Abstract
Purpose
Inflammation is the driving force of many inflammatory and autoimmune diseases, Pyroptosis is a process of cell death in response to excessive inflammation. Punicalin has been reported to have anti-inflammatory effects. However, the anti-pyroptosis is unknown. Hence, this study was aimed to research the inhibition of MG on LPS/ATP-induced pyroptosis in vitro.
Methods
Lipopolysaccharide (LPS)/ATP were used to simulate mouse J774A.1 cells to mimic the inflammatory response and the role of punicalin was examined. The secretion of proinflammatory cytokines was analyzed using enzyme-linked immunosorbent assay (ELISA). The expression of nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), caspase-1, and GSDMD-N in LPS/ATP-stimulated cells were examined by Western blot. N-acetylcysteine (NAC) was used to validate the role of Punicalin.
Results
Punicalin significantly blocked the production of endogenous ROS, reduced LPS/ATP-induced activation of NLRP3, caspase 1, ASC and GSDMD-N, IL-1b and IL-18 protein levels. Furthermore, N-acetylcysteine (NAC), an ROS scavenger, inhibited the LPS/ATP-stimulated activation of NLRP3 inflammasome mediated inflammation and pyroptosis.
Conclusion
Punicalin ameliorates LPS/ATP-induced pyroptosis in J774A.1 macrophages, the mechanism may involve downregulation of the ROS/NLRP3 inflammasome signaling pathway.
Keywords: punicalin, NLRP3, pyroptosis, ROS
Introduction
Inflammation is the driving force of many inflammatory and autoimmune diseases, such as microbial infection, atherosclerosis, type II diabetes, obesity, and rheumatoid arthritis.1 Limiting inflammation helps reduce immunopathology and immune damage. Macrophages are involved in the initiation of inflammatory response. They play a central role in the pathogenesis of many inflammatory diseases by secreting pro-inflammatory mediators and pro-inflammatory cytokines.2
Sensors of innate immune system include pattern-recognition receptors (PRRs) which monitor extracellular and intracellular pathogen-associated molecular patterns (PAMPs), as well as host-derived danger signals, termed danger-associated molecular patterns (DAMPs). PRRs have several subgroups, such as toll-like receptors (TLRs), C-type lectin receptors (CLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), RIG-I-like receptor (RLR) and AIM2-like receptor (ALR). NLRs and TLRs synergistically trigger inflammatory response.3 NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) is an intracellular sensor that detects a broad range of microbial motifs, endogenous danger signals.
Lipopolysaccharide (LPS) activates phagocytosis-related NADPH oxidase, which interacts with Rac1, leading to the initial of ROS.4 ROS further transfers the NLRP3 from the nucleus to the cytoplasm and binds to the dissociative TXNIP.5 The NLRP3/TXNIP complex recruits ASC and procaspase 1 and forms NLRP3 inflammasome, contributing to activation of caspase-1. Activation of inflammasome-associated inflammatory caspases drives cleavage of gasdermin D (GSDMD) to GSDMD-N, which governs the cleavage and activation of IL-18 and IL-1β, and executes pyroptosis.6 ATP triggers NLRP3 inflammasome assembly by inducing K+ efflux through P2X7-dependent pore and increasing intracellular Ca2+ concentration.6
Punicalin (PUN) exists in the leaves of Terminalia catappa L and pomegranate husk, which are combretaceous plants distributed throughout tropical and subtropical beaches.7 Punicalin has shown significant biological activities, including anti-hepatotoxic,8 antibacterial,9 anticancer,10 anti-hepatitis B virus,11 anti-HIV replication,12 and so on. However, it is not clear whether the protective effect of PUN occurs during ROS/NLRP3-mediated pyroptosis.
Materials and Methods
Materials
Cell culture was purchased from GIBCO (USA); The antibodies were as follows: NLRP3 (Cat#AG-20B-0014-C100), and ASC (AG-25B-0006A) were from Adipogen, San Diego, CA, USA, Caspase-1 (Cat. No.: 22915-1-AP) was from Proteintech, Chicago, USA, GSDMD-N (ab209845) was acquired from Abcam (Cambridge, USA); The Cell Whole Protein Extraction Kit and the BCA Protein Content Detection Kit were bought from KeyGEN BioTECH (Beijing, China). Calcein-AM and PI were purchased from Tongren Chemical (Beijing, China); IL-1β and IL-18 kits were purchased from Boster Biological Technology (Wuhan, China). Punicalin (purity 98.4%, batch, 20180706) was from Chengdu Herbpurify Co. Ltd (Chengdu, China); LPS, ATP, and N-Acetylcysteine (NAC) were bought from Netotime (Beijing, China). LPS and NAC were prepared as a 0.5 μg/mL stock solution in PBS (HyClone, USA), filter-sterilized, and stored at −20°C for up to two weeks.
Cell Cultures and Treatment
The mouse mononuclear macrophage J774A.1 cell line (Beijing Union and Cell Resource Center) was cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (HyClone, USA) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 ◦C in a humidified incubator containing 5% CO2.
The experimental grouping was as follows: cells were plated at 4×105 cells/well into 6-well plates (or 1×106 cells/bottle to 25 cm2 cell culture flask), and cultured at 37°C under 5% CO 2 for 12 h. 0, 25, 50, and 100 μM of PUN were added and cultured for 1 h for each group, respectively. For the role of ROS, the experimental cells were divided into four groups, negative control (NC), PUN (100 μM), NAC (5 mM), and PUN (100 μM) + NAC (5 mM) groups and cultured as above. The supernatant was discarded, LPS was added at a concentration at 1 μg/mL and cultured for 5.5 h, and the supernatant was discarded, and 5 mM ATP was added for half an hour for subsequent experiments. Then, cells and supernatants were collected for later analysis.
Cell Viability Assay
Cell viability was detected using MTT assay. 5 mg/mL MTT solution was added into each cell and incubated for 4 hours. The sample was determined at a wavelength of 490 nm microplate reader (Bio-Rad Instrument). The results were expressed as % viability.
LDH Release Assay and Living/Dead Cells Assay by Calcein-AM (Living) and PI (Dead) Double Staining
Cell death was evaluated by lactate dehydrogenase (LDH) release and Calcein-AM and PI double staining. For LDH release, LDH released into cell culture supernatants was detected using LDH assay kit (Nanjing Jiancheng Biology Engineering Institute, Jiangsu, China) according to the manufacturer’s instructions. For Calcein-AM (living cell) and PI (dead cell) double staining, 100 μL of 2 μM Calcein-AM and 4.5 μM PI double staining solution were added into each well and incubated at 37°C for 15 minutes. After washing, the cells were imaged under a laser scanning microscope (Olympus Corporation, Tokyo, Japan). The average fluorescence intensity was assessed using Image Pro advanced 6.0 software.
Intracellular ROS Measurement
ROS were measured with the 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Beyotime). DCF-DA was diluted with a colorless serum-free DMEM medium at a concentration ratio of 1:10,000, 500 μL of DCF-DA dilution was added to each cell well and incubated for 15 minutes at 37°C. The cells were washed three times with serum-free DMEM medium. The fluorescence intensity of the retained DCF-DA was detected at an excitation wavelength of 504 nm and an emission wavelength of 529 nm with a fluorescence microscope (Olympus Corporation, Tokyo, Japan). The average fluorescence intensity was assessed using Image Pro advanced 6.0 software.
IL-1β and IL-18 Measurement
IL-1β and IL-18 in the supernatants were measured using a commercial enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions.
Western Blot Analysis
The whole-cell protein was extracted using a whole-cell protein extraction kit, and concentration of the extracted whole protein was quantified using a protein content detection kit. The sample (20 μg protein) was loaded and separated on 5% concentration gel and 15% separation gel by constant voltage 80V electrophoresis for 120 min. Then, the target protein was transferred film on constant current 250 mA for 120 min. The film was sealed using 5% skimmed milk powder. The primary antibody was incubated overnight at 4°C. The fluorescent secondary antibody was incubated in the dark for 40 min. The Odyssey dual-color infrared laser imaging system is used to scan and imaged. The protein on the film was quantified by gray analysis using Image J software.
Statistical Analysis
All data were expressed as the mean ± standard of three independent replicates. One-way analysis of variance (ANOVA), followed by Turkey test, was performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
PUN Promoted Cell Viability and Decreased the Release of Pro-Inflammatory Cytokines
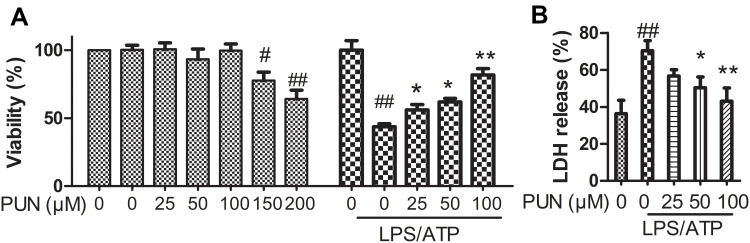
The IC50 of LPS/ATP was identified through MTT cell viability assay. As shown in Figure 1, the maximum non-cytotoxic concentration of PUN was up to 100 μmol/L using MTT assay method (Figure 1A); treatment with 1 μg/mL of LPS for 5.5 h, and followed by 5 mM ATP for half an hour resulted in approximate 50% cell death. PUN pretreatment gradually blocked LPSATP cytotoxicity (Figure 1A).
Figure 1.
The viability and LDH release of cells. (A) Cell viability measured by MTT assay. It is safe to cells’ survival exposed to PUN at concentrations from 0 to 100 μM of (n=5). LPS/ATP induced a nearly 50% cell death, PUN inhibited LPS/ATP-induced cell death in a dose-dependent manner in the range of 0–100 μM (n=5). (B) Pyroptotic cell death determined by LDH activity released into the medium. PUN inhibited LPS/ATP-induced the release of LDH into the medium characterized by decreased LDH activity. # Indicates P<0.05, ##indicates P< 0.01 vs NC; **Indicates P< 0.01, *indicates P< 0.05 vs LPS/ATP stimulated cells, J774A.1 cells were pretreated with indicates concentrations of PUN for 24 hours, or pretreated with indicates concentrations of PUN (0, 25, 50, 100 μM) for one hour, with or without followed by 1 μg/mL of LPS for 5.5 h, and then 5 mM ATP for half an hour.
Damage of the cellular membranes results in the release of injury markers, such as LDH. Thus, LDH was further quantified using LDH activity kit. As shown in Figure 1B, LPS/ATP increased the extracellular LDH content to about 80% compared with negative control cells (NC). PUN pretreatment rescued it from nearly 60% to 50%. The results indicated that PUN pretreatment alleviated LPS/ATP induced J774A.1 macrophage membrane damage and cell death.
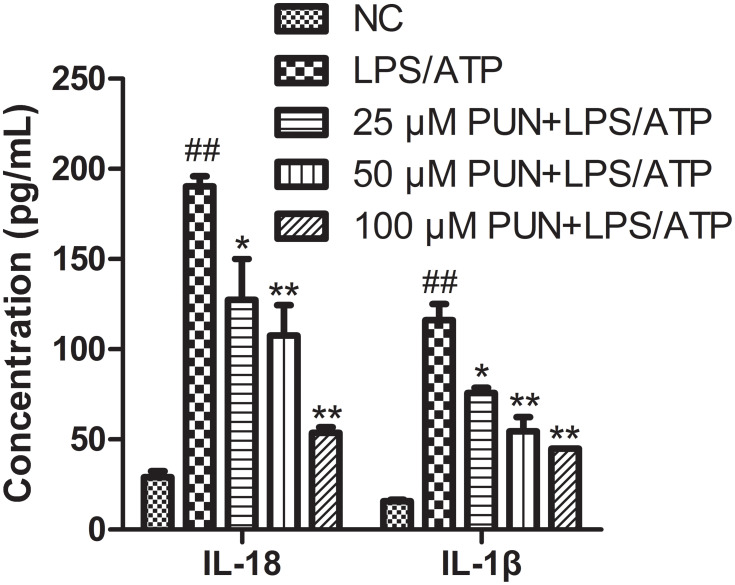
IL-1β and IL-18 release was determined. Compared with NC, the synergistic effect of LPS/ATP increased the mature and secretion of IL-1β as well as IL-18 (Figure 2). When J774A.1 cells were pretreated with different concentrations of PUN, extracellular IL-1β and IL-18 were decreased. These results indicated that PUN inhibited the maturation and release of proinflammatory cytokines triggered by LPS/ATP co-stimulation in a concentration-dependent manner (Figure 2).
Figure 2.
The concentration of extracellular IL-18 and IL-1β in the medium. PUN reduced the release of pro-inflammatory factors into the culture supernatant (n=5). ##Indicate P< 0.01 vs NC; *Indicate P< 0.05, **indicates P< 0.01 vs LPS/ATP stimulated cells.
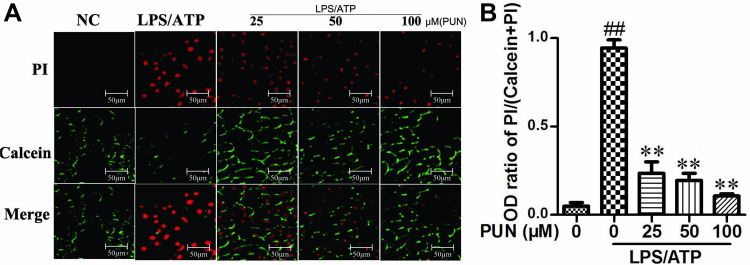
Cellular pyroptosis is usually characterized by pore dysfunction of the cell membrane, loss of membrane integrity and cell swelling and rupture. Calcein-AM and propidium iodide (PI) can stain living and dead cells, respectively. Calcein-AM permeates the cell membrane, and the resulting calcein emits strong green fluorescence in living cells. On the other hand, PI, as a nuclear staining dye only pass through the dead cell membrane, reach and embedded in the cell’s DNA double helix to produce red fluorescence. It was shown that LPS/ATP exposure caused cell pyroptotic death, because the PI-positive cells increased and calcein-AM-positive cells decreased, while PUN pretreatment clearly blocked this trend in a dose-dependent manner (Figure 3).
Figure 3.
The counts for live and dead cells. (A) Fluorescence microscopy images of Calcein-AM (green) and propidium iodide (red). The nucleus of dead cells was stained red by PI, while living cells were stained green by calcein. (B) Relative fluorescence density for Propidium Iodide and Calcein-AM. ##Indicates P<0.01 vs negative control group. **Indicates P<0.01 vs LPS/ATP stimulated group. The cells were pretreated with 0, 25, 50 and 100 μM PUN for one hour, followed by treated with LPS for 5.5 h, ATP for half an hour. Then, the cells were stained with PI and Calcein-AM, respectively; and observed in fluorescence microscope.
Punicalin Blocked NLRP3-Dependent Caspase-1 Maturation and Pyroptosis
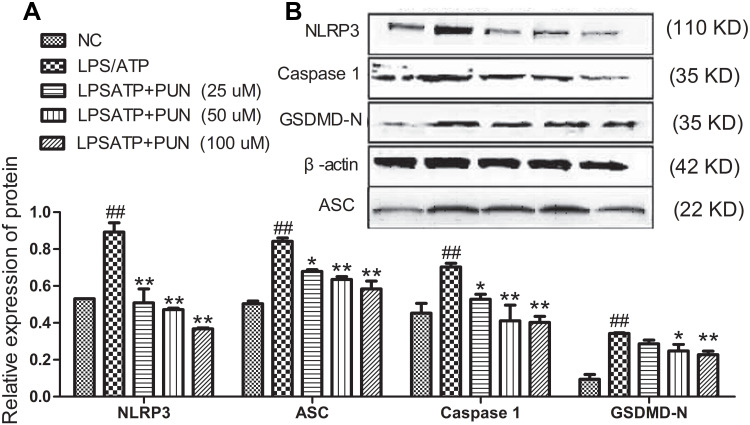
Cellular pyroptosis is a form of caspase-1 mediated death.13 The activation of NLRP3 inflammasome results in the cleavage of pro-caspase-1 into caspase 1, which is responsible for the maturation of GSDMD, pro-IL-1β and pro-IL-18. As shown in Figure 4, LPS/ATP co-stimulation increased the protein levels of NLRP3 and other intracellular pyroptosis-associated speck-like protein (ASC), and caspase-1, and GSDMD-N. PUN pretreatment significantly reduced the increased protein expression of NLRP3, ASC, Caspase-1 and GSDMD-N in LPSATP stimulated J774A.1 cells. The results indicated that PUN alleviated NLRP3 inflammasome activation and pyroptosis induced by LPS/ATP exposure.
Figure 4.
Protein expression levels of NLRP3, Caspase 1, ASC, and GSDMD-N in J774A.1 cells. PUN ameliorated NLRP3 inflammasome activation and pyroptosis. (A) Protein quantitative histogram of NLRP3, Caspase1, ASC, and GSDMD-N in J774A.1 cells. The values presented were the mean ± S.E.M (n=3). ##Indicates P<0.01 vs negative control group. *Indicates P<0.05 and **indicates P<0.01 vs LPS/ATP stimulated group. (B) Representative immunoblots of NLRP3, ASC, caspase-1 and GSDMD-N in J774A.1 cells of different treatment group. The cells were pretreated with 0, 25, 50 and 100 μM PUN for one hour, followed by treated with LPS for 5.5 h, ATP for half an hour, respectively.
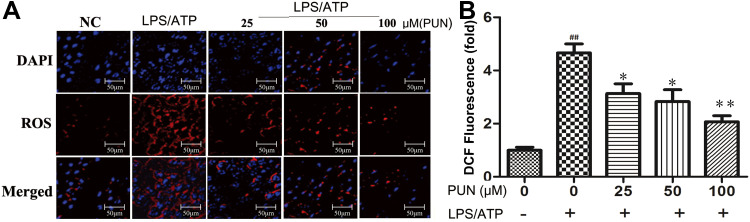
Punicalin Reduced Intracellular Reactive Oxygen Species (ROS)
ROS has a promoted role in NLRP3 inflammasome activation.14 DCFH-DA can pass through the cell membrane and become DCFH, non-fluorescent DCFH can be oxidized by intracellular ROS to fluorescent DCF. In the study, the level of ROS in cells was determined by detecting the fluorescence intensity of DCF. As shown in Figure 5, compared with the negative control group (NC), LPS/ATP stimulation increased endogenous ROS production. However, PUN pretreatment effectively blocked the increase of ROS caused by LPS/ATP stimulation. The quantified ROS levels are shown in Figure 5, which indicated that PUN reversed the increase of ROS in a dose-dependent manner.
Figure 5.
Intracellular ROS concentration determined by fluorescence microscope. PUN attenuated LPS/ATP-induced endogenous ROS production. (A) Intracellular ROS observed under a fluorescence microscope. ROS was stained in red fluorescent DCF, cell nucleus were marked with blue DAPI. (B) Quantitative histogram of ROS. ##Indicates P<0.01 vs NC. *Indicates P<0.05 and **indicates P<0.01 vs LPS/ATP stimulated group. The cells were pretreated with 0, 25, 50 and 100 μM PUN for one hour, followed by LPS for 5.5 h, ATP for half an hour, respectively.
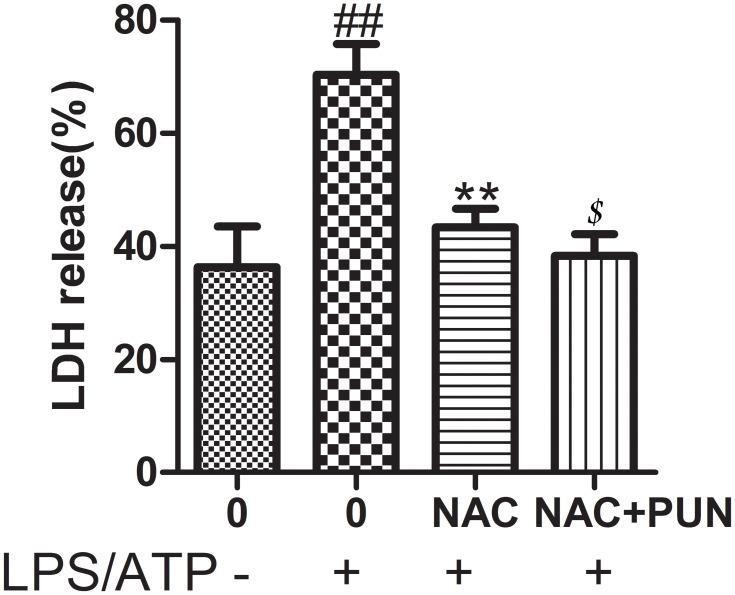
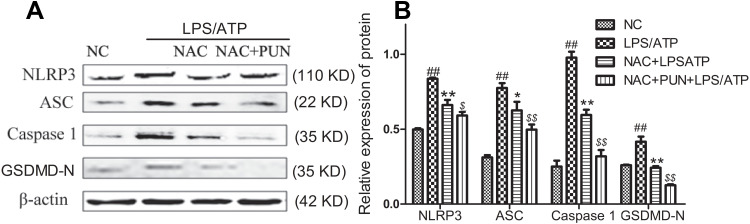
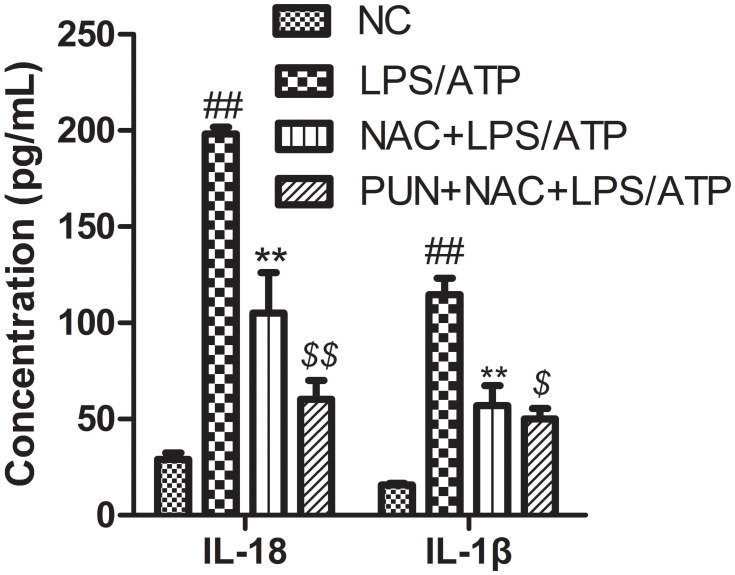
The Role of ROS
Cysteine-containing N-acetyl-L-cysteine-cysteine (NAC), as cell-permeable ROS scavenger, was used to determine the role of PUN in regulating LPS/ATP-induced pyroptosis. Additionally, NAC prevented the LDH leakage (Figure 6), and interfered the activation of NLRP3, ASC, caspase-1, and GSDMD-N consistently (Figure 7), reduced the release of mature IL-1β and IL-18 (Figure 8), and alleviated oxidant stress (Figure 9). The results indicated that ROS was required in the progress of pyroptosis, and the inhibitory effect of PUN on ROS production partially prevented NLRP3 activation, inflammatory factor release, and cellular pyroptosis. ROS scavenger of NAC and PUN exert anti-inflammatory and anti-pyroptotic effects through similar mechanisms.
Figure 6.
Pyroptotic cell death indicates by LDH release; cells were pretreated with NAC (10 mM) or PUN (100 μM) +NAC (10 mM), followed by treated with LPS for 5.5 h, ATP for half an hour. The release of LDH was indicates by LDH activity in medium. ##Indicates P<0.01 vs NC. **Indicates P<0.01 vs LPS/ATP stimulated group. $Indicates P<0.05 vs NAC +LPS/ATP group.
Figure 7.
The protein expression levels of NLRP3, caspase‑1, ASC, and GSDMD-N. (A) Representative western-blot images. (B) Quantified histogram of NLRP3, caspase‑1, ASC, and GSDMD-N. Cells were pretreated with NAC (10 mM) or PUN (100 μM) +NAC (10 mM), followed by treated with LPS for 5.5 h, ATP for half an hour. ##Indicates P<0.01 vs NC. *Indicates P<0.05, and **Indicates P<0.01 vs LPS/ATP stimulated group. $Indicates P<0.05, $$Indicates P<0.01 vs NAC+ LPS/ATP stimulated group.
Figure 8.
The concentrations of IL-1β and IL-18 in the medium determined with ELISA kits. J774A.1 cells were pretreated with NAC (10 mM) or PUN (100 μM) + NAC (10 mM), followed by treated with LPS for 5.5 h, ATP for half an hour. ##Indicates P<0.01 vs NC. **Indicates P<0.01 vs LPS/ATP stimulated cells group. $Indicates P<0.05, $$indicates P<0.01 vs NAC+LPS/ATP stimulated group. J774A.1 cells were pretreated with NAC (10 mM) or PUN (100 μM) +NAC (10 mM), followed by treated with LPS for 5.5 h, ATP for half an hour. The concentrations of IL-1β and IL-18 in the medium were determined with ELISA kits.
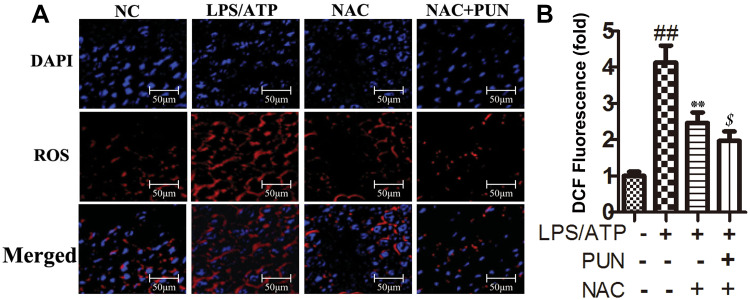
Figure 9.
The intracellular ROS levels. (A) Examined by a fluorescence microscope. (B) The quantified histogram for ROS. ##Indicates P<0.01 vs negative control group. **Indicates P<0.01 vs LPS/ATP stimulated cells group. $Indicates P<0.05 vs NAC plus LPS/ATP stimulated group. J774A.1 cells were pretreated with NAC (10 mM) or PUN (100 μM) +NAC (10 mM), followed by treated with LPS for 5.5 h, ATP for half an hour.
Discussion
Immune dysfunction has been considered one of the key factors in inflammatory disease. Inflammasomes are intracellular multi-protein complexes that can be activated by many exogenous and endogenous factors, such as oxidative stress, potassium efflux, and Ca2+ influx. In particular, ROS generation has been identified as an important mechanism for NLRP3 activation15 Activation of NLRP3 inflammasomes and induction of pyroptosis require a two-step mechanism. Firstly, pro-inflammatory mediators, such as pro-IL-1β, NLRP1/3 and caspase family members, are transcriptionally generated. Secondly, the inflammasomes are assembled and caspase-1 is activated. Active caspase-1 proteolysis matures pro-IL-1a and proIL-18 and therefore induces pyroptosis partially through cleavage of gasdermin D (GSDMD) resulting in the release of pro-inflammatory mediators, including IL-1β, IL-18 and other inflammatory cytokines.16 Inhibiting inflammatory reaction and protecting cells are important strategies to control the inflammatory dysregulation.17 In the study, firstly, it was found that PUN significantly increased the viability of J774A.1 cells from 45% to 80% poisoned by LPS/ATP. The involved mechanism included inhibiting intracellular ROS generation, the activation of NLRP3 inflammasome, the cleavage of GSDMD-N and the mature and release of pro-inflammation cytokines.
NLRP3 inflammasome priming may be promoted by ROS.18 Previous study showed that PUN exerts antioxidant activity.19 In order to confirm the role of PUN’s anti-oxidative stress in the process of NLRP3 inflammasome activation and pyroptosis, NAC as a total ROS scavenger was used to treat J774A.1 cells. The results show that the effect of using NAC alone is similar to that of PUN in combination, NAC and PUN enhance the effect each other; because the reduction of intracellular ROS leads to a decrease in the activation level of NLRP3 inflammasomes and a decrease in the release of inflammatory factors. The result suggests that PUN inhibits NLRP3 activation and pyroptosis by suppressing intracellular ROS generation.
Conclusion
In summary, LPS/ATP induces pyroptosis through the ROS/NLRP3/caspase-1 signaling pathway. PUN inhibits pyroptosis and ROS/NLRP3/caspase-1 signaling pathway. The ROS scavengers also inhibit LPS/ATP-induced caspase-1 activation, proinflammatory cytokine secretion, and pyroptosis. It indicates that PUN inhibits pyroptosis of J774A.1 macrophage cells via suppressing ROS/NLRP3 inflammasomes signal pathway, thereby providing critical insight into the potential cellular and molecular mechanisms involved in the anti-inflammatory effects of PUN.
Acknowledgments
The work was financially supported by the National Key Research and Development Program of China (2016YFD0501307) and China Natural Science Foundation (31372485).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. First author with equal contributions: Ruiting Shen, Peng Yin and Hua Yao.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Zhang WJ, Fang ZM, Liu WQ. NLRP3 inflammasome activation from Kupffer cells is involved in liver fibrosis of Schistosoma japonicum-infected mice via NF-κB. Parasit Vectors. 2019;12(1):29. doi: 10.1186/s13071-018-3223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Bossche J, Saraber DL. Metabolic regulation of macrophages in tissues. Cell Immunol. 2018;330:54–59. PMID: 29395037. doi: 10.1016/j.cellimm.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 3.Swanson KV. NLRP3 inflammasome activation from Kupffer cells is involved in liver fibrosis of Schistosoma japonicum-infected mice via NF-κB. Parasit Vectors. 2019;12(1):477–489. PMID: 31036962. doi: 10.1186/s13071-018-3223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277(25):22131–22139. PMID: 11940570. doi: 10.1074/jbc.M111883200 [DOI] [PubMed] [Google Scholar]
- 5.Li N, Zhou H, Wu H, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. PMID: 31121492. doi: 10.1016/j.redox.2019.101215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. PMID: 21124315. doi: 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 7.Sycheva MV, Kartashova OL, Shchepitova NE, Safronov AA. Antibiotic resistance of enterococci isolated from healthy humans and patients with various pathologies. Antibiot Khimioter. 2016;61(7–8):27–32. PMID: 29533558. [PubMed] [Google Scholar]
- 8.Lin CC, Hsu YF, Lin TC, Hsu HY. Antioxidant and hepatoprotective effects of punicalagin and punicalin on acetaminophen-induced liver damage in rats. Phytother Res. 2013;138(1):437–443. PMID: 11351354. doi: 10.1016/j.foodchem.2012.10.092 [DOI] [PubMed] [Google Scholar]
- 9.Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother. 2018;330(4):54–59. PMID: 11581226. doi: 10.1016/j.cellimm.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 10.Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269(2):262–268. PMID: 18468784. doi: 10.1016/j.canlet.2008.03.043 [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Cai D, Zhang L, et al. Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral Res. 2016;134:97–107. PMID: 27591143. doi: 10.1016/j.antiviral.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martino V, Morales J, Martinez-Irujo JJ, Font M, Monge A, Coussio J. Two ellagitannins from the leaves of Terminalia triflora with inhibitory activity on HIV-1 reverse transcriptase. Phytother Res. 2004;18(8):667–669. PMID: 15472920. doi: 10.1002/ptr.1504 [DOI] [PubMed] [Google Scholar]
- 13.Murthy KN, Reddy VK, Veigas JM, Murthy UD. Study on wound healing activity of Punica granatum peel. J Med Food. 2004;7(2):256–259. PMID: 15298776. doi: 10.1089/1096620041224111 [DOI] [PubMed] [Google Scholar]
- 14.Ren J, Pei Z, Chen X, et al. Inhibition of CYP2E1 attenuates myocardial dysfunction in a murine model of insulin resistance through NLRP3-mediated regulation of mitophagy. Biochim Biophys Acta Mol Basis Dis. 2019;1865(1):206–217. doi: 10.1016/j.bbadis.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 2018;103:115–124. PMID: 30248487. doi: 10.1016/j.molimm.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 16.Gaidt MM, Hornung V. The NLRP3 inflammasome renders cell death pro-inflammatory. J Mol Biol. 2018;430(2):133–141. PMID: 29203171. doi: 10.1016/j.jmb.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 17.Mangan M, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(8):588–606. PMID: 30026524. doi: 10.1038/nrd.2018.97 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Qin Y, Wang T, et al. Pyroptosis induced by enterovirus 71 and coxsackievirus B3 infection affects viral replication and host response. Sci Rep. 2018;8(1):2887. PMID: 29440739. doi: 10.1038/s41598-018-20958-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Zhang H, Liang H, Yuan Q. Purification, antioxidant activity and protein-precipitating capacity of punicalin from pomegranate husk. Food Chem. 2013;138(1):437–443. PMID: 23265509. doi: 10.1016/j.foodchem.2012.10.092 [DOI] [PubMed] [Google Scholar]