Abstract
A new species of Cyrtodactylus is described on the basis of five specimens collected from the karst formations of Zhenkang County, Yunnan Province, China. Cyrtodactylus zhenkangensissp. nov. is recognized by having a unique combination of morphological characters, the most diagnostic being: 12–15 enlarged femoral scales on each thigh; 2–5 femoral pores on each thigh in males, 0–3 pitted scales on each thigh in females; eight or nine precloacal pores in a continuous row or separated by one poreless scale in males, 7–9 pitted scales in females; subcaudals enlarged, arranged alternately as single and double on anterior and mostly single at middle and posterior; dorsal surface of head with obvious reticulations. Phylogenetic analyses show that the new species is a member of the C. wayakonei species group and a sister taxon to a clade consisting of C. wayakonei and C. martini based on Maximum Likelihood analyses and Bayesian Inference and differs from its congeners by at least 12.0% genetic divergence in a fragment of the COI gene.
Keywords: Bent-toed gecko, Cyrtodactylus wayakonei, karst-dwelling, taxonomy, Zhenkang
Introduction
Bent-toed geckos of the genus Cyrtodactylus are one of the most species-diverse genera of gekkonid lizards (Kluge 2001; Uetz 2020), and many of these species are thought to be highly localized with extremely narrow geographic ranges (Nazarov et al. 2012; Luu et al. 2016; Grismer et al. 2018, 2020; Murdoch et al. 2019). At present, the genus contains more than 300 recognized species (Uetz et al. 2020), and approximately 150 new species have been described since 2010 and most of these new discoveries were from Southeast Asia (Schneider et al. 2020).
During our recent fieldwork in Yunnan Province, China, a series of bent-toed geckos was collected from the karst formations of Zhenkang County. Morphological and molecular phylogenetic analyses revealed that the new collection belonged to an unnamed species of Cyrtodactylus. We describe it as a new species.
Materials and methods
Sampling
Fieldwork was conducted at night. Specimens were collected by hand. Photographs were taken to document color pattern in life prior to euthanization. Liver tissues were stored in 99% ethanol and specimens were preserved in 75% ethanol. Specimens were deposited at Kunming Natural History Museum of Zoology, Kunming Institute of Zoology, Chinese Academy of Sciences (KIZ).
Molecular analyses
Molecular data were generated for three specimens and analyzed with the available homologous sequences of the Cyrtodactylus wayakonei species group obtained from GenBank. The new sequences were deposited in GenBank under accession numbers MW593136–MW593138. Sequences of C. cf. interdigitalis Ulber, 1993 and C. elok Dring, 1979 were used as outgroups according to Nguyen et al. (2017) and Schneider et al. (2020).
We used the protocols of Le et al. (2006) for DNA extraction, amplification, and sequencing. DNA extraction used the standard three-step phenol/trichloromethane protocol (Sambrook et al. 1989). A fragment of the mitochondrial gene, cytochrome c oxidase subunit 1 (COI) was amplified in a volume consisted of 25 μl (10 μl of mastermix, 5 μl of water, 2 μl of each primer at 10 pmol/μl and 6 μl of DNA) by the polymerase chain reaction (PCR; 35 cycles of 95 °C for 30 s, 53 °C for 40 s, 72 °C for 90 s) and sequenced using the primer pair VF1-d (TTCTCAACCAACCACAARGAYATYGG) and VR1-d (TAGACTTCTGGGTGGCCRAARAAYCA) (Ivanova et al. 2006). PCR products were cleaned using ExoSAP-IT (Applied Biosystems) and sequenced in both directions by direct double strand cycle sequencing using the BigDye Terminator v. 3.1 Cycle Sequencing Kit on a 3130 DNA Analyzer (Applied Biosystems). Sequences were edited with Sequencher v. 5.4.6 (Gene Codes).
Sequences were aligned using ClustalW (Thompson et al. 1994) integrated in MEGA v. 7 (Kumar et al. 2016) with default parameters. Pairwise distances between species were calculated in MEGA v. 7 with the parameters Transitions + Transversions, Uniform rates, and Pairwise deletion (Kumar et al. 2016). The substitution model GTR+G+I was selected using the corrected Akaike Information Criterion (AICc) in MODELTEST v. 3.7 (Posada and Crandall 1998). Bayesian inference (BI) was performed in MrBayes v. 3.2.6 (Ronquist et al. 2012) based on the selected substitution model. Two runs were performed simultaneously with four Markov chains starting from random tree. The chains were run for 10,000,000 generations and sampled every 1000 generations. The first 25% of the sampled trees was discarded as burn-in after the standard deviation of split frequencies of the two runs reached a value of less than 0.01, and then the remaining trees were used to create a 50% majority-rule consensus tree and to estimate Bayesian posterior probabilities (BPP). Nodes with BPP of 95 and above were considered strongly supported (Huelsenbeck et al. 2001; Wilcox et al. 2002; Alfaro et al. 2003) and nodes with values of 90–94 as well supported (Chomdej et al. 2020). Maximum Likelihood (ML) analysis was performed in RaxmlGUI v. 1.5 (Silvestro and Michalak 2012), and nodal support was estimated by 1,000 rapid bootstrap replicates. Nodes with bootstrap values of 70 and above were considered significantly supported (Alfaro et al. 2003; Sitnikova 1996).
Morphological analyses
Measurements were taken with digital calipers to the nearest 0.1 mm. Bilateral scale counts were given as left/right. The methodology of measurements and meristic counts followed Ngo (2011) and Schneider et al. (2020):
AG axilla to groin distance;
DTR dorsal tubercle rows, number of dorsal, longitudinal rows of tubercles at midbody between the ventrolateral folds;
ED ear diameter, greatest diameter of ear;
EE eye orbit to ear distance, from posterior corner of eye orbit to anterior margin of ear opening;
EFS enlarged femoral scales, number of enlarged femoral scale beneath each thigh;
ForeaL forearm length, from the base of the palm to the elbow;
FP femoral pores;
GSDT granular scales surrounding dorsal midbody tubercles;
HH maximum head height, from occiput to underside of jaws;
HL head length, from tip of snout to posterior margin of ear;
HW maximum head width;
I postrostrals or internasals;
IFL infralabials;
IND internarial distance, measured between inner borders of nostrils;
IOD interorbital distance, measured across narrowest point of frontal bone;
LD4 subdigital lamellae under the fourth finger;
LT4 subdigital lamellae under the fourth toe;
ML mental length;
MW mental width;
OD greatest diameter of orbit;
PAT postcloacal tubercles, number of tubercles on each side of postcloacal region;
PM postmentals, i.e. scales bordering mental shield, except infralabials;
PP precloacal pores;
PVT paravertebral tubercles, counted in a single paravertebral row from the level of the forelimb insertions to the level of the hind limb insertion;
RH rostral heigth;
RW rostral width;
SC5SPL scale rows between fifth supralabials;
SE snout to eye distance, from tip of snout to anterior corner of eye orbit;
SL shank length, from the base of heel to the knee;
SPL supralabials;
SVL snout-vent length, from tip of snout to anterior margin of cloaca;
TaL tail length, from posterior margin of cloaca to tip of tail;
V longitudinal ventral scale rows, counted across the belly between the ventrolateral folds at midbody.
Morphological comparisons and analyses were based on specimen examination and data obtained from the literature (Hoang et al. 2007; Rösler et al. 2008; Bauer et al. 2009, 2010; Ngo and Grismer 2010; Nguyen et al. 2010, 2015, 2017; Sumontha et al. 2010; Teyníe and David 2010; Luu et al. 2011, 2013, 2016; Ngo 2011; Ngo and Chan 2011; Schneider et al. 2011, 2014, 2020; Kunya et al. 2014; Nazarov et al. 2014, 2018; Nguyen et al. 2014; Le 2016; Pham et al. 2019).
Results
Molecular analyses
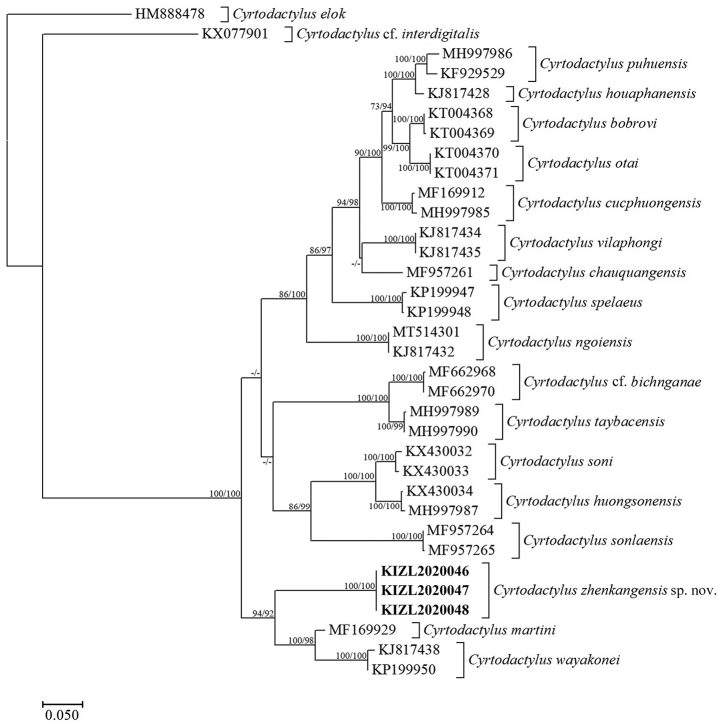
The obtained sequence alignment is 690 bp in length. The topologies derived from ML and BI analyses were similar and basically consistent with those of Nguyen et al. (2017), Pham et al. (2019), and Schneider et al. (2020). The sequences of three specimens collected from Zhenkang County, Yunnan, China were nested them within the Cyrtodactylus wayakonei group and the sister group to a clade consisting of C. wayakonei Nguyen, Kingsada, Rösler, Auer & Ziegler, 2010 and C. martini Ngo, 2011 with strong support in ML and moderate support in BI (Fig. 1). The interspecific uncorrected genetic p-distances between the newly collected specimens and other members of C. wayakonei group ranged from 12.0% to 17.8% (Table 1).
Figure 1.
Bayesian Inference phylogram inferred from partial COI genes. Numbers before slashes indicate bootstrap support for Maximum Likelihood analyses and numbers after slashes indicate Bayesian posterior probabilities. The symbol “–” represents the value below 60.
Table 1.
Mean uncorrected pairwise genetic distances (%) based on 690 base pairs of COI gene sequences.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cyrtodactylus bichnganae | ||||||||||||||||||
| 2 | C. bobrovi | 17.2 | |||||||||||||||||
| 3 | C. chauquangensis | 15.3 | 9.3 | ||||||||||||||||
| 4 | C. cucphuongensis | 16.6 | 6.9 | 8.0 | |||||||||||||||
| 5 | C. houaphanensis | 17.3 | 6.2 | 8.8 | 7.2 | ||||||||||||||
| 6 | C. huongsonensis | 14.7 | 15.8 | 14.5 | 14.9 | 16.0 | |||||||||||||
| 7 | C. martini | 15.6 | 15.1 | 13.7 | 14.1 | 15.6 | 15.0 | ||||||||||||
| 8 | C. ngoiensis | 15.9 | 12.3 | 11.6 | 13.8 | 13.1 | 14.1 | 14.7 | |||||||||||
| 9 | C. otai | 16.7 | 3.8 | 9.3 | 7.2 | 6.2 | 15.4 | 15.9 | 13.1 | ||||||||||
| 10 | C. puhuensis | 18.8 | 7.2 | 10.3 | 8.2 | 3.4 | 17.7 | 16.1 | 15.0 | 7.3 | |||||||||
| 11 | C. soni | 13.6 | 16.7 | 14.5 | 15.8 | 16.4 | 5.3 | 15.4 | 15.0 | 16.3 | 18.7 | ||||||||
| 12 | C. sonlaensis | 15.4 | 16.3 | 16.8 | 16.7 | 17.7 | 13.4 | 14.6 | 15.4 | 17.3 | 18.8 | 14.0 | |||||||
| 13 | C. spelaeus | 16.9 | 10.0 | 11.7 | 12.1 | 10.9 | 16.7 | 15.0 | 13.5 | 11.2 | 11.8 | 15.8 | 15.7 | ||||||
| 14 | C. taybacensis | 5.2 | 16.1 | 14.4 | 15.4 | 16.2 | 15.1 | 14.7 | 15.6 | 16.4 | 17.5 | 14.4 | 16.1 | 15.3 | |||||
| 15 | C. vilaphongi | 16.4 | 9.3 | 8.2 | 9.5 | 8.2 | 15.3 | 14.7 | 12.9 | 9.4 | 10.1 | 15.9 | 17.1 | 11.8 | 15.9 | ||||
| 16 | C. wayakonei | 15.2 | 16.7 | 15.2 | 16.5 | 17.4 | 16.7 | 6.5 | 15.5 | 18.3 | 18.1 | 17.5 | 15.9 | 16.2 | 15.6 | 16.0 | |||
| 17 | Cyrtodactylus zhenkangensis sp. nov. | 17.8 | 15.0 | 14.9 | 15.8 | 16.2 | 16.7 | 12.0 | 14.3 | 16.1 | 16.8 | 17.1 | 17.1 | 15.7 | 16.8 | 16.4 | 13.1 | ||
| 18 | C. interdigitalis | 18.5 | 19.9 | 19.2 | 19.3 | 20.2 | 20.2 | 18.2 | 20.1 | 20.1 | 21.9 | 19.9 | 20.5 | 20.4 | 18.3 | 19.3 | 18.3 | 19.9 | |
| 19 | C. elok | 18.8 | 19.4 | 18.9 | 17.9 | 19.5 | 19.6 | 17.1 | 19.5 | 19.6 | 20.2 | 20.2 | 19.7 | 18.6 | 18.6 | 19.2 | 18.6 | 19.5 | 15.8 |
Taxonomic accounts
Cyrtodactylus zhenkangensis sp. nov.
66B54C01-4996-559C-81B8-86F13C2AF3B1
http://zoobank.org/1CAE09BE-E522-42EF-AD5A-0E3B7A694CDB
Figure 2.
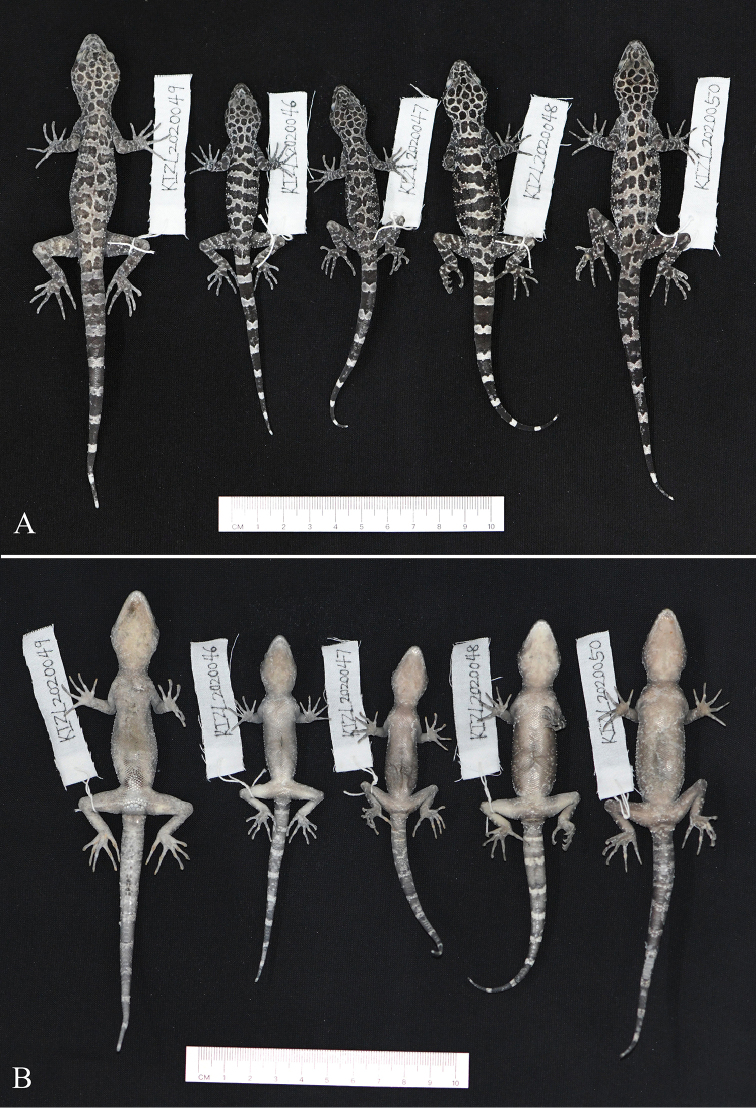
Type series of Cyrtodactylus zhenkangensis sp. nov. in preservative A dorsal view B ventral view.
Figure 3.
Close-up views of the holotype (KIZL20200049) of Cyrtodactylus zhenkangensis sp. nov. in preservative A dorsal view of head B ventral view of head C right side femoral region D precloacal region E left side femoral region F subcaudal scales.
Figure 4.
The holotype (KIZL20200049) of Cyrtodactylus zhenkangensis sp. nov. in life A dorsal view B lateral view.
Figure 5.
The paratypes of Cyrtodactylus zhenkangensis sp. nov. in life A subadult male (KIZL2020046) B subadult female (KIZL2020047) C adult female (KIZL2020048) D adult female (KIZL2020050).
Holotype.
KIZL2020049, adult male, China, Yunnan Province, Lincang City, Zhenkang County, Nansan town, 23°46'32"N, 98°50'28"E, 1060 m elevation, collected on 11 September 2020 by Shuo Liu.
Paratypes.
KIZL2020048 and KIZL2020050, two adult females; KIZL2020046, subadult male; and KIZL2020047, subadult female; all the same collection data as the holotype.
Etymology.
The name refers to Zhenkang County, where the new species was found.
Diagnosis.
Cyrtodactylus zhenkangensis sp. nov. differs from all other congeners by the following combination of characters: medium size (SVL 78.1–87.4 mm); ventrolateral folds present with interspersed tubercles; 12–15 enlarged femoral scales on each thigh; 2–5 femoral pores on each thigh in males, 0–3 pitted scales on each thigh in females; eight or nine precloacal pores in a continuous row or separated by one poreless scale in males, 7–9 pitted scales in females; two or three postcloacal tubercles on each side; 18–21 lamellae under finger IV, 21–23 lamellae under toe IV; subcaudals enlarged, arranged alternately as single and double on anterior and mostly single at middle and posterior; dorsal surface of head with obvious, light-colored reticulations; eight or nine irregular transverse bands on the dorsum of body.
Description of holotype.
Adult male, SVL 87.4 mm; head distinguished from neck, moderately long (HL/SVL 0.27), relatively widened (HW/HL 0.79), slightly depressed (HH/HL 0.48); two supranasals separated by one internasal; nares oval, surrounded by supranasal, rostral, first supralabial, and three or four postnasals; loreal region concave; snout long (SE/HL 0.41), round anteriorly, longer than diameter of orbit (OD/SE 0.70); snout scales small, round, granular, larger than those in frontal and parietal regions; eye large (OD/HL 0.28), pupils vertical; upper eyelid fringe with spinous scales; ear opening oval, obliquely directed, small in size (ED/HL 0.08); rostral wider than high (RH/RW 0.66), medially divided dorsally by a suture, reaching to approximately half-way down rostral, in contact with first supralabial and nostrils laterally, and supranasals and internasal dorsally; mental triangular, narrower than rostral (MW/RW 0.83), wider than high (ML/MW 0.82); two postmentals, enlarged, in contact posteriorly, bordered by mental anteromedially, first infralabial anterolaterally, and an enlarged chin scale posterolaterally; 10/10 supralabials; 10/10 infralabials.
Body slender (AG/SVL 0.41), ventrolateral folds slightly developed with interspersed tubercles; dorsal scales granular; dorsal tubercles round and weakly keeled, four or five times larger than the size of adjoining scales, conical, present on occiput, back and tail base, each surrounded by nine or ten granular scales, in 24 irregular longitudinal rows at the midbody, 29 paravertebral tubercles; ventral scales smooth, larger than those of dorsum, round, subimbricate, largest posteriorly, in 33 longitudinal rows at midbody; gular region with homogenous smooth scales; precloacal groove absent; three rows of enlarged scales present in posterior region of pore-bearing scales; 13/15 enlarged femoral scales beneath thighs continuous with enlarged precloacal scales; femoral pores bearing scales separated from pore-bearing precloacal scales by six poreless or pitted femoral scales on the left side and nine poreless or pitted femoral scales on the right side; 5/5 femoral pores; 5/3 precloacal pores, separated by one poreless scale; most precloacal pores are positioned in the posterior margin of their scales and femoral pores positioned in the center of scales.
Fore and hind limbs moderately slender (ForeaL/SVL 0.17, SL/SVL 0.20); dorsal surface of forelimbs covered by a few weakly developed tubercles; interdigital webbing absent; lamellae under finger IV 20/18, under toe IV 21/23; relative length of fingers I<II<V<III <IV, relative length of toes I<II<III<V<IV.
Tail complete, longer than snout-vent length (TaL/SVL 1.12); 2/3 postcloacal tubercles; dorsal tail base with tubercles; subcaudals smooth, enlarged, arranged alternately in single and double series at anterior and mostly singly at middle and posterior parts.
Color of holotype in life. Head brown with pale-yellow, slightly symmetrical reticulations on either side of the midline, no dark-colored nuchal loop; dorsum of body brown with approximately nine pale-yellow, transverse, irregular bands from forelimb insertions to base of tail and one longitudinal, continuous, narrow vertebral stripe; dorsal surface of limbs brown with some light-yellow, irregularly shaped bands, some small, light-yellow spots on the dorsum of fingers and toes; ventral surface of head, body, and limbs grey with no stripes or spots; tail brownish black with ten yellowish white rings; iris copper-yellow.
Variations. Color pattern variations are shown in Figure 5, and morphometric and meristic differences are presented in Table 2. Morphologically the paratypes resemble the holotype except as follows: KIZL2020046 and KIZL2020047 each has one vertebral stripe like the holotype but it is discontinuous; KIZL2020050 has one continuous vertebral strip and two discontinuous, longitudinal, narrow stripes on the sides of vertebral strip; KIZL2020048 only has transverse bands and no vertebra stripe. All paratypes have continuous precloacal pores (pitted) and fewer femoral pores (pitted).
Table 2.
Measurements (mm) and meristic data for the type series of Cyrtodactylus zhenkangensis sp. nov. Abbreviations defined in Materials and methods.
| KIZL2020049 Holotype | KIZL2020046 Paratype | KIZL2020047 Paratype | KIZL2020048 Paratype | KIZL2020050 Paratype | |
|---|---|---|---|---|---|
| Sex | Male | Subadult male | Subadult female | Female | Female |
| SVL | 87.4 | 64.1 | 66.2 | 78.1 | 85.5 |
| TaL | 98.1 | 73.2 | 76.3 | 86.9 | 96.8 |
| HH | 11.5 | 9.0 | 8.5 | 10.3 | 10.4 |
| HL | 23.9 | 18.6 | 18.6 | 22.0 | 24.2 |
| HW | 18.8 | 13.9 | 14.2 | 17.1 | 17.8 |
| OD | 6.8 | 5.1 | 5.3 | 6.3 | 6.8 |
| SE | 9.7 | 7.9 | 8.0 | 9.3 | 9.9 |
| EE | 7.6 | 5.7 | 5.8 | 6.7 | 7.1 |
| IND | 3.1 | 2.5 | 2.6 | 3.0 | 3.2 |
| IOD | 8.3 | 5.8 | 6.1 | 7.2 | 7.7 |
| ED | 1.8 | 1.4 | 1.3 | 1.3 | 1.8 |
| AG | 35.5 | 25.7 | 25.4 | 33.2 | 36.1 |
| ForeaL | 15.2 | 11.5 | 11.6 | 13.1 | 14.5 |
| SL | 17.8 | 13.0 | 13.5 | 15.9 | 16.7 |
| RW | 4.1 | 3.3 | 3.2 | 3.7 | 4.2 |
| RH | 2.7 | 2.0 | 1.6 | 2.0 | 2.4 |
| MW | 3.4 | 3.1 | 2.7 | 3.2 | 3.8 |
| ML | 2.8 | 2.2 | 2.2 | 2.1 | 2.9 |
| SPL | 10/10 | 10/10 | 10/11 | 11/10 | 10/10 |
| IFL | 10/10 | 8/8 | 10/10 | 9/9 | 8/7 |
| I | 1 | 1 | 1 | 1 | 1 |
| SC5SPL | 37 | 32 | 34 | 28 | 33 |
| PM | 2 | 2 | 2 | 2 | 2 |
| GSDT | 9–10 | 9–10 | 8–9 | 8–10 | 8–9 |
| DTR | 24 | 23 | 21 | 20 | 22 |
| PVT | 29 | 27 | 32 | 33 | 28 |
| V | 33 | 32 | 32 | 34 | 33 |
| EFS | 13/15 | 14/14 | 14/13 | 13/12 | 14/15 |
| PP | 8 | 9 (pitted) | 9 (pitted) | 8 (pitted) | 7 (pitted) |
| FP | 5/5 | 2/2 (pitted) | 1/0 (pitted) | 3/0 (pitted) | 2/2 (pitted) |
| PAT | 2/3 | 2/2 | 2/2 | 3/3 | 2/3 |
| LD4 | 20/18 | 19/20 | 19/18 | 21/20 | 21/19 |
| LT4 | 21/23 | 23/22 | 22/22 | 22/21 | 22/22 |
Distribution.
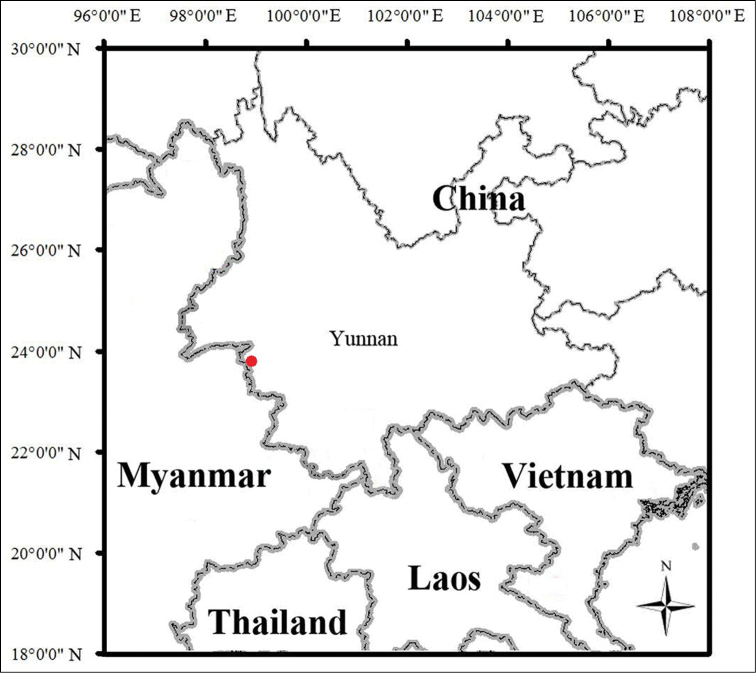
The new species is currently known only from the type locality in Zhenkang County, Yunnan Province, China.
Natural history.
All specimens were found at night between 19:00 and 21:00 on limestone cliffs of the karst formations. The surrounding habitat was primary forestwith a stream nearby. No eggs or juveniles were found.
Comparisons.
Cyrtodactylus zhenkangensis sp. nov. is distinguishable from all other members of the C. wayakonei group by a unique combination of morphological characters. Cyrtodactylus zhenkangensis sp. nov. differs from C. bichnganae Ngo & Grismer, 2010; C. huongsonensis Luu, Nguyen, Do & Ziegler, 2011; and C. sonlaensis Nguyen, Pham, Ziegler, Ngo & Le, 2017 in having fewer femoral pores in males (4–10 vs 15–29).
Cyrtodactylus zhenkangensis sp. nov. differs from C. bobrovi Nguyen, Le, Pham, Ngo, Hoang, Pham & Ziegler, 2015; C. otai Nguyen, Le, Pham, Ngo, Hoang, Pham & Ziegler, 2015; and C. vilaphongi Schneider, Nguyen, Le, Nophaseud, Bonkowski & Ziegler, 2014 in having enlarged subcaudal scales (vs lacking enlarged subcaudals).
Cyrtodactylus zhenkangensis sp. nov. differs from C. chauquangensis Hoang, Orlov, Ananjeva, Johns, Hoang & Dau, 2007; C. cucphuongensis Ngo & Chan, 2011; C. houaphanensis Schneider, Luu, Sitthivong, Teynié, Le, Nguyen & Ziegler, 2020; C. puhuensis Nguyen, Yang, Le, Nguyen, Orlov, Hoang, Nguyen, Jin, Rao, Hoang, Che, Murphy & Zhang, 2014; C. spelaeus Nazarov, Poyarkov, Orlov, Nguyen, Milto, Martynov, Konstantinov & Chulisov, 2014; and C. taybacensis Pham, Le, Ngo, Ziegler & Nguyen, 2019 in having femoral pores in males (vs lacking femoral pores in males).
Cyrtodactylus zhenkangensis sp. nov. differs from C. martini in having femoral pores in males (vs lacking femoral pores in males) and more irregular transverse bands on the dorsum of body (8–9 vs 5–7).
Cyrtodactylus zhenkangensis sp. nov. differs from C. ngoiensis Schneider, Luu, Sitthivong, Teynié, Le, Nguyen & Ziegler, 2020 and C. soni Le, Nguyen, Le & Ziegler, 2016 in its smaller body size (64.1–87.4 mm vs 62.9–103 mm) and having more lamellae under finger IV (18–21 vs 15–19) and toe IV (21–23 vs 18–22).
Figure 6.
Map showing the type locality (red dot) of Cyrtodactylus zhenkangensis sp. nov. in Zhenkang County, Yunnan Province, China.
Cyrtodactylus zhenkangensis sp. nov. differs from C. wayakonei in having enlarged subcaudal scales (vs lacking enlarged subcaudals) and with more irregular transverse bands on the dorsum of body (8–9 vs 5–7).
For other species which were not included in the phylogenetic analyses and resemble Cyrtodactylus zhenkangensis sp. nov. in morphology. Cyrtodactylus zhenkangensis sp. nov. differs from C. auribalteatus Sumontha, Panitvong & Deein, 2010 in having more transverse bands on the dorsum of body (8–9 vs 4–5), obvious reticulations on the dorsum of head (vs no obvious reticulations) and absent dark-colored nuchal loop (vs present).
Cyrtodactylus zhenkangensis sp. nov. differs from C. doisuthep Kunya, Panmongkol, Pauwels, Sumontha, Meewasana, Bunkhwamdi & Dangsri, 2014 in having fewer femoral pores (0–10 vs 12–14), more precloacal pores (7–9 vs 6), and absent dark-colored nuchal loop (vs present).
Cyrtodactylus zhenkangensis sp. nov. differs from C. dumnuii Bauer, Kunya, Sumontha, Niyomwan, Pauwels, Chanhome & Kunya, 2010 in having more lamellae under toe IV (21–23 vs 19), absent dark-colored nuchal loop (vs present), and obvious reticulations on the dorsum of head (vs not obvious or no reticulations).
Cyrtodactylus zhenkangensis sp. nov. differs from C. erythrops Bauer, Kunya, Sumontha, Niyomwan, Panitvong, Pauwels, Chanhome & Kunya, 2009 in having fewer femoral pores in males (4–10 vs 18–20), more lamellae under finger IV (18–21 vs 16) and toe IV (21–23 vs 20), and more transverse bands on the dorsum of body (8–9 vs 6–7).
Figure 7.
Habitat of Cyrtodactylus zhenkangensis sp. nov. at the type locality in Zhenkang County, Yunnan Province, China.
Discussion
According to Pham et al. (2019) and Schneider et al. (2020), the Cyrtodactylus wayakonei species group contains 16 species, namely C. bichnganae, C. bobrovi, C. chauquangensis, C. cucphuongensis, C. houaphanensis, C. huongsonensis, C. martini, C. ngoiensis, C. otai, C. puhuensis, C. soni, C. sonlaensis, C. spelaeus, C. taybacensis, C. vilaphongi, and C. wayakonei. However, we speculate that there are still some other species (e.g., C. auribalteatus, C. doisuthep, C. dumnuii, and C. erythrops) which were not included in the phylogenetic analyses also belong to this species group based on morphology, molecular evidence is needed to clarify these problems.
Although the distribution of the new species is distant from the distributions of C. martini and C. wayakonei, the new species is most similar to the latter two in both morphology and phylogeny. The new species is not found in a protected area; the type locality is just beside the county seat, where there are human activities during the day but usually not at night. This species is nocturnal, so it may be less affected by human activities.
There are many other karst formations in Yunnan, some of which remain insufficiently surveyed. We are continuing to conduct more expeditions in these regions, and it is likely that additional new species of Cyrtodactylus will be found in these karst systems.
Supplementary Material
Acknowledgements
We thank Decai Ouyang for assistance in the field. We are grateful to our workmates for their help and advice. We also thank the reviewers for their valuable comments on the manuscript. This work was supported by Science-Technology Basic Condition Platform from the Ministry of Science and Technology of the People’s Republic of China (grant no. 2005DKA21402) and National Natural Science Foundation Project: Investigation and Classificatory and Phylogenetic Studies on the Lizards of Gekkonidae of China (grant no. NSFC-31970404).
Citation
Liu S, Rao D (2021) A new species of Cyrtodactylus Gray, 1827 (Squamata, Gekkonidae) from Yunnan, China. ZooKeys 1021: 109–126. https://doi.org/10.3897/zookeys.1021.60402
Contributor Information
Shuo Liu, Email: liushuo@mail.kiz.ac.cn.
Dingqi Rao, Email: raodq@mail.kiz.ac.cn.
References
- Alfaro ME, Zoller S, Lutzoni F. (2003) Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Molecular Biology and Evolution 20: 255–266. 10.1093/molbev/msg028 [DOI] [PubMed] [Google Scholar]
- Bauer AM, Kunya K, Sumontha M, Niyomwan P, Panitvong N, Pauwels OSG, Chanhome L, Kunya T. (2009) Cyrtodactylus erythrops (Squamata: Gekkonidae), a new cave-dwelling gecko from Mae Hong Son Province, Thailand. Zootaxa 3811: 251–261. 10.11646/zootaxa.2124.1.4 [DOI] [Google Scholar]
- Bauer A, Kunya K, Sumontha M, Niyomwan P, Pauwels OSG, Chanhome L, Kunya T. (2010) Cyrtodactylus dumnuii (Squamata: Gekkonidae), a new cave-dwelling gecko from Chiang Mai Province, Thailand. Zootaxa 2570: 41–50. 10.11646/zootaxa.2570.1.2 [DOI] [Google Scholar]
- Chomdej S, Suwannapoom C, Pawangkhanant P, Pradit W, Nazarov RA, Grismer LL, Poyarkov NA. (2020) A new species Cyrtodactytlus Gray (Squamata: Gekkonidae) from western Thailand and the phylogenetic placement of C. inthanon and C. doisuthep. Zootaxa 4838: 179–209. 10.11646/zootaxa.4838.2.2 [DOI] [PubMed] [Google Scholar]
- Dring JCM. (1979) Amphibians and reptiles from northern Trengganu, Malaysia, with descriptions of two new geckos: Cnemaspis and Cyrtodactylus. Bulletin of the British Museum (Natural History), Zoology 34: 181–241. [Google Scholar]
- Grismer LL, Wood Jr PL, Thura MK, Zin T, Quah ESH, Murdoch ML, Grismer MS, Lin A, Kyaw H, Ngwe L. (2018) Twelve new species of Cyrtodactylus Gray (Squamata: Gekkonidae) from isolated limestone habitats in east-central and southern Myanmar demonstrate high localized diversity and unprecedented microendemism. Zoological Journal of the Linnean Society 182: 862–959. 10.1093/zoolinnean/zlx057 [DOI] [Google Scholar]
- Grismer LL, Wood Jr PL, Le MD, Quah ESH, Grismer JL. (2020) Evolution of habitat preference in 243 species of bent-toed geckos (genus Cyrtodactylus Gray, 1827) with a discussion of karst habitat conservation. Ecology and Evolution 10: 13717–13730. 10.1002/ece3.6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang QX, Orlov NL, Ananjeva NB, Johns AG, Hoang TN, Dau VQ. (2007) Description of a new species of the genus Cyrtodactylus Gray, 1827 (Squamata: Sauria: Gekkonidae) from the karst of North Central Vietnam. Russian Journal of Herpetology 14: 98–106. [Google Scholar]
- Ivanova NV, Dewaard JR, Hebert PDN. (2006) An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes 6: 998–1002. 10.1111/j.1471-8286.2006.01428.x [DOI] [Google Scholar]
- Kluge AG. (2001) Gekkotan lizard taxonomy. Hamadryad 26: 1–209. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunya K, Panmongkol A, Pauwels OGS, Sumontha M, Meewasana J, Bunkhwamdi W, Dangsri S. (2014) A new forest-dwelling Bent-toed Gecko (Squamata: Gekkonidae: Cyrtodactylus) from Doi Suthep, Chiang Mai Province, northern Thailand. Zootaxa 3811: 251–261. 10.11646/zootaxa.3811.2.6 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. (2001) Bayesian Inference of phylogeny and its impact on evolutionary biology. Science 294: 2310–2314. 10.1126/science.1065889 [DOI] [PubMed] [Google Scholar]
- Le DT, Nguyen TQ, Le MD, Ziegler T. (2016) A new species of Cyrtodactylus (Squamata: Gekkonidae) from Ninh Binh Province, Vietnam. Zootaxa 4162: 268–282. 10.11646/zootaxa.4162.2.4 [DOI] [PubMed] [Google Scholar]
- Le M, Raxworthy CJ, McCord WP, Mertz L. (2006) A molecular phylogeny of tortoises (Testudines: Testudinidae) based on mitochondrial and nuclear genes. Molecular Phylogenetics and Evolution 40: 517–531. 10.1016/j.ympev.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Luu VQ, Bonkowski M, Nguyen TQ, Le MD, Schneider N, Ngo HT, Ziegler T. (2016) Evolution in karst massifs: cryptic diversity among bent-toed geckos along the Truong Son Range with descriptions of three new species and one new country record from Laos. Zootaxa 4107: 101–140. 10.11646/zootaxa.4107.2.1 [DOI] [PubMed] [Google Scholar]
- Luu VQ, Calame T, Nguyen TQ, Soudthichak S, Bonkowski M, Ziegler T. (2013) New country records of reptiles from Laos. Biodiversity Data Journal 1: e1015. 10.3897/BDJ.1.e1015 [DOI] [PMC free article] [PubMed]
- Luu VQ, Nguyen TQ, Do HQ, Ziegler T. (2011) A new Cyrtodactylus (Squamata: Gekkonidae) from Huong Son limestone forest, Hanoi, northern Vietnam. Zootaxa 3129: 39–50. 10.11646/zootaxa.3129.1.3 [DOI] [Google Scholar]
- Murdoch ML, Grismer LL, Wood Jr PL, Neang T, Poyarkov NA, Ngo TV, Nazarov RA, Aowphol A, Pauwels OSG, Nguyen HN, Grismer JL. (2019) Six new species of the Cyrtodactylus intermedius complex (Squamata: Gekkonidae) from the Cardamom Mountains and associated highlands of Southeast Asia. Zootaxa 4554: 1–62. 10.11646/zootaxa.4554.1.1 [DOI] [PubMed] [Google Scholar]
- Nazarov RA, Pauwels OSG, Konstantinov EL, Chulisov AS, Orlov NL, Poyarkov NA. (2018) A new karst-dwelling bent-toed gecko (Squamata: Gekkonidae: Cyrtodactylus) from Xiangkhoang Province, northeastern Laos. Zoological Research 39: 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov RA, Poyarkov NA, Orlov NL, Nguyen SN, Milto KD, Martynov AA, Konstantinov EL, Chulisov AS. (2014) A review of genus Cyrtodactylus (Reptilia: Sauria: Gekkonidae) in fauna of Laos with description of four new species. Proceedings of the Zoological Institute RAS 318: 391–423. [Google Scholar]
- Nazarov RA, Poyarkov NA, Orlov NL, Phung TM, Nguyen TT, Hoang DM, Ziegler T. (2012) Two new cryptic species of the Cyrtodactylus irregularis complex (Squamata: Gekkonidae) from southern Vietnam. Zootaxa 3302: 1–24. 10.11646/zootaxa.3302.1.1 [DOI] [Google Scholar]
- Ngo TV. (2011) Cyrtodactylus martini, another new karst-dwelling Cyrtodactylus Gray, 1827 (Squamata: Gekkonidae) from Northwestern Vietnam. Zootaxa 2834: 33–46. 10.11646/zootaxa.2834.1.3 [DOI] [Google Scholar]
- Ngo TV, Chan KO. (2011) A new karstic cave-dwelling Cyrtodactylus Gray (Squamata: Gekkonidae) from Northern Vietnam. Zootaxa 3125: 51–63. [Google Scholar]
- Ngo TV, Grismer LL. (2010) A new karst dwelling Cyrtodactylus (Squamata: Gekkonidae) from Son La Province, northwestern Vietnam. Hamadryad 35: 84–95. 10.11646/zootaxa.2652.1.1 [DOI] [Google Scholar]
- Nguyen TQ, Kingsada P, Rösler H, Auer M, Ziegler T. (2010) A new species of Cyrtodactylus (Squamata: Gekkonidae) from northern Laos. Zootaxa 2652: 1–16. [Google Scholar]
- Nguyen TQ, Le MD, Pham AV, Ngo HN, Hoang CV, Pham CT, Ziegler T. (2015) Two new species of Cyrtodactylus (Squamata: Gekkonidae) from the karst forest of Hoa Binh Province, Vietnam. Zootaxa 3985: 375–390. 10.11646/zootaxa.3985.3.3 [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Pham AV, Ziegler T, Ngo HT, Le MD. (2017) A new species of Cyrtodactylus (Squamata: Gekkonidae) and the first record of C. otai from Son La Province, Vietnam. Zootaxa 4341: 25–40. 10.11646/zootaxa.4341.1.2 [DOI] [PubMed] [Google Scholar]
- Nguyen SN, Yang JX, Le NT, Nguyen LT, Orlov NL, Hoang CV, Nguyen TQ, Jin JQ, Rao DQ, Hoang TN, Che J, Murphy RW, Zhang YP. (2014) DNA barcoding of Vietnamese bent-toed geckos (Squamata: Gekkonidae: Cyrtodactylus) and the description of a new species. Zootaxa 3784: 48–66. 10.11646/zootaxa.3784.1.2 [DOI] [PubMed] [Google Scholar]
- Pham AV, Le MD, Ziegler T, Nguyen TQ. (2019) A new species of Cyrtodactylus (Squamata: Gekkonidae) from northwestern Vietnam. Zootaxa 4544: 360–380. 10.11646/zootaxa.4544.3.3 [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler H, Vu TN, Nguyen TQ, Ngo TV, Ziegler T. (2008) A new Cyrtodactylus (Squamata: Gekkonidae) from central Vietnam. Hamadryad 33: 48–63. [Google Scholar]
- Schneider N, Luu VQ, Sitthivong S, Teynié A, Le MD, Nguyen TQ, Ziegler T. (2020) Two new species of Cyrtodactylus (Squamata: Gekkonidae) from northern Laos, including new finding and expanded diagnosis of C. bansocensis. Zootaxa 4822: 503–530. 10.11646/zootaxa.4822.4.3 [DOI] [PubMed] [Google Scholar]
- Schneider N, Nguyen TQ, Le MD, Nophaseud L, Bonkowski M, Ziegler T. (2014) A new species of Cyrtodactylus (Squamata: Gekkonidae) from the karst forest of northern Laos. Zootaxa 3835: 80–96. 10.11646/zootaxa.3835.1.4 [DOI] [PubMed] [Google Scholar]
- Schneider N, Nguyen TQ, Schmitz A, Kingsada P, Auer M, Ziegler T. (2011) A new species of karst dwelling Cyrtodactylus (Squamata: Gekkonidae) from northwestern Laos. Zootaxa 2930: 1–21. 10.11646/zootaxa.2930.1.1 [DOI] [Google Scholar]
- Silvestro D, Michalak I. (2012) raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution 12: 335–337. 10.1007/s13127-011-0056-0 [DOI] [Google Scholar]
- Sitnikova T. (1996) Bootstrap method of interior-branch test for phylogenetic trees. Molecular Biology and Evolution 13: 605–611. 10.1093/oxfordjournals.molbev.a025620 [DOI] [PubMed] [Google Scholar]
- Sumontha M, Panitvong N, Deein G. (2010) Cyrtodactylus auribalteatus (Squamata: Gekkonidae), a new cave-dwelling gecko from Phitsanulok Province, Thailand. Zootaxa 2370: 53–64. 10.11646/zootaxa.2370.1.3 [DOI] [Google Scholar]
- Teyníe A, David P. (2010) Voyages naturalists au Laos. Les reptiles. Revoir Editions, Chamalières, France, 315 pp. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P. (2020) The Reptile Database. http://www.reptile-database.org [Accessed on: 2020-11-5]
- Ulber T. (1993) Bemerkungen über cyrtodactyline Geckos aus Thailand nebst Beschreibungen von zwei neuen Arten (Reptilia: Gekkonidae). Mitteilungen aus dem Zoologischen Museum in Berlin 69: 187–200. 10.1002/mmnz.19930690202 [DOI] [Google Scholar]
- Wilcox TP, Zwickl DJ, Heath TA, Hillis DM. (2002) Phylogenetic relationships of the dwarf boas and a comparison of Bayesian and bootstrap measures of phylogenetic support. Molecular Phylogenetics and Evolution 25: 361–371. 10.1016/S1055-7903(02)00244-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.