Abstract
Emotional acceptance is an important emotion regulation strategy promoted by most psychotherapy approaches. We adopted the Activation Likelihood Estimation technique to obtain a quantitative summary of previous fMRI (functional Magnetic Resonance Imaging) studies of acceptance and test different hypotheses on its mechanisms of action. The main meta-analysis included 13 experiments contrasting acceptance to control conditions, yielding a total of 422 subjects and 170 foci of brain activity. Additionally, subgroups of studies with different control conditions (react naturally or focus on emotions) were identified and analysed separately. Our results showed executive areas to be affected by acceptance only in the subgroup of studies in which acceptance was compared to natural reactions. In contrast, a cluster of decreased brain activity located in the posterior cingulate cortex (PCC)/precuneus was associated with acceptance regardless of the control condition. These findings suggest that high-level executive cortical processes are not a distinctive feature of acceptance, whereas functional deactivations in the PCC/precuneus constitute its specific neural substrate. The neuroimaging of emotional acceptance calls into question a key tenet of current neurobiological models of emotion regulation consisting in the necessary involvement of high-level executive processes to actively modify emotional states, suggesting a complementary role for limbic portions of the default system.
Keywords: acceptance, emotion regulation, meta-analysis, ALE, Default Mode Network, mindfulness, posterior cingulate
Introduction
Recognition of the value of emotional acceptance for emotional well-being has philosophical and religious roots that date back thousands of years (Graver, 2008). In the context of the scientific investigation, acceptance has been conceptualized as a regulation strategy based on an open and welcoming attitude towards emotions, thoughts or events (Williams and Lynn, 2010). It consists of a mental stance characterized by openness and a non-judgemental attitude towards on-going emotional experiences (Grecucci et al., 2015; Goldin et al., 2019), without trying to control, change, suppress or avoid them. Acceptance also refers to a sense of curiosity about emotions. This entails a perspective that does not see emotions and thoughts as threatening, but rather as mental phenomena that are temporary, transient, interesting or at least neutral, sources of information about one’s current mental state, and not to be feared, changed or expunged. Early mentions of theoretical issues relevant to acceptance emerged from the thought suppression literature, which argued for the paradoxical pathogenetic effects of attempts at mental contents control (Wegner et al., 1987; Wenzlaff and Wegner, 2000). More recently, the revival within clinical psychology of ancient Buddhist traditions of mindfulness meditation—a set of techniques that raise awareness by paying attention in a non-judgmental way to mental activity (Kabat-Zinn, 2013)—has led to a growing interest in the adaptive value of non-judgemental attitudes towards emotions as a means to achieving positive mental health effects. This line of inquiry has produced evidence that emotional acceptance may be effective in diminishing emotional reactivity and physiological arousal in response to aversive emotions (Campbell-Sills et al., 2006; Hofmann et al., 2009; Wolgast et al., 2011; Grecucci et al., 2015). Moreover, self-reported acceptance may positively influence perceived daily stress (Catalino et al., 2017) and is associated with better mental health outcomes (Aldao et al., 2010; Berking and Wupperman, 2012). Based on the findings on its effect on coping with negative emotions and stress, acceptance may be considered as an emotion regulation strategy (Hofmann and Asmundson, 2008; Wolgast et al., 2013).
The relevance of emotional acceptance to mental health is also widely recognized in clinical practice. It occupied a position of primary importance in early humanistic–experiential therapy approaches (Perls et al., 1951; Rogers, 1959; Berne, 1961). More recently, acceptance-based techniques have been proposed as a specific therapeutic factor in mindfulness-based interventions (where they play a prominent role, Kabat-Zinn, 2013), dialectical behaviour therapy (Linehan, 1993), acceptance and commitment therapy (Hayes et al., 1999; Grecucci et al., 2018), schema therapy (Dadomo et al., 2016, 2018), short-term dynamic therapies (Frederickson, 2013; Grecucci et al., 2020) and emotion-focused therapy (Greenberg, 2011; Grecucci et al., 2020). Acceptance-based treatments have been shown to be effective in the treatment of several forms of psychopathology (Segal et al., 2002; Hayes et al., 2006; Bowen et al., 2011; Feliu Soler et al., 2018; Grecucci et al., 2018). More generally, in most psychotherapy approaches, patients are encouraged to freely observe and explore their own mental activity. Hence, acceptance may be considered a non-specific, common outcome across different psychotherapy approaches (Martin, 1997; Sambin and Messina, 2017).
The unquestionable clinical relevance of acceptance, however, is in contrast with the poor empirical investigation of this emotion regulation strategy in the clinical and affective neurosciences, at least when compared to other strategies (such as reappraisal). A reason of this relative neglect can be attributed to the fact that acceptance was not originally considered by influential models of emotion regulation (such as Gross’s process model, Gross, 1998). In this respect, the concept of acceptance breaks with the established tradition in affective neuroscience that identifies emotion regulation with cognitive control, as this latter is recruited ‘to actively modify an emotional state in terms of quality, strength, length, or frequency of emotion’ (Gross, 2015). Within the affective neurosciences, evidence for this view of emotion regulation has been sought in neuroimaging studies of reappraisal, in which participants were exposed to negative emotional stimuli and were instructed to modify (the opposite of acceptance) their emotional reactions (Ochsner et al., 2002). In these studies, findings of increased activation of cortical areas associated with working memory and executive function—such as the dorsolateral prefrontal cortex, the dorsal anterior cingulate and the ventrolateral prefrontal cortex—together with decreased activity in subcortical areas (the amygdala) have been marshalled in support of a dual-process model that describes emotion regulation as the result of interactions between prefrontal cognitive control systems accounting for ‘top-down’ modulation and subcortical systems that support ‘bottom-up’ emotional reactivity (Ochsner and Gross, 2005; Ochsner et al., 2012; Messina et al., 2015). Even if some acceptance instructions included attentional components, which would account for the involvement of controlled processes (see Table 1), they always precluded any deliberate attempt to modify emotional states. Therefore, the resulting response comes much closer to a ‘let it be’ non-interference attitude towards emotions, thoughts and bodily reactions associated with emotions, which consequently differs substantially from emotional control. In the following, we will refer to emotion regulation strategies based on changing affect aroused by stimuli as the ‘traditional’ strategies to distinguish them from acceptance-based strategies.
Table 1.
Overview of acceptance studies included in the meta-analysis
| Studies | N | Design | Stimuli | Acceptance instruction | Control condition instruction | N foci A > C | N foci C > A |
|---|---|---|---|---|---|---|---|
| 1. Kross et al. (2009) | 24 healthy (15 F) | Blocks within subjects | Negative autobiographical memories (cue phrases) | ‘… to recognize that the feelings they experienced during recollection were passing mental events that were psychologically distant from the self and did not control them.’ | ‘… to focus on the specific feelings that naturally flowed through their mind as they thought about their recalled experiences.’ (CFE) | 0 | 3 |
| 2. Lutz et al. (2014) | 46 healthy (15 F) | Between-group design | Negative pictures | ‘Try to consciously be aware of yourself, of what happens to you at this moment. Do this while expecting the picture and while looking at it. Do not judge; remain conscious and attentive to your present state. You may focus on thoughts, on emotions or on bodily sensations.’ | ‘… to expect and perceive the emotional stimuli.’ (CNR) | 3 | 2 |
| 3. Smoski et al. (2015a) | 19 healthy (12 F) | Event-related within subjects | Sad pictures | ‘To accept, your task is to notice what you are thinking and feeling, and to allow those thoughts and feelings to be there. So rather than try to push the feeling or thought away or try to feel differently, you just acknowledge it, perhaps saying, “That’s just how it is right now,” “This feeling will come and go,” or “I can accept this thought.” Note that acceptance doesn’t mean that you have to like the feeling, or that you are resigning yourself to the feeling. It is reminding yourself that it’s ok to feel what you feel without having to change it’ | ‘… not to regulate their emotion response.’ (CNR) | 8 | – |
| 4. Smoski et al. (2015b) | 18 Remitted MDD (14 F) | 5 | – | ||||
| 5. Murakami et al. (2015) | 21 healthy (11 F) | Event-related within subjects | Negative IAPS | ‘… to observe objectively and describe their subjective feelings or thoughts in their minds, and physiological changes in bodies, not with voice but just mentally, and to not suppress the emotions that are evoked by viewing the negative pictures.’ | ‘Simply look at the neutral or negative pictures and respond naturally.’ (CNR) | 22 | – |
| 6. Lebois et al. (2015) | 30 healthy (15 F) | Blocks within subjects | One-sentence stressful scenarios | ‘… to remain aware of their current physical location while thinking about the scenarios. They were further asked to notice the kinds of reactions that they normally have during immersion, but rather than “living” the event, they were instructed to simply observe their thoughts and reactions to it in the present moment. Participants were asked to perceive their thoughts about the stimuli as transitory mental states, not as parts of the scenarios, but as their psychological responses to them.’ | ‘Become completely absorbed in the experience of the scenarios, as if they were happening in the moment. They were to mentally time travel and experience the sensory details, physical sensations, feelings, emotions, and bodily states associated with engaging in the scenario vividly.’ (CFE) | 14 | 18 |
| 7. Westbrook et al. (2013) | 47 smokers (31 F) | Event-related within subjects | Craving stimuli | ‘… to actively focus on their responses to the picture, including thoughts, feelings, memories and bodily sensations, while maintaining a nonjudgmental attitude toward those responses’ | ‘… simply to relax and view the picture as naturally as possible’ (CNR) | 0 | 1 |
| 8. Ellard et al. (2017) | 21 GAD (21 F) | Blocks within subjects | Worry statements | ‘Observe and accept. “Observing” means instead of trying to do something about the distress you feel when you read statements about things you are worried about, try to observe your reaction in an objective way[…] “Accept” means allowing yourself to feel distress, knowing that this distress will peak and fade, and that it is ok to have this initial reaction.’ | ‘… just let yourself respond in whatever way you might naturally respond when you think about these topics. Don’t try and stop yourself from worrying, just let yourself worry as you might usually do.’ (CFE) | 2 | 10 |
| 9. Kober et al. (2019a) | 17 healthy (5 F) | Blocks within subjects | Negative IAPS | ‘… to attend to and accept their experience as it is. This instruction was modeled after the two-component […] (i) attention to present moment sensation, coupled with (ii) non-judgmental acceptance of the sensation as it is, allowing it to exist without trying to avoid it or react to it.’ | ‘React naturally, whatever your response might be’ (CNR) | 0 | 3 |
| 10. Kober et al. (2019b) | Pain | 0 | 9 | ||||
| 11. Goldin et al. (2019) | 35 healthy (15 F) | Event-related within subjects | Negative self-belief statements | ‘… simply observing and accepting without judgment from moment to moment (and not attempting to modify or change) any responses, including thoughts, emotions, memories, images, and physical sensations.’ | ‘… reacting to negative self-beliefs by considering how it reflected something true about themselves’ (CFE) | 11 | 4 |
| 12. Dixon et al. (2020a) | 113 SAD (61 F) | Blocks within subjects | Negative self-belief statements | ‘to regulate their reaction by ccepting their reactions, which involved a nonjudgmental monitoring of thoughts, memories, emotions, and sensations as they appeared and dissolved, without modifying or avoiding them.’ | ‘to react to the negatives statements by reflecting on how the NSB may describe something true about themselves and to let themselves feel the sting of the statement’ (CFE) | 2 | 9 |
| 13. Dixon et al. (2020b) | 35 healthy (22 F) | 0 | 6 |
N foci A > C = Number of foci for acceptance versus control condition.
N foci C > A = Number of foci for control condition versus acceptance.
IAPS = International Affective Pictures System.
GAD = Generalized Anxiety Disorder.
F = female.
Due to the relevance of top-down control in traditional emotion regulation models, a first question of this study regarded the involvement of executive areas in acceptance-based regulation. Neuroimaging studies of acceptance have provided mixed evidence in this respect. In some studies, prefrontal activations of the dorsal attention network have been reported in acceptance, consistently with recruitment of cognitive control as in traditional emotion regulation strategies (Lebois et al., 2015; Goldin et al., 2019). In other studies, even if prefrontal activations were reported, they were less than in traditional strategies and/or located more medially (Murakami et al., 2015; Smoski et al., 2015; Ellard et al., 2017). Finally, yet other studies reported that acceptance of emotions occurred in the absence of detectable increases in prefrontal cortical areas associated with top-down control (Kross et al., 2009; Westbrook et al., 2013; Kober et al., 2019; Dixon et al., 2020).
Besides executive areas associated with cognitive control, the neuroimaging literature has drawn attention to the possible involvement of the Default Mode Network (DMN) (Ellard et al., 2017), a set of areas usually deactivated during attention-demanding tasks (Raichle, 2015) associated with mind-wandering (Mason et al., 2007; Fox et al., 2015; Mittner et al., 2016). Since mind-wandering has been considered as the opposite of mindfulness (Mrazek et al., 2012), it has been suggested that the ‘interruption’ of ruminative processing that characterizes mindfulness-based emotion regulation strategies may modulate the DMN activity (Ellard et al., 2017). This suggestion is consistent with findings of hyper-activation of the DMN in depression (Sheline et al., 2009; Messina et al., 2016a), anxiety (Zhao et al., 2007) and more in general in conditions of emotional dysregulation (Whitfield-Gabrieli and Ford, 2012; Buckner et al., 2019). A further reason to investigate the involvement of the DMN is its relationship with semantic association areas (Binder et al., 2009). In the context of emotion regulation, the DMN and the surrounding association cortex have been hypothesized to be involved in regulation by modulating semantic processing of emotional stimuli (Viviani, 2013; Messina et al., 2016b, 2020).
In summary, the strategy of acceptance to regulate emotions calls into question a key tenet of current neurobiological models of emotion regulation, consisting in the involvement of top-down control associated with recruitment of cortical areas associated with working memory and executive processes. Available studies have produced mixed evidence concerning the involvement of substrates of the executive system. An alternative hypothesis consists of the modulation of the activity of the DMN. To shed light on this issue, we adopted a meta-analytic approach, which allowed us to combine data across neuroimaging studies with a similar experimental design. In the present study, the coordinates-based Activation Likelihood Estimation (ALE) method (Laird et al., 2005) was used to obtain an objective, systematic and quantitative summary of previous neuroimaging studies of emotion regulation which have considered acceptance as emotion regulation strategy.
Method
Data sources
Neuroimaging studies on acceptance were collected through advanced searches in Google Scholar (http://scholar.google.com/databases) and PubMed (https://www.ncbi.nlm.nih.gov/pubmed) of all articles that mentioned in the title the terms ‘acceptance’ and/or ‘mindfulness’ together with the terms ‘fMRI’ (functional Magnetic Resonance Imaging) or ‘neuroimaging’. Additional studies were obtained reviewing the references of papers using the Google Scholar database. We excluded from the retrieved studies those that investigated neural effects of meditation or meditation trainings, that involved meditators participants and that investigated acceptance and/or mindful attitude as a personality trait. We included all studies that used fMRI to investigate the neural correlates of acceptance using a typical emotion regulation design in which participants were exposed to emotional stimuli and instructed to use acceptance to regulate emotional reactions compared to a control condition (see Table 1 for a review of stimuli and task instructions). As shown in Table 1, when operationalizing acceptance, researchers use different instructions, stressing one or more components of acceptance, such as willingness to take in emotions, being present (mindfulness), cognitive defusion, self as a context, and concentration on values and commitment. This variety of instructions has been previously observed also in reviews of behavioural studies (Kohl et al., 2012; Wojnarowska et al., 2020). Following these criteria, a total of 10 papers and more than 400 participants were examined (the main features of included studies are shown in Table 1). All reported significant reductions of negative emotions in the acceptance condition, compared to the control condition (with the exception of Kross et al., 2009 that did not report behavioural results). Studies with two different experimental conditions (Kober et al., 2019) or with different groups of participants analysed separately (Smoski et al., 2015; Dixon et al., 2020) were considered as separate samples (these studies are reported in Table 1 as ‘a’ and ‘b’ to mention different conditions), yielding a total of 13 studies. From these studies, we extracted foci reported in three-dimensional (3D) coordinates (x, y, z) in stereotactic space that resulted significantly activated for the contrast between an acceptance regulation condition vs a control condition (increased brain activity in acceptance) and control condition vs acceptance regulation (decreased brain activity in acceptance). Verbatim instructions of acceptance regulation and control conditions are reported in Table 1.
Among the selected studies, we noted that two subgroups of studies differed in the control condition used in the experimental design. In the first subgroup (Contrast to Natural Reaction—CNR), in the control condition, participants were instructed to naturally ‘look’ or ‘react’ to the experimental stimuli. In the second subgroup (Contrast to Focus on Emotions—CFE), more complex instructions were given to increase the focus of participants on their emotional experience. Example of these instructions was ‘to focus on the specific feelings’ (Kross et al., 2009) or ‘become completely absorbed by feeling’ (Lebois et al., 2015). Due to possible systematic discrepancies in the patterns of brain activity across studies attributable to differences in control conditions used as baseline for contrast analysis, we also conducted explorative meta-analyses of CNR and CFE subgroups considered separately. Among the 13 studies, 7 experiments were included in the CNR subgroup, and 6 experiments were included in the CFE subgroup.
Meta-analytic procedure
To conduct the meta-analyses, the ALE method for coordinate-based meta-analysis of neuroimaging data was used (Eickhoff et al., 2009). This method is based on the evaluation of the overlap between foci of brain activity reported in different studies and treats the reported foci not as single points, but as centres for 3D Gaussian probability distributions capturing their spatial uncertainty. An algorithm is used to identify clusters of brain activity that show a convergence of activation across experiments and determine if the clusters obtained occur more frequently than in the null distribution arising from random spatial distribution of foci across the experiments. The procedure weights foci of brain activity reported in single studies by the number of participants, yielding a quantitative estimate of the probability of activation identifying common activations across studies (Laird et al., 2005). Significance values were obtained with permutation tests. The observed values in the ALE distribution are then compared to the null distribution in order to assign probability estimates to the observed data.
ALE meta-analyses were carried out using GingerALE 2.3.2 software as distributed by the BrainMap project (http://www.brainmap.org/ale/). The ‘non-additive’ method was used, which models each focus with a Gaussian function defined by a full width at half-maximum kernel size empirically determined by finding the maximum across each focus’s Gaussian. The non-additive method allows the modelling of the spatial uncertainty of each focus arising from inter-subject and inter-study variability. The meta-analyses were performed in Montreal Neurological Institute (MNI) space. Coordinates reported in studies in Talairach coordinates space were transformed into MNI using the Lancaster transform, icbm2tal algorithm in Ginger ALE (Laird et al., 2005). The probability maps were threshold at P < 0.05 and corrected for multiple testing using the false discovery rate approach (Genovese et al., 2002). SurfIce software (https://www.nitrc.org/plugins/mwiki/index.php/surfice:MainPage) was used to obtain brain renderings.
Results
Meta-analysis of all acceptance studies
The analysis of the contrast between acceptance vs all control conditions included the data collected from 13 experiments, for a total of 422 subjects, yielding 61 foci of brain activations. No significant clusters of increased brain activity emerged from this analysis.
The analysis of the contrast between the control conditions vs acceptance included the data collected from 10 experiments (3 experiments did not analyse this contrast; see Table 1 for information concerning reported foci of brain activity), for a total of 364 subjects, yielding a total of 109 foci of significant brain activity. A significant cluster of decreased brain activity in the acceptance condition relative to control emerged from this analysis. This cluster was located in the posterior cingulate cortex/precuneus (PCC), insula and limbic subcortical areas such as the thalamus and the parahippocampal gyrus (see Table 2; Figure 1).
Table 2.
Foci of significant brain activity in control conditions compared to acceptance condition
| Cluster | Areas (Brodmann’s areas) | MNI coordinates | ALE score | Z | P | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|---|
| Y | Y | Z | ||||||
| 1 | Culmen (BA 30) | −4 | −54 | 6 | 0.013 | 3.99 | <0.001 | 20 344 |
| Posterior cingulate (BA 31) | −6 | −56 | 26 | 0.012 | 3.83 | <0.001 | ||
| Thalamus | −10 | −32 | 6 | 0.010 | 3.47 | <0.001 | ||
| Thalamus | −18 | −34 | 14 | 0.010 | 3.38 | <0.001 | ||
| Lingual gyrus (BA 27) | 0 | −34 | 2 | 0.010 | 3.36 | <0.001 | ||
| Posterior cingulate (BA 29) | 6 | −52 | 12 | 0.009 | 3.15 | <0.001 | ||
| Parahippocampal gyrus (BA 30) | −12 | −44 | −2 | 0.009 | 3.14 | <0.001 | ||
| Parahippocampal gyrus (BA 30) | 14 | −38 | 4 | 0.009 | 3.11 | <0.001 | ||
| Thalamus | 24 | −34 | 2 | 0.009 | 3.09 | <0.001 | ||
| Cuneus (BA 18) | −12 | −72 | 18 | 0.009 | 3.08 | 0.001 | ||
| Posterior cingulate (BA 30) | 10 | −54 | 18 | 0.009 | 3.07 | 0.001 | ||
| Posterior insula (BA 13) | −38 | −14 | 12 | 0.008 | 3.06 | 0.001 | ||
| Lingual gyrus (BA 17) | −8 | −70 | 6 | 0.008 | 3.03 | 0.001 | ||
| Thalamus | −24 | −22 | 10 | 0.008 | 2.97 | 0.002 | ||
| Culmen (BA 27) | 2 | −46 | 0 | 0.008 | 2.88 | 0.002 | ||
BA, Brodmann’s areas.
Fig. 1.
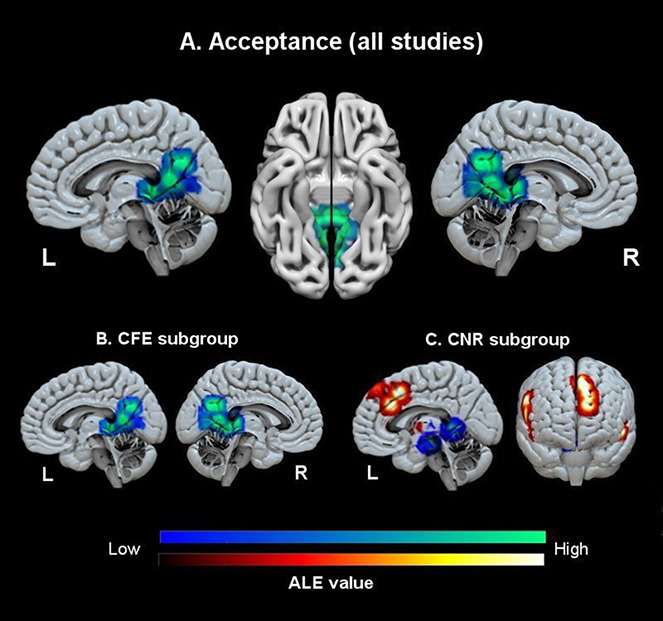
Decreased (cold colours) and increased (warm colours) brain activity associated with all acceptance studies (A), CFE studies (B) and CNR studies (C).
Explorative subgroup analyses
CNR subgroup.
Seven experiments reported results of the contrast acceptance vs natural reactions, yielding a total of 164 subjects, and 38 foci of brain activations. Four clusters of increased brain activity were found for acceptance relative to control. These clusters were located bilaterally in the inferior frontal gyrus, extending to the anterior insula and the putamen on the left side and to the frontal pole and the medial prefrontal cortex/anterior cingulate cortex (see Table 3).
Table 3.
Significant clusters of brain activity in CNR and CFE subgroups
| Cluster | Areas (Brodmann’s areas) | MNI coordinates | ALE score | Z | P | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|---|
| Y | Y | Z | ||||||
| Acceptance vs CNR | ||||||||
| 1 | Anterior insula (BA 13) | −36 | 26 | −4 | 0.012 | 4.40 | <0.001 | 16 792 |
| Inferior frontal gyrus (BA 47) | −52 | 16 | −6 | 0.009 | 3.64 | <0.001 | ||
| Anterior insula (BA 13) | −32 | 16 | 12 | 0.009 | 3.64 | <0.001 | ||
| Putamen | −15 | 3 | 6 | 0.002 | 1.97 | 0.024 | ||
| 2 | Inferior frontal gyrus (BA 45) | 54 | 24 | 6 | 0.009 | 3.84 | <0.001 | 10 520 |
| Inferior frontal gyrus (BA 45) | 58 | 24 | 14 | 0.009 | 3.64 | <0.001 | ||
| Inferior frontal gyrus (BA 9) | 60 | 18 | 30 | 0.008 | 3.56 | <0.001 | ||
| Inferior frontal gyrus (BA 47) | 50 | 30 | −6 | 0.008 | 3.53 | <0.001 | ||
| 3 | Superior frontal gyrus (BA 8) | −10 | 52 | 46 | 0.009 | 3.86 | <0.001 | 8800 |
| Superior frontal gyrus (BA 8) | −6 | 46 | 52 | 0.009 | 3.64 | <0.001 | ||
| Superior frontal gyrus (BA 8) | −14 | 58 | 36 | 0.008 | 3.61 | <0.001 | ||
| Superior frontal gyrus (BA 9) | −14 | 66 | 24 | 0.008 | 3.53 | <0.001 | ||
| 4 | Medial frontal gyrus (BA 32) | 10 | 16 | 42 | 0.009 | 4.01 | <0.001 | 8544 |
| Medial frontal gyrus (BA 6) | 6 | 18 | 44 | 0.009 | 3.97 | <0.001 | ||
| Anterior cingulate gyrus (BA 32) | 4 | 28 | 30 | 0.008 | 3.56 | <0.001 | ||
| CNR vs acceptance | ||||||||
| 1 | Lingual gyrus (BA 27) | −5 | −36 | 5 | 0.009 | 1.68 | 0.046 | 15 872 |
| 2 | Hippocampus | 22 | −14 | −12 | 0.009 | 4.26 | <0.001 | 12 824 |
| Hippocampus | 12 | −18 | −14 | 0.008 | 3.86 | <0.001 | ||
| 3 | Superior temporal gyrus (BA 48) | 51 | −24 | 18 | 0.008 | 3.75 | <0.001 | 10 776 |
| Superior temporal gyrus (BA 48) | 70 | −33 | 24 | 0.007 | 3.34 | <0.001 | ||
| CFE vs acceptance | ||||||||
| 1 | Precuneus (BA 30) | −4 | −54 | 6 | 0.013 | 4.07 | <0.001 | 19 928 |
| Precuneus (BA 23) | −6 | −56 | 26 | 0.012 | 3.93 | <0.001 | ||
| Lingual gyrus (BA 27) | 0 | −34 | 2 | 0.009 | 3.39 | <0.001 | ||
| Thalamus | −18 | −34 | 14 | 0.009 | 3.39 | <0.001 | ||
| Thalamus | −10 | −34 | 4 | 0.009 | 3.30 | <0.001 | ||
| Posterior cingulate gyrus (BA 30) | 6 | −52 | 12 | 0.009 | 3.21 | <0.001 | ||
| Parahippocampal gyrus (BA 27) | −12 | −44 | −2 | 0.009 | 3.21 | <0.001 | ||
| Lingual gyrus (BA 18) | 18 | −54 | 2 | 0.009 | 3.19 | <0.001 | ||
| Parahippocampal gyrus (BA 27) | 14 | −38 | 4 | 0.009 | 3.18 | <0.001 | ||
| Thalamus | 24 | −34 | 2 | 0.009 | 3.16 | <0.001 | ||
| Cuneus (BA 18) | −12 | −72 | 18 | 0.009 | 3.15 | <0.001 | ||
| Posterior cingulate gyrus (BA 30) | 10 | −54 | 18 | 0.009 | 3.14 | <0.001 | ||
| Lingual gyrus (BA 17) | −8 | −70 | 6 | 0.008 | 3.10 | <0.001 | ||
| Thalamus | −24 | −20 | 10 | 0.008 | 3.01 | 0.001 | ||
Four experiments reported results of the contrast natural reaction vs, acceptance, yielding a total of 106 subjects, and 15 foci of brain activations. Significant clusters of decreased brain activity in acceptance relative to natural reaction were located in the posterior cingulate cortex/precuneus (PCC), right hippocampus and right superior temporal gyrus (see Table 3).
CFE subgroup.
With regard to the analysis of the contrast acceptance vs focus on emotions (increased brain activity for acceptance), 6 experiments reported significant results and were included in the analysis, yielding a total of 258 subjects, and 23 foci of brain activations. No significant clusters emerged from this analysis.
In the contrast focus on emotions vs acceptance, 6 experiments reported significant results, yielding a total of 258 subjects, and 94 foci of brain activations. One significant clusters of decreased brain activity in acceptance relative to focus on emotion was located in the posterior cingulate cortex/precuneus (PCC), thalamus and parahippocampal gyrus.
Discussion
In the present meta-analytic study, we provided a synthesis of functional neuroimaging studies investigating the neural correlates of regulating emotions through acceptance-based strategies, in which participants were instructed to take on a non-judgemental attitude towards on-going emotional experiences, contrasted to naturally react or focus on negative emotions control conditions. Due to their relevance for neurobiological models of emotion processing and regulation, we were interested in clarifying the involvement of executive areas and of the DMN in the acceptance strategy.
When we tested the presence of increased brain activity in all studies included in the present meta-analysis (the main contrast acceptance vs control conditions)—despite decreased activity in limbic regions in acceptance (thalamus and hippocampus/parahippocampal gyrus) accounting for regulation efficacy (Frank et al., 2014; Morawetz et al., 2017)—no areas associated with executive processes resulted to be significantly activated by acceptance (or indeed any other cortical area). This result is in line with previous studies that reported the absence of increased activity in executive areas, and more generally with reports of the absence of increased brain activity in association with acceptance in the whole brain (Kross et al., 2009; Westbrook et al., 2013; Kober et al., 2019; Dixon et al., 2020). However, when we explored the involvement of substrates of executive functions in two different subgroups of studies differing in the control condition contrasted to acceptance, we found a significant increased activity in executive areas only in the subgroup of studies characterized by the use of natural reaction as the control condition (Contrast to Natural Reactions—CNR; e.g. ‘react naturally’). In contrast, no significant clusters of increased brain activity were observed in studies that used more complex instructions related to increasing the focus on emotional experience (Contrast to Focus on Emotions subgroup—CFE; e.g. ‘become completely absorbed’).
The involvement of executive areas is consistent with previous attempts to conceptualize acceptance as a specific variety of reappraisal (Webb et al., 2012; Wolgast et al., 2013) and with the presence of attentional element in acceptance instructions (see Table 1). However, this effect was not significant when acceptance was compared to control conditions requiring some amounts of cognitive load (CFE), even if these control conditions did not include instructions to down-regulate emotional response. The increased activity of executive areas only in the CNR subgroup may result from a difference in cognitive effort between the acceptance and the natural reaction control conditions, rather than a specific regulation effect. Indeed, the prefrontal effects of acceptance in the comparison with the natural reaction control condition in the CNR group overlapped with those reported in a meta-analysis of the effects of task difficulty across tasks (Radua et al., 2014).
Beyond the effects of acceptance in executive areas associated with top-down control, a remarkable aspect of many of these studies is given by frequent reports of decreased activity in acceptance relative to the control conditions, even in studies in the CNR subgroup (Lebois et al., 2015; Ellard et al., 2017; Dixon et al., 2020). These findings are upheld by the second result of the present study, which was the decrease of brain activity in the posterior cingulate cortex posterior cingulate cortex (PCC)/precuneus, posterior insula and limbic lobe in the acceptance compared to control conditions. There are two reasons for attributing this effect to acceptance, rather than to the control conditions. First, this finding was obtained regardless of the control condition. The PCC cluster was significant also when considering the two CNR and CFE subgroups separately, even if these groups differed in the control conditions they used. Second, previous meta-analyses of traditional emotion regulation studies (Buhle et al., 2014; Messina et al., 2015), did not report effects in this area in any of the considered contrasts, even if these meta-analyses were conducted on a larger database of studies than the present one.
However, effects in the PCC have been reported in individual studies in the emotion regulation literature. Activations of PCC have been reported in emotion regulation processes that, on the contrary of acceptance, have included aspects of emotional avoidance. For instance, PCC modulations have been reported in self-distancing from negative emotional pictures (Koenigsberg et al., 2010), in down-regulating the reaction to a negative stimulus by self-distraction (Kanske et al., 2011), in association with amount of eye movements to directing gaze so as to avoid aversive emotional stimuli (van Reekum et al., 2007) and in association with individual differences in spontaneous avoidance of emotional words (Benelli et al., 2012). PCC activity modulation has also been observed after psychotherapy (Buchheim et al., 2013). Thus, emotional avoidance and acceptance studies seem to show two sides of the same coin, possibly highlighting a neglected component of emotion regulation which has its neural correlate in the PCC. Future studies, specifically designed to test the association of PCC activity modulation with effective down-regulation of negative affect, may provide further evidence in support of this hypothesis.
With regard to the nature of the contribution of PCC to emotion regulation, the literature offers several hypotheses. A first interpretation emerges from considering brain activity as the result of activation and deactivation patterns in large-scale connected networks (Fox et al., 2015). The PCC is a key part of the DMN, associated with mind-wandering (Mason et al., 2007; Fox et al., 2015; Mittner et al., 2016) and ruminative processes (Cooney et al., 2010; Berman et al., 2011). Hence, the present findings would be consistent with the hypothesis that modulations of PCC activity during acceptance may be associated with the interruption of ruminative, self-reflective processes over emotions (especially when acceptance is compared to focus on emotion, which can be viewed as related to ruminative processes). Also, the posterior insula may contribute to this process, due to its role of bringing visceral sensation to the posterior network (Taylor et al., 2009; Cauda et al., 2011). Note, however, that ruminative processes, which may be expected to be most prevalent during the baseline or fixation phase rather than in a control condition, were not directly investigated in the studies included in the present meta-analysis.
A second alternative interpretation of the present findings posits that PCC would be normally activated in response to emotional stimuli and the use of acceptance strategy may modulate this activation appearing as decreased activity in the contrast with control conditions. This interpretation also accounts for the fact that in the meta-analysis, only PCC, and not the whole DMN as in mind-wandering, was found to be modulated by acceptance. However, a study specifically looking at this issue found that PCC was deactivated while appraising emotional material relative to the fixation baseline (Benelli et al., 2012).
A third interpretative approach, which is also compatible with the hypothesis of interruption of ruminative processes associated with DMN modulations, recognizes deactivation of PCC as the neural substrate of acceptance. The idea of functional deactivations related to stimulus processing is not new in the literature (Shulman et al., 2007). Research and theories are converging on the notion of brain function as computing a hierarchical predictive model of the world in which functional processing hierarchies are not confined to sensorimotor systems but terminate in heteromodal association areas where predictions are generated (Huntenburg et al., 2018). The most innovative aspect of these models is to realize that these heteromodal association areas are organized around a core constituted by the DMN (Margulies et al., 2016), which is usually deactivated by the task. Indeed, together with cortical limbic areas, heteromodal association areas are precisely those where functional deactivations are observed (Viviani et al., 2020), justifying the current interest on neural inhibition as a mechanism to select representations at the top of the sensory processing hierarchy (Hunt and Hayden, 2017). This suggests that a simple dichotomy between top-down and bottom-up processes may not adequately describe emotion regulation processes as a whole (Messina et al., 2016b).
In line with this notion, the role of cortical limbic areas, including the PCC, has been recently redefined considering their involvement in representing sensory input based on past experience, placing them at the top of the predictive hierarchy (Chanes and Barrett, 2016). Mental processes implicated in avoidance vs acceptance of emotional reactions may be associated with recruitment of specific hubs of the DMN, in which deactivations of PCC are functionally related to acceptance. Hence, emotion regulation may act at this level of cortical processes influencing the impact of emotional events independently of the involvement of high-level executive cortical processes, instead modulating emotion processing through the recruitment of emotional and schematic representations (Viviani, 2013, 2014; Messina et al., 2016b).
The insights gained in the present study may be useful to understand the mechanism of psychotherapy. The traditional conceptualization of emotion regulation as a form of cognitive control is only partially compatible with models of psychotherapy. Considering the neural substrates of emotional and schematic representations may help elucidate the mechanisms of cognitive restructuring of dysfunctional cognitions that characterize cognitive-behavioural therapies (Beck et al., 1979), where this restructuring has been shown to be a mediator of the therapeutic outcome (Wishman, 1993; Clark, 1999). In contrast, the concept of cognitive control is not consistent with the putative mechanism of action of ‘expressive’ psychotherapy approaches, which encourage individuals to experience their emotions, related thoughts and bodily sensations fully to put them in contact with their own internal experiences (instead of controlling emotions). These mechanisms also appear to apply to psychodynamic therapies (Grecucci et al., 2018; Messina et al., 2020) and ‘third-wave’ behavioural therapies (Hayes et al., 1999, 2004). In this context, we have argued for limiting the importance of executive functions only to specific forms of emotion regulation, emphasizing the importance of enlarging neurobiological models of emotion regulation beyond cognitive control (Messina et al., 2015, 2016b). Moving the focus of emotion regulation models from executive to semantic processes may bridge the gap between clinical models of psychotherapeutic interventions and affective neuroscience. Regardless of the specific psychotherapy model, psychotherapy theories are often concerned with changes in semantic representations of the self, past experiences interpersonal situations (including the therapeutic relationship) that function as ‘schemas’ (Beck et al., 1979) or internal working models (Bowlby, 1988) to organize and interpret the emotional significance of everyday emotional experiences.
The present study has some limitations. First, the number of studies that have investigated acceptance-based regulation strategies is relatively exiguous. Due to the scarcity of available studies, we were not able to statistically compare the interaction between acceptance and the CNR and CFE subgroups (contrast analysis is unlikely to have enough statistical power to show significant differences with less than about 15 experiments in each data set). Second, the analyses provided here using the ALE methodology were based solely on reported peak activation coordinates. Therefore, we were unable to take into account information on the whole extent of estimated effects. Third, the definition of acceptance, together with instructions provided to the participants, remains somewhat inconsistent in the literature. For example, some definitions emphasize the attention to body sensations, whereas others are more focused on emotions and thoughts. All these issues should be considered in future studies.
Contributor Information
Irene Messina, Universitas Mercatorum, Rome 00186, Italy.
Alessandro Grecucci, Department of Psychology and Cognitive Sciences, University of Trento, Trento 38068, Italy.
Roberto Viviani, Institute of Psychology, University of Innsbruck 6020; Austria—Psychiatry and Psychotherapy Clinic III, University of Ulm, Ulm 89075, Germany.
Conflict of interest
None declared.
References
- Aldao A., Nolen-Hoeksema S., Schweizer S. (2010). Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychology Review, 30, 217–37. [DOI] [PubMed] [Google Scholar]
- Beck T.A., Rush A.J., Shaw B.F., Emery G. (1979). Cognitive Therapy of Depression. New York, NY: Guildford Press. [Google Scholar]
- Benelli E., Mergenthaler E., Walter S., et al. (2012). Emotional and cognitive processing of narratives and individual appraisal styles: recruitment of cognitive control networks vs. modulation of deactivations. Frontiers in Human Neuroscience, 6, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M., Wupperman P. (2012). Emotion regulation and mental health: recent findings, current challenges, and future directions. Current Opinion in Psychiatry, 25(2), 128–34. [DOI] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne E. (1961). Transactional Analysis in Psychotherapy. New York, NY: Grove Press. [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19, 2767–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S., Chawla N., Marlatt G.A. (2011). Mindfulness-Based Relapse Prevention for Addictive Behaviors: A Clinician’s Guide. New York, NY: Guilford Press. [Google Scholar]
- Bowlby J. (1988). A Secure Base: Parent-Child Attachment and Healthy Human Development. London, Routledge: Tavistock professional book. [Google Scholar]
- Buchheim A., Labek K., Walter S., Viviani R. (2013). A clinical case study of a psychoanalytic psychotherapy monitored with functional neuroimaging. Frontiers in Human Neuroscience, 7, 677. doi: 10.3389/fnhum.2013.00677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., DiNicola L.M. (2019). The brain’s default network: updated anatomy, physiology and evolving insights nature reviews. Neuroscience, 20(10), 593–608. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L., Barlow D.H., Brown T.A., Hofmann S.G. (2006). Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behaviour Research and Therapy, 44(9), 1251–63. [DOI] [PubMed] [Google Scholar]
- Catalino L.I., Arenander J., Epel E., Puterman E. (2017). Trait acceptance predicts fewer daily negative emotions through less stressor-related rumination. Emotion, 17(8), 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., D’Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. (2011). Functional connectivity of the insula in the resting brain. Neuroimage, 55(1), 8–23. [DOI] [PubMed] [Google Scholar]
- Chanes L., Barrett L.F. (2016). Redefining the role of limbic areas in cortical processing. Trends in Cognitive Sciences, 20(2), 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.M. (1999). Anxiety disorders: why they persist and how to treat them. Behaviour Research and Therapy, 37, S5–27. [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugène F., Dennis E.L., Gotlib I.H. (2010). Neural correlates of rumination in depression. Cognitive, Affective & Behavioral Neuroscience, 10(4), 470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadomo H., Grecucci A., Giardini I., Ugolini E., Carmelita A., Panzeri M. (2016). Schema therapy for emotional dysregulation: theoretical implication and clinical application. Frontiers in Psychology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadomo H., Panzeri M., Caponcello D., Carmelita A., Grecucci A. (2018). Schema therapy for emotional dysregulation in personality disorders: a review. Current Opinion in Psychiatry, 31(1), 43–9. [DOI] [PubMed] [Google Scholar]
- Dixon M.L., Moodie C.A., Goldin P.R., Farb N., Heimberg R.G., Gross J.J. (2020). Emotion regulation in social anxiety disorder: reappraisal and acceptance of negative self-beliefs. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(1), 119–29. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard K.K., Barlow D.H., Whitfield-Gabrieli S., Gabrieli J.D.E., Deckersbach T. (2017). Neural correlates of emotion acceptance vs worry or suppression in generalized anxiety disorder. Social Cognitive and Affective Neuroscience, 12(6), 1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliu Soler A., Montesinos F., Gutiérrez-Martínez O., Scott W., McCracken L.M., Luciano J.V. (2018). Current status of acceptance and commitment therapy for chronic pain: a narrative review. Journal of Pain Research, 11, 2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K.C.R., Spreng R.N., Ellamil M., Andrews-Hanna J.R., Christoff K. (2015). The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage, 111, 611–21. [DOI] [PubMed] [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., et al. (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–11. [DOI] [PubMed] [Google Scholar]
- Frederickson J. (2013). Co-creating Change: Effective Dynamic Therapy Techniques. Kansas City, MO: Seven Leaves Press. [Google Scholar]
- Frederickson J., Messina I., Grecucci A. (2018). Dysregulated Anxiety and dysregulating defenses: toward an emotion regulation informed dynamic psychotherapy. Frontiers in Psychology, 9, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage, 15(4), 870–8. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., Moodie C.A., Gross J.J. (2019). Acceptance versus reappraisal: behavioral, autonomic, and neural effects. Cognitive, Affective & Behavioral Neuroscience, 19(4), 927–44. [DOI] [PubMed] [Google Scholar]
- Graver M. (2008). Stoicism and Emotion. Chicago: University of Chicago Press. [Google Scholar]
- Grecucci A., Pappaianni E., Siugzdaite R., Theuninck A., Job R. (2015). Mindful emotion regulation: exploring the neurocognitive mechanisms behind mindfulness. BioMed Research International, 2015, 670–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A., Messina I., Amodeo L., et al. (2020). A dual route model for regulating emotions: comparing models, techniques and biological mechanisms. Frontiers in Psychology, 11, 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A., Messina I., Dadomo H. (2018). Decoupling internalized dysfunctional attachments: a combined ACT and schema therapy approach. Frontiers in Psychology, 9, 2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A., Sulpizio S., Tommasello E., Vespignani F., Job R. (2019). Seeing emotions, reading emotions: Behavioral and ERPs evidence of the regulation of pictures and words. PLoS One, 14(5), e0209461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg L.S. (2011). Emotion-focused therapy. American Psychological Association. [Google Scholar]
- Gross J.J. (1998). The emerging field of emotion regulation: an integrative review. Review of General Psychology, 2(3), 271–99. [Google Scholar]
- Gross J.J. (2015). Emotion regulation: current status and future prospects. Psychological Inquiry, 26(1), 1–26. [Google Scholar]
- Hayes S.C., Strosahl K., Wilson K.G. (1999). Acceptance and Commitment Therapy: Understanding and Treating Human Suffering. [Google Scholar]
- Hayes S.C. (2004). Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies. Behavior Therapy. New York: Guilford Press, 35(4), 639–65. [DOI] [PubMed] [Google Scholar]
- Hayes S.C., Luoma J.B., Bond F.W., Masuda A., Lillis J. (2006). Acceptance and commitment therapy: model, processes and outcomes. Behaviour Research and Therapy, 44, 1–25. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Asmundson G.J.G. (2008). Acceptance and mindfulness-based therapy: new wave or old hat? Clinical Psychology Review, 28(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Heering S., Sawyer A.T., Asnaani A. (2009). How to handle anxiety: the effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behaviour Research and Therapy, 47(5), 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L.T., Hayden B.Y. (2017). A distributed, hierarchical and recurrent framework for reward-based choice. Nature Reviews Neuroscience, 18(3), 172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntenburg J.M., Bazin P.-L., Margulies D.S. (2018). Large-scale gradients in human cortical organization. Trends in Cognitive Sciences, 22(1), 21–31. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. (2013). Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, NY: Bantam Dell. [Google Scholar]
- Kanske P., Heissler J., Schönfelder S., Bongers A., Wessa M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21, 1379–88. [DOI] [PubMed] [Google Scholar]
- Kober H., Buhle J., Weber J., Ochsner K.N., Wager T.D. (2019). Let it be: mindful acceptance down-regulates pain and negative emotion. Social Cognitive and Affective Neuroscience, 14(11), 1147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Fan J., Ochsner K.N., et al. (2010). Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia, 48, 1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl A., Rief W., Glombiewski J.A. (2012). How effective are acceptance strategies? A meta-analytic review of experimental results. Journal of Behavior Therapy and Experimental Psychiatry, 43(4), 988–1001. [DOI] [PubMed] [Google Scholar]
- Kross E., Davidson M., Weber J., Ochsner K. (2009). Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry, 65(5), 361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Price C.J., et al. (2005). ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping, 25, 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois L.A.M., Papies E.K., Gopinath K., et al. (2015). A shift in perspective: decentering through mindful attention to imagined stressful events. Neuropsychologia, 75, 505–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan M.M. (1993). Dialectical behavior therapy for treatment of borderline personality disorder: implications for the treatment of substance abuse. NIDA Research Monograph, 137, 201–201. [PubMed] [Google Scholar]
- Lutz J., Herwig U., Opialla S., et al. (2014). Mindfulness and emotion regulation—an fMRI study. Social Cognitive and Affective Neuroscience, 9(6), 776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D.S., Ghosh S.S., Goulas A., et al. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences, 113(44), 12574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.R. (1997). Mindfulness: a proposed common factor. Journal of Psychotherapy Integration Publication Discontinued, 7(4), 291–312. [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315(5810), 393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I., Bianco F., Cusinato M., Calvo V., Sambin M. (2016a). Abnormal default system functioning in depression: implications for emotion regulation. Frontiers in Psychology, 7, 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I., Bianco S., Sambin M., Viviani R. (2015). Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Frontiers in Psychology, 6, 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I., Grecucci A., Marogna C., Calvo V. (2020). Relational exposure as mechanisms of change in psychodynamic psychotherapy: convergences between psychotherapy research and affective neuroscience. TPM—testing. Psychometrics, Methodology in Applied Psychology, 27(1), 1–14. [Google Scholar]
- Messina I., Sambin M., Beschoner P., Viviani R. (2016b). Changing views of emotion regulation and neurobiological models of the mechanism of action of psychotherapy. Cognitive, Affective & Behavioral Neuroscience, 16(4), 571–87. [DOI] [PubMed] [Google Scholar]
- Mittner M., Hawkins G.E., Boekel W., Forstmann B.U. (2016). A neural model of mind wandering. Trends in Cognitive Sciences, 20(8), 570–8. [DOI] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Derntl B., Heekeren H.R. (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews, 72, 111–28. [DOI] [PubMed] [Google Scholar]
- Mrazek M.D., Smallwood J., Schooler J.W. (2012). Mindfulness and mind-wandering: finding convergence through opposing constructs. Emotion, 12(3), 442. [DOI] [PubMed] [Google Scholar]
- Murakami H., Katsunuma R., Oba K., et al. (2015). Neural networks for mindfulness and emotion suppression. PLoS One, 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perls F., Hefferline G., Goodman P. (1951). Gestalt therapy. New York, 64(7), 19–313. [Google Scholar]
- Radua J., Del Pozo N.O.D., Gómez J., Guillen-Grima F., Ortuño F. (2014). Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia, 58, 14–22. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. (2015). The brain’s default mode network. Annual Review of Neuroscience, 38, 433–47. [DOI] [PubMed] [Google Scholar]
- Rogers C.R. (1959). A Theory of Therapy, Personality, and Interpersonal Relationships: As Developed in the Client-centered Framework, Vol. 3. New York, NY: McGraw-Hill, 184–256. [Google Scholar]
- Sambin M., Messina I. (2017). Rivoluzione mindfulness: fino a che punto? Giornale Italiano di Psicologia, 44(2), 333–8. [Google Scholar]
- Segal Z.V., Williams J.M.G., Teasdale J.D. (2002). Mindfulness-Based Cognitive Therapy for Depression. New York, NY: Guilford Press. [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., et al. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences, 106(6), 1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., Astafiev S.V., McAvoy M.P., d’Avossa G., Corbetta M. (2007). Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cerebral Cortex, 17(11), 2625–33. [DOI] [PubMed] [Google Scholar]
- Smoski M.J., Keng S.-L., Ji J.L., Moore T., Minkel J., Dichter G.S. (2015). Neural indicators of emotion regulation via acceptance vs reappraisal in remitted major depressive disorder. Social Cognitive and Affective Neuroscience, 10(9), 1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K.S., Seminowicz D.A., Davis K.D. (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping, 30(9), 2731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum C.M., Johnstone T., Urry H.L., et al. (2007). Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage, 36(3), 1041–55. [DOI] [PubMed] [Google Scholar]
- Viviani R. (2013). Emotion regulation, attention to emotion, and the ventral attentional network. Frontiers in Human Neuroscience, 7, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani R. (2014). Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Frontiers in Psychiatry, 5, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani R., Dommes L., Bosch J.E., Labek K. (2020). Segregation, connectivity, and gradients of deactivation in neural correlates of evidence in social decision making. Neuroimage, 223, 117339. doi: 10.1016/j.neuroimage.2020.117339 [DOI] [PubMed] [Google Scholar]
- Webb T.L., Miles E., Sheeran P. (2012). Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138, 775–808. [DOI] [PubMed] [Google Scholar]
- Wegner D.M., Schneider D.J., Carter S.R., White T.L. (1987). Paradoxical effects of thought suppression. Journal of Personality and Social Psychology, 53(1), 5. [DOI] [PubMed] [Google Scholar]
- Wenzlaff R.M., Wegner D.M. (2000). Thought suppression. Annual Review of Psychology, 51(1), 59–91. [DOI] [PubMed] [Google Scholar]
- Westbrook C., Creswell J.D., Tabibnia G., Julson E., Kober H., Tindle H.A. (2013). Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience, 8(1), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Williams J.C., Lynn S.J. (2010). Acceptance: an historical and conceptual review. Imagination, Cognition and Personality, 30(1), 5–56. [Google Scholar]
- Whisman M.A. (1993). Mediators and moderators of change in cognitive therapy of depression. Psychological Bulletin, 114(2), 248. [DOI] [PubMed] [Google Scholar]
- Wojnarowska A., Kobylinska D., Lewczuk K. (2020). Acceptance as an emotion regulation strategy in experimental psychological research: what we know and how we can improve that knowledge. Frontiers in Psychology, 11, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgast M., Lundh L.-G., Viborg G. (2011). Cognitive reappraisal and acceptance: an experimental comparison of two emotion regulation strategies. Behaviour Research and Therapy, 49(12), 858–66. [DOI] [PubMed] [Google Scholar]
- Wolgast M., Lundh L.-G., Viborg G. (2013). Cognitive restructuring and acceptance: an empirically grounded conceptual analysis. Cognitive Therapy and Research, 37(2), 340–51. [Google Scholar]
- Zhao X.-H., Wang P.-J., Li C.-B., et al. (2007). Altered default mode network activity in patient with anxiety disorders: an fMRI study. European Journal of Radiology, 63(3), 373–8. [DOI] [PubMed] [Google Scholar]


