Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a newly identified and serious health condition associated with SARS-CoV-2 infection. Clinical manifestations vary widely among patients with MIS-C, and the aim of this study was to investigate factors associated with severe outcomes.
Methods
In this retrospective surveillance study, patients who met the US Centers for Disease Control and Prevention (CDC) case definition for MIS-C (younger than 21 years, fever, laboratory evidence of inflammation, admitted to hospital, multisystem [≥2] organ involvement [cardiac, renal, respiratory, haematological, gastrointestinal, dermatological, or neurological], no alternative plausible diagnosis, and either laboratory confirmation of SARS-CoV-2 infection by RT-PCR, serology, or antigen test, or known COVID-19 exposure within 4 weeks before symptom onset) were reported from state and local health departments to the CDC using standard case-report forms. Factors assessed for potential links to severe outcomes included pre-existing patient factors (sex, age, race or ethnicity, obesity, and MIS-C symptom onset date before June 1, 2020) and clinical findings (signs or symptoms and laboratory markers). Logistic regression models, adjusted for all pre-existing factors, were used to estimate odds ratios between potential explanatory factors and the following outcomes: intensive care unit (ICU) admission, shock, decreased cardiac function, myocarditis, and coronary artery abnormalities.
Findings
1080 patients met the CDC case definition for MIS-C and had symptom onset between March 11 and Oct 10, 2020. ICU admission was more likely in patients aged 6–12 years (adjusted odds ratio 1·9 [95% CI 1·4–2·6) and patients aged 13–20 years (2·6 [1·8–3·8]), compared with patients aged 0–5 years, and more likely in non-Hispanic Black patients, compared with non-Hispanic White patients (1·6 [1·0–2·4]). ICU admission was more likely for patients with shortness of breath (1·9 [1·2–2·9]), abdominal pain (1·7 [1·2–2·7]), and patients with increased concentrations of C-reactive protein, troponin, ferritin, D-dimer, brain natriuretic peptide (BNP), N-terminal pro B-type BNP, or interleukin-6, or reduced platelet or lymphocyte counts. We found similar associations for decreased cardiac function, shock, and myocarditis. Coronary artery abnormalities were more common in male patients (1·5 [1·1–2·1]) than in female patients and patients with mucocutaneous lesions (2·2 [1·3–3·5]) or conjunctival injection (2·3 [1·4–3·7]).
Interpretation
Identification of important demographic and clinical characteristics could aid in early recognition and prompt management of severe outcomes for patients with MIS-C.
Funding
None.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a rare but severe hyperinflammatory condition in children and adolescents that typically occurs 2–6 weeks after acute SARS-CoV-2 infection.1, 2 Also known as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), MIS-C was first described in Europe in April, 2020, and can affect multiple organ systems, including cardiac, gastrointestinal, haematological, dermatological, neurological, respiratory, and renal systems.
Hospital admission has been explicitly included as a requirement in MIS-C case definitions3, 4 or used as a criterion to identify patients with MIS-C.5, 6, 7 A wide range of clinical manifestations and outcomes have been identified. Most patients are discharged after hospital admission for MIS-C, but approximately 60% of patients are admitted to intensive care and approximately 2% die.8 Cardiovascular complications such as shock, myocarditis, decreased cardiac function, and coronary artery dilatation have been reported in 47–100% of children with MIS-C.9 However, not all patients have cardiac involvement and, even among those with cardiac involvement, some have milder cardiac disease than others.8, 10, 11, 12 Whether pre-existing factors or clinical features exist that might be associated with more severe outcomes from MIS-C is unclear.
Understanding factors associated with more severe outcomes of patients with MIS-C might inform prognosis and early treatment decisions. We aimed to identify patient factors—demographics, obesity, clinical features, and laboratory results—that might be associated with severe clinical outcomes of MIS-C.
Research in context.
Evidence before this study
Because multisystem inflammatory syndrome in children (MIS-C; alternately known as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 [PIMS-TS]) has only been identified since April, 2020, information on factors that are potentially linked to severe clinical outcomes has been scarce. We searched PubMed for publications between April 28 and Dec 12, 2020, using the following phrases or combinations of phrases: “multisystem inflammatory syndrome”; “Kawasaki disease”; “hyperinflammatory”; “hyperinflammation”; “toxic shock syndrome”; “shock” with “children” or “pediatric”; “cytokine” with “children” or “pediatric”; “inflammation” or “inflammatory” with “syndrome” and “children” or “pediatric”. We found many descriptive studies that contained demographic and clinical data on patients with MIS-C, including individual case studies, small case series, and some large cohorts of patients with MIS-C. While some cohort studies presented clinical outcomes stratified by specific factors (eg, age group, SARS-CoV-2 testing, Kawasaki disease criteria), no studies attempted multivariable analyses to identify independent risk factors for more severe outcomes.
Added value of this study
The US Centers for Disease Control and Prevention MIS surveillance database contains data on the largest MIS-C cohort described so far, providing substantial statistical power for detailed analyses. These data allowed us to assess associations between severe outcomes and many potential explanatory factors, controlling for relevant pre-existing patient factors. We found that children older than 5 years, boys, and non-Hispanic Black children were more likely to have severe outcomes. We also identified signs and symptoms (shortness of breath and abdominal pain) and increasingly abnormal laboratory markers as factors significantly associated with certain severe outcomes.
Implications of all the available evidence
These results could potentially aid in the clinical management of patients with MIS-C. For patients admitted to hospital with suspicion of MIS-C, those with specific patient factors described in this study could be identified as high risk for severe outcomes, providing impetus for rapid imaging studies and more aggressive treatment. These results also highlight the importance of specific laboratory tests for the assessment of severe outcomes in patients with MIS-C. Further research is needed to assess the efficacy of treatments for patients with MIS-C, particularly for those likely to be at highest risk of severe outcomes.
Methods
Study design and participants
The US Centers for Disease Control and Prevention (CDC) has been monitoring potential cases of MIS-C since May 14, 2020. State and local health departments reported cases to the CDC using the standard MIS-C case-report form. Abstraction varied by jurisdiction and might have been done by clinicians, hospital staff, or health department staff; in some cases, full medical records were sent to the CDC and abstracted by some of the study authors (MEO, SEG-C, and EDB). In this retrospective surveillance study, case reports were assessed to decide whether they met the CDC MIS-C case definition, which was (1) younger than 21 years, (2) fever, (3) laboratory evidence of inflammation, (4) hospital admission, (5) multisystem (≥2) organ involvement (cardiac, renal, respiratory, haematological, gastrointestinal, dermatological, or neurological), (6) no alternative plausible diagnosis, and (7) either laboratory confirmation of SARS-CoV-2 infection by RT-PCR, serology, or antigen test, or known COVID-19 exposure within 4 weeks before symptom onset.13 Information collected included patient demographics, obesity, clinical manifestations, complications, treatments, imaging studies, laboratory test results, and outcomes. Patients meeting the CDC MIS-C case definition who were reported to the CDC as of Oct 19, 2020, for whom information on clinical signs and symptoms, laboratory results, and intensive care unit (ICU) admission was available were included in the study.
Procedures
The potential explanatory variables that were investigated were (1) demographic characteristics (sex, age, and race or ethnicity); (2) obesity (either as obesity noted as present on the case-report form or above the 95th percentile of body-mass index by age and sex); (3) clinical signs and symptoms (cough, shortness of breath, abdominal pain, vomiting, diarrhoea, any rash, mucocutaneous lesions, and conjunctival injection); (4) peak laboratory values for fibrinogen, D-dimer, troponin, brain natriuretic peptide (BNP), N-terminal pro B-type BNP (proBNP), C-reactive protein, ferritin, and interleukin-6 (IL-6); and (5) lowest platelet and lymphocyte counts. The timing of most clinical variables, including laboratory measurements, was not available for analysis. Comorbidities other than obesity were not common enough for inclusion in the analyses. Because MIS-C case identification and treatment strategies changed over time, timing of MIS-C symptom onset (as a binary value for whether or not onset occurred before June 1, 2020, roughly the midpoint of symptom onset for included cases) was also assessed as a potential explanatory factor.
Outcomes
Reported data were analysed to investigate associations between severe outcomes and potential demographic or clinical factors. The severe outcomes of interest were admission to an ICU, decreased cardiac function, coronary artery abnormalities (aneurysm or dilatation), shock, and myocarditis. These outcomes were reported as present or not present on the case-report form, and no additional criteria were used to define outcomes. Death was not included as an outcome of interest because only few patients died.
Statistical analysis
Multivariable logistic regression was used to investigate associations between potential explanatory variables and outcomes in patients with MIS-C. Two separate study aims were assessed. The first aim was to identify which pre-existing factors might be linked to severe outcomes in patients with MIS-C. For this research question, all variables preceding MIS-C onset (demographics, obesity, and date of MIS-C onset) were selected. These variables were entered into regression models together, and adjusted odds ratios (aORs) and 95% CIs were calculated for associations with each outcome.
The second study aim was to investigate which clinical findings might be predictive of severe outcomes in patients with MIS-C. For this research question, variables assessed during clinical care (laboratory tests and clinical signs and symptoms) were entered into regression models. Similar to the first study aim, aORs and 95% CIs were calculated for each variable controlling for pre-existing factors (demographics, obesity, and date of MIS-C onset). Clinical signs and symptoms were analysed as binary variables (ie, presence or absence). Continuous laboratory marker values were log-transformed and standardised (converted to a distribution with a mean of 0 and SD of 1) to account for non-normal distributions and widely varying ranges. To aid in interpretation of associations between laboratory values and outcomes, aORs were generated over a range of observed values compared with a reference value (selected as roughly the upper limit of normal values for laboratory markers that are typically increased in patients with MIS-C, and roughly the lower limit of normal values for laboratory markers that are typically reduced in patients with MIS-C). For patients who might have presented with the severe outcomes of interest, assessment of clinical factors would be merely descriptive and could not be predictive of progression to severe disease; therefore, analyses assessing clinical findings were restricted to patients who were not admitted to the ICU on the same day as hospital admission.
All analyses were done using R (version 4.0.2). This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy.14
Role of the funding source
There was no funding source for this study.
Results
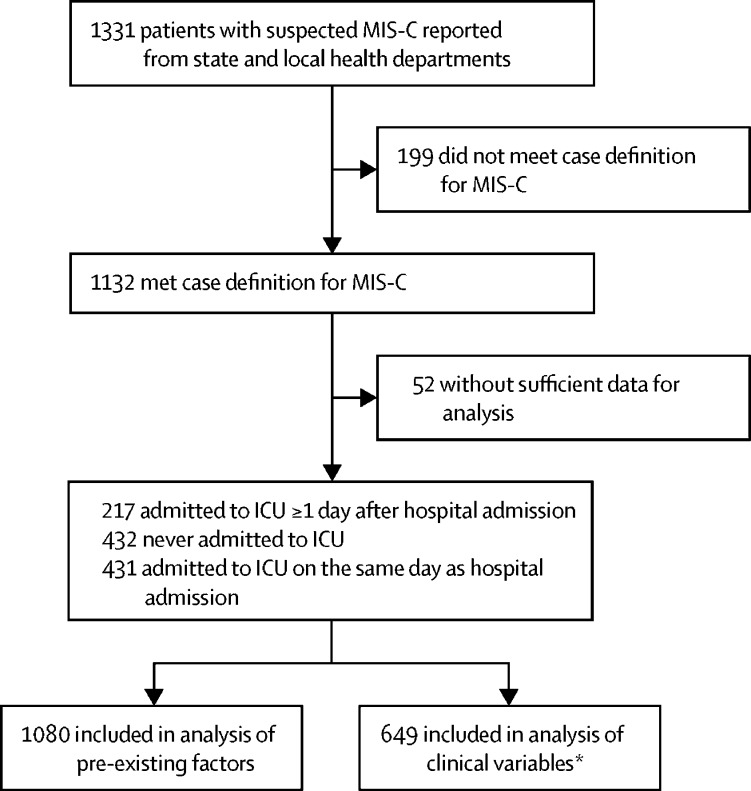
1331 patients with suspected MIS-C were reported from state and local health departments with dates of onset between March 11 and Oct 10, 2020. Of those, 1132 met the MIS-C case definition and 1080 (95%) of these patients had sufficient clinical data for analysis of pre-existing factors (figure 1 ).
Figure 1.
Study profile
MIS-C=multisystem inflammatory syndrome in children. ICU=intensive care unit. *Analysis of clinical variables excluded patients admitted to ICU on the same as day as hospital admission.
Of the 1080 patients included, 602 (56%) patients were male, the median age was 8 years (IQR 4–12; range 10 days to 21 years), 724 [77%] of 945 patients were either Hispanic or non-Hispanic Black, and 286 (26%) had obesity (table ). 648 (60%) patients were admitted to an ICU, of whom 431 (67%) were admitted to the ICU on the same day as hospital admission and 217 (33%) were admitted to the ICU on a date after admission to the hospital. For those 217 patients, the median time between hospital admission and ICU admission was 4 days (IQR 2–5). 303 patients (28%) had decreased cardiac function, 185 (17%) had a coronary artery abnormality, 392 (36%) had shock, 192 (18%) had myocarditis, and 18 (2%) died.
Table.
Patient demographic and clinical characteristics
| All patients (n=1080) |
Patients admitted to ICU |
Patients never admitted to ICU (n=432) | ||
|---|---|---|---|---|
| Admitted to ICU on same day as hospitalisation (n=431) | Admitted to ICU ≥1 day after hospitalisation (n=217) | |||
| Sex (n=1078) | ||||
| Female | 476 (44%) | 194 (45%) | 81 (37%) | 201 (47%) |
| Male | 602 (56%) | 236 (55%) | 136 (63%) | 230 (53%) |
| Age, years (n=1078) | ||||
| 0–5 | 373 (35%) | 123 (29%) | 52 (24%) | 198 (46%) |
| 6–12 | 456 (42%) | 185 (43%) | 113 (52%) | 158 (37%) |
| 13–20 | 249 (23%) | 123 (29%) | 52 (24%) | 74 (17%) |
| Race or ethnicity (n=945) | ||||
| Non-Hispanic White | 133 (14%) | 49 (13%) | 26 (14%) | 58 (15%) |
| Non-Hispanic Black | 339 (36%) | 154 (40%) | 67 (37%) | 118 (31%) |
| Hispanic | 385 (41%) | 148 (39%) | 73 (40%) | 164 (43%) |
| Other race or ethnicity | 88 (9%) | 30 (8%) | 15 (8%) | 43 (11%) |
| Comorbidities | ||||
| Obesity | 286 (26%) | 119 (28%) | 70 (32%) | 97 (22%) |
| Date of MIS-C onset | ||||
| Onset before June 1, 2020 | 439 (41%) | 179 (42%) | 106 (49%) | 154 (36%) |
| Clinical signs and symptoms | ||||
| Cough | 322 (30%) | 134 (31%) | 58 (27%) | 130 (30%) |
| Shortness of breath | 287 (27%) | 146 (34%) | 67 (31%) | 74 (17%) |
| Abdominal pain | 693 (64%) | 285 (66%) | 162 (75%) | 246 (57%) |
| Vomiting | 684 (63%) | 284 (66%) | 139 (64%) | 261 (60%) |
| Diarrhoea | 573 (53%) | 241 (56%) | 108 (50%) | 224 (52%) |
| Rash | 584 (54%) | 229 (53%) | 117 (54%) | 238 (55%) |
| Mucocutaneous lesions | 313 (29%) | 116 (27%) | 61 (28%) | 136 (31%) |
| Conjunctival injection | 534 (49%) | 225 (52%) | 111 (51%) | 198 (46%) |
| Laboratory values | ||||
| Fibrinogen, mg/dL (n=721) | 544 (438–662) | 577 (466–695) | 547 (434–660) | 510 (419–631) |
| D-dimer, mg/L (n=747) | 2·4 (1·2–4·4) | 2·9 (1·6–5·5) | 3 (1·5–5) | 1·5 (0·8–3·3) |
| Troponin, ng/mL (n=793) | 0·05 (0·01–0·27) | 0·11 (0·03–0·52) | 0·09 (0·02–0·34) | 0·01 (0·01–0·08) |
| BNP, pg/mL (n=381) | 566 (146–1364) | 907 (368–1933) | 795 (345–1677) | 195 (48–694) |
| proBNP, ng/L (n=371) | 2054 (310–7778) | 4796 (1164–16 324) | 3341 (439–7320) | 558 (212–2605) |
| C-reactive protein, mg/dL (n=870) | 18 (10–26) | 22 (15–29) | 21 (14–28) | 13 (6–21) |
| Ferritin, ng/mL (n=790) | 463 (225–911) | 626 (350–1171) | 628 (333–1146) | 289 (156–529) |
| Interleukin-6, pg/mL (n=221) | 67 (17–208) | 81 (23–262) | 101 (26–322) | 43 (9–128) |
| Platelets, ×103 cells per μL (n=622) | 136 (94–211) | 121 (82–167) | 120 (83–184) | 172 (112–268) |
| Lymphocytes, cells per μL (n=501) | 950 (504–1945) | 858 (400–1600) | 705 (428–1308) | 1300 (790–2715) |
| Outcomes | ||||
| Admitted to ICU | 648 (60%) | 431 (100%) | 217 (100%) | 0 (0%) |
| Decreased cardiac function | 303 (28%) | 177 (41%) | 84 (39%) | 42 (10%) |
| Shock | 392 (36%) | 271 (63%) | 100 (46%) | 21 (5%) |
| Myocarditis | 192 (18%) | 108 (25%) | 53 (24%) | 31 (7%) |
| Coronary artery abnormality | 185 (17%) | 81 (19%) | 38 (18%) | 66 (15%) |
| Death | 18 (2%) | 10 (2%) | 4 (2%) | 4 (1%) |
Data are n (%) or median (IQR). Denominators for sex, age, race, and laboratory values represent the number with reported values; all other percentages are based on all 1080 patients. BNP=brain natriuretic peptide. ICU=intensive care unit. MIS-C=multisystem inflammatory syndrome in children. proBNP=N-terminal pro B-type BNP.
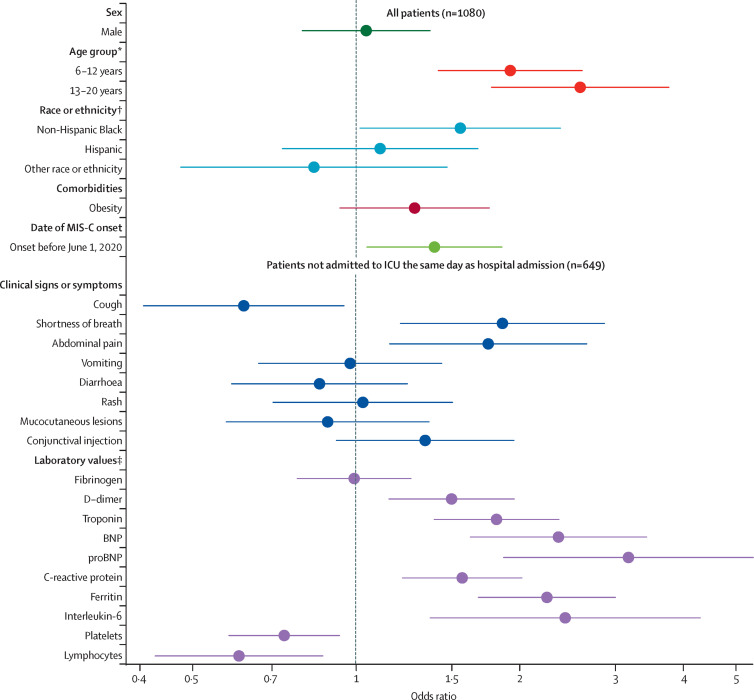
Figure 2 shows aORs for associations between potential explanatory factors and ICU admission. Compared with MIS-C patients aged 0–5 years, ICU admission was more likely in patients aged 6–12 years (aOR 1·9 [95% CI 1·4–2·6]) and patients aged 13–20 years (2·6 [1·8–3·8]). Non-Hispanic Black patients were more likely to be admitted to an ICU compared with non-Hispanic White patients (1·6 [1·0–2·4]), as well as patients with a date of MIS-C onset before June 1, compared with those with onset after June 1 (1·4 [1·0–1·9]). Odds of ICU admission were greater in patients with shortness of breath (1·9 [1·2–2·9]) and abdominal pain (1·7 [1·2–2·7]), but lower in patients with cough (0·6 [0·4–0·9]). ICU admission was associated with increased D-dimer, troponin, BNP, proBNP, C-reactive protein, ferritin, and IL-6 concentrations and decreased counts of platelets and lymphocytes.
Figure 2.
Associations between potential explanatory factors and ICU admission
Odds ratios were adjusted for sex, age group, race and ethnicity, obesity, and date of MIS-C onset. All potential explanatory factors were binary (yes or no) except for laboratory values, which were continuous (log-transformed and standardised). Elucidation of those odds ratios is shown in figure 4. BNP=brain natriuretic peptide. ICU=intensive care unit. MIS-C=multisystem inflammatory syndrome in children. proBNP=N-terminal pro B-type BNP. *Reference 0–5 years. †Reference Non-Hispanic White. ‡For laboratory values, odds ratios above 1 indicate that higher values increase the odds of the outcome and odds ratios below 1 indicate that lower values increase the odds of the outcome.
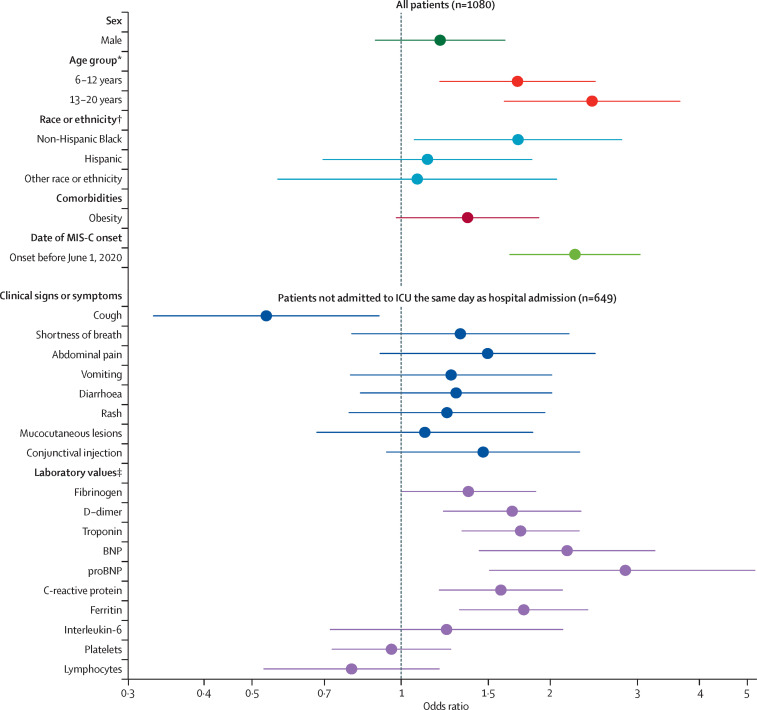
Associations between potential explanatory factors and decreased cardiac function (figure 3 ), shock (appendix p 1), and myocarditis (appendix p 2) were broadly similar to associations for ICU admission. Older age generally increased odds of these outcomes. Compared with patients aged 0–5 years, patients aged 6–12 years had higher odds of shock (aOR 1·7 [1·2–2·3]; appendix p 1) and decreased cardiac function (1·7 [1·2–2·5]; figure 3), and patients aged 13–20 years had substantially higher odds of shock (2·5 [1·8–3·7]; appendix p 1), decreased cardiac function (2·4 [1·6–3·7]; figure 3), and myocarditis (2·6 [1·6–4·2]; appendix p 2). Onset before June 1, 2020, was associated with decreased cardiac function (2·2 [1·7–3·0]) and myocarditis (2·2 [1·6–3·2; figure 3; appendix p 2). Decreased cardiac function was more common among non-Hispanic Black children compared with non-Hispanic White children (1·7 [1·1–2·8]; figure 3). Obesity was linked to decreased cardiac function (1·4 [1·0–1·9]; figure 3). Patients with shock were more likely to have reported abdominal pain and shortness of breath but less likely to have reported cough (appendix p 1). Increased D-dimer and ferritin concentrations were associated with shock and decreased cardiac function (appendix p 1; figure 3), and increased IL-6 concentrations were associated with shock (figure 3; appendix p 1). Decreased counts of lymphocytes and platelets were associated with shock (appendix p 1). Increased concentrations of troponin, BNP, proBNP, and C-reactive protein were associated with shock, decreased function, and myocarditis (figure 3; appendix pp 1–2). Low to moderate correlations were observed between these outcomes: Pearson's correlation coefficient was 0·37 for myocarditis and decreased cardiac function, 0·33 for decreased cardiac function and shock, and 0·19 for shock and myocarditis (p<0·0001 for all). Only 70 [9%] of 770 patients without decreased cardiac function had myocarditis, and 122 [40%] of 303 patients with decreased cardiac function had myocarditis.
Figure 3.
Associations between potential explanatory factors and having decreased cardiac function
Odds ratios were adjusted for sex, age group, race or ethnicity, obesity, and date of MIS-C onset. All potential explanatory factors were binary (yes or no) except for laboratory values, which were continuous (log-transformed and standardised). Elucidation of those odds ratios is shown in figure 3. BNP=brain natriuretic peptide. proBNP=N-terminal pro b-type BNP. ICU=intensive care unit. MIS-C=multisystem inflammatory syndrome in children. *Reference 0–5 years. †Reference Non-Hispanic White. ‡For laboratory values, odds ratios above 1 indicate that higher values increase the odds of the outcome and odds ratios below 1 indicate that lower values increase the odds of the outcome.
Associations between potential explanatory factors and coronary artery abnormalities are shown in the appendix (p 3). Male sex was associated with coronary artery abnormalities (aOR 1·5 [95% CI 1·1–2·1]; appendix p 3). Patients with mucocutaneous lesions (2·2 [1·3–3·5]) and conjunctival injection (2·3 [1·4–3·7]) were more likely to have coronary artery abnormalities compared with patients without these symptoms (appendix p 3). The only laboratory markers associated with coronary artery abnormalities were proBNP and IL-6 (appendix p 3). Patients with coronary artery abnormalities who had available Z scores (n=38 [26%]), had a median Z score of 3·4 (IQR 2·8–4·9). Echocardiograms were not done for 112 (10%) patients.
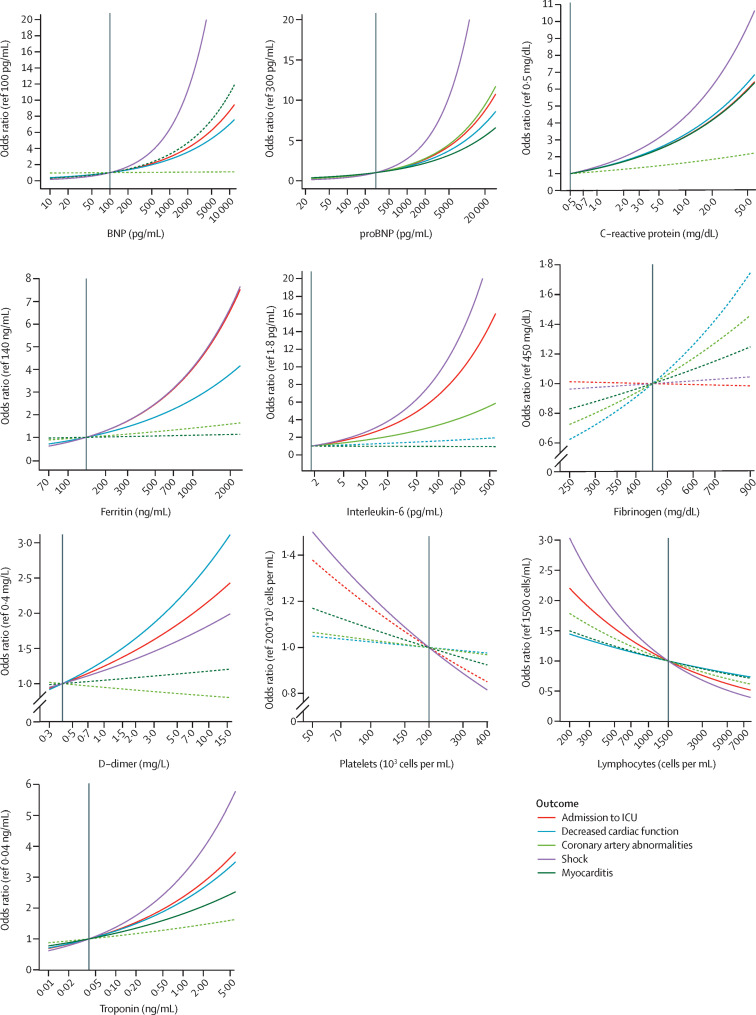
Figure 4 shows aORs over different measured values of laboratory markers for each outcome. For most laboratory markers of inflammation and cardiac damage, increasing laboratory values were associated with increasing odds of ICU admission, shock, or decreased cardiac function (figure 4). For example, compared with a patient with a troponin value of 0·04 ng/mL (the reference), a patient with an increased troponin value of 1·0 ng/mL would have 3 times the odds of shock, and a patient with a troponin value of 5·0 ng/mL would have 5·5 times the odds of shock (figure 4). Conversely, lower values of platelets and lymphocytes were associated with shock and ICU admission (figure 4). Troponin, BNP, proBNP, C-reactive protein, ferritin, IL-6, platelet, and lymphocyte concentrations were all more strongly associated with shock than with any other outcome (figure 4).
Figure 4.
Estimated adjusted odds ratios for outcomes of interest over different values of laboratory markers compared with a reference value
Odds ratios were calculated from logistic regression using data from patients not admitted to an ICU on the same day as hospital admission, controlling for sex, age group, race or ethnicity, obesity, and date of MIS-C onset. Solid lines represent odds ratios that significantly differed from the null value of 1, and dashed lines were not statistically significant. Vertical black lines are reference values. BNP=brain natriuretic peptide. ICU=intensive care unit. MIS-C=multisystem inflammatory syndrome in children. proBNP=N-terminal pro B-type BNP.
Discussion
We identified key demographic and clinical characteristics that were associated with severe outcomes among patients with MIS-C. The CDC MIS-C database is a large national database comprised of patients reported from state and local health departments that allows for rigorous statistical investigation of many potential explanatory factors and outcomes. Age older than 5 years was strongly associated with more severe outcomes, and non-Hispanic Black children also seem to be more affected than non-Hispanic White children. Patients reporting shortness of breath or abdominal pain were more likely to be admitted to an ICU, compared with those without these symptoms. Consistent with patients with non-SARS-CoV-2-related sepsis, increased concentrations of troponin, BNP, and proBNP were strongly indicative of ICU admission, shock, and decreased cardiac function,15, 16 and high values for D-dimer, C-reactive protein, and ferritin also increased the odds of these outcomes. Reduced counts of platelets and lymphocytes were associated with ICU admission and shock.
These results might have important clinical utility in identifying which patients presenting to the emergency department or admitted to the general ward are likely to have severe outcomes during their admission. Taking repeated laboratory measurements during the course of illness might be useful in identifying patients at risk and is especially true among children older than 5 years. However, whether earlier identification and, in turn, earlier treatment, of patients at higher risk of severe outcomes leads to better outcomes is unknown. Further investigation using temporal associations between laboratory markers and outcomes might help to clarify the predictive potential of the markers.
Notably, not many factors were associated with the development of coronary artery abnormalities; proBNP and IL-6 were the only laboratory values that were associated with this outcome. Unlike for other outcomes, those aged 13–20 years had lower (albeit non-significantly) odds of coronary artery abnormalities. Mucocutaneous lesions and conjunctival injections were also associated with coronary artery abnormalities. This clinical picture is suggestive of Kawasaki disease. Given the overlapping clinical features of MIS-C and Kawasaki disease and the absence of a diagnostic test for either condition, some patients captured by MIS-C surveillance might have had Kawasaki disease.9 Predictive models exist for risk of coronary artery abnormalities among patients with Kawasaki disease,17, 18 and further analyses could explore the effectiveness of these models for patients with MIS-C. Similarly, some patients with acute COVID-19 might have met the case definition for MIS-C and have been included in our cohort. Patients in our study with reported cough generally had lower odds for the outcome assessed, and some of these patients might have had acute COVID-19, which would be less likely to progress to the severe outcomes investigated in this study.
The 112 patients who did not receive echocardiograms were not excluded from analyses assessing associations with decreased cardiac function and coronary artery abnormalities. Although inclusion of these patients might have led to some missed diagnoses, the greater concern was that these patients represented those for whom cardiac imaging was not deemed necessary and their exclusion would lead to loss of information on milder MIS-C cases. Assessment of patients without echocardiograms supported this conclusion because these patients had substantially lower concentrations of troponin, BNP, and proBNP; shorter duration of hospitalisation; and were less likely to be admitted to an ICU, compared with patients with echocardiograms.
Similar to studies showing more severe outcomes in Black children with COVID-19,19 this study showed higher odds for ICU admission and decreased cardiac function among non-Hispanic Black patients compared with non-Hispanic White patients. Although the exact cause of these disparities might be unknown, factors such as insufficient access to health care, increased prevalence of underlying medical conditions, and increased exposure to environmental pollutants can affect a wide range of health risks and outcomes.20 Addressing social determinants of health that disproportionately affect certain racial and ethnic groups, while improving access to health care, is crucial to reducing the additional burden of MIS-C.
The MIS-C case-report form did not include information on temporality for most clinical findings and laboratory tests; therefore, whether some potential explanatory variables preceded or occurred during or after the development of outcomes of interest was not clear. Thus, we cannot rule out the possibility that some laboratory findings might be markers, not predictors, of severe outcomes. We limited the analyses to patients with MIS-C who were not admitted to the ICU on the same day as hospitalisation, in order to assess patients for whom clinical findings were described before the development of severe outcomes. Signs and symptoms selected for this analysis were those typically known to be noted at presentation, such that they would probably have preceded the severe outcomes of interest. However, without explicit information on the date of most clinical findings, patient clinical course was not definitively known.
This study has several other limitations. Potential differences between health-care sites and clinician practices in a number of areas and over the course of the COVID-19 pandemic (eg, treatment protocols, criteria for ICU admission, laboratory testing methodology, type of troponin tested, diagnosis of clinical findings, and reporting of suspected MIS-C cases) could have affected findings. In particular, we did not prespecify definitions of the outcomes on the case-report forms, which might have led to variations in the identification and reporting of these outcomes. The types of people abstracting medical records (eg, clinicians, hospital staff, health department staff) might have been different across health jurisdictions. Certain potential explanatory factors, including other comorbidities, were not included because of small numbers. Similarly, explanatory factors for mortality were not assessed. Some mild symptoms might possibly not have been consistently reported in patients with more severe clinical course. Although the MIS-C case-report form included treatment information, the observational nature of the study meant that associations between treatments and clinical outcomes might have been more of an indicator of disease severity as opposed to a reliable measure of treatment effectiveness.
In conclusion, clinical manifestations vary among children with MIS-C. Age older than 5 years and certain laboratory markers—particularly concentrations of troponin, BNP, proBNP, ferritin, C-reactive protein, and D-dimer—can be helpful in identifying children who might have an increased risk of severe disease outcomes, including ICU admission, shock, and decreased cardiac function. By identifying characteristics of patients who are likely to develop severe outcomes, these findings could help to inform the management of children admitted to hospital with MIS-C.
Data sharing
Patient data are not available to be shared publicly. Please contact Joseph Abrams (jabrams@cdc.gov) for questions about study protocols. Basic summary data (overall numbers as well as age, sex, race, and geographical distribution) are available at https://www.cdc.gov/mis-c/cases/index.html.
Acknowledgments
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Contributors
EDB, JYA, MEO, and SEG-C conceived of and designed the study. JYA conducted all statistical analyses and produced figures and tables, and JYA and MEO co-wrote the initial draft. SEG-C, BB, JWL, CAT, TJP, and JLK helped to procure and clean the data. All authors had access to the data, and JYA, JWL, CAT, and JLK have accessed and verified the data underlying the study. All authors edited the manuscript, provided feedback on the study, and approved the final manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Feldstein LR, Rose EB, Horwitz SM. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufort EM, Koumans EH, Chow EJ. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2020 health advisory #16: updated reporting requirements for multisystem inflammatory syndrome in children associated with covid-19 (formerly pediatric multisystem inflammatory syndrome). New York City Department of Health. June 3, 2020. https://www1.nyc.gov/assets/doh/downloads/pdf/han/advisory/2020/covid-19-providers-mis-c.pdf
- 4.New York State Department of Health Health advisory: pediatric multi-system inflammatory syndrome potentially associated with coronavirus disease (COVID-19) in children. June 5, 2020. https://www.health.ny.gov/press/releases/2020/docs/2020-05-06_covid19_pediatric_inflammatory_syndrome.pdf
- 5.Toubiana J, Poirault C, Corsia A. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L, Mazza A, Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker E, Bamford A, Kenny J. Clinical Characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfred-Cato S, Bryant B, Leung J. COVID-19-associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams JY, Godfred-Cato SE, Oster ME. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45–54. doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belhadjer Z, Meot M, Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 11.Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2020;180:307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blondiaux E, Parisot P, Redheuil A. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020 doi: 10.1148/radiol.2020202288. published online June 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention Health Alert Network; May 14, 2020. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19)https://emergency.cdc.gov/han/2020/han00432.asp [Google Scholar]
- 14.Goñi F, Mathiason CK, Yim L. Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease. Vaccine. 2015;33:726–733. doi: 10.1016/j.vaccine.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klouche K, Pommet S, Amigues L. Plasma brain natriuretic peptide and troponin levels in severe sepsis and septic shock: relationships with systolic myocardial dysfunction and intensive care unit mortality. J Intensive Care Med. 2014;29:229–237. doi: 10.1177/0885066612471621. [DOI] [PubMed] [Google Scholar]
- 16.Scheer C, Fuchs C, Rehberg S. Biomarkers in severe sepsis and septic shock: just listen to the heart? Crit Care Med. 2016;44:849–850. doi: 10.1097/CCM.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 17.Nakano H, Ueda K, Saito A. Scoring method for identifying patients with Kawasaki disease at high risk of coronary artery aneurysms. Am J Cardiol. 1986;58:739–742. doi: 10.1016/0002-9149(86)90348-6. [DOI] [PubMed] [Google Scholar]
- 18.Honkanen VE, McCrindle BW, Laxer RM, Feldman BM, Schneider R, Silverman ED. Clinical relevance of the risk factors for coronary artery inflammation in Kawasaki disease. Pediatr Cardiol. 2003;24:122–126. doi: 10.1007/s00246-002-0063-1. [DOI] [PubMed] [Google Scholar]
- 19.Swann OV, Holden KA, Turtle L. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Centers for Disease Control and Prevention . US Centers for Disease Control and Prevention; 2020. Social determinants of health: know what affects health.https://www.cdc.gov/socialdeterminants [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient data are not available to be shared publicly. Please contact Joseph Abrams (jabrams@cdc.gov) for questions about study protocols. Basic summary data (overall numbers as well as age, sex, race, and geographical distribution) are available at https://www.cdc.gov/mis-c/cases/index.html.