Abstract
Objective
To report symptoms, disability, and rehabilitation referral rates after coronavirus disease 2019 (COVID-19) hospitalization in a large, predominantly older population.
Design
Cross-sectional study, with postdischarge telemonitoring of individuals hospitalized with confirmed COVID-19 at the first month after hospital discharge, as part of a comprehensive telerehabilitation program.
Setting
Private verticalized health care network specialized in the older population.
Participants
Individuals hospitalized because of COVID-19. We included 1696 consecutive patients, aged 71.8±13.0 years old and 56.1% female. Comorbidities were present in 82.3% of the cases (N=1696).
Interventions
Not applicable.
Main Outcome Measures
Dependence for basic activities of daily living (ADL) and instrumental activities of daily living (IADL) using the Barthel Index and Lawton's Scale. We compared the outcomes between participants admitted to the intensive care unit (ICU) vs those admitted to the ward.
Results
Participant were followed up for 21.8±11.7 days after discharge. During postdischarge assessment, independence for ADL was found to be lower in the group admitted to the ICU than the ward group (61.1% [95% confidence interval (CI), 55.8%-66.2%] vs 72.7% [95% CI, 70.3%-75.1%], P<.001). Dependence for IADL was also more frequent in the ICU group (84.6% [95% CI, 80.4%-88.2%] vs 74.5%, [95% CI, 72.0%-76.8%], P<.001). Individuals admitted to ICU required more oxygen therapy (25.5% vs 12.6%, P<.001), presented more shortness of breath during routine (45.2% vs 34.5%, P<.001) and nonroutine activities (66.3% vs 48.2%, P<.001), and had more difficulty standing up for 10 minutes (49.3% vs 37.9% P<.001). The rehabilitation treatment plan consisted mostly of exercise booklets, which were offered to 65.5% of participants. The most referred rehabilitation professionals were psychologists (11.8%), physical therapists (8.0%), dietitians (6.8%), and speech-language pathologists (4.6%).
Conclusions
Individuals hospitalized because of COVID-19 present high levels of disability, dyspnea, dysphagia, and dependence for both ADL and IADL. Those admitted to the ICU presented more advanced disability parameters.
Keywords: Activities of daily living, COVID-19, Deglutition disorders, Disabled persons, Dyspnea, Mental disorders, Rehabilitation, Telerehabilitation
List of abbreviations: ADL, activities of daily living; CI, confidence interval; COVID-19, coronavirus disease 2019; IADL, instrumental activities of daily living; ICU, intensive care unit; IQR, interquartile range
Postacute symptoms and persistent disability after COVID-19 discharge are still unclear.1 Currently available data suggest that at the time of discharge, individuals present high levels of physical and mental health disability, as well as fatigue, respiratory, cardiac, renal, neuropsychological, speech and swallow, nutritional, and vocational impairments that persist for at least 6 months.2, 3, 4, 5, 6, 7, 8, 9 Those impairments seem to be more pronounced in those with more severe disease.2 , 6 , 9 , 10 In a cohort from China, individuals that required high-flow nasal cannula, noninvasive ventilation, or invasive ventilation presented more frequent mobility impairment, pain, anxiety, and depression 6 months after discharge than those hospitalized without oxygen therapy.2 Data from the United Kingdom show that individuals admitted to the intensive care unit (ICU), when compared with those admitted to the ward, presented higher rates of fatigue (72% vs 60%), breathlessness (66% vs 43%), and neuropsychological impairments (47% vs 24%) in the first 2 months after discharge.6 However, data on different populations and demographics are needed to better understand disability after COVID-19, as well as the potential effect of ICU admission.
Given the increasing number of cases and its potential disability rates, COVID-19 is placing an enormous strain on rehabilitation services worldwide.11, 12, 13, 14 To muster the appropriate resources to respond to this disability epidemic, rehabilitation services require data about frequency of persistent symptoms, disability rates, and rehabilitation referral needs in this population, which are currently scarce.1 Identifying risk factors for disability is also important for an appropriate response plan. ICU admission correlates to more severe disease and prevalent disability rates in those hospitalized because of COVID-19.10 Our goal was to provide data from a comprehensive telerehabilitation program on postdischarge symptoms and disability, as well as rehabilitation referral needs, comparing those admitted to the ICU and ward. We hypothesized that individuals admitted to the ICU had higher prevalence of symptoms, disability, and rehabilitation referral needs.
Methods
This retrospective cross-sectional study was performed at Prevent Senior, a verticalized Brazilian private health care network specialized in the older population,15 currently caring for over 500,000 lives. Reporting of this article was performed following Strengthening the Reporting of Observational Studies in Epidemiology guidelines.16 The present study was approved by the ethics committee.
Participants
Eligibility criteria
From March 15-August 27, 2020, all individuals hospitalized because of COVID-19 at Prevent Senior in the city of São Paulo were screened for eligibility. Participants were included if COVID-19 was the cause of admission, confirmed by positive molecular diagnosis (real-time polymerase chain reaction) for SARS-CoV-2 infection, and if they were discharged alive. Participants hospitalized with asymptomatic COVID-19 and those who presented symptoms only after hospitalization were excluded from the present study (n=9). We did not exclude individuals that were readmitted to the hospital.
Outcomes
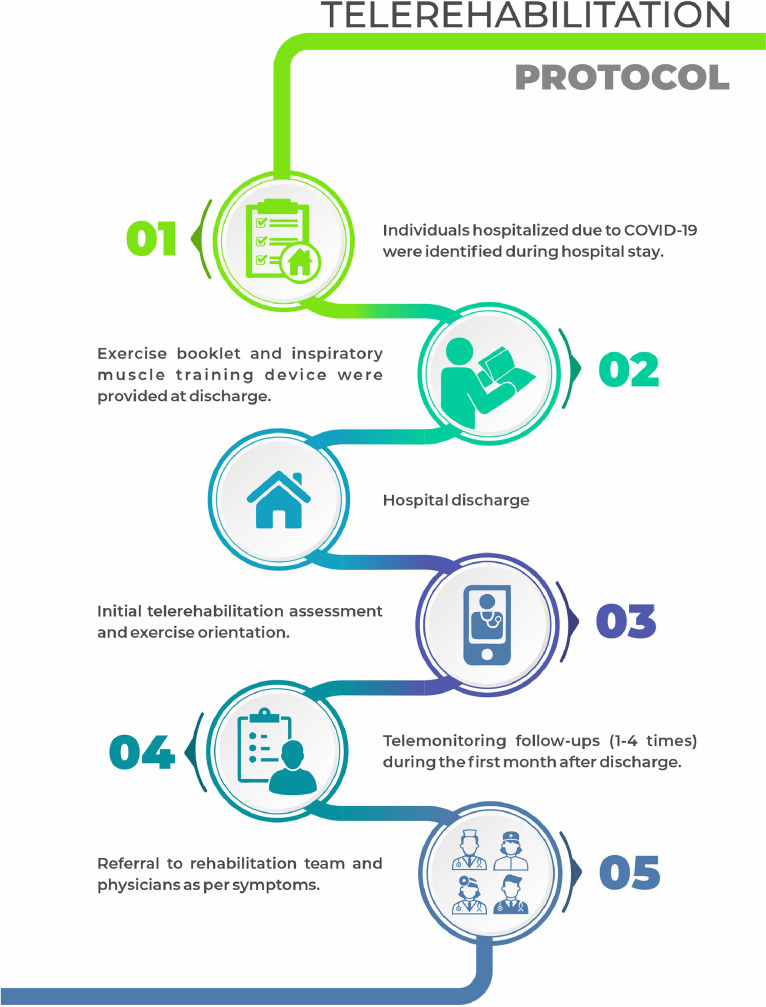
Data were obtained from a comprehensive telerehabilitation program implemented for individuals who were discharged after COVID-19 hospitalization (fig 1 ). Our telerehabilitation team had access to a central list containing all individuals hospitalized because of COVID-19 in our verticalized health care network. Each patient was individually and manually monitored using our electronic health record. Once we identified that the patient had been discharged, telephone contact was made by a physical therapist. We elected the physical therapist for this role based on 2 main reasons: (1) we expected the highest impairments to be in the motor and respiratory functions and (2) because of the number of available professionals at that time owing to the suspension of outpatient sessions. The objective of the first telephone contact was to identify symptoms and disability and provide early referral to telerehabilitation services.
Fig 1.
Telerehabilitation protocol for COVID-19.
Demographics, comorbidities, and hospitalization data were retrieved using electronic health records. Radiological severity was assessed by the Tomographic score for COVID-19 (RAD-COVID score) at admission, which uses chest computerized tomography scans to stratify overall pulmonary parenchyma involvement in <25%, 25%-50%, and >50%, resulting in scores 1-3, respectively.17
During the initial telephone contact, data were gathered using a structured form specifically designed for identifying disability and rehabilitation needs in individuals discharged after COVID-19 in our institution (supplemental appendix S1, available online only at http://www.archives-pmr.org/). This was the only time point at which data were gathered from the individuals in this study. The structured form used in the telerehabilitation program assessed individuals’ physical and respiratory symptoms, mobility impairments, measures of independence and affect, nutritional, and swallowing symptoms. Individuals were also asked to report any other symptoms not addressed by the form. The structured form assessed the following self-reported variables:
Respiratory symptoms
Shortness of breath was assessed as a binomial variable (yes/no). We assessed current shortness of breath in 3 different situations: at rest, during routine activities, and during nonroutine activities. Routine activities included ADL that were part of that individual's routine prior to COVID-19 infection, such as bathing, dressing, walking inside home, or climbing stairs (for those individuals with stairs at home). Nonroutine activities included any other activities, such as exercising, climbing stairs (in case there were no stairs at home), and walking outside the home.
Current use of oxygen therapy was assessed as a binomial variable (yes/no). Thus, participants who were currently using oxygen therapy for a few hours or for any specific activities were considered as users of oxygen therapy.
Physical symptoms
We assessed current energy levels using a 0-10 numeric rating scale (0=no energy whatsoever, 10=best energy possible). Current overall pain level (on any location) was rated using a 0-10 numeric rating scale (0=no pain, 10=worst imaginable pain). We also asked if there was any current numbness or tingling sensation present (yes/no).
Mobility impairment
We asked if the individuals were having any difficulty standing up for over 10 minutes unassisted (yes/no), if they had difficulty moving any limb (yes/no), and if they had any falls since hospital discharge (yes/no). We also assessed if they required any gait assistance devices, such as a cane, walker, or wheelchair (yes/no).
Measures of independence
Independence for ADL (feeding, bathing, grooming, dressing, bowel/bladder management, toilet use, transfers, mobility, use of stairs) was assessed using the Barthel Index. Barthel Index was scored from 0-100, with individual items scoring 0-10, where 0 meant dependent, 5 partially dependent, and 10 independent. Transfers and mobility were scored 0-15 each, where 0 meant dependent, 5 and 10 referred to different degrees of partial dependence, and 15 mean independent.18 , 19
Independence for IADL (telephone use, shopping, food preparation, housekeeping, mode of transportation, responsibility for medication, ability to hand finances) was assessed using Lawton's Scale scored from 7-21, with individual items scoring 1-3 (1=unable, 2=needs assistance, 3=independent).20 Dependence was defined when scoring <21.
Measures of affect
We assessed for perception of anxious and or depressive symptoms by asking, “Have you been feeling anxious or depressed lately?” (yes/no).
Nutrition/eating
We asked if the individuals presented weight loss with inappetence (yes/no). We also assessed for dysphagia to liquids or solids by asking, “Do you have any trouble swallowing food or liquids?” (yes/no).
Follow-up and telerehabilitation protocol
During the initial telephone contact, individuals were stratified based on their current Barthel Index score18: level 1 (0-39, dependent), level 2 (40-79, partially dependent) and level 3 (80-100, independent) as shown in table 1 . All participants were provided with a physical exercise guide in booklet and/or video format, based in a previous publication.21 The physical therapist then decided if the patient would perform unsupervised home exercises onl or if a referral to an online physical therapy group was necessary. Those performing unsupervised home exercises were followed up by telemonitoring (telephone contact) for a month, either weekly, biweekly, or monthly, for level 1, 2, or 3, respectively. The objective was to assess and promote adherence to exercises, correct any doubts regarding exercises, and assess any other rehabilitation needs (data from telemonitoring follow-ups were not recorded).
Table 1.
Follow-up protocol recommendations for telerehabilitation following hospitalization for COVID-19 using the dependence score on the Barthel Index
| Barthel Index | Subgroup | Follow-up |
|---|---|---|
| 0-40 | Level 1 | Referral to a level 1 online physical therapy group or home exercises+weekly telemonitoring (total of 4) |
| 41-80 | Level 2 | Referral to a level 2 online physical therapy group or home exercises+biweekly telemonitoring (total of 2) |
| 81-100 | Level 3 | Referral to a level 3 online physical therapy group or home exercises+monthly telemonitoring (total of 1) |
Criteria for referral to other rehabilitation professionals were the following:
-
•
Dysphagia: referral to a speech-language pathologist.
-
•
Issues regarding oxygen therapy (eg, dosage, how to wean down), worsened dyspnea, or impaired blood pressure or heart rate: referral to a cardiologist specialized in cardiac rehabilitation.
-
•
Complaints regarding fine motor control or cognition: referral to occupational therapy.
-
•
Pain rated as >5 (0-10 numeric scale) or any numbness/tingling or difficulty moving their limbs: referral to a physiatrist.
-
•
Weight loss or inappetence: referral to a dietitian.
-
•
Anxious or depressive symptoms: referral to a psychologist.
Statistical analysis
Categorical data were reported as frequency, percentage, and 95% confidence intervals (CIs) estimated by the exact method. Continuous variables were reported as mean, SD, median, and interquartile range (IQR). Shapiro-Wilk test showed that all continuous variables did not present normal distribution. Continuous variables were compared among groups using a Mann-Whitney U test. Categorical variables were compared using Pearson chi-square test, with statistical power of 80% and ɑ=5%. No data imputation method was used. We used Stata 13.0a for the analyses.
This is a descriptive and exploratory study, and sample size calculation was not performed. We used all available data from the telerehabilitation program at the time of protocol writing.
Results
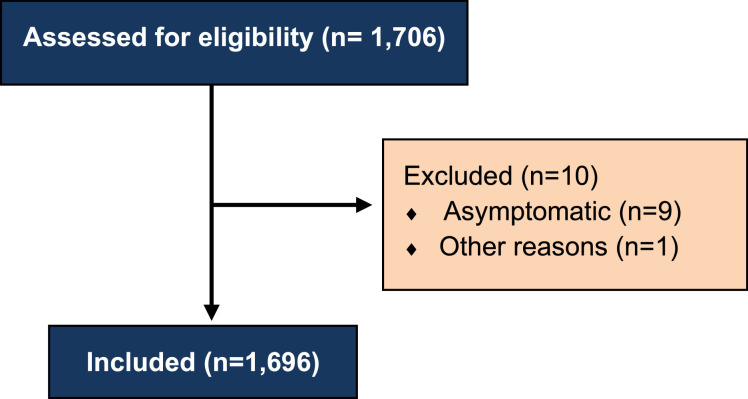
A total of 1733 individuals were screened, and 1696 were included (fig 2 ). A total of 357 individuals (21.0%) were admitted to the ICU at any point during their hospitalization. Individuals admitted to the ICU and the ward had similar characteristics (table 2 ), although those admitted to the ICU were slightly younger (median age, 71y [IQR, 63-78y] vs 73y [IQR, 64-82y] P=.006). Individuals were institutionalized after discharge in 4.5% and 7.8% of the cases admitted to the ICU and ward, respectively. As expected, those admitted to the ICU presented longer length of hospital stay (median, 16d [IQR, 11-2d] vs 6d [IQR, 4-9d] P<.0001) and higher radiological severity (RAD-COVID 3 in 39.2% [95% CI, 34.1%-44.4%] vs 10.5% [95% CI, 8.9%-12.3%]) (table 3 ). After discharge, telephone contact was made with all individuals. Those admitted to the ICU presented worse postdischarge outcomes than those in the ward: higher incidence of shortness of breath during routine (45.1% [95% CI, 39.9%-50.4%] vs 34.5% [95% CI, 32.0%-37.1%]) and nonroutine activities (46.8% [95% CI, 41.5%-52.1%] vs 38.2% [95% CI, 35.6%-40.8%]); higher prevalence of pain (33.9% [95% CI, 29.0%-39.1%] vs 27.1% [95% CI, 24.7%-29.6%]); numbness/tingling (20.2% [95% CI, 16.1%-24.7%] vs 11.3% [95% CI, 9.6%-13.1%]); and need for gait assistance devices (39.8% [95% CI, 34.7%-45.1%] vs 28.8% [95% CI, 26.3%-31.3%]) (table 4 ). Independence for ADL was lower in the ICU group (61.1% [95% CI, 55.8%-66.2%] vs 72.7% [95% CI, 70.3%-75.1%]). Dependence for IADL was also more frequent in the ICU group (84.6%, [95% CI, 80.4%-88.2%] vs 74.5% [95% CI, 72.0%-76.8%]). Postdischarge telerehabilitation treatment plan consisted mostly of exercise booklets (table 5 ), which were offered to 65.5% of the individuals after discharge, whereas the remaining individuals received those booklets during hospital stay. Patients were most frequently referred to psychologists (11.8%), physical therapists (8.0%), and dietitians (6.8%). Our rehabilitation treatment plan was declined by patients and/or families in 2.5% of the cases at time of referral (see table 5) for different reasons: “she needs to rest,” “online rehabilitation will not strengthen him,” “we are afraid she will be infected again,” and “we are afraid that our family will be infected.” We have not assessed if the remaining 97.5% of those referred to telerehabilitation followed our recommendations and scheduled their therapy sessions.
Fig 2.
Flowchart of patient selection according to the eligibility criteria.
Table 2.
Demographics, comorbidities, marital status, and living situation in the first month after hospitalization because of COVID-19
| Variables | Total (N=1696) | ICU (n=357) | Ward (n=1339) | P Value |
|---|---|---|---|---|
| Female, n (%) 95% CI |
951 (56.1) 53.7%-58.4% |
183 (51.3) 45.9%-56.6% |
768 (57.4) 54.7%-60.0% |
.039⁎ |
| Age (y), median (IQR) | 73 (64-81) | 71 (63-78) | 73 (64-82) | .006⁎ |
| Mean ± SD | 71.8±13.0 | 70.3±11.9 | 72.2±13.2 | - |
| Age strata, n (%) 95% CI |
<.001⁎ | |||
| <60 y | 253 (14.9) 13.3%-16.7% |
56 (15.7) 12.0%-19.9% |
197 (14.7) 12.9%-16.7% |
- |
| 60-80 y | 930 (54.8) 52.4%-57.2% |
225 (63.0) 57.8%-68.0% |
705 (52.7) 49.9%-55.4% |
- |
| >80 y | 513 (30.2) 28.1%-32.5% |
76 (21.3) 17.1%-25.9% |
437 (32.6) 30.1%-35.2% |
- |
| Comorbidities, n (%) 95% CI |
||||
| Hypertension | 1082 (63.8) 61.5%-66.1% |
224 (62.8) 57.5%-67.8% |
858 (64.1) 61.4%-66.7% |
.642 |
| Coronary artery disease | 321 (18.9) 17.1%-20.9% |
75 (21.0) 16.9%-25.6% |
246 (18.4) 16.3%-20.6% |
.258 |
| Pulmonary | 262 (15.5) 13.8%-17.3% |
57 (16.0) 12.3%-20.2% |
205 (15.3) 13.4%-17.3% |
.760 |
| Neurologic | 284 (16.7) 15.0%-18.6% |
41 (11.5) 8.4%-15.3% |
243 (18.2) 16.1%-20.3% |
.003⁎ |
| Immunosuppressed | 49 (2.9) 2.1%-3.8% |
13 (3.6) 2.0%-6.1% |
36 (2.7) 1.9%-3.7% |
.340 |
| Cancer | 83 (4.9) 3.9%-6.0% |
15 (4.2) 2.4%-6.8% |
68 (5.1) 4.0%-6.4% |
.495 |
| None | 300 (17.7) 15.9%-19.6% |
60 (16.8) 13.1%-21.1% |
240 (17.9) 15.9%-20.1% |
.623 |
| Smoking status, n (%) 95% CI |
.177 | |||
| Never | 1136 (67.0) 64.7%-69.2% |
231 (64.7) 59.5%-69.7% |
905 (67.5) 65.0%-70.1% |
- |
| Current | 26 (1.5) 1.0%-2.2% |
9 (2.5) 1.2%-4.7% |
17 (1.3) 0.7%-2.0% |
- |
| Former | 534 (31.5) 29.3%-33.8% |
117 (32.8) 27.9%-37.9% |
417 (31.1) 26.7%-33.7% |
- |
| Alcohol use, n (%) 95% CI |
.900 | |||
| Never | 1581 (93.2) 91.9%-94.3% |
331 (92.7) 89.5%-95.2% |
1250 (93.4) 91.9%-94.6% |
- |
| Regular | 47 (2.8) 2.0%-3.7% |
11 (3.1) 1.5%-5.4% |
36 (2.7) 1.9%-3.7% |
- |
| Former | 68 (4.0) 3.1%-5.1% |
15 (4.2) 2.4%-6.8% |
53 (4.0) 3.0%-5.1% |
- |
| Marital status, n (%) 95% CI |
.054 | |||
| Single | 141 (8.3) 7.0%-9.7% |
31 (8.7) 6.0%-12.1% |
110 (8.2) 6.8%-9.8% |
- |
| Married | 861 (50.8) 48.4%-53.2% |
198 (55.5) 50.1%-60.7% |
663 (49.5) 46.7%-52.2% |
- |
| Divorced | 145 (8.6) 7.3%-10.0% |
34 (9.5) 6.7%-13.1% |
111 (8.3) 6.9%-9.9% |
- |
| Widowed | 549 (32.4) 30.1%-34.7% |
94 (26.3) 21.8%-31.2% |
455 (34.0) 31.4%-36.6% |
- |
| Living situation, n (%) 95% CI |
.196 | |||
| Alone | 177 (10.4) 9.0%-12.0% |
32 (9.0) 6.2%-12.4% |
145 (10.9) 9.2%-12.6% |
- |
| Spouse | 422 (24.9) 22.8%-27.0% |
94 (26.3) 21.8%-31.2% |
328 (24.5) 22.2%-26.9% |
- |
| Spouse and children | 409 (24.1) 22.1%-26.2% |
94 (26.3) 21.8%-31.2% |
315 (23.5) 21.3%-25.9% |
- |
| Children | 396 (23.4) 21.4%-25.4% |
81 (22.7) 18.4%-27.3% |
315 (23.5) 21.3%-25.9% |
- |
| SNF | 121 (7.1) 6.0%-8.5% |
16 (4.5) 2.6%-7.2% |
105 (7.8) 6.5%-9.4% |
- |
| Other | 171 (10.1) 8.7%-11.6% |
40 (11.2) 8.1%-14.9% |
131 (9.8) 8.2%-11.5% |
- |
Abbreviation: SNF, skilled nursing facility.
Statistical significance.
Table 3.
Length of hospital stay, symptoms, and radiological severity at admission because of COVID-19
| Variables | Total (N=1696) | ICU (n=357) | Ward (n=1339) | P Value |
|---|---|---|---|---|
| Length of hospital stay (d), median (IQR) | 7 (4-12) | 16 (11-25) | 6 (4-9) | <.001⁎ |
| Mean ± SD | 9.7±8.6 | 19.3±12.1 | 7.2±5.0 | - |
| Symptoms at admission, n (%) 95% CI |
- | |||
| Coughing | 1046 (61.7) 59.3%-64.0% |
221 (61.9) 56.6%-70.0% | 825 (61.6) 58.9%-64.2% | .920 |
| Fever | 1029 (60.7) 58.3%-63.0% | 217 (60.8) 55.5%-65.9% | 812 (60.6) 58.0%-63.3% | .961 |
| Malaise | 1106 (65.2) 62.9%-67.5% | 232 (64.5) 59.8%-69.9% | 874 (65.3) 62.7%-67.8% | .920 |
| Shortness of breath | 948 (55.9) 53.5%-58.3% | 226 (63.3) 58.1%-68.3% | 722 (53.9) 51.2%-56.6% | .002⁎ |
| Hypo/anosmia | 469 (27.7) 25.5%-29.8% | 114 (31.9) 27.1%-37.0% | 355 (26.5) 24.1%-28.9% | .042⁎ |
| Hypo/ageusia | 619 (36.5) 34.2%-38.8% | 135 (37.8) 32.8%-43.1% | 484 (36.2) 33.6%-38.8% | .561 |
| Radiological severity at admission, n (%) 95% CI |
<.001⁎ | |||
| RAD-COVID 1 | 593 (35.0) 32.7%-37.2% | 70 (19.6) 15.6%-24.1% | 523 (39.1) 36.4%-41.7% | - |
| RAD-COVID 2 | 884 (46.2) 49.7%-54.5% | 136 (38.1) 33.0%-43.4% | 648 (49.4) 45.7%-51.1% | - |
| RAD-COVID 3 | 281 (16.9) 14.8%-18.4% | 140 (39.2) 34.1%-44.4% | 141 (10.5) 8.9%-12.3% | - |
Statistical significance.
Table 4.
Symptoms on different systems and measures of disability in the first month after hospitalization because of COVID-19
| Variables | Total (N=1696) | ICU (n=357) | Ward (n=1339) | P Value |
|---|---|---|---|---|
| Time from discharge to follow-up (d), median (IQR) | 21 (14-27) | 20 (14-26) | 21 (14-27) | .022⁎ |
| Mean ± SD | 21.8±11.7 | 20.7±11.6 | 22.1±11.8 | - |
| Respiratory symptoms, n (%) 95% CI |
- | |||
| Shortness of breath at rest | 281 (16.6) 14.8%-18.4% | 69 (19.3) 15.4%-23.8% |
212 (15.8) 13.9%-17.9% |
.112 |
| Shortness of breath during routine activities | 623 (36.7) 34.3%-39.1% |
161 (45.1) 39.9%-50.4% | 462 (34.5) 32.0%-37.1% | <.001⁎ |
| Shortness of breath during nonroutine activities | 678 (51.7) 37.6%-42.4% | 167 (46.8) 41.5%-52.1% |
511 (38.2) 35.6%-40.8% | <.001⁎ |
| Oxygen therapy | 260 (15.3) 13.6%-17.1% |
91 (25.5) 21.0%-30.3% |
169 (12.6) 10.9%-14.5% |
<.001⁎ |
| Physical symptoms | - | |||
| Energy level, (0-10), median (IQR) | 7 (5-9) | 7 (5-8) | 7 (6-9) | <.0001⁎ |
| Mean ± SD | 6.9±2.4 | 6.5±2.4 | 7.0±2.4 | |
| Pain prevalence, n (%) 95% CI |
484 (28.5) 26.4%-30.8% | 121 (33.9) 29.0%-39.1% | 363 (27.1) 24.7%-29.6% | .012⁎ |
| Median (IQR) | 5 (4-7) | 6 (4-7) | 5 (4-7) | .0696 |
| Mean ± SD | 5.5±2.2 | 5.8±2.2 | 5.4±2.3 | - |
| Numbness/tingling sensation, n (%) 95% CI |
223 (13.1) 11.6%-14.9% | 72 (20.2) 16.1%-24.7% | 151 (11.3) 9.6%-13.1% | <.001⁎ |
| Mobility impairment, n (%) 95% CI |
- | |||
| Difficulty standing still for >10 min | 683 (40.3) 37.9%-42.7% | 176 (49.3) 44.0%-54.6% |
507 (37.9) 35.3%-40.5% |
<.001⁎ |
| Difficulty moving any limb | 407 (24.0) 22.0%-26.1% | 114 (31.9) 27.1%-37.0% |
293 (21.9) 19.7%-24.2% |
.007⁎ |
| Need for gait assistance devices | 527 (31.1) 28.9%-33.3% | 142 (39.8) 34.7%-45.1% |
385 (28.8) 26.3%-31.3% |
<.001⁎ |
| Recent falls | 107 (6.3) 5.2%-7.6% |
20 (5.6) 3.5%-8.5% |
87 (6.5) 5.2%-8.0% |
.536 |
| Measures of independence | ||||
| ADL total score, median (IQR) | 100 (70-100) | 90 (55-100) | 100 (75-100) | <.0001 |
| Mean ± SD | 80.4±29.7 | 75.6±29.7 | 81.6±29.5 | - |
| Total dependence (level 1), n (%) 95% CI |
232 (13.7) 12.1%-15.4% |
55 (15.4) 11.8%-19.6% |
177 (13.2) 11.4%-15.2% |
<.001⁎ |
| Partial dependence (level 2), n (%) 95% CI |
272 (16.0) 14.3%-17.9% |
84 (23.5) 19.2%-28.3% |
188 (14.0) 12.2%-16.0% |
<.001⁎ |
| Independence (level 3), n (%) 95% CI |
1192 (70.3) 68.0%-72.5% |
218 (61.1) 55.8%-66.2% |
974 (72.7) 70.3%-75.1% |
<.001⁎ |
| IADL total score, median (IQR) | 16 (10-20) | 14 (10-18) | 17 (10-21) | <.0001⁎ |
| Mean ± SD | 15.0 (5.2) | 14.1 (4.7) | 15.2 (5.3) | - |
| Dependence, n (%) 95% CI |
1299 (76.6) 74.5%-78.6% |
302 (84.6) 80.4%-88.2% | 997 (74.5) 72.0%-76.8% | <.001⁎ |
| Measures of affect, n (%) 95% CI |
||||
| Anxious/depressive symptoms | 632 (37.3) 35.0%-39.6% | 139 (38.9) 33.8%-44.2% | 493 (36.8) 34.2%-39.5% | .462 |
| Nutrition/eating, n (%) 95% CI |
||||
| Weight loss with inappetence | 566 (33.4) 31.1%-35.7% | 143 (40.1) 34.9%-45.3% | 423 (31.6) 29.1%-34.2% | .003⁎ |
| Dysphagia | 215 (12.7) 11.1%-14.4% | 45 (12.6) 9.3%-16.5% |
170 (12.7) 11.0%-14.6% | .963 |
Statistical significance.
Table 5.
Exercise orientation and telerehabilitation referral needs during telemonitoring after COVID-19 hospitalization
| Variables, n (%) 95% CI |
Total (N=1696) | ICU (n=357) | Ward (n=1339) | P Value |
|---|---|---|---|---|
| Orientation | ||||
| Exercise booklet | 1111 (65.5) 63.2%-67.7% | 213 (59.7) 54.3%-64.8% |
898 (67.1) 64.5%-69.6% |
.009⁎ |
| Exercise video | 21 (1.2) 0.8%-1.9% |
7 (2.0) 0.8%-4.0% |
14 (1.0) 0.5%-1.7% |
.165 |
| Referrals | - | |||
| Telehealth physical therapist | 98 (5.8) 4.7%-7.0% |
21 (5.9) 3.7%-8.9% |
77 (5.8) 4.6%-7.1% |
.006⁎ |
| In-home physical therapist | 37 (2.2) 1.5%-3.0% |
15 (4.2) 2.4%-6.9% |
22 (1.6) 1.0%-2.5% |
.003⁎ |
| Occupational therapist | 17 (1.0) 0.6%-1.6% |
4 (1.1) 0.3%-2.8% |
13 (1.0) 0.5%-1.7% |
.801 |
| Speech-language pathologist | 78 (4.6) 3.7%-5.7% |
16 (4.5) 2.6%-7.2% |
62 (4.6) 3.6%-5.9% |
.905 |
| Psychologist | 200 (11.8) 10.3%-13.4% |
48 (13.5) 10.1%-17.4% |
152 (11.4) 9.7%-13.2% |
.276 |
| Dietitian | 116 (6.8) 5.7%-8.1% |
36 (10.1) 7.2%-13.7% |
80 (6.0) 4.8%-7.4% |
.006⁎ |
| Physiatrist | 12 (0.7) 0.3%-1.2% |
6 (1.7) 0.6%-3.6% |
6 (0.5) 0.2%-0.9% |
.014⁎ |
| Cardiologist | 13 (0.8) 0.4%-1.3% |
5 (1.4) 0.5%-3.2% |
8 (0.6) 0.3%-1.1% |
.122 |
| Patient declined | 43 (2.5) 1.8%-3.4% |
5 (1.4) 0.5%-3.2% |
38 (2.8) 2.0%-3.9% |
.125 |
Statistical significance.
Discussion
We have showed high prevalence of symptoms and disability rates after COVID-19. As anticipated, individuals admitted to the ICU had higher disability levels than those admitted to the ward. Persistent symptoms and disability after COVID-19 have been previously reported,2 , 4, 5, 6, 7, 8 , 10 and other authors have observed worse outcomes in those admitted to the ICU (table 6 ). According to data from the United Kingdom, breathlessness at rest in the first 2 months after discharge was higher in those admitted to the ICU (28.1% vs 19.3% in our sample) than the ward group (19.1% vs 15.8% in our sample).6 That study also showed more prevalent fatigue, posttraumatic stress disorder symptoms, and decrement in quality of life in the ICU group compared with the ward group. Differences between ICU and ward groups are likely multifactorial and could be partially explained by disease severity.2 , 9 , 10 SARS-CoV-2 infection can cause pulmonary abnormalities; thrombocytopathy; endotheliopathy; hepatic, renal, and nervous system injuries, some due to viral infection and others likely due to excessive immune response.2 , 22 , 23 Recovery time of those injuries is still uncertain because a study with matched controls found that the majority of survivors of COVID-19 persisted with magnetic resonance imaging abnormalities in the lungs, brain, heart, liver, and/or kidneys 2 to 3 months after discharge.9 Besides disease severity, consequences of ICU stay could partially explain our findings because long-term effect of ICU stay has been previously demonstrated for acute respiratory distress syndrome due to SARS-CoV infection, as well as for other nonrespiratory critical illnesses.24, 25, 26 ICU-acquired weakness is a neuromuscular dysfunction that consists of polyneuropathy, myopathy, and/or muscle atrophy that results of critical illness and can be magnified by conditions during ICU stay.26 Potential risk factors for ICU-acquired weakness include use of corticosteroids and continuous neuromuscular blockade, which were present in more than 40% and 80%, respectively, of those admitted to the ICU because of COVID-19.26 , 27
Table 6.
Current available literature on postdischarge symptoms after COVID-19
| Variables | Current Study | Carfi et al4 | Chopra et al5 | Halpin et al6 | Huang et al2 | Mandal et al8 |
|---|---|---|---|---|---|---|
| Sample size | 1696 | 143 | 488 | 100 | 1733 | 384 |
| Country | Brazil | Italy | USA | United Kingdom | China | United Kingdom |
| Time after discharge (d) | 21 (14-27) | 36.1±12 | 60 | 48±10.3 | 153 (143-160) | 54 (47-59) |
| Age (y) | ICU=71 (63-78) Ward=73 (64-82) |
56.5±14.6 | 62 (50-72) | ICU=58.5 (34-84)* Ward=70.5 (20-93)* |
57 (47-65) | 59±16.1 |
| ICU admission (%) | 21.0 | 12.6 | 13.2 | 32 | 4 | 14.5 |
| LOS (d) | ICU=16 (11-25) Ward=6 (4-9) |
13.5±9.7 | 5 (3-8) | ICU=12 (10-16) Ward=6.5 (4-14) |
14 (10-19) | 6.5 (4-10.75) |
| Breathlessness (%) | ICU=45.1† Ward=34.5† |
43.4 | 16.6 | ICU=65.6 Ward=42.6 | 26 | 54.8-63.3‡ |
| Oxygen therapy at follow-up (%) | ICU=25.5 Ward=12.6 |
NR | 6.6 | NR | NR | NR |
| Fatigue (%) | NR | 53.1 | NR | ICU=72.0 Ward=60.3 |
63 | 67.3-76.9‡ |
NOTE. Data expressed as median (IQR) or mean ± SD.
Abbreviations: LOS, length of stay; NR, not reported.
Median and range.
Breathlessness during routine activities (eg, climbing stairs).
Data provided by subgroups only.
To our knowledge, our study is the first to report disability for ADL and IADL after COVID-19 infection in the Brazilian population. Individuals in our study presented high rates of dependence for both ADL and IADL, which cannot be completely attributed to COVID-19 hospitalization. Because of the absence of baseline values, we cannot ascertain if such symptoms and disability rates were already present prior to COVID-19 hospitalization. Disability in the general population could be estimated from a population-based study with 1451 community-living older Brazilians, which reported dependence for ADL and IADL of 36.0% and 34.0%,28 respectively, compared with our rates of 38.9% and 84.6% for the ICU group and 27.3% and 74.5% for the ward group. Despite that, we cannot ascertain whether the individuals who were admitted because of COVID-19 in our network were representative of the general population or if they represented a subset with higher (or lower) disability. Obtaining disability rates before and after COVID-19 hospitalization in the same population would provide a clearer image of its effect. Additional factors may have affected the generalizability of our findings: (1) 35% of the participants were instructed to perform home exercises during hospitalization, which may have reduced disability rates. (2) Outcome assessment relied on self-reporting. Reliability of self-reported Barthel Index on older adults has been reported to be >80% for eating, toileting, and transferring and 63% for bathing and dressing, with frequent underestimation of disability on self-reporting. Therefore, it is possible that actual rates of ADL dependence are higher.29 (3) Physical distancing measures during the pandemic may have overestimated dependency for IADL, particularly for the categories “using transportation” and “shopping.”
Telemonitoring, as part of a comprehensive telerehabilitation program, was feasible in our population. We managed to telemonitor 100% of individuals after discharge, resulting in early identification of persistent symptoms and disability, as well as early referral to telerehabilitation with low refusal rates at the time of referral. The rehabilitation treatment plan was affected by the pandemic and physical distancing measures, and, thus, we have focused on providing exercise booklets and videos. Exercise booklets were provided to all participants, and 65.5% of them received those exercises during telemonitoring. Those with more rehabilitation needs were referred to telerehabilitation using a device with camera (eg, smartphone, tablet). In-place therapy was provided only for in-home physical therapy. Referral rates for occupational therapy were low (1%), considering that disability for ADL and IADL were 29.7% and 76.6%, respectively, and referral rate to physical therapy was 8%. Possible causes for this disparity include (1) overestimation of IADL disability because of physical distancing measures, which created barriers unrelated to body functions or structures that may have affected categories such as shopping and (2) underdiagnosis of triggers for occupational therapy referral (cognitive and/or fine motor impairments) because they were not screened in the telemonitoring protocol and required active complaint by the individual.
Study limitations
We did not assess variables relevant to our study topic, such as obesity, prevalence and duration of mechanical ventilation, and use of continuous neuromuscular blockade.26 , 30 We have not assessed prevalence of fatigue, which has been previously reported as a persistent symptom in this population (see table 6).
Obtaining data on individual categories for both ADL (Barthel Index) and IADL (Lawton's Index) would provide more comprehensive information than the aggregate score. That would improve the understanding of which activities need to be rehabilitated in this population. Dysphagia and anxious/depressive symptoms were identified without using validated assessment tools and are prone to measurement bias.
Our findings are limited by the absence of a control group and baseline values for the outcomes. Therefore, we cannot ascertain if the participants already presented those symptoms and disabilities nor if individuals hospitalized for conditions other than COVID-19 would present such findings. When assessing difference between outcomes in individuals admitted to the ICU vs ward, we did not take measures to avoid third variable effects. Therefore, it is possible there are other factors influencing the higher rates of disability in this subgroup. Using statistical methods that take those effects into account could minimize this issue.
Conclusions
We have reported high rates of pain, shortness of breath, anxious and depressive symptoms, dysphagia, need for oxygen therapy, and dependence for both ADL and IADL in a predominantly older population, with worse outcomes in the ICU group. We also provided data on rehabilitation referral needs to address disability in this population. Our study corroborates and expands on the current body of evidence regarding high rates of disability after COVID-19 hospitalization. Future studies should explore individuals longitudinally, ideally with preadmission assessments as well as validated assessment tools for ADL and IADL disability, pulmonary function, sarcopenia, cardiopulmonary fitness, cognition, dysphagia, neuropsychiatric effects (eg, cognition, mood disorder, posttraumatic stress disorder, substance abuse). Our team is currently conducting a prospective study assessing pre- and post-COVID functionality with patient-reported outcomes and objective assessments with follow-ups until 12 months after discharge, which could contribute to our understanding of this subject.
Supplier
-
a.
Stata 13.0; StataCorp.
Footnotes
Disclosures: none
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.apmr.2021.03.001.
Appendix. Supplementary materials
References
- 1.Ceravolo MG, Arienti C, De Sire A, et al. Rehabilitation and COVID-19: the Cochrane Rehabilitation 2020 rapid living systematic review. Eur J Phys Rehabil Med. 2020;56:642–651. doi: 10.23736/S1973-9087.20.06501-6. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curci C, Pisano F, Bonacci E, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 rehabilitation unit and proposal of a treatment protocol. A cross-sectional study. Eur J Phys Rehabil Med. 2020;56:633–641. doi: 10.23736/S1973-9087.20.06339-X. [DOI] [PubMed] [Google Scholar]
- 4.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020 Nov 11:M20–5661. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2020;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 7.Roberts P, Wertheimer J, Park E, Nuño M, Riggs R. Identification of functional limitations and discharge destination in patients with COVID-19. Arch Phys Med Rehabil. 2020;102:351–358. doi: 10.1016/j.apmr.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal S, Barnett J, Brill SE, et al. Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020 Nov 10 doi: 10.1136/thoraxjnl-2020-215818. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leigh AE, McCall J, Burke RV, Rome R, Raines AM. Predictors of functional dependence after COVID-19: a retrospective examination among veterans. Am J Phys Med Rehabil. 2021;100:34–38. doi: 10.1097/PHM.0000000000001614. [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Kumble S, Patel B, et al. Managing the rehabilitation wave: rehabilitation services for COVID-19 survivors. Arch Phys Med Rehabil. 2020;101:2243–2249. doi: 10.1016/j.apmr.2020.09.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldrini P, Bernetti A, Fiore P. Impact of COVID-19 outbreak on rehabilitation services and physical and rehabilitation medicine physicians’ activities in Italy. An official document of the Italian PRM Society (SIMFER) Eur J Phys Rehabil Med. 2020;56:316–318. doi: 10.23736/S1973-9087.20.06256-5. [DOI] [PubMed] [Google Scholar]
- 13.Kiekens C, Boldrini P, Andreoli A, et al. Rehabilitation and respiratory management in the acute and early post-acute phase. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur J Phys Rehabil Med. 2020;56:323–326. doi: 10.23736/S1973-9087.20.06305-4. [DOI] [PubMed] [Google Scholar]
- 14.Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99:470–474. doi: 10.1097/PHM.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herzlinger RE, Malik AM, Zogbi P. Prevent Senior: a new paradigm for growth in the health care sector? HBS Case. 2017:317–373. [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro TFG, Rstom RA, Barbosa PNVP, et al. Tomographic score (RAD-COVID score) to assess the clinical severity of infection with the novel coronavirus. Research Square; 2020. [DOI] [PMC free article] [PubMed]
- 18.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 19.Minosso JSM, Amendola F, Alvarenga MRM, MAdC Oliveira. Validation of the Barthel Index in elderly patients attended in outpatient clinics, in Brazil. Acta Paul Enferm. 2010;23:218–223. [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 21.Ambrose A. COVID-19 patient guide to at-home exercises. J Int Soc Phys Rehabil Med. 2020;3:32–34. [Google Scholar]
- 22.Gu SX, Tyagi T, Jain K, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto V, Bolanos JF, Fiallos J, et al. COVID-19: review of a 21st century pandemic from etiology to neuro-psychiatric implications. J Alzheimers Dis. 2020;77:459–504. doi: 10.3233/JAD-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 26.Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest. 2016;150:1129–1140. doi: 10.1016/j.chest.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M, Hajage D, Demoule A, et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farías-Antúnez S, Lima NP, Bierhals IO, Gomes AP, Vieira LS, Tomasi E. Disability related to basic and instrumental activities of daily living: a population-based study with elderly in Pelotas, Rio Grande do Sul, 2014. Epidemiol Serv Saúde. 2018:27. doi: 10.5123/S1679-49742018000200005. [DOI] [PubMed] [Google Scholar]
- 29.Sager MA, Dunham NC, Schwantes A, Mecum L, Halverson K, Harlowe D. Measurement of activities of daily living in hospitalized elderly: a comparison of self-report and performance-based methods. J Am Geriatr Soc. 1992;40:457–462. doi: 10.1111/j.1532-5415.1992.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 30.Muscogiuri G, Pugliese G, Barrea L, Savastano S, Colao A. Commentary: obesity: the "Achilles heel" for COVID-19? Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.