Abstract
Root–shoot communication has a critical role in plant adaptation to environmental stress. Grafting is widely applied to enhance the abiotic stress tolerance of many horticultural crop species; however, the signal transduction mechanism involved in this tolerance remains unknown. Here, we show that pumpkin- or figleaf gourd rootstock-enhanced cold tolerance of watermelon shoots is accompanied by increases in the accumulation of melatonin, methyl jasmonate (MeJA), and hydrogen peroxide (H2O2). Increased melatonin levels in leaves were associated with both increased melatonin in rootstocks and MeJA-induced melatonin biosynthesis in leaves of plants under cold stress. Exogenous melatonin increased the accumulation of MeJA and H2O2 and enhanced cold tolerance, while inhibition of melatonin accumulation attenuated rootstock-induced MeJA and H2O2 accumulation and cold tolerance. MeJA application induced H2O2 accumulation and cold tolerance, but inhibition of JA biosynthesis abolished rootstock- or melatonin-induced H2O2 accumulation and cold tolerance. Additionally, inhibition of H2O2 production attenuated MeJA-induced tolerance to cold stress. Taken together, our results suggest that melatonin is involved in grafting-induced cold tolerance by inducing the accumulation of MeJA and H2O2. MeJA subsequently increases melatonin accumulation, forming a self-amplifying feedback loop that leads to increased H2O2 accumulation and cold tolerance. This study reveals a novel regulatory mechanism of rootstock-induced cold tolerance.
Subject terms: Abiotic, Jasmonic acid, Plant signalling
Introduction
As sessile organisms, plants frequently face challenges from various environmental factors throughout their life cycle. In particular, cold stress is one of the most destructive abiotic stresses due to its adverse effects on plant growth and development and subsequent negative impacts on crop productivity1. To adapt and survive cold exposure, plants have evolved sophisticated defense mechanisms. When a plant senses that temperature decreases via molecular sensors, the production of secondary messengers is triggered, and a set of transcriptional regulators are subsequently activated to regulate plant tolerance2.
At the whole-organism level, root-to-shoot communication is crucial for the increased survival of plants under environmental stress. Under drought conditions, roots produce more abscisic acid (ABA), which is then transported to the leaves to reduce water loss via stomatal closure3. In many horticultural crop species, grafting is widely applied to enhance plant tolerance to various environmental stresses, such as soil-borne pathogens, salt, and low temperature. Grafting-induced plant tolerance is associated with the inherent resistance of rootstocks and some rootstock-sourced signals that are transported to shoots and subsequently regulate shoot responses4. Therefore, grafting is also a useful tool to reveal the signaling mechanisms related to root–shoot communication. For instance, by using cucumber scions grafted onto heat-tolerant luffa rootstock, Li et al.5 revealed that root-produced ABA as a long-distance signal could alter the expression of heat shock protein (HSP) 70 and subsequently improve heat tolerance of the shoots.
Melatonin (N-acetyl-5-methoxytryptamine) was initially identified as an essential animal hormone that has regulatory roles in various biological processes6. In 1995, melatonin was identified in vascular plants for the first time7,8. A number of subsequent studies have shown phytomelatonin to be an essential regulator in plant growth and development; postharvest physiology; and defense against various environmental stresses, such as pathogen infection, drought, salinity, nutrient deficiency, and heavy metals9–11. In particular, the recent identification of the first phytomelatonin receptor (CAND2/PMTR1) in Arabidopsis has opened the door to consider melatonin a new phytohormone12,13. Increasing numbers of studies have indicated that melatonin enhances the cold tolerance of various plant species, including melon, tomato, watermelon, and Arabidopsis14. Moreover, some evidence has shown melatonin to be a novel long-distance signal that can be transported from roots to shoots15–17. Therefore, it would be interesting to investigate the involvement of melatonin in grafting-induced cold tolerance.
In plants, jasmonates (JAs) such as jasmonic acid (JA) and its methyl ester (methyl jasmonate, MeJA) act as important phytohormones that regulate multiple plant processes, such as seed germination, root growth, flowering, leaf senescence, and defense responses to various biotic and abiotic stresses18,19. Accumulating amounts of data have shown that JAs have a positive role in inducing plant tolerance to cold stress19. Cold exposure rapidly induces JA biosynthesis-related genes and subsequent JA accumulation20. Moreover, exogenous application of MeJA enhances Arabidopsis tolerance to cold, while mutants defective in JA biosynthesis or signaling exhibit hypersensitivity to cold stress19. Recent studies involving grafting experiments have shown that JA is involved in root and shoot communication to fine-tune plant responses to shoot wounding and root-knot nematodes21,22. However, little is known about the involvement of JA in root–shoot communication under cold stress.
Watermelon is a widely cultivated vegetable crop species worldwide and is very sensitive to cold stress23. Grafting onto pumpkin can induce watermelon tolerance to cold24,25. Our recent study suggests that melatonin application to the roots can confer cold tolerance to the shoot via xylem transport, and such induction involves the expression of several genes involved in JA signaling and the production of H2O217, an important secondary messenger in the cold response26. This raises the possibility that melatonin has an important role in rootstock-induced shoot tolerance against the cold by interacting with JA and H2O2. To test this assumption, the present study evaluated the roles of melatonin, MeJA, and H2O2 and their interaction in rootstock-induced cold tolerance. Our results provide convincing evidence that melatonin-induced cold tolerance of grafted watermelon plants essentially involves MeJA and H2O2 signaling. This study provides novel insight into the mechanism of rootstock-induced cold tolerance in cucurbits.
Materials and methods
Plant materials
Three cucurbit species, namely, watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai cv. Nongkeda No. 5, Cl), pumpkin (Cucurbita moschata Duch cv. Weizhen No. 1, Cm), and figleaf gourd (Cucurbita ficifolia Bouché, Cf), were used in the current study. Germinated seeds of Cl, Cm, and Cf as rootstocks were sown into plastic pots (7 × 7 × 7.5 cm (length × width × height, respectively)) filled with 210 cm3 of commercial peat-based compost, and 7 d later, germinated seeds of Cl (as scions) were sown. Top insertion grafts were performed when the cotyledons of watermelon (as scions) had fully expanded27. The resulting three groups of grafted seedlings were designated Cl/Cl, Cl/Cm, and Cl/Cf; Cl/Cl plants were used as control. The plants were grown in growth chambers with a temperature of 25/18 °C (day/night), a 12-h photoperiod, and photosynthetic photon flux density (PPFD) of 400 μmol m−2 s−1. The seedlings were watered every 2 d and supplied with Hoagland’s nutrient solution every 3 d.
Experimental design
Experiment 1. To evaluate the effects of different rootstocks on scion tolerance to cold stress, Cl/Cl, Cl/Cm, and Cl/Cf plants with four true leaves were transferred into growth chambers maintained at 25 °C for the control treatment or 4 °C for cold treatment. At 0, 6, 12, 18, and 24 h after cold treatment, the relative expression levels of C-REPEAT BINDING FACTOR 1 (ClCBF1) and ClCBF2 were measured. At 36 h after cold treatment, the maximum photochemical efficiency of PSII (Fv/Fm) and the relative electrical conductivity (REC) were measured. At 12 h after cold treatment, root or leaf samples were harvested for biochemical assays.
Experiment 2. To determine the effects of exogenous melatonin, MeJA, and H2O2 on the cold tolerance of Cl/Cl seedlings, the leaves were first sprayed with distilled water (as a control), melatonin at 150 μM17, MeJA at 200 μM19, or H2O2 at 2 mM5. Melatonin (Sigma-Aldrich, St. Louis, MO, USA) or MeJA (Sigma-Aldrich) was dissolved in ethanol followed by dilution with distilled water at a ratio of 1/10,000 (v/v). Each plant was sprayed with 20 mL of the respective chemical solution or distilled water (as a control). At 12 h after pretreatment with melatonin, MeJA, or H2O2, the plants were subjected to 4 °C temperature. To block the synthesis or accumulation of melatonin, JA, or H2O2 in Cl/Cl plants, the leaves were pretreated with 100 μM p-chlorophenylalanine (CPA, a melatonin synthesis inhibitor)28,29, 5 mM diethyldithiocarbamic acid (DIECA, a JA biosynthesis inhibitor)30, or 20 μM diphenylene iodonium (DPI, an inhibitor of NADPH oxidase, which produces H2O2)31. Eight hours later, the plants were sprayed with melatonin or MeJA, and 12 h later, they were exposed to 4 °C.
Experiment 3. To assess the involvement of melatonin, MeJA, and H2O2 and their interactions in rootstock-induced cold tolerance, the leaves of Cl/Cm and Cl/Cf seedlings were sprayed with CPA, DIECA, or DPI 8 h prior to cold exposure. The leaves were sampled at 12 h, whereas the Fv/Fm and REC were measured at 36 h after cold exposure.
Cold tolerance assays
The Fv/Fm was measured using an imaging pulse-amplitude-modulated (PAM) chlorophyll fluorometer (Heinz Walz, GmbH, Effeltrich, Germany) according to the method described by Li et al.5. The REC was determined and calculated as described by Hong et al.32.
Melatonin measurements
Melatonin was extracted and measured as described previously33. Frozen samples (0.5 g) were homogenized in 5 mL of acetone–methanol buffer (acetone/methanol/water = 89/10/1) on ice. After centrifugation (5,000 g, 4 °C) for 10 min, 0.5 mL of trichloric acid (1%) was added to the supernatant for protein precipitation. After centrifugation at 10,000×g for 10 min at 4 °C, the supernatants were used to quantify melatonin levels using an ELISA kit (Shanghai Lanpai Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions.
Quantification of MeJA
MeJA was extracted as described by Pan et al.34. For the extraction of MeJA, 0.5 g of frozen leaf samples was homogenized in 5 mL of 1-propanol/H2O/concentrated HCl (2/1/0.002, v/v/v) and subsequently incubated overnight. The extracts and 5 mL of dichloromethane were then mixed together, after which the mixture was shaken at 4 °C for 30 min. After centrifugation, the obtained lower phase was dried under a stream of N2 gas. The residue was then dissolved in methanol. MeJA concentrations were analyzed using an ELISA kit (China Agricultural University, Beijing, China) following the manufacturer’s instructions.
Analysis of H2O2
H2O2 in the leaf samples was measured as described by Bellincampi et al.35. Briefly, the leaf material (0.3 g) was homogenized in 2 mL of 0.2 M HClO4. After centrifugation (10,000×g, 4 °C) for 15 min, an aliquot of supernatant (0.5 mL) was added to 0.5 mL of assay reagent consisting of 500 μM ammonium ferrous sulfate, 200 μM xylenol orange, 50 mM H2SO4, and 200 mM sorbitol. After incubation for 1 h, the absorbance at OD560 was recorded. DAB staining of H2O2 was performed following the protocol described by Thordal-Christensen et al.36.
qRT-PCR analysis
Total RNA was extracted from the leaves using an RNA extraction kit (AxGen, Union City, CA, USA). Residual DNA was removed using a DNase Mini Kit (Qiagen, Hilden, Germany). The isolated total RNA (1 μg) was reverse transcribed using a ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). qRT-PCR was then performed using an iCycler iQ Multicolor PCR Detection System (Bio-Rad, Hercules, CA, USA) as described by Li et al.17. The primers used for qRT-PCR are listed in Supplementary Table S1. The relative expression levels were standardized to those of watermelon β-ACTIN and were calculated as described by Livak and Schmittgen37,38.
Statistical analysis
The experiment was carried out in accordance with a completely randomized design, with three independent biological replicates. Each replicate included 15 plants. For statistical analysis, the data were analyzed using variance (ANOVA), and P values < 0.05 were considered statistically significant according to Tukey’s test.
Results
Pumpkin and figleaf gourd rootstocks induced cold tolerance in watermelon shoots
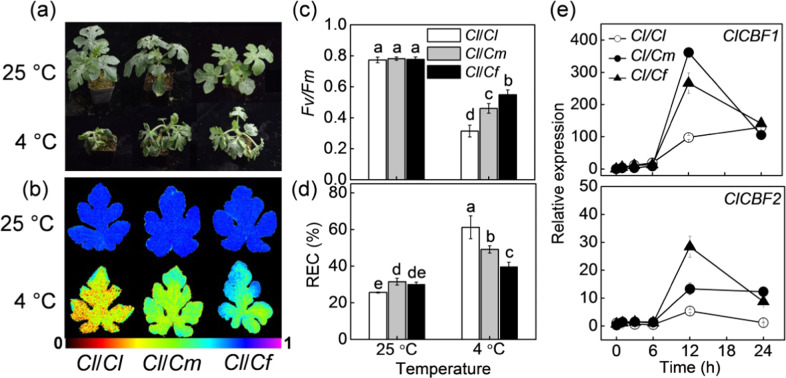
We first compared the leaf phenotypes, Fv/Fm values, and REC of watermelon plants grafted onto the rootstocks of watermelon (Cl/Cl), pumpkin (Cl/Cm), and figleaf gourd (Cl/Cf). Under normal conditions at 25 °C, no significant differences in plant growth or Fv/Fm were observed among the different grafted plants, although the REC of the Cl/Cm and Cl/Cf plants was slightly higher than that of the Cl/Cl plants (Fig. 1a–d). Cold stress caused severe leaf wilting, a significant decrease in Fv/Fm, and an increase in REC of the Cl/Cl plants. However, pumpkin and especially figleaf gourd as rootstocks alleviated the cold-induced wilting, Fv/Fm decrease, and REC increase in watermelon leaves. For example, the Fv/Fm of Cl/Cm and Cl/Cf plants were 0.46 and 0.55, respectively, which was much higher than that (0.31) of Cl/Cl plants at 36 h after cold exposure.
Fig. 1. Pumpkin or figleaf gourd rootstocks induced cold tolerance and the C-REPEAT BINDING FACTOR (CBF) transcripts in watermelon shoots.
Watermelon plants grafted with roots of watermelon (Cl/Cl), pumpkin (Cl/Cm), and figleaf gourd (Cl/Cf) were treated with cold at 4 °C for 36 h. a Chilling phenotypes. b Images of the maximum photochemical efficiency of PSII (Fv/Fm). The false-color code depicted at the bottom of the image ranges from 0 (black) to 1 (purple). c The average values of Fv/Fm. d Relative electrical conductivity (REC). The data from a to d were measured at 36 h after cold treatment. e Changes in the relative expression of ClCBF1 and ClCBF2 at 0, 6, 12, 18, and 24 h, respectively, after cold treatment. The data are the means of three replicates (±SDs). The means denoted with different letters differed significantly at P < 0.05
The relative expression of ClCBF1 and ClCBF2 was upregulated after cold treatment, and the highest peak occurred at 12 h in nearly all of the grafted plants; however, the highest expression of ClCBF1 in the Cl/Cl plants occurred at 24 h after cold exposure (Fig. 1e). Importantly, Cl/Cm and Cl/Cf plants showed more significant increases in transcript levels of ClCBF1 and ClCBF2 than did Cl/Cl plants after cold exposure. For example, ClCBF1 transcript levels were upregulated 98.1-, 361.6-, and 266.4-fold in Cl/Cl, Cl/Cm, and Cl/Cf plants, respectively, while the ClCBF2 transcripts were upregulated 5.3-, 13.3-, and 28.4-fold, respectively, at 12 h after cold stress.
Different rootstocks induced melatonin accumulation differently in leaves of watermelon plants under cold stress
At the optimum growth temperature, the melatonin contents in pumpkin and figleaf gourd roots were similar to and lower than those in watermelon roots, respectively (Fig. 2). However, both pumpkin and figleaf gourd as rootstocks increased the melatonin contents and increased the relative expression of N-ACETYLSEROTONIN O-METHYLTRANSFERASE (ClASMT), a key gene involved in melatonin biosynthesis, in the leaves. Cold stress-induced melatonin accumulation in both the roots and leaves and upregulated the relative expression of ClASMT in the leaves of all grafted seedlings at 12 h after cold treatment. Interestingly, the melatonin contents in the roots of Cl/Cm and especially Cl/Cf were obviously higher than those in the Cl/Cl roots after cold exposure. Moreover, the levels of melatonin and ClASMT transcripts in the Cl/Cm and Cl/Cf leaves were also higher than those in the Cl/Cl leaves.
Fig. 2. The transcript abundance of N-ACETYLSEROTONIN O-METHYLTRANSFERASE (ClASMT) and melatonin accumulation in response to different rootstocks and cold stress.

The seedlings were treated as described in Fig. 1. Root and leaf samples were harvested at 12 h after the cold exposure. The data are presented as the means of three replicates (±SDs). The means denoted with different letters differ significantly at P < 0.05
Pumpkin and figleaf gourd rootstocks induced MeJA and H2O2 accumulation in leaves of watermelon plants under cold stress
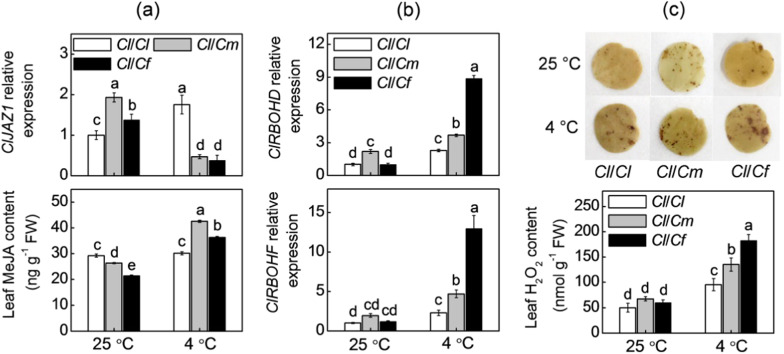
Under normal conditions at 25 °C, pumpkin or figleaf gourd as rootstocks decreased the MeJA contents but increased the relative expression of JASMONATE ZIM-DOMAIN 1 (ClJAZ1, a repressor gene involving in JA signaling) in watermelon leaves (Fig. 3a). The MeJA content in Cl/Cl leaves was almost unchanged after exposure to cold stress; however, that in Cl/Cm and Cl/Cf leaves significantly increased. The MeJA contents in the Cl/Cm and Cl/Cf leaves were 40.4% and 19.7% higher than those in the Cl/Cl leaves, respectively, at 12 h after cold stress. The relative expression of ClJAZ1 was upregulated in the Cl/Cl leaves but downregulated in the Cl/Cm and Cl/Cf leaves of plants under cold stress. Unexpectedly, no significant differences were observed in root MeJA content among the different grafted plants under cold stress (Supplementary Fig. S1).
Fig. 3. Accumulation of methyl jasmonate (MeJA) and H2O2 in leaves in response to different rootstocks and cold stress.
The treatments were the same as those described in Fig. 1. Leaf samples were harvested at 12 h after the cold exposure. a Transcript abundances of JASMONATE ZIM-DOMAIN (JAZ) 1 and MeJA contents. b Transcript abundances of RESPIRATORY BURST OXIDASE HOMOLOG (RBOH) D and RBOHF. c DAB staining and H2O2 contents. The data are presented as the means of three replicates (±SDs). The means denoted with different letters differ significantly at P < 0.05
The H2O2 generated by RESPIRATORY BURST OXIDASE HOMOLOG (RBOH) functions as an important signaling molecule in regulating plant tolerance to cold stress. Thus, we investigated whether H2O2 is involved in rootstock-induced cold tolerance. At optimum growth temperatures, pumpkin as a rootstock induced the accumulation of ClRBOHD transcripts in watermelon leaves compared to watermelon rootstocks (Fig. 3b). However, there were no significant differences in ClRBOHF transcripts or H2O2 content among any of the grafted plants (Fig. 3b, c). Exposure to 4 °C increased H2O2 accumulation and ClRBOHD and ClRBOHF transcript levels in all grafted plants. The leaves of Cl/Cm and especially Cl/Cf plants showed higher H2O2 levels and ClRBOHD/F transcripts than did those of Cl/Cm under cold stress. For example, the H2O2 contents in the Cl/Cm and Cl/Cf leaves were 41.8% and 91.3% higher than those in the Cl/Cl leaves, respectively, after cold exposure.
Role of melatonin in rootstock-induced accumulation of MeJA and H2O2 and cold tolerance
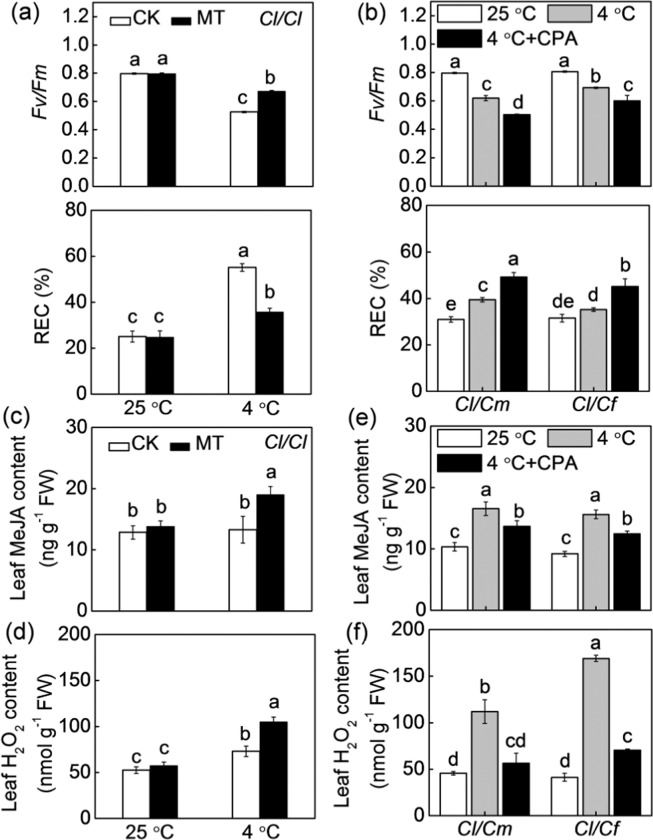
To evaluate the role of melatonin in rootstock-induced cold tolerance, we first analyzed the effect of exogenous melatonin on the cold tolerance of Cl/Cl plants. Melatonin (150 μM) pretreatment alleviated the cold-induced Fv/Fm decrease and REC increase (Fig. 4a). In melatonin-pretreated plants, the Fv/Fm was 14.8% higher while the REC was 35.0% lower after exposure to cold stress than in the control plants. As CPA application can prevent melatonin biosynthesis29, we thus evaluated the effect of CPA on the cold tolerance of Cl/Cm and Cl/Cf plants. Pretreatment with CPA attenuated the pumpkin- and figleaf gourd rootstock-induced alleviation of cold stress, as reflected by the Fv/Fm decrease and REC increase (Fig. 4b).
Fig. 4. Involvement of melatonin in pumpkin or figleaf gourd rootstock-induced cold tolerance and accumulation of MeJA and H2O2 in watermelon leaves.
a Changes in the Fv/Fm and REC of watermelon plants grafted onto watermelon (Cl/Cl). b Changes in the Fv/Fm and REC of watermelon plants grafted onto pumpkin (Cl/Cm) or figleaf gourd (Cl/Cf). c MeJA and d H2O2 contents in Cl/Cl plants. e MeJA and f H2O2 contents in Cl/Cm and Cl/Cf plants. For a, c, and d, the leaves were sprayed with melatonin (150 μM) 12 h prior to cold exposure at 4 °C. For b, e, and f, the leaves were sprayed with p-chlorophenylalanine (CPA, 100 μM) 8 h prior to cold exposure at 4 °C. The data from a and b were determined at 36 h after cold exposure. MeJA and H2O2 were measured at 12 h after cold treatment. The data are the means of three replicates (±SDs). The means denoted with different letters differ significantly at P < 0.05
At the optimum growth temperatures, exogenous melatonin application did not induce significant changes in MeJA and H2O2 levels in Cl/Cl leaves (Fig. 4c, d). However, the contents of MeJA and H2O2 increased by 42.9% and 44.1%, respectively, in response to melatonin under cold stress. More importantly, pretreatment with CPA attenuated or completely blocked pumpkin- and figleaf gourd rootstock-induced increases in MeJA and H2O2 accumulation in the leaves (Fig. 4e, f). These data suggest that melatonin has an important role in pumpkin- or figleaf gourd rootstock-induced accumulation of MeJA and H2O2 and cold tolerance.
Roles of MeJA and H2O2 in melatonin- or rootstock-induced cold tolerance
To evaluate the roles of MeJA and H2O2 in cold tolerance acquired by grafting and melatonin, we first evaluated the effects of foliar applications of MeJA or H2O2 on the cold tolerance of Cl/Cl plants. Both MeJA (200 μM) and H2O2 (2 mM) application alleviated the cold-induced decrease in Fv/Fm and increase in REC of Cl/Cl plants (Fig. 5a). Pretreatment with DIECA (an inhibitor of JA biosynthesis, 5 mM) or DPI (an inhibitor of H2O2 production, 20 μM) attenuated or abolished the pumpkin- or figleaf gourd rootstock-induced alleviation of cold stress, as reflected by a decline in Fv/Fm and an increase in REC (Fig. 5b). Furthermore, DIECA or DPI application also prevented the melatonin-induced Fv/Fm increase and REC decrease after exposure to cold stress (Fig. 5c). Taken together, these results suggest that MeJA and H2O2 are involved in melatonin- or rootstock-induced cold tolerance.
Fig. 5. Involvement of MeJA and H2O2 in melatonin- or pumpkin or figleaf gourd rootstock-induced cold tolerance in watermelon leaves.
a Changes in the Fv/Fm and REC of watermelon plants grafted onto watermelon (Cl/Cl). Plant leaves were sprayed with MeJA (200 μM) or H2O2 (2 mM) 12 h prior to cold exposure at 4 °C. b Changes in the Fv/Fm and REC in watermelon plants grafted onto pumpkin (Cl/Cm) or figleaf gourd (Cl/Cf). Plant leaves were sprayed with diethyldithiocarbamic acid (DIECA, 5 mM) or diphenylene iodonium (DPI, 20 μM) 8 h prior to cold exposure at 4 °C. c Changes in the Fv/Fm and REC of Cl/Cl plants. The plant leaves were sprayed with DIECA or DPI 8 h prior to melatonin treatment. Twelve hours later, the plants were exposed to cold at 4 °C. The data are presented as the means of three replicates (±SDs). The means denoted with different letters differ significantly at P < 0.05
Role of MeJA in rootstock-induced accumulation of melatonin and H2O2
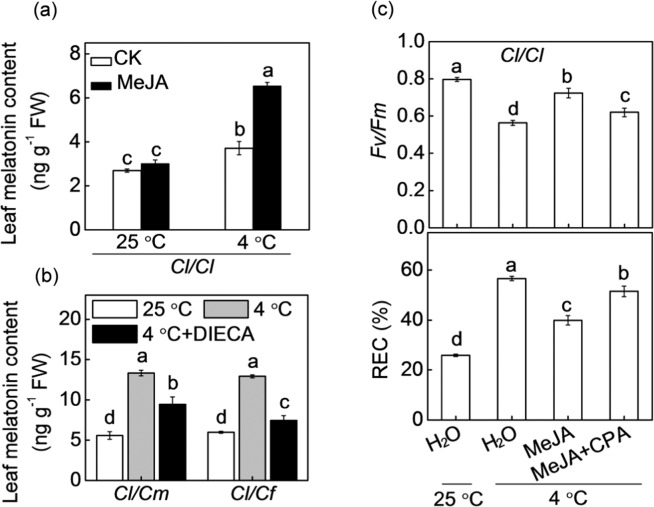
To evaluate whether MeJA regulates melatonin biosynthesis in a feedback manner, we analyzed the response of melatonin to MeJA. The application of MeJA significantly increased melatonin accumulations in the Cl/Cl leaves after cold stress (Fig. 6a), while DIECA attenuated the pumpkin- or figleaf gourd rootstock-induced increase in melatonin contents (Fig. 6b). Moreover, CPA pretreatment attenuated the MeJA-induced Fv/Fm increase and REC decrease of Cl/Cl leaves of plants under cold stress (Fig. 6c).
Fig. 6. Involvement of melatonin in MeJA-induced cold tolerance of grafted watermelon plants.
a Changes in melatonin contents in watermelon plants grafted onto watermelon rootstock (Cl/Cl). Seedlings were pretreated with MeJA (200 μM) and then subjected to 4 °C for 12 h. b Changes in melatonin contents in watermelon plants grafted onto pumpkin (Cl/Cm) or figleaf gourd (Cl/Cf). Leaves were pretreated with DIECA 8 h prior to cold exposure at 4 °C for 12 h. c Changes in the Fv/Fm and REC of Cl/Cl plants. Plants were pretreated with CPA, and 8 h later, the plants were sprayed with MeJA. Twelve hours later, the plants were subjected to 4 °C for 36 h. The data are presented as the means of three replicates (±SDs). The means denoted with different letters differ significantly at P < 0.05
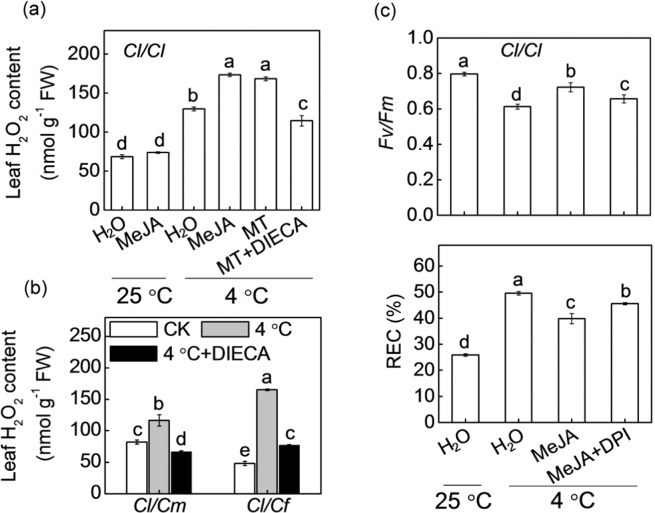
To further characterize the role of H2O2 in MeJA-enhanced cold tolerance, we first evaluated the effects of MeJA on H2O2 accumulation in Cl/Cl plants. MeJA application induced an increase in H2O2 accumulation under cold stress (Fig. 7a), while DIECA pretreatment completely abolished the melatonin-induced increase in H2O2. Similarly, DIECA also completely blocked the pumpkin- and figleaf gourd rootstock-induced increase in H2O2 accumulation under cold stress (Fig. 7b). Furthermore, DPI pretreatment attenuated the MeJA-induced alleviation of cold stress, as reflected by the Fv/Fm decrease and REC increase (Fig. 7c).
Fig. 7. Involvement of H2O2 in MeJA-induced cold tolerance of grafted watermelon plants.
a Changes in H2O2 contents in watermelon plants grafted onto watermelon (Cl/Cl). Seedlings were pretreated with or without DIECA, and 8 h later, the plants were sprayed with MeJA or melatonin. After 12 h, the plants were subjected to 4 °C for 12 h. b Changes in H2O2 contents in watermelon plants grafted onto pumpkin (Cl/Cm) or figleaf gourd (Cl/Cf). Plant leaves were sprayed with DIECA, and 8 h later, the plants were subjected to 4 °C for 12 h. c Changes in Fv/Fm and REC of Cl/Cl plants. Plants were pretreated with DPI, and 8 h later, the plants were sprayed with MeJA. After 12 h, the plants were subjected to 4 °C for 36 h. The data are presented as the means of three replicates (±SDs). The means denoted with different letters differ significantly at P < 0.05
Discussion
Melatonin is involved in rootstock-induced cold tolerance
Grafting onto tolerant rootstocks is well known to enhance plant tolerance to various environmental stresses, such as soil-borne pathogens, cold, and salinity. Consistent with the findings of previous studies24,25, we found that the rootstocks of pumpkin or figleaf gourd enhanced watermelon tolerance to cold stress (Fig. 1). By using RNA-seq analysis, Xu et al.39 found that pumpkin rootstocks could alter the expression of COLD-RESPONSIVE (COR) genes in shoots of watermelon plants under chilling stress. CBFs, the major activators of a subset of COR genes, have essential roles in modulating plant responses to cold stress40. In the present study, pumpkin or figleaf gourd as rootstocks significantly increased the transcripts of ClCBF1 and ClCBF2 in watermelon leaves after cold exposure (Fig. 1e). Therefore, it is plausible that some long-distance signal(s) originating from rootstocks are involved in inducing the shoot response to cold stress.
Increasing amounts of evidence have demonstrated that melatonin can induce cold tolerance in various plant species, and such induction is associated with the upregulated expression of CBFs41,42. Here, we observed that pumpkin or figleaf gourd rootstocks increased the accumulation of melatonin in leaves of watermelon plants under cold stress (Fig. 2). Furthermore, exogenous melatonin improved the cold tolerance of Cl/Cl plants, while inhibition of melatonin biosynthesis by CPA attenuated pumpkin- or figleaf gourd rootstock-induced cold tolerance (Fig. 4a, b). These results suggest that melatonin has an important role in pumpkin- or figleaf gourd rootstock-induced cold tolerance. It is worth noting that the increased melatonin contents in Cl/Cm or Cl/Cm leaves were accompanied by greater increases in melatonin contents in pumpkin or figleaf gourd rootstocks, respectively, after exposure to cold stress. Our previous study provided evidence that, as a long-distance signal, melatonin can be transported from roots to shoots via the xylem, subsequently inducing shoot tolerance to cold stress17. These data thus suggest that under cold stress, pumpkin- or figleaf gourd rootstock-sourced melatonin may act as a long-distance signal that induces melatonin accumulation and cold tolerance in watermelon leaves.
Melatonin and MeJA function together in a self-amplifying feedback loop in rootstock-induced cold tolerance
Like melatonin, JAs have important roles in regulating plant tolerance to cold stress43. Under cold stress, JAs trigger the degradation of JAZ proteins, which releases INDUCER OF CBF EXPRESSION (ICE) from repression and then activates CBF-mediated transcriptional regulatory cascades20. In the current study, we observed that pumpkin and figleaf gourd rootstocks led to increased MeJA accumulation but decreased ClJAZ1 transcript levels in leaves of watermelon plants under cold stress (Fig. 3a). Moreover, exogenous MeJA improved the cold tolerance of Cl/Cl plants (Fig. 5a), while inhibition of JA biosynthesis by DIECA decreased the cold tolerance of Cl/Cm and Cl/Cf plants (Fig. 5b), suggesting that MeJA is involved in rootstock-induced cold tolerance.
Recently, crosstalk between melatonin and MeJA in plant defense against biotic stress has been revealed in some studies. For instance, Liu et al.44 revealed that melatonin induces tomato fruit resistance to Botrytis cinerea by activating the JA signaling pathway. However, the crosstalk between melatonin and MeJA in abiotic stress responses remains largely unknown. Our results showed that melatonin and MeJA increased the accumulation of each other in the leaves of Cl/Cl plants under cold stress (Figs. 4c and 6a), while pretreatment with CPA or DIECA attenuated the pumpkin- or figleaf gourd rootstock-induced increase in MeJA or melatonin, respectively (Figs. 4e and 6b). Furthermore, melatonin- and MeJA-induced cold tolerance was attenuated or completely blocked by DIECA and CPA, respectively, in Cl/Cl plants (Figs. 5c and 6c). Taken together, these results suggest that melatonin and MeJA function together in a self-amplifying feedback loop, in which melatonin induces MeJA accumulation and MeJA subsequently increases melatonin accumulation during the cold response of Cl/Cm and Cl/Cf plants. Such crosstalk between melatonin and MeJA during the cold response is similar to that between ABA and H2O2 in plant responses to heat and oxidative stresses45.
MeJA mediates melatonin- and rootstock-induced H2O2 accumulation and subsequent cold tolerance
As an important secondary messenger, the H2O2 generated by RBOH has an essential role in regulating plant tolerance against various abiotic stresses, including cold stress26,46. In our study, pumpkin or figleaf gourd as rootstocks increased the accumulation of H2O2, as well as the levels of ClRBOHD and ClRBOHF transcripts under cold stress (Fig. 3b, c). Additional experiments showed that exogenous application of H2O2 enhanced the cold tolerance of Cl/Cl plants, while inhibition of H2O2 generation by DPI prevented pumpkin- or figleaf gourd rootstock-induced cold tolerance (Fig. 5a, b). These results indicate that H2O2 is involved in rootstock-induced cold tolerance, and these findings are consistent with previous findings that H2O2 is involved in rootstock-induced heat tolerance of cucumber plants5.
Melatonin is a well-documented antioxidant that can effectively remove reactive oxygen species (ROS) and consequently alleviate oxidative stress47. However, accumulating studies have shown that melatonin can increase H2O2 accumulation in plant tissues, and as a key signaling molecule, H2O2 mediates melatonin-induced lateral root formation, stomatal closure, and tolerance to environmental stresses12,48,49. In agreement with these findings, our findings also showed that melatonin increased the H2O2 accumulation in Cl/Cl leaves, while pretreatment with CPA completely abolished the pumpkin- or figleaf gourd rootstock-induced increase in H2O2 under cold stress (Fig. 4d, f). Moreover, DPI pretreatment completely blocked the melatonin-induced cold tolerance of Cl/Cl plants (Fig. 5c). Thus, it is apparent that H2O2 mediates melatonin-induced cold tolerance of Cl/Cm and Cl/Cf plants.
It has been well established that JA and H2O2 function synergistically to regulate multiple physiological processes, such as leaf senescence, drought tolerance, stomatal closure, and wound-induced responses, and JA functions upstream of H2O250,51. Consistent with the data of these studies, our data show that MeJA mediates the melatonin-induced cold tolerance of Cl/Cm and Cl/Cf plants by inducing H2O2 accumulation. This conclusion is based on the following evidence: (1) foliar application of MeJA increased H2O2 accumulation in Cl/Cl plants in response to cold (Fig. 7a), while H2O2 application failed to induce an increase in MeJA accumulation (Supplementary Fig. S2); (2) pretreatment with DIECA prevented melatonin- or rootstock-induced H2O2 accumulation (Fig. 7a, b); and (3) application of DPI attenuated the MeJA-induced cold tolerance of Cl/Cl plants (Fig. 7c).
To date, the mechanisms underlying rootstock-induced shoot tolerance to cold stress are unclear; however, we have demonstrated in the present study that, as a potential root-to-shoot signal, melatonin is involved in rootstock-induced cold tolerance. The rootstock-promoted melatonin accumulation in leaves induces MeJA accumulation, which in turn increases melatonin accumulation, forming a self-amplifying feedback loop. MeJA further triggers H2O2 generation and subsequently enhances cold tolerance (Fig. 8). Thus, melatonin-induced increases in MeJA and subsequent H2O2 have an essential role in rootstock-scion communication in response to cold stress.
Fig. 8. Model depicting the role of methyl jasmonate (MeJA) in melatonin-induced H2O2 accumulation and cold tolerance in watermelon plants grafted onto pumpkin or figleaf gourd rootstocks.
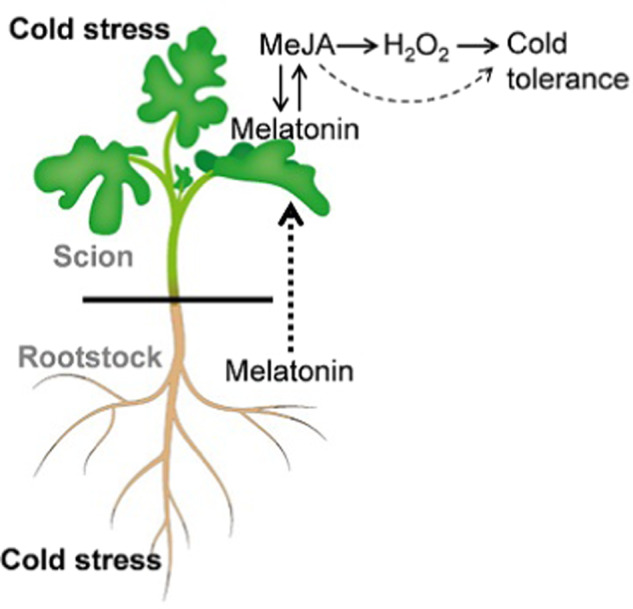
When grafted watermelon plants suffer cold stress, rootstock-sourced melatonin may act as a long-distance signal that induces melatonin accumulation in leaves. Melatonin accumulation in leaves induces MeJA accumulation, which in turn increases melatonin accumulation, forming a self-amplifying feedback loop. MeJA further triggers H2O2 generation and subsequently enhances cold tolerance
Supplementary information
Supporting information Figure S1, Figure S2, Table S1
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFD1000800), the National Natural Science Foundation of China (31801884, 31972479), the Earmarked Fund for Modern Agroindustry Technology Research System of China (CARS-25), and the Tang Scholar of Northwest A&F University.
Author contributions
H.L. and X.Z. planned and designed the research; H.L., Y.G., Z.L., K.X., J.C., J.M., and C.W. performed the experiments and analyzed the data; and H.L., G.A., and X.Z. wrote the manuscript. H.L. and Y.G. contributed equally to this work.
Conflict of interest
The authors declare no competing interests.
Footnotes
These authors contributed equally: Hao Li, Yanliang Guo.
Supplementary information
The online version contains supplementary material available at 10.1038/s41438-021-00496-0.
References
- 1.Rahman A. Auxin: a regulator of cold stress response. Physiol. Plant. 2013;147:28–35. doi: 10.1111/j.1399-3054.2012.01617.x. [DOI] [PubMed] [Google Scholar]
- 2.Guo XY, Liu DF, Chong K. Cold signaling in plants: Insights into mechanisms and regulation. J. Int. Plant Biol. 2018;60:745–756. doi: 10.1111/jipb.12706. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson S, Davies WJ. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 2010;33:510–525. doi: 10.1111/j.1365-3040.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YH, et al. Chill-induced decrease in capacity of RuBP carboxylation and associated H2O2 accumulation in cucumber leaves are alleviated by grafting onto figleaf gourd. Ann. Bot. 2007;100:839–848. doi: 10.1093/aob/mcm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, et al. Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ. 2014;37:2768–2780. doi: 10.1111/pce.12360. [DOI] [PubMed] [Google Scholar]
- 6.Calvo JR, González-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 2013;55:103–120. doi: 10.1111/jpi.12075. [DOI] [PubMed] [Google Scholar]
- 7.Dubbels R, et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995;18:28–31. doi: 10.1111/j.1600-079X.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 8.Hattori A, et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995;35:627–634. [PubMed] [Google Scholar]
- 9.Arnao MB, Hernández-Ruiz J. Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014;19:789–797. doi: 10.1016/j.tplants.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Li, H. et al. Alkanes (C29 and C31)-mediated intracuticular wax accumulation contributes to melatonin- and ABA-induced drought tolerance in watermelon. J. Plant Growth Regul. 10.1007/s00344-020-10099-z (2020).
- 11.Sun, Y. D. et al. Melatonin treatment improves the shelf-life and postharvest quality of table grape (Vitis labrusca L. cv. ‘Fengzao’). J. Berry Res. 10.3233/JBR-200569 (2020).
- 12.Wei J, et al. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018;65:e12500. doi: 10.1111/jpi.12500. [DOI] [PubMed] [Google Scholar]
- 13.Arnao MB, Hernández-Ruiz J. Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci. 2018;24:38–48. doi: 10.1016/j.tplants.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Bose SK, Howlader P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: a review. Environ. Exp. Bot. 2020;176:104063. doi: 10.1016/j.envexpbot.2020.104063. [DOI] [Google Scholar]
- 15.Tan DX, et al. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J. 2007;21:1724–1729. doi: 10.1096/fj.06-7745com. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S, et al. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant. 2014;152:714–728. doi: 10.1111/ppl.12218. [DOI] [PubMed] [Google Scholar]
- 17.Li H, et al. Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Sci. Rep. 2017;8:40858. doi: 10.1038/srep40858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, et al. Jasmonate action in plant growth and development. J. Exp. Bot. 2017;68:1349–1359. doi: 10.1093/jxb/erw495. [DOI] [PubMed] [Google Scholar]
- 19.Yu XX, et al. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019;46:197–212. doi: 10.1071/FP18106. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, et al. Jasmonate regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 cascade and freezing tolerance in Arabidopsis. Plant Cell. 2013;25:2907–2924. doi: 10.1105/tpc.113.112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, et al. Systemic root-shoot signaling drives jasmonate-based root defense against nematodes. Curr. Biol. 2019;29:3430–3438. doi: 10.1016/j.cub.2019.08.049. [DOI] [PubMed] [Google Scholar]
- 22.Schulze A, et al. Wound-induced shoot-to-root relocation of JA-Ile precursors coordinates Arabidopsis growth. Mol. Plant. 2019;12:1383–1394. doi: 10.1016/j.molp.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Rivero RM, et al. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–321. doi: 10.1016/S0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu HY, et al. Study on relationship between physiological changes and chilling tolerance in grafted watermelon seedlings under low temperature stress. Agric. Sin. Chin. 2003;36:1325–1329. [Google Scholar]
- 25.Shi XF, et al. iTRAQ-based quantitative proteomics analysis of cold stress-induced mechanisms in grafted watermelon seedlings. J. Proteom. 2019;192:311–320. doi: 10.1016/j.jprot.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis AR, et al. Cucurbit grafting. Crit. Rev. Plant Sci. 2008;27:50–74. doi: 10.1080/07352680802053940. [DOI] [Google Scholar]
- 28.Wen D, et al. Promoting roles of melatonin in adventitious root development of Solanumly copersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016;7:718. doi: 10.3389/fpls.2016.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park WJ. Melatonin as an endogenous plant regulatory signal: debates and perspectives. J. Plant Biol. 2011;54:143–149. doi: 10.1007/s12374-011-9159-6. [DOI] [Google Scholar]
- 30.Rajendran SK, et al. Differential activation of sporamin expression in response to abiotic mechanical wounding and biotic herbivore attack in the sweet potato. BMC Plant Biol. 2014;14:112. doi: 10.1186/1471-2229-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia XJ, et al. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009;150:801–814. doi: 10.1104/pp.109.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong SW, Lee U, Vierling E. Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol. 2003;132:757–767. doi: 10.1104/pp.102.017145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okazaki M, Ezura H. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. J. Pineal Res. 2009;46:338–343. doi: 10.1111/j.1600-079X.2009.00668.x. [DOI] [PubMed] [Google Scholar]
- 34.Pan X, Welti R, Wang X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010;5:986–992. doi: 10.1038/nprot.2010.37. [DOI] [PubMed] [Google Scholar]
- 35.Bellincampi D, et al. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000;122:1379–1385. doi: 10.1104/pp.122.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thordal-Christensen H, et al. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- 37.Kong QS, et al. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS ONE. 2014;9:e90612. doi: 10.1371/journal.pone.0090612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Xu JH, et al. Comparative transcriptome profiling of chilling stress responsiveness in grafted watermelon seedlings. Plant Physiol. Biochem. 2016;109:561–570. doi: 10.1016/j.plaphy.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Liu JY, Shi YT, Yang SH. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Int. Plant Biol. 2018;60:780–795. doi: 10.1111/jipb.12657. [DOI] [PubMed] [Google Scholar]
- 41.Shi H, et al. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015;59:334–342. doi: 10.1111/jpi.12262. [DOI] [PubMed] [Google Scholar]
- 42.Zhang N, et al. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015;66:647–656. doi: 10.1093/jxb/eru336. [DOI] [PubMed] [Google Scholar]
- 43.Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20:219–229. doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Liu CX, et al. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019;67:6116–6124. doi: 10.1021/acs.jafc.9b00058. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, et al. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014;65:4371–4383. doi: 10.1093/jxb/eru217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahammed GJ, et al. Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2–mediated stomatal closure. Environ. Exp. Bot. 2020;171:103960. doi: 10.1016/j.envexpbot.2019.103960. [DOI] [Google Scholar]
- 47.Zhang HM, Zhang YQ. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 48.Gong B, et al. Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiol. Plant. 2017;160:396–409. doi: 10.1111/ppl.12581. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, et al. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot. 2018;121:1127–1136. doi: 10.1093/aob/mcx207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suhita D, et al. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate-and abscisic acid-induced stomatal closure. Plant Physiol. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nazir F, Fariduddin Q, Khan TA. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere. 2020;252:126486. doi: 10.1016/j.chemosphere.2020.126486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information Figure S1, Figure S2, Table S1