Abstract
Aims
The irregular atrial electrical activity during atrial fibrillation (AF) is associated with a variable left ventricular (LV) systolic function. The mechanisms determining LV function during AF remain incompletely understood. We aimed at elucidating how changes in RR-interval and LV preload affect LV function during AF.
Methods and results
Beat-to-beat speckle-tracking echocardiography was performed in 10 persistent AF patients. We evaluated the relation between longitudinal LV peak strain and preceding RR-interval during AF. We used the CircAdapt computational model to evaluate beat-to-beat preload and peak strain during AF for each patient by imposing the patient-specific RR-interval sequences and a non-contractile atrial myocardium. Generic simulations with artificial RR-interval sequences quantified the haemodynamic changes induced by sudden irregular beats. Clinical data and simulations both showed a larger sensitivity of peak strain to changes in preceding RR-interval at slow heart rate (HR) (cycle length, CL <750 ms) than at faster HR. Simulations explained this by a difference in preload of the current beat. Generic simulations confirmed a larger sensitivity of peak strain to preceding RR-interval at fast HR (CL = 600 ms: Δ peak strain = 3.7% vs. 900 ms: Δ peak strain = 0.3%) as in the patients. They suggested that longer LV activation with respect to preceding RR-interval is determinant for this sensitivity.
Conclusions
During AF, longitudinal LV peak strain is highly variable, particularly at fast HR. Beat-to-beat changes in preload explain the differences in LV systolic function. Simulations revealed that a reduced diastolic LV filling time can explain the increased variability at fast HR.
Keywords: Atrial fibrillation, Speckle-tracking echocardiography, Left ventricular function, Computer simulations, Haemodynamics
What’s new?
In this article, we investigate how the irregularity of ventricular rate during atrial fibrillation (AF) relates to beat-to-beat variability of left ventricular (LV) systolic function.
Using a novel combined computational–clinical approach, we identify a larger sensitivity of LV peak strain to preceding RR-interval at fast heart rates (HRs).
We quantify how both beat-to-beat changes in RR-interval and HR history influence the sensitivity of peak strain to sudden changes of RR-interval through preload changes.
In silico analyses reveal that reduced diastolic LV filling time explains this variability in LV function during AF.
Introduction
Atrial fibrillation (AF) is a common sustained arrhythmia characterized by an irregular atrial electrical activity, generating irregular activations of the ventricle. AF is also characterized by mechanical remodelling of the atria, decreasing their active contribution to ventricular filling,1,2 resulting in reduced left ventricular (LV) performance, fatigue, and reduced exercise tolerance.3 However, the relative effects of the reduced ‘atrial kick’ and the irregular ventricular activation on LV systolic pump function remains unclear and therefore the determinants of LV systolic function during AF are incompletely understood. Beat-to-beat variations in LV function have been associated with changes in preload, interval force relation, or afterload4,5 but their actual contributions to LV function in the context of AF remains unclear. Previously, Gosselink et al.6 investigated beat-to-beat systolic ventricular function during AF using nuclear probe imaging and analysed the relationship between LV performance and RR-interval. They concluded that beat-to-beat changes in preload contributed to the variability of LV systolic function, with a smaller influence after long preceding intervals compared to short preceding intervals. However, the haemodynamic interactions responsible for these observations and the mechanisms modulating LV function sensitivity to RR-interval remained unclear.
Here, we hypothesize that both AF-related beat-to-beat changes in RR-interval and the average heart rate (HR) contribute to variability in ventricular loading conditions, leading to variations in LV systolic function. We imaged patients during AF episodes using speckle-tracking echocardiography (STE) to assess longitudinal peak strain, as a measure of LV function, and recorded the sequences of irregular RR-intervals. Combining this with a computational approach, we used the well-validated CircAdapt computer model of the heart and circulation7–9 to simulate ventricular function in these patients. Using the ability offered by computer modelling to control potential determinants of LV performance separately, we were able to pinpoint the variations in haemodynamics and mechanical loading responsible for the variability in peak strain and LV systolic function observed in these patients. We investigated how AF-induced acute changes in RR-intervals and average HR relate to beat-to-beat variability of LV systolic function, in terms of LV longitudinal peak strain.
Methods
Speckle-tracking echocardiography
Ten patients with persistent AF were recruited at the outpatient clinic of the Cardiology department from Maastricht University Medical Center+ between April and May 2019. All patients underwent routine echocardiography for standard clinical follow-up. Echocardiographic studies with poor image quality or presence of regional wall motion abnormalities were excluded. Speckle-tracking strain analysis was conducted offline by one experienced observer, using dedicated vendor-independent software (2D CPA; TomTec Imaging Systems, Unterschleissheim, Germany). For each individual patient, peak strain was measured in 100 consecutive cardiac cycles during breath hold. Measurements were performed in the apical four-chamber view only. The cardiac cycles (RR-interval) were manually defined based on the electrocardiogram recording. The regions of interest were manually outlined by marking the endocardial and epicardial borders in the LV end-systolic frame. The software automatically tracks myocardial speckle patterns frame-by-frame during one cardiac cycle. Suboptimal tracking was manually adjusted. Limited baseline characteristics were retrospectively collected from the digital patient record system.
Computer simulations of AF
In order to investigate the determinants of LV function during AF we used the CircAdapt computer model of the heart and circulation, allowing the simulation of beat-to-beat volumes and pressures in the cardiac chambers as well as blood flow through the valves.7–9 We imposed the irregular sequences of RR-intervals recorded in the patients to the model. In order to model the mechanical remodelling of the atria in AF, we simulated non-contractile atrial myocardium by setting atrial contractility to zero in the model {this led to a left atrial volume of 102 mL in agreement with the clinical data of our cohort [median left atrial volume 120 mL (interquartile range 101–131)]}. This led to the loss of atrial mechanical contribution to ventricular filling. For each virtual patient simulation, ventricular myocardial contractility was reduced so that simulated mean peak LV myofibre strain was in the range of measured peak longitudinal LV strain measured in the patient. LV end-diastolic volume (EDV) was quantified as measure of LV preload for each simulated cardiac cycle.
In addition to patient-specific simulations, generic simulations were performed to isolate the effects of irregular RR-interval changes on ventricular function by using artificial sequences of RR-intervals. The EDV changes induced by a short or a long beat (RR-interval ± 100 ms) were simulated for a range of cycle lengths (CLs) (from CL = 500 ms to CL = 1000 ms). The sequences of RR intervals simulated were: a series of beats with constant RR-interval interrupted by a single beat that is 100 ms shorter or longer, followed by several beats at the initial RR-interval. Finally, we repeated these generic simulations with prolonged duration of LV activation (increase of 10% of the initial RR-interval). More precisely, active force development was prolonged, mimicking an increased duration of myocardial twitch at the tissue level. This reduced the time available for ventricular filling and allowed us to test the hypothesis that filling time is the limiting factor explaining the large sensitivity of LV systolic function to preceding RR-interval at high HR. The filling time was quantified as the duration of the mitral flow wave in each beat.
Statistical analysis
Continuous variables with normal distribution are expressed as mean ± standard deviation (SD), otherwise as median with interquartile range. Categorical variables are presented as observed number with percentage. Normally distributed data were compared using t-tests. Non-normally distributed data were compared using the Mann–Whitney U-test. Statistical significance was assumed when P < 0.05.
Results
Study population
We examined 10 patients with persistent AF and the baseline characteristics are shown in Table 1. Mean age of the patients was 72 years (± 9) and seven patients were men (70%). Majority of these patients were diagnosed with coronary artery disease (70%) and half of the patients had hypertension. All patients were adequately treated with anti-coagulants and seven patients (70%) were treated with betablockers. Overall, routine echocardiographic measurements show a trend towards reduced LV ejection fraction [44% (38–62)] and an enlarged left atrial volume index [60 mL/m2 (50–77)].
Table 1.
Baseline characteristics of the 10 AF patients analysed
| Baseline characteristic | Total (n = 10) |
|---|---|
| Male | 7 (70%) |
| Age (years) | 71 ± 9 |
| BMI (kg/m2) | 29 ± 4 |
| History | |
| Hypertension | 5 (50%) |
| Diabetes mellitus | 2 (20%) |
| Coronary artery disease | 7 (70%) |
| PCI | 7 (70%) |
| CABG | 0 (0%) |
| Cerebrovascular accident | 1 (10%) |
| TIA | 1 (10%) |
| Medication | |
| Betablocker | 7 (70%) |
| Verapamil/diltiazem | 0 (0%) |
| Anti-coagulants | 10 (100%) |
| NOAC | 7 (70%) |
| Acenocoumarol | 3 (30%) |
| Echocardiography | |
| Left atrial volume (mL) | 120 (101–131) |
| Left atrial volume index (mL/m2) | 60 (50–77) |
| LVEDD (mm) | 54 ± 8 |
| IVSD (mm) | 10 ± 1 |
| PWD (mm) | 9 ± 1 |
| LVEF (%) (Teichholtz method) | 44 (38–62) |
| Septal E/e′ | 9.0 ± 3.1 |
| Lateral E/e′ | 7.1 ± 2.4 |
BMI, body mass index; CABG, coronary artery bypass grafting; IVSD, interventricular septum diameter during diastole; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; NOAC, non-vitamin-K-antagonist oral anti-coagulant; PCI, percutaneous coronary intervention; PWD, posterior wall diameter during diastole; TIA, transient ischaemic attack.
Peak strain exhibits a large sensitivity to a change of preceding RR-interval at high average heart rate
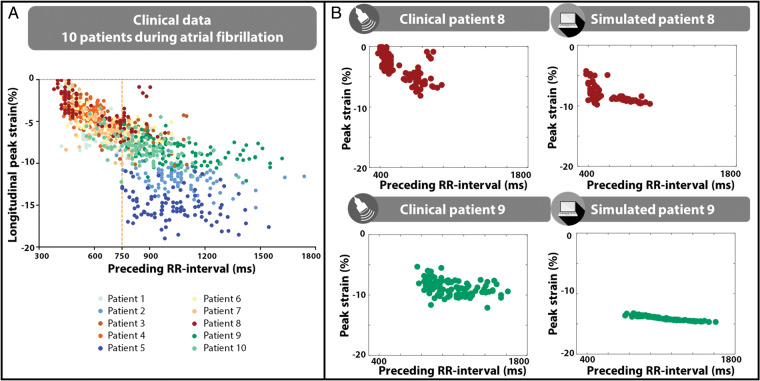
Figure 1A shows the clinical data obtained from STE in all ten patients during AF. The sensitivity of longitudinal LV peak strain to changes in preceding RR-interval was more pronounced at fast HR (CL < 750 ms) than at CL ≥750 ms. There was considerable variability in the range of CL spanned by the individual patients, with some patients having only RR-intervals ≥750ms (e.g. patients 6, 7, or 8) and others only RR-intervals <750ms (e.g. patients 2, 5, 9, or 10) (Figure 1A).
Figure 1.
(A) Longitudinal peak strain (%) vs. preceding RR-interval (ms) in 10 patients in AF. (B) Clinical data and computer simulation of two patients in AF (Patient 8, top; Patient 9, bottom). AF, atrial fibrillation.
By introducing the patient-specific sequences of RR-intervals and a mechanically remodelled atrial myocardium, the computer model was able to reproduce the relation between LV peak strain and preceding RR-interval for each patient (Supplementary material online, S1). Figure 1B highlights the clinical and simulated data for two distinct patients, spanning different RR-interval ranges. Patient 8 had a relatively high average HR (CL = 570 ± 217 ms) and exhibited a large sensitivity of LV peak strain to preceding RR-interval both in the model and the patient data (Figure 1B, top). Patient 9 had a low average HR (CL = 970 ± 388 ms) and showed less dependence of longitudinal peak strain to preceding RR-interval, as simulated with the model (Figure 1B, bottom). Interestingly, the LV and left atrial tissue properties in these simulations were the same for both patients, demonstrating that the exact same contractile and passive tissue function may translate in different average LV peak strain (Patient 8: −8.3% vs. Patient 9: −13.9%) and beat-to-beat variability of LV peak strain (SD Patient 8 = 1.2%, SD Patient 9 = 0.36%), depending on where in the range of HR the heart is operating.
Ventricular failure was simulated by a reduction in myocardial contractility in LV, septal and right ventricular walls. This led to a lower overall peak strain and a loss of peak strain sensitivity to preceding RR-interval at short RR-intervals (Supplementary material online, S2).
Beat-to-beat changes in RR-interval contribute to changes in LV systolic function through changes in preload
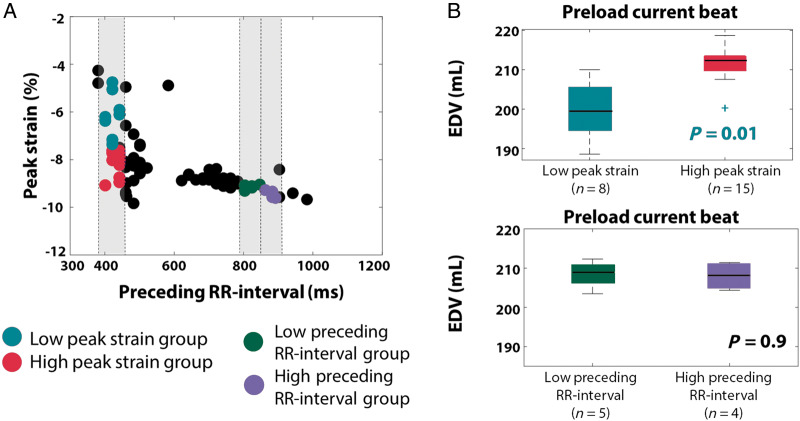
Figure 2 highlights the preload differences in different groups of beats from Patient 8 (Figure 2A). The red and light green groups exhibited similar preceding RR-intervals (411 ± 115 ms vs. 417 ± 8ms, P = 0.67, U-test) but significant differences in peak strain (−5.6 ± 0.81% vs. −7.8 ± 0.67, P = 0.009, U-test). The group with high absolute peak strain (red) showed significantly larger preload (defined as LVEDV) in the current beat, in which the strain was measured (196 ± 3.9 mL vs. 208 ± 4 mL, P = 0.01, U-test) (Figure 2B), in agreement with the Frank–Starling law of the myocardium by which an increased preload leads to larger force generation. The green and purple groups exhibited differences in preceding RR-interval but no difference in peak strains. In this case, the current preload difference between the groups was not significant (209 ± 3 mL vs. 207 ± 3, P = 0.9, t-test) (Figure 2B).
Figure 2.
Comparison of preload of the current beat in different beat groups of Patient 8. (A) Two groups are selected with similar preceding RR-intervals but significantly different current peak strain (light green, red). Two other groups with different preceding RR-intervals but similar current peak strains are selected (green, purple). (B) Preload in the current beat is compared for these different groups. EDV, end-diastolic volume.
In order to investigate how cardiomyocyte sensitivity to preload (i.e. the cellular basis of the Frank–Starling mechanism) may affect this relationship, we made use of the control offered by the computer model to directly vary the sensitivity of the length-dependent activation (LDA). Results showed that LDA modulated the sensitivity of peak strain to preceding RR-interval, with an increased LDA sensitivity leading to a higher variability in peak strain, both at short and long CL (Supplementary material online, S3, Figures S3.1). On the contrary, a reduced LDA sensitivity led to a reduction of peak strain sensitivity to preceding RR-interval, and a reduction in average peak strain (Supplementary material online, S3, Figures S3.2).
Average heart rate also influences the sensitivity of peak strain to changes in preceding RR-interval, through diastolic LV filling time
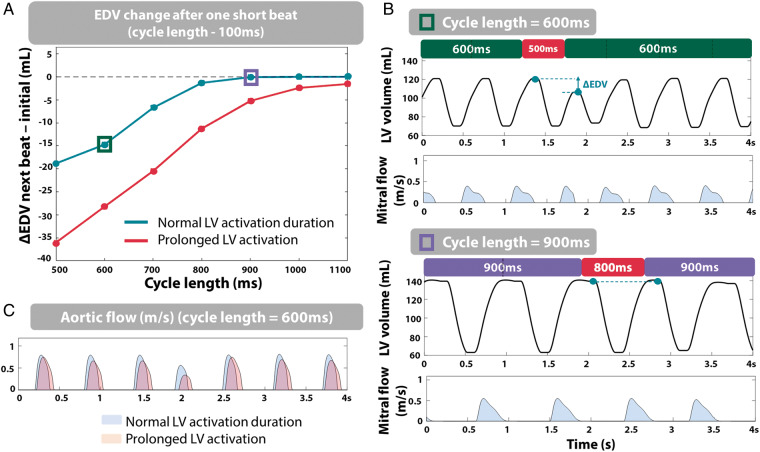
We made use of the ability offered by computer modelling to investigate the effect of irregular beats (long or short) on ventricular haemodynamic parameters at various average HR independently from any other confounding factor (from 500 ms to 1100 ms). As described in the Methods section, the average HR, or CL, refers to the RR-interval of the initial series of beats that were simulated prior to the abrupt RR-interval change (with no other prior history of RR-interval changes). We quantified the preload (EDV) of the beat following this sudden RR-interval change. Figure 3A (green) illustrates the changes in EDV observed after a short beat (100 ms reduction compared to the initial RR-interval) for various values of average HR. At a CL ≥750 ms, a sudden short beat led to little variation in EDV (ΔEDV < -2.5%). On the contrary, at a CL <750 ms, changes were more pronounced (ΔEDV = −18 mL at CL = 500 ms). This is highlighted by the LV volume traces in the two situations in Figure 3B. Qualitatively similar findings were also observed when decreasing the RR-interval by 10% of the initial beat duration (instead of 100 ms) to avoid the influence of RR-interval duration (Supplementary material online, S4). In terms of peak strain, this translated in a larger sensitivity at CL = 600 ms (Δ peak strain = 3.7%) than at CL = 900 ms (Δ peak strain = 0.3%). In addition, these simulations revealed that LV systolic function was most compromised at CL <750 ms because of insufficient diastolic LV filling time to allow the passive filling wave to finish. Indeed, Figure 3B displays the truncated mitral flow pattern observed at a CL of 600 ms, compared to the complete flow pattern observed at CL = 900 ms. The difference in filling time between the initial beats and the beat following the abrupt change in RR-interval was 106 ms at CL = 600 ms (vs. 2 ms at CL = 900 ms), illustrating the overall reduction of LV filling time at fast HRs. At slow HR, filling time reserve is long enough to prevent large variability in LV systolic function.
Figure 3.
(A) Deviation of LV EDV from initial EDV (mL) after a short beat (RR-interval—100 ms) for varying CL, in the case of normal systolic duration (green) and prolonged LV activation duration (red). (B) LV volume and mitral flow pattern traces at CL = 600 ms (top) and 900 ms (bottom). (C) Aortic flow at CL = 600 ms for normal LV activation duration (blue) and prolonged LV activation duration (orange). CL, cycle length; EDV, end-diastolic volume; LV, left ventricle.
Prolonging LV activation duration in the computer model allowed us to control LV ejection time and therefore LV diastolic filling time. With a prolonged LV activation duration (Figure 3A, red), the limiting effect on LV systolic function shifted to longer CL, and the same preload change occurred at a longer CL (normal systolic duration: ΔEDV = −5 mL for CL = 700 ms vs. prolonged LV activation: ΔEDV = −5 mL for CL = 900 ms). This was illustrated by a longer duration of aortic flow (i.e. prolonged ejection time) (Figure 3C). These results indicate that the more severe changes in preload following a short beat at fast average HR were explained by a prolonged ejection time, limiting LV diastolic filling time. This was also in agreement with the simulations performed with a reduced length-sensitivity of activation duration, showing a reduced sensitivity of peak strain to RR-interval changes (Supplementary material online, S3).
Discussion
In this article, we used for the first time a combination of echocardiographic imaging and computer simulations to investigate the determinants of beat-to-beat systolic ventricular function during AF, where ventricular RR-interval is highly variable. Both patient and simulation data showed that LV peak strain is related to and improves with preceding RR-interval. However, this relationship is characterized by more variability in LV peak strain at fast HR (CL < 750 ms) than at slower HR. More pronounced beat-to-beat differences in preload could explain the larger variability of LV peak strain at fast HR. In silico experiments also revealed that LV systolic function is most compromised at CL <750 ms because of an insufficient reserve capacity of LV diastolic filling time, preventing the passive filling wave to be finished. Simulations also showed that this limiting effect on LV systolic function shifts to longer preceding RR-intervals when the duration of ventricular activation is prolonged, highlighting that LV diastolic filling time modulates the sensitivity of LV peak strain to sudden changes of RR-interval.
Longitudinal peak strain is more sensitive to changes in preceding RR-interval at fast HR during AF
First, our computer model, informed by the patient-specific sequence of RR-intervals, was able to accurately reproduce the peak strain—preceding RR-interval relationship in AF patients, suggesting that the model captures the underlying haemodynamic mechanisms. Our combined clinical–computational approach highlighted that longitudinal peak strain is sensitive to changes in preceding RR-intervals, with this sensitivity being more pronounced at fast HR. This corroborates a previous study6 investigating the relationship between LV function and RR-interval.
We were able to simulate the characteristic relationship between LV peak strain and preceding RR-interval observed in the patients by only imposing the patient-specific irregular RR-interval sequence and mechanically remodelled atria to the model. This suggests that the irregularity of RR-interval is a key determinant in the beat-to-beat variations observed in systolic ventricular function. Moreover, the LV and left atrial tissue properties in these simulations were the same for both patients, which exhibited very different sensitivities of LV peak strain to a sudden change in RR-interval, suggesting that myocardial tissue properties are not the sole determinant of LV function during AF. Clinically, this suggests that two patients exhibiting very different peak strain measurements may nonetheless have the same underlying tissue properties.
Interestingly, less variability was observed in the simulation data compared to the clinical data. Among other factors, this can be explained by the absence of measurement noise in the computer model. The absence of measurement noise in the model simulations also reinforces the fact that the peak strain variations observed in the patients are due to haemodynamic interactions, because they are reproduced rather well by the model. Beat-by-beat regulation of the autonomic nervous system may be another factor present in the patients that subsequently affects RR-intervals and contractility, as well as their variability. Finally, although the clinical measurements were obtained during breath hold, variability in the measurements due to movement and respiration artefacts may be present in the clinical data, contrary to the simulations. In their study, Gosselink et al.6 also noticed an influence of the pre-preceding RR-interval on the variability in peak strain. In our work, pre-preceding RR-interval did not exhibit such a relationship (not shown). This may be due to differences in LV function measurement (LV peak strain vs. nuclear probe imaging), patient characteristics, or sequences of RR intervals measured in the patients.
Both acute beat-to-beat changes in RR-interval, as well as RR-interval history, are determinants of the variations in LV systolic function
Computer simulations showed that the large peak strain sensitivity observed at fast HR was driven by preload differences. Beats with higher peak strain were associated with a larger preload in the current beat. This was in agreement with the Frank–Starling law of the myocardium and was previously proposed as potential mechanism behind variable LV function in AF.6,10
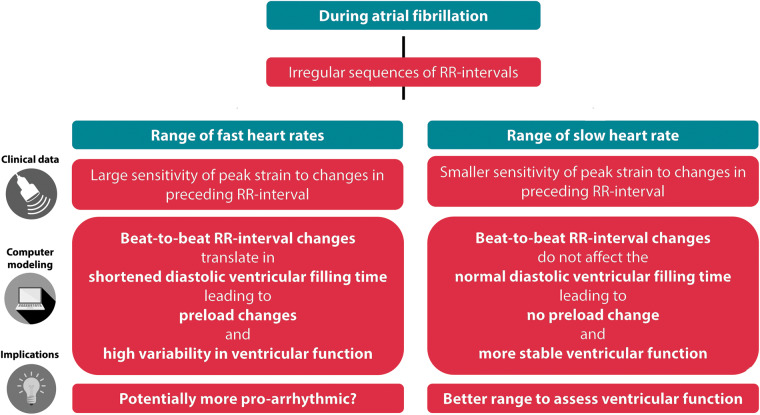
This beat-to-beat sensitivity was larger at fast HR. This was hypothesized in earlier studies11,12 where it was suggested that cardiac index was inversely correlated with the RR variability produced by short cycles but not with the SD of all RR-intervals. Our in silico experiments, allowing a perfect control of the RR-interval sequence, confirmed this hypothesis. We showed that at short CL, the change of EDV following a short beat was more pronounced, suggesting that not only the beat-to-beat change in RR-interval but also where in the range of RR-interval this change happens is important. Our simulations showed that this could be explained by the relative prolonged ventricular activation duration, leaving less time for passive filling. The truncated mitral flow patterns observed at short initial RR-intervals reveal that the reduction in diastolic LV filling reserve explains the sensitivity of peak strain to RR-interval changes. Figure 4 summarizes the proposed mechanisms leading to LV systolic function variability during AF.
Figure 4.
Mechanistic hypothesis of how both beat-to-beat changes in RR-interval and diastolic LV filling time affect ventricular function during atrial fibrillation, based on a clinical-computational approach, and its potential clinical implications. LV, left ventricle.
Altogether, our findings suggest two levels at which beat-to-beat RR-interval changes related to AF rhythm may influence LV systolic function: an acute sensitivity of LV function to beat-to-beat changes in RR-interval, as well as a modulation of this sensitivity by the history of RR-intervals determining the range of RR-interval in which the patient operates.
Potential clinical implications and future work
These findings may impact the way strain is clinically measured in patients in AF. Indeed, our results showed a large variability in peak strain at short CL (<750 ms in our cohort). This implies that measuring peak strain at longer CL may reduce the variability in peak strain and lead to a more robust measurement of LV systolic function. At the same time, however, this comes with practical limitations, as it is clinically challenging to induce longer CL in patients in which these CL ranges are never reached. Whether this shows that applying rate control medication before the echocardiographic measurement, or analysing beats with longer RR-intervals is a better approach to determine LV function in AF patients remains to be determined. Our results also suggest that the effect of variations in cardiac loading differs depending on the HR history, with a larger sensitivity of cardiac function at fast HR. In these cases, the reduced LV diastolic time may impair proper filling of the LV therefore leading to ventricular dysfunction. This may be a potential mechanism underlying the adverse cardiovascular outcomes observed in AF patients with fast HRs.13 Moreover, clinical data showed that some patients exhibited a large sensitivity of peak strain, even at long CL. Our results showed that this might be explained by a reduced sensitivity of LDA in these patients. Peak strain variability during AF may therefore contain diagnostic information on the sensitivity of the myocardium to preload (Frank–Starling reserve) and more research is needed to investigate this diagnostic potential.
In this study, atrial and ventricular volumes were not fitted for each patient, to limit the degree of freedom introduced in the model, and focus on the influence of irregularity in RR-intervals only. Future work may focus on developing personalized models, including fitting the geometry of each patient. Finally, in future studies, modelling of additional AF dynamics (e.g. flutters, allowing the propagation of some atrial contractions towards the ventricle) may be implemented to explore the effects of other AF-induced changes on ventricular function and haemodynamics. The coupling of this model to electrophysiological models will also allow to link AF-induced changes in calcium handling to the haemodynamic mechanisms mentioned in this study.
Conclusions
Using a combined clinical–computational approach, we investigated the determinants of beat-to-beat systolic LV function in AF. We showed a larger sensitivity of peak strain to preceding RR-interval at fast HR. Differences in preload of the current beat could explain the beat-to-beat variations in peak strain. In addition, in silico experiments suggested that a reduced capacity of LV diastolic filling time at fast HR could explain this larger sensitivity at overall faster HR. Our results provide insight in the dynamic haemodynamic interactions in AF, suggesting that the effect of cardiac loading may vary depending on both acute beat-to-beat changes in RR-interval, as well as the average HR preceding these changes. This may have impact on measurement of LV peak strain in AF and management of AF patients.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by the Netherlands Organization for Scientific Research (NWO- ZonMw, VIDI grant 016.176.340) and the Dutch Heart Foundation (ERA-CVD JTC2018 grant 2018T094; Dr Dekker Programme grant 2015T082). This paper is part of a supplement supported by an unrestricted grant from the Theo-Rossi di Montelera (TRM) foundation.
Conflict of interest: none declared.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
References
- 1. Schotten U, Duytschaever M, Ausma J, Eijsbouts S, Neuberger H-R, Allessie M. Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation 2003;107:1433–9. [DOI] [PubMed] [Google Scholar]
- 2. Schotten U, Ausma J, Stellbrink C, Sabatschus I, Vogel M, Frechen D et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation 2001;103:691–8. [DOI] [PubMed] [Google Scholar]
- 3. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- 4. Belenkie I. Beat-to-beat variability of echocardiographic measurements of left ventricular end diastolic diameter and performance. J Clin Ultrasound 1979;7:263–8. [DOI] [PubMed] [Google Scholar]
- 5. Braunwald E Frye R L Aygen M M Gilbert J W. Studies on starling's law of the heart. III. Observations in patients with mitral stenosis and atrial fibrillation on the relationships between left ventricular end-diastolic segment length, filling pressure, and the characteristics of ventricular contraction *. J Clin Invest 1960;39:1874–84. 10.1172/JCI104211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gosselink ATM, Blanksma PK, Crijns HJGM, Van Gelder IC, de Kam P-J, Hillege HL et al. Left ventricular beat-to-beat performance in atrial fibrillation: contribution of Frank-Starling mechanism after short rather than long RR intervals. J Am Coll Cardiol 1995;26:1516–21. [DOI] [PubMed] [Google Scholar]
- 7. Arts T, Delhaas T, Bovendeerd P, Verbeek X, Prinzen FW. Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. Am J Physiol Heart Circ Physiol 2005;288:H1943–1954. [DOI] [PubMed] [Google Scholar]
- 8. Walmsley J, Arts T, Derval N, Bordachar P, Cochet H, Ploux S et al. Fast simulation of mechanical heterogeneity in the electrically asynchronous heart using the MultiPatch module. PLoS Comput Biol 2015;11:e1004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lumens J, Delhaas T, Kirn B, Arts T. Three-wall segment (TriSeg) model describing mechanics and hemodynamics of ventricular interaction. Ann Biomed Eng 2009;37:2234–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Popović ZB, Yamada H, Mowrey KA, Zhang Y, Wallick DW, Grimm RA et al. Frank-Starling mechanism contributes modestly to ventricular performance during atrial fibrillation. Heart Rhythm 2004;1:482–9. [DOI] [PubMed] [Google Scholar]
- 11. Herbert WH. Cardiac output and the varying R-R interval of atrial fibrillation. J Electrocardiol 1973;6:131–5. [DOI] [PubMed] [Google Scholar]
- 12. Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol 1997;30:1039–45. [DOI] [PubMed] [Google Scholar]
- 13. Andrade JG, Roy D, Wyse DG, Tardif J-C, Talajic M, Leduc H et al. Heart rate and adverse outcomes in patients with atrial fibrillation: a combined AFFIRM and AF-CHF substudy. Heart Rhythm 2016;13:54–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.