Scholar]
- Ferreira M, Simões C, Matos MG, Ramiro L, & Diniz JA (2012). The role of social and emotional competence on risk behaviors in adolescence. The International Journal of Emotional Education, 4(1), 43–55. [Google Scholar]
- Fett A-KJ, Shergill SS, Gromann PM, Dumontheil I, Blakemore S-J, Yakub F, & Krabbendam L (2014). Trust and social reciprocity in adolescence–a matter of perspective-taking. Journal of Adolescence, 37(2), 175–184. 10.1016/j.adolescence.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Fluharty ME, Heron J, & Munafo MR (2018). Longitudinal associations of social cognition and substance use in childhood and early adolescence: Findings from the Avon Longitudinal Study of Parents and Children. Eur Child Adolesc Psychiatry, 27(6), 739–752. 10.1007/s00787-017-1068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, & White SF (2008). Research review: The importance of callous- unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry, 49(4), 359–375. 10.1111/j.1469-7610.2007.01862.x [DOI] [PubMed] [Google Scholar]
- Frith C (2008). Social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1499), 2033–2039. 10.1098/rstb.2008.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C, & Frith U (2005). Theory of mind [Review]. Current Biology : CB., 15(17), R644–646. 10.1016/j.cub.2005.08.041 [DOI] [PubMed] [Google Scholar]
- Frith CD (2007). The social brain? Philos Trans R Soc Lond B Biol Sci, 362(1480), 671–678. 10.1098/rstb.2006.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky AD, Ku G, & Wang CS (2005). Perspective-taking and self-other overlap: Fostering social bonds and facilitating social coordination. Group Processes & Intergroup Relations, 8(2), 109–124. 10.1177/1368430205051060 [DOI] [Google Scholar]
- Gambin M, & Sharp C (2016). The differential relations between empathy and internalizing and externalizing symptoms in inpatient adolescents. Child Psychiatry & Human Development, 47(6), 966–974. 10.1007/s10578-016-0625-8 [DOI] [PubMed] [Google Scholar]
- Geary DC (2002). Sexual selection and sex differences in social cognition. Biology, society, and behavior: The development of sex differences in cognition, 21, 23–53. [Google Scholar]
- Harrer M, Cuijpers P, Furukawa T, & Ebert DD (2019). dmetar: Companion R Package For The Guide 'Doing Meta-Analysis in R'. R package version 0.0.9000. http://dmetar.protectlab.org [Google Scholar]
- Herpers PCM, Klip H, Rommelse NNJ, Greven CU, & Buitelaar JK (2016). Associations between high callous–unemotional traits and quality of life across youths with non-conduct disorder diagnoses. European Child & Adolescent Psychiatry, 25(5), 547–555. 10.1007/s00787-015-0766-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, & Sterne JA (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BmJ, 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Finn P, Hummer TA, Leibenluft E, Ball B, Gichina V, & Anand A (2013). Cortical activation deficits during facial emotion processing in youth at high risk for the development of substance use disorders [Article]. Drug & Alcohol Dependence, 131(3), 230–237. 10.1016/j.drugalcdep.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imuta K, Henry JD, Slaughter V, Selcuk B, & Ruffman T (2016). Theory of mind and prosocial behavior in childhood: A meta-analytic review. Developmental Psychology, 52(8), 1192. 10.1037/dev0000140 [DOI] [PubMed] [Google Scholar]
- Jones AP, Happé FGE, Gilbert F, Burnett S, & Viding E (2010). Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry, 51(11), 1188–1197. 10.1111/j.1469-7610.2010.02280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H-J, Sacco P, Bright CL, & Camlin EA (2015). Relations among internalizing and externalizing symptoms and drinking frequency during adolescence. Substance use & misuse, 50(14), 1814–1825. 10.3109/10826084.2015.1058826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Vanyukov M, Reynolds M, & Habeych M (2004). Relation between cognitive distortions and neurobehavior disinhibition on the development of substance use during adolescence and substance use disorder by young adulthood: A prospective study. Drug Alcohol Depend, 76(2), 125–133. 10.1016/j.drugalcdep.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Laghi F, Bianchi D, Pompili S, Lonigro A, & Baiocco R (2019). Cognitive and affective empathy in binge drinking adolescents: Does empathy moderate the effect of self-efficacy in resisting peer pressure to drink? Addictive Behaviors, 89, 229–235. 10.1016/j.addbeh.2018.10.015 [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, & Singer T (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54(3), 2492–2502. 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Li W, Mai X, & Liu C (2014). The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci, 8, 74. 10.3389/fnhum.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney BR, Frick PJ, Clements CB, Ellis ML, & Kerlin K (2003). Callous-unemotional traits, impulsivity, and emotional processing in adolescents with antisocial behavior problems. Journal of Clinical Child and Adolescent Psychology, 32(1), 66–80. 10.1207/S15374424JCCP3201_07 [DOI] [PubMed] [Google Scholar]
- Lüdecke D (2019). esc: Effect Size Computation for Meta Analysis (Version 0.5.1). 10.5281/zenodo.1249218 [DOI] [Google Scholar]
- Luengo MA, Otero JM, Carrillo-de-la-Peña MT, & Mirón L (1994). Dimensions of antisocial behaviour in juvenile delinquency: A study of personality variables. Psychology, Crime & Law, 1(1), 27–37. 10.1080/10683169408411934 [DOI] [Google Scholar]
- Massey SH, Newmark RL, & Wakschlag LS (2017). Explicating the role of empathic processes in substance use disorders: A conceptual framework and research agenda. Drug and Alcohol Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter BT, Besett-Alesch TM, Horibata J, & Gat I (2002). Loneliness in high risk adolescents: The role of coping, self-esteem, and empathy. Journal of Youth Studies, 5(1), 69–84. 10.1080/13676260120111779 [DOI] [Google Scholar]
- Mitchell JP (2005). The false dichotomy between simulation and theory-theory: The argument's error. Trends in Cognitive Sciences, 9(8), 363–364. 10.1016/j.tics.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. 10.1371/journal.pmed.1000097 [DOI] [PubMed] [Google Scholar]
- Muratori P, Lochman JE, Manfredi A, Milone A, Nocentini A, Pisano S, & Masi G (2016). Callous unemotional traits in children with disruptive behavior disorder: Predictors of developmental trajectories and adolescent outcomes. Psychiatry Research, 236, 35–41. 10.1016/j.psychres.2016.01.003 [DOI] [PubMed] [Google Scholar]
- O'Brien JW, & Hill SY (2017). Neural predictors of substance use disorders in young adulthood. Psychiatry Research: Neuroimaging, 268, 22–26. 10.1016/j.pscychresns.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechorro P, da Silva DR, Andershed H, Rijo D, & Abrunhosa Goncalves R (2016). The Youth Psychopathic Traits Inventory: Measurement Invariance and Psychometric Properties among Portuguese Youths. Int J Environ Res Public Health, 13(9). 10.3390/ijerph13090852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechorro P, da Silva DR, Rijo D, Gonçalves RA, & Andershed H (2017). Psychometric properties and measurement invariance of the Youth Psychopathic Traits Inventory—Short version among Portuguese youth. Journal of Psychopathology and Behavioral Assessment, 39(3), 486–497. 10.1007/s10862-017-9597-7 [DOI] [Google Scholar]
- Pechorro P, Gonçalves RA, Andershed H, & DeLisi M (2017). Female psychopathic traits in forensic and school context: Comparing the antisocial process screening device self-report and the Youth Psychopathic Traits Inventory-Short. Journal of Psychopathology and Behavioral Assessment, 39(4), 642–656. 10.1007/s10862-017-9605-y [DOI] [Google Scholar]
- Pechorro P, Hawes SW, Gonçalves RA, & Ray JV (2017). Psychometric properties of the inventory of callous-unemotional traits short version (ICU-12) among detained female juvenile offenders and community youths. Psychology, Crime & Law, 23(3), 221–239. 10.1080/1068316X.2016.1239724 [DOI] [Google Scholar]
- Pechorro P, Ray JV, Barroso R, Maroco J, & Abrunhosa Goncalves R (2016). Validation of the Inventory of Callous-Unemotional Traits Among a Portuguese Sample of Detained Juvenile Offenders. Int J Offender Ther Comp Criminol, 60(3), 349–365. 10.1177/0306624x14551256 [DOI] [PubMed] [Google Scholar]
- Pu W, Luo Q, Jiang Y, Gao Y, Ming Q, & Yao S (2017). Alterations of Brain Functional Architecture Associated with Psychopathic Traits in Male Adolescents with Conduct Disorder. Sci Rep, 7(1), 11349. 10.1038/s41598-017-11775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. URL https://www.R-project.org/. [Google Scholar]
- Ray JV, Thornton LC, Frick PJ, Steinberg L, & Cauffman E (2016). Impulse Control and Callous-Unemotional Traits Distinguish Patterns of Delinquency and Substance Use in Justice Involved Adolescents: Examining the Moderating Role of Neighborhood Context. J Abnorm Child Psychol, 44(3), 599–611. 10.1007/s10802-015-0057-0 [DOI] [PubMed] [Google Scholar]
- Russell TA, Tchanturia K, Rahman Q, & Schmidt U (2007). Sex differences in theory of mind: A male advantage on Happé's “cartoon” task. Cognition and Emotion, 21(7), 1554–1564. 10.1080/0269993Q601117096 [DOI] [Google Scholar]
- Rutherford HJ, Mayes LC, & Potenza MN (2010). Neurobiology of adolescent substance use disorders: Implications for prevention and treatment. Child and Adolescent Psychiatric Clinics, 19(3), 479–492. 10.1016/j.chc.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SR, Stanger C, Thostenson J, Whitmore JJ, & Budney AJ (2013). The impact of disruptive behavior disorder on substance use treatment outcome in adolescents. Journal of Substance Abuse Treatment, 44(5), 506–514. 10.1016/j.jsat.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JT, Dalwani MS, Mikulich-Gilbertson SK, Raymond K, McWilliams S, Tanabe J, Rojas D, Regner M, Banich MT, & Crowley TJ (2017). Imaging decision about whether to benefit self by harming others: Adolescents with conduct and substance problems, with or without callous-unemotionality, or developing typically. Psychiatry Research: Neuroimaging, 263(Supplement C), 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenck C, Mergenthaler J, Keller K, Zech J, Salehi S, Taurines R, Romanos M, Schecklmann M, Schneider W, & Warnke A (2012). Empathy in children with autism and conduct disorder: Group- specific profiles and developmental aspects. Journal of Child Psychology and Psychiatry, 53(6). 651–659. 10.1111/j.1469-7610.2011.02499.x [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, & Perry D (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–627. 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- Stickle TR, Marini VA, & Thomas JN (2012). Gender differences in psychopathic traits, types, and correlates of aggression among adjudicated youth. Journal of Abnormal Child Psychology, 40(4), 513–525. 10.1007/s10802-011-9588-1 [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Overbye K, Ferschmann L, Fjell AM, Walhovd KB, Blakemore S-J, & Dumontheil I (2018). Social perspective taking is associated with self-reported prosocial behavior and regional cortical thickness across adolescence. Developmental Psychology, 54(9), 1745–1757. 10.1037/dev0000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LC, Frick PJ, Ray JV, Wall Myers TD, Steinberg L, & Cauffman E (2019). Risky Sex, Drugs, Sensation Seeking, and Callous Unemotional Traits in Justice-Involved Male Adolescents. Journal of Clinical Child & Adolescent Psychology, 48(1), 68–79. 10.1080/15374416.2017.1399398 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, & Zeffiro TA (2002). Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage, 16(3), 765–780. 10.1006/nimg.2002.1131 [DOI] [PubMed] [Google Scholar]
- Umbach R, Raine A, Gur RC, & Portnoy J (2017). Neighborhood disadvantage and neuropsychological functioning as part mediators of the race–antisocial relationship: A serial mediation model [journal article]. Journal of Quantitative Criminology, 1–32. 10.1007/s10940-017-9343-z [DOI] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, & Clark L (2008). Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews, 32(4), 777–810. 10.1016/j.neubiorev.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Volkow ND, Koob GF, & McLellan AT (2016). Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine, 374(4), 363–371. 10.1056/nejmra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, & Baler R (2010). Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays, 32(9), 748–755. 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Baskin-Sommers AR, & Hyde LW (2018). Examining predictors of callous unemotional traits trajectories across adolescence among high-risk males. Journal of Clinical Child and Adolescent Psychology, 47(3), 444–457. 10.1080/15374416.2015.1102070 [DOI] [PubMed] [Google Scholar]
- Wang CS, Kenneth T, Ku G, & Galinsky AD (2014). Perspective-Taking Increases Willingness to Engage in Intergroup Contact. PLoS One, 9(1), e85681. 10.1371/journal.pone.0085681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Spengler S, Bermpohl F, Singer T, & Kanske P (2017). Social cognition in aggressive offenders: Impaired empathy, but intact theory of mind. Scientific Reports, 7(1), 670. 10.1038/s41598-017-00745-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters DE, Wu W, & Fukui S (2020). Longitudinal Effects of Cognitive and Affective Empathy on Adolescent Substance Use. Substance Use & Misuse, 1–7. 10.1080/10826084.2020.1717537 [DOI] [PubMed] [Google Scholar]
- Wymbs BT, McCarty CA, King KM, McCauley E, Vander Stoep A, Baer JS, & Waschbusch DA (2012). Callous-unemotional traits as unique prospective risk factors for substance use in early adolescent boys and girls. J Abnorm Child Psychol, 40(7), 1099–1110. 10.1007/s10802-012-9628-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, & Braver TS (2009). BOLD correlates of trial-by-trial reaction time variability in gray and white matter: a multi-study fMRI analysis. PLoS One, 4(1), e4257. 10.1371/journal.pone.0004257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Lahey BB, & Decety J (2016). Callous traits in children with and without conduct problems predict reduced connectivity when viewing harm to others. Scientific Reports, 6(1), 20216. 10.1038/srep20216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, & Wang Z (2011). The neurobiology of pair bonding: insights from a socially monogamous rodent. Frontiers in neuroendocrinology, 32(1), 53–69. 10.1016/j.yfrne.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


