Abstract
The control of brucellosis across sub-Saharan Africa is hampered by the lack of standardized testing and the use of tests with poor performance. This study evaluated the performance and costs of serological assays for human brucellosis in a pastoralist community in northern Tanzania. Serum collected from 218 febrile hospital patients was used to evaluate the performance of seven index tests, selected based on international recommendation or current use. We evaluated the Rose Bengal test (RBT) using two protocols, four commercial agglutination tests and a competitive enzyme-linked immunosorbent assay (cELISA). The sensitivity, specificity, positive predictive value, negative predictive value, Youden’s index, diagnostic accuracy, and per-sample cost of each index test were estimated. The diagnostic accuracy estimates ranged from 95.9 to 97.7% for the RBT, 55.0 to 72.0% for the commercial plate tests, and 89.4% for the cELISA. The per-sample cost range was $0.69–$0.79 for the RBT, $1.03–$1.14 for the commercial plate tests, and $2.51 for the cELISA. The widely used commercial plate tests performed poorly and cost more than the RBT. These findings provide evidence for the public health value of discontinuing the use of commercial agglutination tests for human brucellosis in Tanzania.
Subject terms: Bacteriology, Infectious-disease diagnostics, Policy and public health in microbiology, Laboratory techniques and procedures, Infection, Bacterial infection, Bacterial infection, Diagnosis, Health care economics, Health policy, Epidemiology, Population screening
Introduction
Brucellosis is considered to be the most widespread bacterial zoonosis of both veterinary and public health importance globally1. Over 500,000 new human cases are reported annually worldwide, mostly in low- and middle-income countries (LMICs)2. This figure is suspected to be an underestimation of the true incidence of the disease2, 3. Brucellosis is caused by bacteria of the genus Brucella and, in humans, the disease is most commonly caused by B. abortus, B. melitensis, and B. suis4. Human brucellosis cases are characterized by acute febrile illness that can progress to a chronic disease characterized by flu-like symptoms and musculoskeletal pain5. The occurrence of clinical signs in humans is highly variable, but an estimated 78% of patients experience fever and around 50% experience chronic musculoskeletal symptoms6. Up to 5% of acute cases suffer severe life-threatening complications1.
Given the wide variety of clinical manifestations of human brucellosis, diagnosis cannot be made solely on clinical grounds7–9. The United States Centers for Disease Control and Prevention (CDC) case definition for a confirmed brucellosis case is a clinically compatible illness with definitive laboratory evidence7. Definitive laboratory evidence is defined as a positive culture and identification of Brucella spp. from clinical samples or a four-fold or greater rise in Brucella antibody titer between acute and convalescent-phase serum samples collected at least two weeks apart6,9. In addition, probable cases are defined by the CDC as a clinically compatible illness with at least one of the following: epidemiologically linked to a confirmed Brucella case or having presumptive laboratory evidence. Presumptive laboratory evidence is defined as an antibody titer of ≥ 1:160 by serum agglutination test (SAT) or Brucella micro-agglutination test in one or more serum specimens obtained after onset of symptoms9. There are multiple case definitions in guidelines published by international organizations, in national surveillance programs, or in the scientific literature10–15. None of these case definitions allows the identification of all true brucellosis cases because of the imperfect performance of the recommended tests and variation in their performance at different stages of disease.
Healthcare facilities and laboratories in low-resource settings where brucellosis is endemic face several challenges in the diagnosis of human brucellosis. The recommended diagnostic methods (e.g. culture and serological testing of paired sera using the SAT) are technically demanding, have relatively slow turnaround times, are expensive, and are often not available in many endemic settings. Many commercially available plate agglutination tests, also known as rapid or febrile antigen Brucella agglutination tests, are widely used in human health facilities in the East Africa region, likely due to their perceived affordability and simplicity16–19. A small number of studies have shown that several commercial plate agglutination tests are inadequate for the diagnosis of brucellosis, but many different tests are currently available and in use across the region16–18. If the performance of all or many of the different tests of this type is as poor as the small number of existing studies suggest, the potential consequences of misdiagnosis are considerable. Thus, more evidence is needed to accurately quantify the performance characteristics of the range of kits in use and their affordability16,20,21. Although infections caused by B. abortus and B. melitensis cannot be differentiated with standard serological assays, i.e. those detecting antibodies to the smooth lipopolysaccharide (sLPS) of Brucella spp., some commercially available kits include separate abortus and melitensis suspensions, incorrectly suggesting that they can be used for this purpose22,23.
The Rose Bengal plate test (RBT), used in conjunction with confirmatory assays, such as culture or additional serological tests, is recommended by multiple international organizations for the diagnosis of human brucellosis6,7,9. There is considerable existing literature confirming the value of RBT in multiple contexts19,24–29. False negativity due to prozones (partial or no agglutination at low serum dilutions and complete agglutination at higher serum dilutions) and inability to detect non-agglutinating antibodies (IgA/IgG) have been raised as concerns with the RBT20,30. However, previous studies have found limited impact of prozones31,32. Although the RBT has been shown to be highly specific, there are recognized challenges for its use in clinical settings. False positivity can occur due to cross-reactivity with non-target pathogens or due to detection of antibodies attributable to previous exposure rather than being related to current illness, which is a significant challenge in brucellosis-endemic areas31. Other serological tests that employ the O-polysaccharide antigen (e.g. complement fixation test, SAT, and some ELISA kits) share some of these challenges. Application of the RBT with serial dilutions and use of a 1:8 titer cut-off (RBT 1:8) as opposed to reading the result using an undiluted sample (RBT 1:2) has been shown to improve the specificity of the test in healthy contacts of cases, without any significant decrease in sensitivity or increase in complexity, time for completion or cost31.
Competitive format ELISA tests have previously shown high sensitivity (98.3%) and specificity (99.7%) for detecting human brucellosis33 and have the advantage of allowing the use of the same assay to detect antibodies in various livestock hosts34 as well as in humans33,35,36. In comparison to rapid format tests, ELISA tests often show good performance, but the higher cost and infrastructural requirements mean they are often used as a second-line test in low-resource settings37,38. Several previous studies in Tanzania and the broader region have used the competitive ELISA (cELISA) evaluated in this study in combination with the RBT to test for brucellosis in humans, particularly in pastoralist communities39–42. The cELISA evaluated in this study has no recommended reference cut-off for use in human testing, having been developed and validated for livestock testing. The use of this specific kit for human testing therefore requires evaluation of the cut-off in different human populations and contexts.
Several measures can be used to characterize the diagnostic performance of serological tests, namely the sensitivity and specificity, positive and negative predictive values, diagnostic accuracy (the percentage of cases correctly diagnosed), Receiver Operating Characteristic (ROC) curves, and the Youden’s Index43. Different measures relate to the different aspects of the diagnostic process, such as the ability to correctly discriminate between samples from diseased and non-diseased individuals (sensitivity, specificity, Youden’s index (YI), area under the curve), and predictive ability (diagnostic accuracy, predictive values)43,44. The positive and negative predictive values and the diagnostic accuracy of a test all vary according to the prevalence of disease43,45. This variation by prevalence limits estimates of these metrics to the specific population evaluated and complicates extrapolations to other populations. While the sensitivity, specificity, YI, and area under the curve do not vary by prevalence and can be extrapolated to other populations, estimates of these metrics are also affected by population-specific disease dynamics, limiting inference between populations43,44. Diagnostic accuracy is commonly used alongside cost estimates to assess the cost-effectiveness of diagnostic tests in a specific geographical area46–48. Interpretation of the test accuracy metric should always be weighed considering other measures of prevalence-independent diagnostic performance45,48,49.
The cost of brucellosis diagnosis is an important element of the total public and private impacts of the disease6,40,43. Repeat visits to health facilities due to recurring illness, coupled with repeat testing due to poor test accuracy, substantially add to the costs of diagnosis, which are borne by the patient in most LMICs43,46. The running costs of currently available test options in northern Tanzania have not been fully evaluated. A comprehensive evaluation of these costs is needed to improve the cost-effectiveness of brucellosis diagnosis in the region, which is essential to mitigate some of the impacts of the disease.
The national surveillance guidelines for brucellosis in Tanzania recommend that all patients presenting to health facilities with brucellosis-consistent symptoms should be tested with RBT followed by a confirmatory serological test10,38,50. However, the RBT is not widely used in health facilities. Instead, a range of other tests commercially available on the Tanzanian market is used51,52. This study aimed to evaluate the diagnostic performance characteristics and running costs of the tests that are currently in use for human brucellosis in northern Tanzania and the wider region. Here, we include four commercial plate agglutination tests, the recommended RBT, and a cELISA kit. The outcomes of this assessment are expected to inform policy for the diagnosis and management of human brucellosis in Tanzania and other similar settings.
Methods
Study design
This study estimated the diagnostic performance of seven assays (henceforth referred to as index tests) using a set of sera from a study conducted to determine the prevalence of brucellosis amongst patients presenting to hospital with febrile illness. Patients were considered brucellosis cases if they met the CDC’s case definition for either a probable or confirmed case15. The case population thus included cases defined by culture positivity, SAT seroconversion, or high SAT titre (of ≥ 1:160 in acute, convalescent or both serum samples). Acute samples from all participants were collected at the time of hospital presentation when all individuals had documented fever. Full details of the patient population are described elsewhere15 and full details of the diagnostic testing performed for all participants are given in the accompanying data file (see Data Availability section). All samples used in this study were derived from blood samples collected at presentation to hospital, prior to any clinical intervention. All the tests were performed at the Kilimanjaro Clinical Research Institute—Biotechnology Laboratory in Moshi, Tanzania.
Study population
Febrile patients presenting at the Endulen Hospital in the Ngorongoro Conservation Area between August 2016 and November 2017 were eligible to enroll in the previous prevalence study15. Inclusion criteria were: (1) age of two years or older and (2) reported fever within the past 72 h or a tympanic temperature of ≥ 38 °C at presentation. In total, 14 (6.1%) of 230 consecutively enrolled participants met the study definition for a probable or confirmed brucellosis case. Full details of the patient population, enrolment processes, patient testing and treatment are given elsewhere15.
Data collection and tests evaluated
Out of 230 previously collected acute-phase serum samples, 218 had sufficient volume for completion of all evaluated tests and were included in this study. All samples excluded due to insufficient volume were collected from participants classified as negative for brucellosis case status15. In the population of 218 individuals evaluated for this study, (1) culture was performed in 186, eight (4.3%) of which had a positive result, and (2) SAT was performed in all (in both acute and convalescent-phase sera), twelve (6.4%) of which were positive (with a SAT titer ≥ 1:160). Of these twelve, one patient (0.5%) sero-converted (four-fold or greater rise in titer)15. Two cases that did not meet the SAT criteria in the case definition (either a SAT titer ≥ 1:160 or a four-fold or greater rise) were identified through culture. Out of the ten patients that met the SAT criteria in the case definition and had culture performed, six had a positive culture result.
The index tests for this study were performed by individuals who were blinded to the results of the previous testing and patient clinical information. The index tests evaluated were the standard RBT protocol (RBT 1:2)31, the RBT modified protocol with a 1/4 serum pre-dilution (RBT 1:8)31, four commercial plate agglutination tests available on the local market in Tanzania, and a cELISA kit used previously for human brucellosis testing studies in the region. For RBT 1:2, the test was performed following standard guidelines, testing serum samples with an equal volume of antigen31 (Rose Bengal antigen, RA 0060, Animal and Plant Health Agency (APHA)-Scientific, Weybridge-UK). For all samples classified as positive with RBT 1:2, doubling dilutions of serum (in buffered saline) were made from neat (1/1, reported as RBT 1:2) to 1/128 and each dilution tested with an equal volume (30 μL) of the Rose Bengal antigen. Diluted sera and antigen were mixed with a sterile wooden toothpick and gently rocked at room temperature for eight minutes. Any sample with visible agglutination observed at a titer of 1:8 was considered positive by the modified RBT 1:8 test. Positive and negative controls (APHA RAB1003—Brucella abortus positive control serum and RAB0701—Brucella abortus negative control serum) were run in parallel with all RBT test batches.
The manufacturer’s details of the four commercial plate agglutination tests evaluated were as follows: Amitech (Amitech Diagnostics, Ontario-Canada); Arkray (Arkray Healthcare Pvt., Surat-India); Eurocell (Euromedi Equip, Middlesex-UK); and, Fortress (Fortress Diagnostics, Antrim-UK). The four commercial plate agglutination tests were run as per kit instructions for the rapid, qualitative (screening) and semi-quantitative slide assays. The plate agglutination test protocols were identical except for the volumes of serum and antigen used. In all cases, equal volumes of serum and antigen were mixed. When a kit contained more than one antigen, sera were tested with each antigen included (Amitech B. abortus antigen at 50 μL serum and antigen volumes; Arkray stained B. abortus [15SA402-05] and B. melitensis [15SA403-05] suspension at 20 μL serum and antigen volumes; Eurocell B. abortus antigen at 50 μL serum and antigen volumes; Fortress Febrile B. abortus and B. melitensis [FEBAMP05] at 80 μL serum and antigen volumes). All kit protocols refer to controls, but only the Arkray and Fortress kits included controls when purchased from local suppliers. For this study, the positive controls provided with the Arkray and Fortress kits, the APHA B. abortus positive control serum used in the RBT tests, and a negative control were run on every test plate (i.e. four common controls in all test runs). For each combination of serum and antigen, equal volumes were mixed on a clean, white tile and rocked for one minute (except for the Fortress test, which was read after two minutes) at room temperature as per kit instructions. For each antigen, sera showing agglutination were further subjected to semi-quantitative titer testing with that same antigen as per kit instructions. Briefly, 80, 40, 20, 10, and five μL of serum were mixed with one drop of antigen (using the kit-provided dropper in each case) on a clean, white tile. Each reaction was rocked for one minute (except for the Fortress test, which was read after two minutes) at room temperature. Although samples were tested with each antigen included in the kits, data were analyzed in terms of Brucella spp. antibody detection only. A sample was classified as positive for Brucella spp. antibody detection by a given commercial plate agglutination test kit if agglutination was observed with a serum volume of 20 μL or less, as per kit instructions.
The cELISA kit evaluated was the COMPELISA400 that uses B. melitensis 16 M sLPS antigen (APHA Scientific), which was run as per kit instructions, as described elsewhere53. The optical density (OD) was read on an automated ELISA microplate reader (MultiSkan FC, Thermo Scientific, Germany) at 450 nm wavelength. A Receiver Operating Characteristic (ROC) curve analysis was carried out as described elsewhere33,45,54 using the R package ‘ROCit’55 to determine a suitable cut-off of the cELISA as applied to this human population. A two-graphs ROC was also produced (S1). A sample was considered cELISA positive for anti-Brucella antibodies if the sample OD was less than or equal to the optimal percentage of the OD of the four conjugate control wells (the cut-off), as estimated by the ROC analysis.
Data analysis
All test results were compiled in Microsoft Excel. All data analyses were performed using R statistical software 3.6.156. Given the pre-defined brucellosis case status for each sample, the results for each of the seven index tests were classified as one of the following: true positive (TP), false positive (FP), true negative (TN), and false negative (FN).
Several measures of diagnostic test performance were calculated for each of the index tests. These measures, recorded in percentage values (except for the Youden’s Index, which is expressed between 0 and 1), were calculated as follows:
Sensitivity = ;
Specificity = ;
Positive predicted value (PPV) = ;
Negative predicted value (NPV) = ;
Youden’s Index (YI) = sensitivity + specificity − 1;
Diagnostic accuracy = .
For each measure, except the YI, 95% confidence intervals were computed using the exact method for binomial distributions57. For the YI, 95% confidence intervals were calculated using the R package ‘ThresholdROC’58. The exact binomial test for differences in the sensitivity or specificity of pairwise combinations of index tests was performed with the R package ‘DTComPair’59.
The cost of running each of the index tests was calculated using a tool developed by the WHO60. To calculate the cost of each test, we assumed that each test was run independently for all samples and the same general conditions for test usage (e.g. number of batches run per week, number of samples tested per batch). The costs were estimated for the following:—reagents and consumables;—equipment;—personnel;—facilities; and,—quality control. The estimates and sources of the prices used for these calculations are given in the supplementary material (S2). The key assumptions made for the calculation of the cost per sample for each test were based on the premise that testing would be performed in a clinical setting, with rapid feedback of results required and thus small sample numbers per testing batch. These assumptions were as follows:—time to run one testing batch of 60 min, except for RBT 1:2 (30 min), RBT 1:8 (35 min) and cELISA (120 min);—laboratory working hours per day (eight);—laboratory working days per year (312);—laboratory working weeks per year (52);—testing schedule (number of batches tested per week; six);—number of samples per batch (five); and, percentage of samples retested (10). For cELISA, additional estimates of cost per sample were calculated assuming 30 samples per batch (one batch per week) and 60 samples per batch (one batch per two weeks). Given the influence of these key assumptions upon outcome values, a probabilistic sensitivity analysis with 1000 iterations was carried out to assess the level of variability in the outcome measures with variation in these assumptions. The distributions and values explored in this analysis are reported in the supplementary material (S3).
Research clearance and ethics
Approval to conduct the study was granted by the Tanzania Commission for Science and Technology, Tanzania Wildlife Research Institute and the Ngorongoro Conservation Area Authority. Ethical approval was granted by the Kilimanjaro Christian Medical Centre (KCMC) Ethics Committee (698), National Institute of Medical Research (NIMR), Tanzania (NIMR/HQ/R.8c/Vol. I/1140), University of Otago Human Ethics Committee (H17/052), and University of Glasgow College of Medical, Veterinary and Life Sciences Ethics Committee (200140149). The research was performed in accordance with the guidelines and regulations prescribed by the above organizations. Written informed consent for study participation was obtained from each participant and/or their legal guardian, using forms translated into Swahili and verbal translation into Maa when needed. All procedures were conducted according to recommended international standards and following manufacturer’s instructions.
Results
Participants
Of the 218 individuals included in this study, 93 (42.7%) were males. The age of participants ranged from 2 to 78 years, with a median of 27 years. Among 183 study participants for whom there were clinical diagnosis records, 76 (40.4%) presented with respiratory symptoms. Brucellosis was included in the initial diagnosis made at presentation for 36 (19.6%) of these 183 participants.
Diagnostic performance characteristics
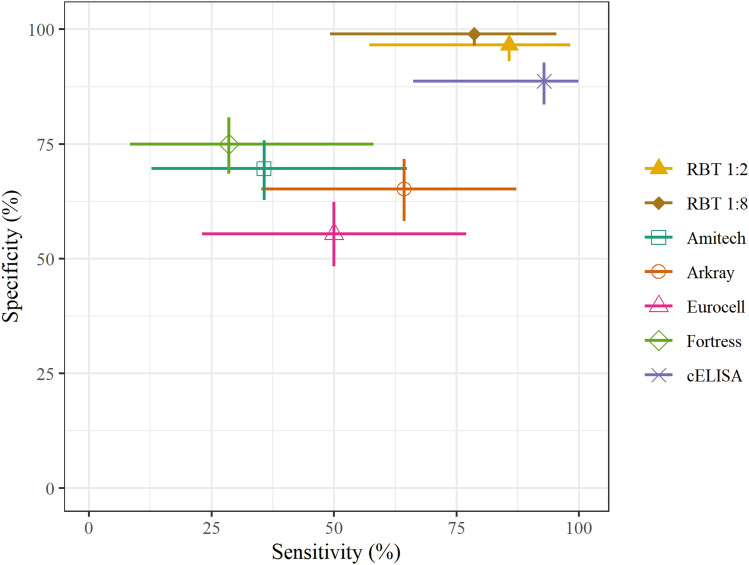
The cross tabulation for each index test compared with the previously defined brucellosis case status of each individual is presented in Table 1. The optimal OD cut-off point for this population estimated using the ROC curve analysis was 56% of the OD of the conjugate control wells (S1), and this 56% cut-off was used to define sample cELISA results for this study. The results for all samples included in the study with all index tests and also additional RBT dilutions are given in the accompanying data file (see Data Availability section). The RBT 1:2 and RBT 1:8 had diagnostic accuracy estimates of 95.9% and 97.7%, respectively (Table 2). The four plate agglutination tests had diagnostic accuracy estimates ranging from 55.0 to 72.0%. The estimated accuracy of the cELISA was 89.4%. The estimated sensitivity and specificity for each index test are shown in Fig. 1. The sensitivity, specificity, PPV, NPV, YI, and diagnostic accuracy estimates for each index test are given in Table 2. The statistical significance of differences between the estimated sensitivity and specificity of each assay pair is shown in supplementary material (S4). According to this statistical analysis, the RBT 1:2, RBT 1:8, and cELISA had higher specificity than the four commercial agglutination tests. The RBT 1:2 had higher sensitivity than two (Amitech and Fortress) of the commercial agglutination tests. The cELISA had higher sensitivity than three of the commercial agglutination tests but not the Arkray test. The index and reference test results for each sample are shown in supplementary material (S5).
Table 1.
Cross tabulation of the index test results by patient brucellosis case status (n = 218, of which 14 were brucellosis cases and 204 were non-brucellosis cases).
| Index test | Index test result | Brucellosis case status | |||
|---|---|---|---|---|---|
| Non-brucellosis case | Brucellosis case | Total | Percentage positive by test (95% CI) | ||
| RBT 1:2 | Negative | 197 | 2 | 199 | 8.7 (5.3–13.3) |
| Positive | 7 | 12 | 19 | ||
| RBT 1:8 | Negative | 202 | 3 | 205 | 6.0 (3.2–10.0) |
| Positive | 2 | 11 | 13 | ||
| Amitech | Negative | 142 | 9 | 151 | 30.7 (24.7–37.3) |
| Positive | 62 | 5 | 67 | ||
| Arkray | Negative | 133 | 5 | 138 | 36.7 (30.3–43.5) |
| Positive | 71 | 9 | 80 | ||
| Eurocell | Negative | 113 | 7 | 120 | 45.0 (38.2–51.8) |
| Positive | 91 | 7 | 98 | ||
| Fortress | Negative | 153 | 10 | 163 | 25.2 (19.6–31.5) |
| Positive | 51 | 4 | 55 | ||
| cELISA | Negative | 182 | 1 | 183 | 16.1 (11.4–21.6) |
| Positive | 22 | 13 | 35 | ||
RBT the Rose Bengal test, cELISA competitive enzyme-linked immunosorbent assay, CI confidence interval.
Table 2.
Performance characteristic estimates for each index test.
| Test | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | YI (95% CI) | Accuracy % (95% CI) |
|---|---|---|---|---|---|---|
| RBT 1:2 | 85.7 (57.2–98.2) | 96.6 (93.1–98.6) | 63.2 (38.4–83.7) | 99.0 (96.4–99.9) | 0.82 (0.81–0.84) | 95.9 (92.3–98.1) |
| RBT 1:8 | 78.6 (49.2–95.3) | 99.0 (96.5–99.9) | 84.6 (54.6–98.1) | 98.5 (95.8–99.7) | 0.78 (0.75–0.80) | 97.7 (94.7–99.3) |
| Amitech | 35.7 (12.8–64.9) | 69.6 (62.8–75.8) | 7.5 (2.5–16.6) | 94.0 (89.0–97.2) | 0.05 (0.02–0.09) | 67.4 (60.8–73.6) |
| Arkray | 64.3 (35.1–87.2) | 65.2 (58.2–71.7) | 11.3 (5.3–20.3) | 96.4 (91.7–98.8) | 0.29 (0.26–0.33) | 65.1 (58.4–71.4) |
| Eurocell | 50.0 (23.0–77.0) | 55.4 (48.3–62.3) | 7.1 (2.9–14.2) | 94.2 (88.4–97.6) | 0.05 (0.02–0.09) | 55.0 (48.2–61.8) |
| Fortress | 28.6 (8.4–58.1) | 75.0 (68.5–80.8) | 7.3 (2.0–17.6) | 93.9 (89.0–97.0) | 0.04 (0.01–0.07) | 72.0 (65.6–77.9) |
| cELISA | 92.9 (66.1–99.8) | 89.2 (84.1–93.1) | 37.1 (21.5–55.1) | 99.5 (97.0–100.0) | 0.82 (0.81–0.83) | 89.4 (84.6–93.2) |
PPV positive predictive value, NPV negative predictive value, YI Youden’s index reported to two decimal places, RBT the Rose Bengal test, cELISA competitive enzyme-linked immunosorbent assay, CI confidence interval.
Figure 1.
Point estimates of the sensitivity and specificity with 95% confidence intervals (horizontal and vertical lines) for each index test (n = 218). RBT: the Rose Bengal test. cELISA: competitive enzyme-linked immunosorbent assay.
Diagnostic test costs
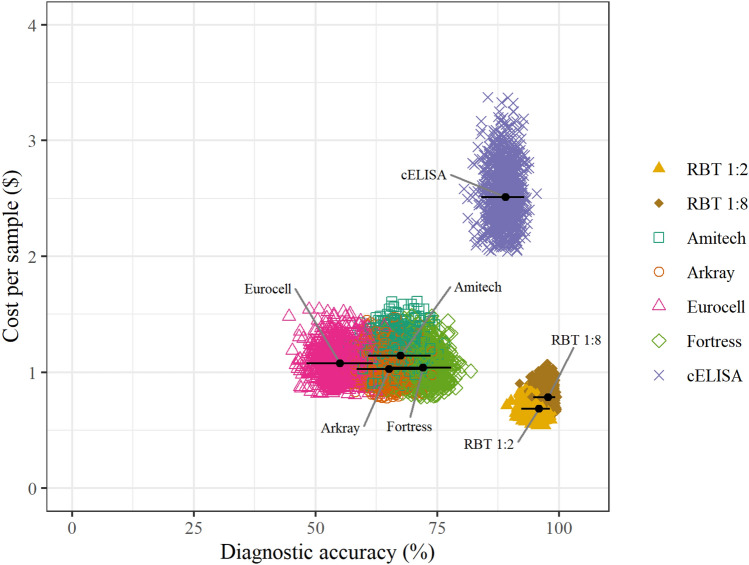
The estimated cost per sample of the seven index test options ranged from $0.69 for RBT 1:2 to $2.51 for cELISA (Table 3). The greatest proportion of component costs were made up by consumables and personnel. The higher cost per sample of the cELISA reflects longer test runtimes, requirement for specialized equipment, and higher cost per kit. All the plate agglutination assays were cheaper relative to the cELISA, with cost variation largely dependent on kit-specific consumables. Figure 2 shows the relationship between test diagnostic accuracy and cost per sample for the seven index test options evaluated. The probabilistic sensitivity analysis showed that plausible variation in the estimates of component costs did not affect the overall conclusions about the relative costs of these tests, based on their use as frontline options and the corresponding costing assumptions made. The RBT 1:2 and RBT 1:8 assays showed the highest accuracy and lowest cost (Fig. 2).
Table 3.
Cost per sample in United States dollars and diagnostic accuracy estimates for each index test evaluated (with 95% confidence intervals; 95% CI).
| Test | Cost per sample ($) | Accuracy % (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Consumables | Equipment | Personnel | Facilities | Quality control | Total | ||
| RBT 1:2 | 0.34 | < 0.001 | 0.19 | 0.07 | 0.09 | 0.69 | 95.9 (92.3–98.1) |
| RBT 1:8 | 0.39 | < 0.001 | 0.22 | 0.09 | 0.09 | 0.79 | 97.7 (94.7–99.3) |
| Amitech | 0.53 | 0.001 | 0.38 | 0.15 | 0.09 | 1.14 | 67.4 (60.8–73.6) |
| Arkray | 0.41 | 0.001 | 0.38 | 0.15 | 0.09 | 1.03 | 65.1 (58.4–71.4) |
| Eurocell | 0.46 | 0.001 | 0.38 | 0.15 | 0.09 | 1.08 | 55.0 (48.2–61.8) |
| Fortress | 0.42 | 0.001 | 0.38 | 0.15 | 0.09 | 1.04 | 72.0 (65.6–77.9) |
| cELISA | 1.36 | 0.003 | 0.76 | 0.30 | 0.09 | 2.51 | 89.4 (84.6–93.2) |
| cELISA30 | 1.09 | 0.001 | 0.13 | 0.05 | 0.09 | 1.36 | “ |
| cELISA60 | 1.07 | < 0.001 | 0.06 | 0.02 | 0.09 | 1.24 | “ |
The first seven rows indicate costs assuming testing of five samples per batch, six batches per week, and 52 weeks per year. Rows for cELISA30 and cELISA60 represent costs assuming testing of 30 samples per batch, one batch per week and 60 samples per batch, one batch per two weeks, for cELISA, respectively. CI: confidence interval. RBT: the Rose Bengal test. cELISA: competitive enzyme-linked immunosorbent assay.
Figure 2.
Probabilistic sensitivity analysis of the cost per sample of each of the index tests (estimates indicated by colored symbols) with their corresponding diagnostic accuracy estimate (black dots; 95% confidence intervals represented with black lines). RBT the Rose Bengal test, cELISA competitive enzyme-linked immunosorbent assay.
Discussion
Our data show that all the rapid commercial plate assays evaluated had poor diagnostic accuracy. In comparison, the RBT 1:2 and RBT 1:8 assays both had high diagnostic accuracy and also had lower costs per sample when applied to diagnose brucellosis in this population of Tanzanian pastoralists. The cELISA had high diagnostic accuracy but a higher cost per sample when evaluated as a frontline test. This study provides a strong rationale for replacing the rapid commercial plate assays with the RBT for frontline brucellosis testing in Tanzanian health facilities.
Our findings in this Tanzanian pastoralist population corroborate the results of earlier studies carried out elsewhere, where excellent diagnostic performance of RBT 1:2 (high sensitivity and specificity estimates within the 85–100% interval) was reported24,27,29,61–64. RBT specificity estimates may be underestimated in contexts where a positive test can occur due to previous exposure to Brucella spp., rather than active infection, or an active infection caused by a cross-reacting pathogen (e.g. Y. enterocolitica O:9, Vibrio cholerae O:1, Francisella tularensis or Escherichia coli O157)20. Compared to our reference case standard (including SAT and blood culture results), RBT 1:2 and RBT 1:8 both displayed high specificity. With RBT 1:8, the point estimate for specificity increased from 96.6 to 99.0%. However, the 95% confidence intervals on these estimates overlap. Five out of the seven false positive test results observed with RBT 1:2 were classified as true negatives with RBT 1:8. This leads to an increase of 21.4% in the PPV (from 63.2% for RBT 1:2 to 84.6% for RBT 1:8) (Table 2). The precision of these estimates is limited by the relatively small sample size available for this study, but a true difference between the two RBT protocols is likely to be important in clinical practice. Particularly in contexts where access to confirmatory tests is limited, a high PPV is a crucial attribute of a frontline test. The PPV determines the confidence with which health practitioners start patients on targeted treatments. For brucellosis, high PPV is particularly important, given the long duration of recommended treatment regimens, adverse effects of these regimens for patients, frequent involvement of restricted drugs, and frequent treatment failures5,65,66. A full evaluation of the cut-off used for the RBT was not performed as part of this study, in part due to the small proportion of positive individuals and thus limited data to robustly compare results at different dilutions. However, the data for all RBT results at serial dilution are shown in the accompanying data file (see Data Availability section). Further evaluation of the field performance of the RBT with different dilution cut-offs at scale could resolve this query. Future studies could also aim to inform selection of a preferred testing protocol for this context and shed light on the impacts of current misdiagnosis.
Our results showed that the widely used commercial plate agglutination tests have significantly lower specificity and diagnostic accuracy as compared to the RBT protocols. These findings agree with the small number of published evaluations of similar tests16–18. We estimate that the PPV of each of the commercial plate agglutination tests is at least six times lower than that of the RBT 1:2 (63.2%) and RBT 1:8 (84.6%). Given the relatively small sample size and low brucellosis case prevalence in this sample set, the sensitivity estimates obtained in this study have wide confidence intervals. However, the point estimates for sensitivity indicate that between 28.6% (Fortress) and 64.3% (Arkray) of the pre-defined brucellosis cases were classified as positive by the commercially available plate agglutination tests. Estimating the performance of RBT 1:2 and RBT 1:8 using the commercial plate agglutination tests as reference further highlights the difference in performance between these tests: (1) if the Eurocell test (the rapid commercial plate assay with highest percentage of samples positive and lowest estimated accuracy) was used as the reference for true case status, the estimated accuracy of both RBT 1:2 and 1:8 would be 53.7% (95% CI 46.8–60.4); (2) if the Fortress test (the rapid commercial plate assay with lowest percentage of samples positive and highest estimated accuracy) was used as the reference for true case status instead, the accuracy of RBT 1:2 and RBT 1:8 would be 69.7% (95% CI 63.2–75.7) and 72.5% (95% CI 66.0–78.3), respectively. Given the considerable existing literature on the performance of the RBT (1:2 and 1:8), these accuracy estimates are not plausible. These data further illustrate that the results of the commercial plate agglutination tests cannot be regarded as accurate indicators of true brucellosis case status. The proportion of individuals testing positive by the four commercial plate agglutination tests (Table 1) are implausibly high, when evaluated alongside the other tests and the existing literature on the brucellosis prevalence expected in this and other comparable populations15,51,67. These estimates are unlikely to be explained by previous exposure in this population68–71, and are more likely due to the low specificity of these tests. The higher sensitivity of RBT protocols (as compared to these commercial plate agglutination tests) is likely to be explained, at least partially, by the standardization of the antigen to OIE specification and the acid buffer used to suspend Rose Bengal stained Brucella cells. The acid buffering improves the ability of RBT to detect agglutinating and non-agglutinating antibodies irrespective of the stage of disease evolution30. Information on the pH of the buffers used with the commercially available plate agglutination tests is not included in the test kits. Our data provide further rationale for replacement of the poorly performing plate agglutination tests that are currently used in Tanzanian health facilities with RBT (RBT 1:2 or RBT 1:8), as recommended in national and international guidelines6,7,9.
Using the estimated optimal cut-off for human testing, the cELISA evaluated in this study was highly sensitive and specific in this population. The kit recommended cut-off for this cELISA, which has been applied for human testing previously39–41 uses a cut-off value of 60% of the OD obtained with conjugate control wells. This threshold value was originally optimized for livestock testing, and its application to human samples requires formal evaluation33,34,72–74. The estimated cut-off point based on the assay readings for this population and the pre-defined brucellosis case status (56% of the conjugate blank OD) fell close to the kit recommended value (60%). The high estimates of sensitivity and specificity generated from a small sample set provide a strong justification for a full validation of the cELISA, specifically including cut-off evaluation in a larger dataset that ideally also includes well-characterized patient samples known to span the different clinical stages of presentation of human brucellosis.
There are no publicly available data on the per-sample running costs of the RBT or alternative test options in northern Tanzania75. The cost of a diagnostic test can negatively impact its utility20,37,46, especially in rural, low-resource settings5,8,20. Our data suggest that RBT 1:2 is the cheapest option for frontline use among the evaluated tests. The RBT 1:8 has marginally increased costs as compared to the RBT 1:2 due to the additional time and consumables required for serum dilution, but this cost difference is trivial (Fig. 2). In addition to the poor diagnostic performance of the commercially available plate agglutination tests, they also cost more per sample as compared to the RBT 1:2 or RBT 1:8 (Fig. 2). The cELISA costs more per sample than any of plate agglutination tests evaluated under the common assumptions specified. However, the costs per sample for the cELISA are substantially reduced when samples are batched for testing (Table 3). The application of the cELISA, with batching of samples, is more likely to occur when used as a frontline test in larger health facilities. In this study, our primary aim was to assess the suitability of available options specifically for frontline use in a clinical setting, hence, assuming a small number of samples per batch. Under these circumstances, RBT 1:2 and RBT 1:8 were more affordable (and accurate) than any of the other evaluated test options.
The availability and use of a rapid, cheap, and accurate test for the diagnosis of human brucellosis are vital to minimize some of the impacts of brucellosis. The higher the test accuracy in particular, the lower the risk of delays in diagnosing true cases and, consequently, the lower the multiple downstream impacts of missed diagnoses. Among the population of individuals tested for brucellosis but who are not true cases, a higher test accuracy could also contribute to faster exclusion of brucellosis as a likely cause of illness. The large-scale deployment of a cheap and accurate test for brucellosis would also be key to strengthening surveillance capacity, therefore improving the quality of the data needed to plan, design, and deliver brucellosis control strategies. Our findings indicate that the RBT is a good candidate for national roll-out in Tanzania. Further evaluation of RBT implementation at scale is needed to assess, among other factors, reliability of the reagent supply chain, ability to ensure and maintain antigen quality in field conditions76,77 and overall test performance under field conditions. A regional or national scale evaluation could also provide evidence to inform the selection of the best candidate test for confirmatory testing in this context.
This study has several limitations. First, given the limited sample size and proportion of brucellosis cases in the population used for this study, the confidence intervals on many of the estimates of test sensitivity are wide and overlap in many cases. Second, we used serum of febrile patients from a pastoralist community, some of whom may have had previous exposure to Brucella39,67,78. We evaluated the performance of the index tests in this study with reference to sample status defined by SAT and culture tests that are estimated to have lower sensitivity than the RBT and some cELISA assays20,31. As a consequence, our estimates of the specificity and PPV of the index tests evaluated might be underestimated in comparison to their unobserved true performance in this population. Third, for the commercial plate agglutination tests, we used the semi-quantitative dilution protocols described in the test kit materials in all cases. In practice, these dilution protocols are rarely applied in health facilities, and test results are performed with neat serum testing only51,52. For this reason, our data may well over-estimate the specificity of the commercial plate agglutination tests as compared to their common use in practice. Finally, all of the diagnostic test data presented were generated in a research laboratory, and we have not evaluated the field performance of these tests.
Conclusions
This evaluation of the diagnostic performance characteristics of tests for human brucellosis provides robust estimates of the markedly poor diagnostic performance of the commercial plate agglutination tests currently available and widely used in Tanzania. Our results suggest that data generated based on these currently used tests are likely to be highly inaccurate and that the systematic use of RBT (either RBT 1:2 or RBT 1:8) as the frontline test for human brucellosis in northern Tanzania would provide more accurate data on human brucellosis than is currently available. In addition, the per-sample costs of RBT 1:2 and RBT 1:8 were lower than any other test evaluated. Future studies to evaluate the feasibility and cost-effectiveness of national roll-out of RBT as the frontline brucellosis test in Tanzania are recommended. Standardized application of RBT for human brucellosis testing across Tanzania could have enormous value for both patient management and also for understanding the current distribution and burden of disease by improving disease surveillance data10,50.
Supplementary Information
Acknowledgements
We thank the patients and staff at the Endulen Hospital for providing the samples used in this study, the field team for their assistance in data collection and the laboratory teams at Kilimanjaro Clinical Research Institute (KCRI) and Animal & Plant Health Agency (APHA), UK, for diagnostic analyses. We also thank the Ngorongoro Conservation Area Authority (NCAA) for approvals to collect data within the Ngorongoro Conservation Area. A.S.L, C.M and R.R.K are supported by the DELTAS Africa Initiative Afrique One-ASPIRE scholarship scheme (Afrique One-ASPIRE/DEL-15-008, http://afriqueoneaspire.org). Â.J.M is supported by The University of Glasgow’s Lord Kelvin/Adam Smith (LKAS) PhD scholarship. R.F.B received scholarship support from the UK Biotechnology and Biological Sciences Research Council (BBSRC), Department for International Development (DFID), the Economic & Social Research Council, the Medical Research Council, the Natural Environment Research Council and the Defence Science & Technology Laboratory, under the Zoonoses and Emerging Livestock Systems – Associated Studentship (ZELS-AS) programme (grant number BB/N503563/1). This study was also supported by the Zoonoses and Emerging Livestock Systems program grant numbers BB/L018845 and BB/L017679 http://www.bbsrc.ac.uk/).
Author contributions
A.S.L., Â.J.M., R.F.B, J.A.M., C.M, V.P.M., N.A.M., M.P.R., G.M.S., C.J.K., R.R.K., J.E.B.H, B.T.M. designed the study. A.S.L., Â.J.M., R.F.B., J.A.M., D.D.S., M.P.R., P.S., K.M.T., J.E.B.H. performed data collection/generation. A.S.L, Â.J.M, J.A.M, J.E.B.H. performed data analysis. A.S.L., Â.J.M, J.A.M, J.E.B.H, B.T.M wrote the main manuscript. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available in the Enlighten research data repository of the University of Glasgow (10.5525/gla.researchdata.1119).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82906-w.
References
- 1.Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: A systematic review of disease frequency. PLoS Negl. Trop. Dis. 2012;6:66. doi: 10.1371/journal.pntd.0001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect. Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 3.Al-Dahouk S, Sprague LD, Neubauer H. New developments in the diagnostic procedures for zoonotic brucellosis in humans. Sci. Tech. Rev. Off. Int. des Epizoot. 2013;32:177–188. doi: 10.20506/rst.32.1.2204. [DOI] [PubMed] [Google Scholar]
- 4.Jamil T, et al. Brucella abortus: Current research and future trends. Curr. Clin. Microbiol. Rep. 2017;4:1–10. doi: 10.1007/s40588-017-0052-z. [DOI] [Google Scholar]
- 5.Rubach PM, Halliday JEB, Cleaveland S, Crump AJ. Brucellosis in low-income and middle-income countries. Curr. Opin. Infect. Dis. 2014;26:404–412. doi: 10.1097/QCO.0b013e3283638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbel, M. J., Food and Agriculture Organization of the United Nations, World Health Organization & World Organisation for Animal Health. Brucellosis in Humans and Animals. World Health Organization (WHO Press, 2006).
- 7.CDC. Brucellosis (Brucella spp.) 2010 Case Definition (2010).
- 8.Dean AS, et al. Clinical manifestations of human brucellosis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2012;6:67. doi: 10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Disease Control and Prevention. Brucellosis Reference Guide: Exposures, Testing and Prevention. 1–35 (2017).
- 10.United Republic of Tanzania (URT). Guidelines for Surveillance of Prioritized Zoonotic Diseases for Human and Animal Health in the United Republic of Tanzania. Government Report vol. 1 (2018).
- 11.The European Commission. Commission Implementing Decision 2018/945 On the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. Annex II. Official Journal of the European Union 1–74 (2018).
- 12.Yagupsky P, Morata P, Colmenero JD. Laboratory diagnosis of human Brucellosis Pablo. Clin. Microbiol. Rev. 2020;33:1–54. doi: 10.1128/CMR.00073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouley AJ, et al. Brucellosis among hospitalized febrile patients in northern Tanzania. Am. J. Trop. Med. Hyg. 2012;87:1105–1111. doi: 10.4269/ajtmh.2012.12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cash-Goldwasser S, et al. Risk factors for human brucellosis in northern Tanzania. Am. J. Trop. Med. Hyg. 2017;19:135–140. doi: 10.4269/ajtmh.17-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodenham FR, et al. Prevalence and speciation of acute brucellosis in febrile patients from a pastoralist community of Tanzania. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-62849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Glanville WA, et al. Poor performance of the rapid test for human brucellosis in health facilities in Kenya. PLoS Negl. Trop. Dis. 2017;11:1–15. doi: 10.1038/s41598-020-62849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiambi SG, Fèvre EM, Omolo J, Oundo J, de Glanville WA. Risk factors for acute human brucellosis in Ijara, north-eastern Kenya. PLoS Negl. Trop. Dis. 2020;14:e0008108. doi: 10.1371/journal.pntd.0008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alumasa L, et al. Hospital-based evidence on cost-effectiveness of brucellosis diagnostic tests and treatment in Kenyan hospitals. PLoS Negl. Trop. Dis. 2021;891:1–19. doi: 10.1371/journal.pntd.0008977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz R, Maravi Poma E, Rivero A. Comparison of counter immunoelectrophoresis with other serological tests in the diagnosis of human brucellosis. Bull. World Health Organ. 1976;53:417–424. [PMC free article] [PubMed] [Google Scholar]
- 20.Al Dahouk S, Nöckler K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev. Anti. Infect. Ther. 2011;9:833–845. doi: 10.1586/eri.11.55. [DOI] [PubMed] [Google Scholar]
- 21.Kunda J, et al. Health-seeking behaviour of human brucellosis cases in rural Tanzania. BMC Public Health. 2007;7:1–7. doi: 10.1186/1471-2458-7-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson L. Diagnosis and treatment of infection with Brucella abortus, biotype 5. J. Clin. Pathol. 1967;20:199–203. doi: 10.1136/jcp.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roop RM, Preston-Moore D, Bagchi T, Schurig GG. Rapid identification of smooth Brucella species with a monoclonal antibody. J. Clin. Microbiol. 1987;25:2090–2093. doi: 10.1128/JCM.25.11.2090-2093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oomen LJA, Waghela S. The Rose Bengal plate test in human brucellosis. Trop. Geogr. Med. 1974;26:300–302. [PubMed] [Google Scholar]
- 25.Caces E, De Lauture H, Vol S, Tichet J, Boulard P. The systematic detection of human brucellosis by the Rose Bengal test on agricultural people after a study in the middle west of France on 89000 workers. Comp. Immunol. Microbiol. Infect. Dis. 1978;1:107–114. doi: 10.1016/0147-9571(78)90017-6. [DOI] [PubMed] [Google Scholar]
- 26.Cernyseva MI, Knjazeva EN, Egorova LS. Study of the plate agglutination test with rose bengal antigen for the diagnosis of human brucellosis. Bull. World Health Organ. 1977;55:669–674. [PMC free article] [PubMed] [Google Scholar]
- 27.Russell AO, Patton CM, Kaufmann AF. Evaluation of the card test for diagnosis of human brucellosis. J. Clin. Microbiol. 1978;7:454–458. doi: 10.1128/jcm.7.5.454-458.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saz JV, et al. Enzyme-linked immunosorbent assay for diagnosis of brucellosis. Eur. J. Clin. Microbiol. 1987;6:71–74. doi: 10.1007/BF02097200. [DOI] [PubMed] [Google Scholar]
- 29.Maichomo MW, McDermott JJ, Arimi SM, Gathura PB. Assessment of the Rose-Bengal plate test for the diagnosis of human brucellosis in health facilities in Narok district, Kenya. East Afr. Med. J. 1998;75:219–222. [PubMed] [Google Scholar]
- 30.Alton GG, Jones LM, Pietz DE. Laboratory techniques in brucellosis. Monogr. Ser. World Health Organ. 1975;47:1–163. [PubMed] [Google Scholar]
- 31.Díaz R, Casanova A, Ariza J, Moriyón I. The rose Bengal test in human brucellosis: A neglected test for the diagnosis of a neglected disease. PLoS Negl. Trop. Dis. 2011;5:1–7. doi: 10.1371/journal.pntd.0000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz-Mesa JD, et al. Rose Bengal test: Diagnostic yield and use for the rapid diagnosis of human brucellosis in emergency departments in endemic areas. Clin. Microbiol. Infect. 2005;11:221–225. doi: 10.1111/j.1469-0691.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- 33.Lucero NE, Foglia L, Ayala SM, Gall D, Nielsen K. Competitive enzyme immunoassay for diagnosis of human brucellosis. J. Clin. Microbiol. 1999;37:3245–3248. doi: 10.1128/JCM.37.10.3245-3248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrett LL, McGiven JA, Brew SD, Stack JA. Evaluation of competitive ELISA for detection of antibodies to Brucella infection in domestic animals. Croat. Med. J. 2010;51:314–319. doi: 10.3325/cmj.2010.51.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucero NE, et al. Unusual clinical presentation of brucellosis caused by Brucella canis. J. Med. Microbiol. 2005;54:505–508. doi: 10.1099/jmm.0.45928-0. [DOI] [PubMed] [Google Scholar]
- 36.Lucero NE, Escobar GI, Ayala SM, Paulo PS, Nielsen K. Fluorescence polarization assay for diagnosis of human brucellosis. J. Med. Microbiol. 2003;52:883–887. doi: 10.1099/jmm.0.05217-0. [DOI] [PubMed] [Google Scholar]
- 37.Dieckhaus KD, Kyebambe PS. Human Brucellosis in Rural Uganda: Clinical manifestations, diagnosis, and comorbidities at Kabale Regional Referral Hospital, Kabale, Uganda. Open Forum Infect. Dis. 2017;4:1–6. doi: 10.1093/ofid/ofx237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United Republic of Tanzania (URT), United States Department of Defense (DoD), Defense Threat Reduction Agency (DTRA), Cooperative Threat Reduction (CTR) & Cooperative Biological Engagement Program (CBEP). The United Republic of Tanzania One Health Strategic Plan 2015–2020. http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/health/Key_Sector_Documents/Tanzania_Key_Health_Documents/FINAL_URT_One_Health_Strategy_Plan_20151021.pdf (2015).
- 39.Shirima GM, Kunda JS. Prevalence of brucellosis in the human, livestock and wildlife interface areas of Serengeti National Park, Tanzania. Onderstepoort J. Vet. Res. 2016;83:2–5. doi: 10.4102/ojvr.v83i1.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.John K, et al. Quantifying risk factors for human brucellosis in Rural Northern Tanzania. PLoS ONE. 2010;5:66. doi: 10.1371/journal.pone.0009968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabriel SM. The Epidemiology of Brucellosis in Animals and Humans in Arusha and Manyara Regions of Tanzania. Glasgow: The University of Glasgow; 2005. [Google Scholar]
- 42.Nasinyama G, et al. Brucella sero-prevalence and modifiable risk factors among predisposed cattle keepers and consumers of un-pasteurized milk in Mbarara and Kampala districts, Uganda. Afr. Health Sci. 2014;14:790–796. doi: 10.4314/ahs.v14i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Šimundić A-M. Measures of diagnostic accuracy: Basic definitions. Ejifcc. 2009;19:203–211. [PMC free article] [PubMed] [Google Scholar]
- 44.Okeh U, Okoro C. Evaluating measures of indicators of diagnostic test performance: Fundamental meanings and formulars. J. Biom. Biostat. 2012;03:1–10. [Google Scholar]
- 45.Metz CE. Basic principles of ROC analysis. Semin. Nucl. Med. 1978;8:283–298. doi: 10.1016/S0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 46.Chanda P, Castillo-Riquelme M, Masiye F. Cost-effectiveness analysis of the available strategies for diagnosing malaria in outpatient clinics in Zambia. Cost Eff. Resour. Alloc. 2009;7:1–12. doi: 10.1186/1478-7547-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R, Wang G, Zhang N, Li X, Liu Y. Clinical evaluation and cost-effectiveness analysis of serum tumor markers in lung cancer. Biomed Res. Int. 2013;2013:66. doi: 10.1155/2013/195692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assefa LM, et al. Diagnostic accuracy and cost-effectiveness of alternative methods for detection of soil-transmitted helminths in a post-treatment setting in Western Kenya. PLoS Negl. Trop. Dis. 2014;8:66. doi: 10.1371/journal.pntd.0002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutjes AWS, et al. Evidence of bias and variation in diagnostic accuracy studies. CMAJ. 2006;174:469–476. doi: 10.1503/cmaj.050090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The United Republic of Tanzania (URT). National Strategy for Prevention and Control of Brucellosis in Humans and Animals 2018–2023. Prime Ministers Office vol. 56 (2018).
- 51.Orsel K, et al. Brucellosis serology as an alternative diagnostic test for patients with malaria-like symptoms. Tanzan. J. Health Res. 2015;17:1–10. [Google Scholar]
- 52.Mngumi EB, Mirambo MM, Wilson S, Mshana SE. Predictors of specific anti-Brucella antibodies among humans in agro-pastoral communities in Sengerema district, Mwanza, Tanzania: The need for public awareness. Trop. Med. Health. 2016;44:64. doi: 10.1186/s41182-016-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.APHA Scientific. COMPELISA 160 & 400 A Competitive ELISA Kit for the Detection of Antibodies Against Brucella in Serum Samples Instructions For Use (For In-Vitro and Animal Use Only). 1–4 (2014).
- 54.Greiner M, Sohr D, Göbel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-P. [DOI] [PubMed] [Google Scholar]
- 55.Khan, M. R. A. & Brandenburger, T. ROCit: Performance Assessment of Binary Classifier with Visualization. R package version 2.1.1. 1–21 (2020).
- 56.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ (2019).
- 57.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- 58.Perez-jaume, S., Pallares, N. & Skaltsa, K. Optimum Threshold Estimation. in Package ‘ThresholdROC’ F (R CRAN, 2019).
- 59.Stock, A. C., Hielscher, T. & Stock, M. C. Package ‘ DTComPair, Comparison of Binary Diagnostic Tests in a Paired Study Design’ (2015).
- 60.World Health Organization (WHO). Laboratory Test Costing Tool User Manual/Training Manual. 17 (2019).
- 61.Andriopoulos P, et al. Prevalence of Brucella antibodies on a previously acute brucellosis infected population: sensitivity, specificity and predictive values of Rose Bengal and Wright standard tube agglutination tests. Infection. 2015;43:325–330. doi: 10.1007/s15010-015-0748-z. [DOI] [PubMed] [Google Scholar]
- 62.Rahman AKMA, Saegerman C, Berkvens D. Latent class evaluation of three serological tests for the diagnosis of human brucellosis in Bangladesh. Trop. Med. Health. 2016;44:1–6. doi: 10.1186/s41182-016-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ron-Román J, et al. Bayesian evaluation of three serological tests for detecting antibodies against brucella spp. Among humans in the Northwestern Part of Ecuador. Am. J. Trop. Med. Hyg. 2019;100:1312–1320. doi: 10.4269/ajtmh.18-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konstantinidis A, et al. Evaluation and comparison of fluorescence polarization assay with three of the currently used serological tests in diagnosis of human brucellosis. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26:715–721. doi: 10.1007/s10096-007-0363-8. [DOI] [PubMed] [Google Scholar]
- 65.Kazak E, et al. Brucellosis: A retrospective evaluation of 164 cases. Singap. Med. J. 2016;57:624–629. doi: 10.11622/smedj.2015163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pappas G, Solera J, Akritidis N, Tsianos E. New approaches to the antibiotic treatment of brucellosis. Int. J. Antimicrob. Agents. 2005;26:101–105. doi: 10.1016/j.ijantimicag.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Makala R, et al. Seroprevalence of Brucella infection and associated factors among pregnant women receiving antenatal care around human, wildlife and livestock interface in Ngorongoro ecosystem, Northern Tanzania. A cross-sectional study. BMC Infect. Dis. 2020;20:1–7. doi: 10.1186/s12879-020-4873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ducrotoy MJ, Bardosh KL. How do you get the Rose Bengal Test at the point-of-care to diagnose brucellosis in Africa? The importance of a systems approach. Acta Trop. 2017;165:33–39. doi: 10.1016/j.actatropica.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Njeru J, et al. Human brucellosis in febrile patients seeking treatment at remote hospitals, northeastern Kenya, 2014–2015. Emerg. Infect. Dis. 2016;22:2160–2164. doi: 10.3201/eid2212.160285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muturi M, et al. Risk factors for human brucellosis among a pastoralist community in South-West Kenya, 2015 11 Medical and Health Sciences 1117 Public Health and Health Services. BMC Res. Notes. 2018;11:1–6. doi: 10.1186/s13104-018-3961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osoro EM, et al. Strong association between human and animal brucella seropositivity in a linked study in Kenya, 2012–2013. Am. J. Trop. Med. Hyg. 2015;93:224–231. doi: 10.4269/ajtmh.15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGiven JA, et al. Validation of FPA and cELISA for the detection of antibodies to Brucella abortus in cattle sera and comparison to SAT, CFT, and iELISA. J. Immunol. Methods. 2003;278:171–178. doi: 10.1016/S0022-1759(03)00201-1. [DOI] [PubMed] [Google Scholar]
- 73.Stack JA, Perrett LL, Macmillan AP. Competitive ELISA for Bovine Brucellosis suitable for testing poor quality samples. Vet. Rec. 1999;145:735–736. doi: 10.1136/vr.145.25.735. [DOI] [PubMed] [Google Scholar]
- 74.Organisation International Epizoonoses (OIE). Chapter 3.6.1—Development and optimisation of antibody detection assays. in OIE Validation Recommendations 1–13 (2014). 10.1787/c88edbcd-en.
- 75.Kunda JS. The Epidemiology of Human Brucellosis in the Context of Zoonotic Diseases in Tanzania. Edinburgh: University of Edinburgh; 2005. [Google Scholar]
- 76.Gall D, Nielsen K. Serological diagnosis of bovine brucellosis: A review of test performance and cost comparison. OIE Rev. Sci. Technol. 2004;23:989–1002. doi: 10.20506/rst.23.3.1545. [DOI] [PubMed] [Google Scholar]
- 77.Ducrotoy M, et al. Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Trop. 2017;165:179–193. doi: 10.1016/j.actatropica.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 78.Assenga JA, Matemba LE, Muller SK, Malakalinga JJ, Kazwala RR. Epidemiology of Brucella infection in the human, livestock and wildlife interface in the Katavi-Rukwa ecosystem, Tanzania. BMC Vet. Res. 2015;11:1–11. doi: 10.1186/s12917-015-0504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the Enlighten research data repository of the University of Glasgow (10.5525/gla.researchdata.1119).