Abstract
Purpose:
To evaluate the long-term outcomes of uveitic macular edema
Design:
Longitudinal follow-up of a cohort of participants in a randomized clinical trial
Participants:
248 eyes of 177 participants with uveitic macular edema enrolled in the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study
Methods:
Optical coherence tomography (OCT) measurements, taken at baseline and annually, were graded by reading-center graders masked to clinical data. Macular edema was defined as a center point macular thickness (CMT) ≥240 μm on time-domain OCT or time-domain OCT equivalent. Resolution of macular edema was defined as normalization of macular thickness on OCT. Relapse of macular edema was defined as increase in macular thickness to ≥240 μm in an eye that previously had resolution. Visual acuity was measured at each visit with logarithmic visual acuity charts.
Main outcome measures:
Resolution and relapse of macular edema. Visual acuity.
Results:
Among 227 eyes with macular edema followed ≥1 year, the cumulative percent of eyes with macular edema resolving at any point during 7 years was 94% (95% confidence interval [CI]: 89%, 97%). Epiretinal membranes on OCT were associated with a lower likelihood of macular edema resolution (hazard ratio [HR] 0.74; 95% CI 0.55, 1.01; P=0.05). Among 177 eyes with resolved macular edema, the cumulative percent with relapse within 7 years was 43% (95% CI: 32%, 51%). Eyes in which macular edema resolved gained a mean of 6.24 letters (95% CI: 4.40, 8.09, P< 0.001) compared to eyes that remained free from macular edema during the 1-year follow-up intervals, whereas eyes where macular edema did not resolve experienced no gain in vision (mean change −1.30 letters; 95% CI: −2.70, 0.09, P=0.065), and eyes that developed macular edema during the year (either incident or relapsed) experienced a mean loss of −8.65 letters (95% CI: −11.5, −5.84, P< 0.001).
Conclusions:
Given sufficient time and treatment, nearly all uveitic macular edema resolves, but episodes of relapse were common. Visual acuity results were better among eyes with resolved macular edema, suggesting that control of inflammation and resolution of macular edema might be visually-relevant treatment targets.
PRECIS
Among eyes with treated uveitic macular edema, 94% resolved, and 43% relapsed by 7 years of follow-up. Eyes with resolved macular edema had better visual acuity outcomes than those with non-resolved macular edema.
Uveitis refers to a collection of over 30 diseases characterized by intraocular inflammation.1 Uveitic diseases typically are classified anatomically as an anterior, intermediate, posterior, or panuveitis.1 Among the uveitides, particularly intermediate, posterior and panuveitides, macular edema affects about 40% of patients and is the most frequent cause of visual impairment.2–7 Macular edema may respond to primary treatment of the ocular inflammation with a decrease in macular thickness and improvement in vision, but often adjunctive regional corticosteroid injections (given either periocularly or intravitreally) are needed to treat the edema.6–8
Uveitic macular edema may resolve, improve without resolving, or not improve.7,9,10 The latter two situations are considered persistent macular edema.7 In the Multicenter Uveitis Steroid Treatment (MUST) Trial, 46% of patients had macular edema in one or both eyes at enrollment; and among eyes with uveitic macular edema at enrollment, 40% had persistent or relapsed macular edema at 2 years of follow-up.7 The MUST Follow-up Study followed participants enrolled in the MUST Trial for an additional 5 years, permitting assessment of long-term outcomes up to 7–10 years of patients with uveitis and of its ocular complications. In this analysis we evaluated the long-term outcomes of uveitic macular edema, with particular attention paid to the issues of resolution and relapse of the macular edema.
Methods
The MUST Trial was a randomized, comparative effectiveness trial of the 0.59 mg fluocinolone acetonide implant (Retisert®, Bausch & Lomb, Inc., Tampa, FL, USA) versus oral corticosteroids and immunosuppression for the treatment of active or recently active non-infectious intermediate, posterior, and panuveitides.11 The MUST Follow-up Study extended participant follow-up from the minimum of 2 years up to 7–10 years, depending upon the date of enrollment. Eligibility, enrollment, treatment, follow-up procedures, and 2- and 7-years outcomes have been described previously.11–13 Participants were treated with either the fluocinolone acetonide implant or oral corticosteroids for the uveitis with the goal of suppression of the inflammation. Regional corticosteroid injections (either periocular or intravitreal) were used as supplemental treatment for macular edema as clinically indicated. At enrollment, participants gave a detailed medical and ophthalmic history, had best corrected visual acuity measured using logarithmic visual acuity charts,11,14 and underwent a complete eye examination, including slit lamp examination, tonometry, and examination of the retina through a dilated pupil using indirect ophthalmoscopy. Time-domain optical coherence tomography (OCT, Stratus 3, Carl Zeiss Meditec, Jena, Germany) was performed at baseline, six months, and annually thereafter.15 After the last participant completed 2-years of follow-up in the MUST Trial, 84% of participants (93% of participants still under follow-up) were enrolled in the MUST Follow-up Study, during which OCTs were performed on an annual basis. During the MUST Follow-up Study, spectral domain OCT was introduced. Overall in the Trial and Follow-up Study, 72% of OCT images were obtained by time-domain OCT, and 28% by spectral-domain OCT. As clinical centers converted to spectral domain OCT, spectral-domain OCT thickness values were converted to those equivalent to the thicknesses that would have been measured on a Stratus 3 system to permit direct comparison of measurements throughout the study.16
All clinical and resource centers obtained and maintained institutional review board (IRB) approval for both the MUST Trial and the MUST Follow-up Study. All participants gave written, informed consent for both the MUST Trial and MUST Follow-up Study. The MUST Trial and Follow-up Study and their procedures adhered to the principles of the Declaration of Helsinki.
Optical coherence tomograms were evaluated by graders at a centralized image reading center, who were unaware of treatment assignment and of clinical characteristics.7,15,16 For the purposes of this study, macular edema was defined as macular thickening on OCT (center point macular thickness [CMT] ≥240 μm), regardless of the presence or absence of cystoid spaces. Resolution of macular edema was defined as a decrease in macular thickening to a thickness within the normal range (<240 μm) on OCT, regardless of whether all cystoid spaces resolved.7,10
Statistical analysis
All eyes that were identified as having macular edema during the MUST Trial and Follow-up study were included in the analysis. In the 2-year analysis of macular edema response to treatment, there were no significant differences between the treatment groups in the response of macular edema to treatment.7 Therefore, for these analyses all eyes were analyzed together regardless of treatment assignment. Eyes that had macular edema at enrollment were considered ‘prevalent’ cases and eyes that developed new-onset macular edema during follow-up were considered ‘incident’ cases. Results are based on analyses pooling the data from the prevalent and incident macular edema cases except for those analyses in which a significant test of interaction indicated that the risk differed between those eyes with prevalent and those eyes with incident macular edema. For these analyses follow-up was initiated at the visit at which macular edema was identified for each eye. Generalized estimating equations were used to compare the baseline characteristics of eyes with incident and prevalent macular edema while adjusting for correlation due to bilateral disease.
Time to resolution is defined as the time from the identification of macular edema until the retinal thickness returned to normal levels. Time to relapse is defined as the time from the first resolution until a return to abnormal thickness. Kaplan-Meier estimates of the survival function were used to estimate medians and cumulative proportions for time-to-event outcomes (e.g. resolution, relapse). Cox proportional hazards models with a random intercept to account for between-eye correlation were used to identify risk factors associated with time-to-event outcomes. Risk factors were based upon characteristics evaluated at the time of macular edema identification and the time of the first resolution for the assessment of resolution and relapse, respectively. Results of the pooled analyses for risk factors are presented except for those cases in which a significant difference in the risk was identified based upon a test of interaction.
Visual acuity analyses included all 1-year intervals during the first 7 years since diagnosis. Linear mixed effects models were used to assess the longitudinal patterns of visual acuity over time as well as the impact of changes in macular edema status. A random intercept was included for each individual to account for between eye correlations and an exchangeable correlation structure was included to account for repeated measurements within each eye over time. The pattern of macular edema status for each eye during each interval was classified as stable without disease (no macular edema at the beginning or end of the interval), resolved (macular edema resolved during the interval), interval-onset (eye developed macular edema during the interval, either “incident” or relapsed macular edema), and non-resolved (macular edema present at the beginning and end of the interval).
Robust standard errors were computed for all models. P-values less than 0.05 were considered statistically significant. Statistical analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.5; SAS, Inc., Cary, NC) and R (The R Project for Statistical Computing, Version 3.3.1, available at: http://www.r-project.org).
Results
Overall there were 248 eyes of 177 participants with macular edema in the MUST Trial and Follow-up Study, which comprises the study population for these analyses. The majority of eyes (62.5%) had prevalent macular edema at baseline (155 eyes, 123 patients), and just over one-third of eyes (37.5%) developed incident macular edema (93 eyes, 82 patients) during follow-up. Participants could have prevalent macular edema in one eye and incident edema in the other. The baseline characteristics, as well as a comparison of prevalent and incident cases, are shown as Table 1. The incident cases differed from the prevalent cases by being younger, less likely to have active uveitis, having a longer duration of uveitis, having less macular thickening, being less likely to have fluorescein angiographic leakage, less likely to be phakic, more likely to be on glaucoma medications, and more likely to have had glaucoma surgery. Nevertheless, visual acuity at the time of macular edema diagnosis (enrollment for prevalent cases or diagnosis of macular edema for incident cases) was similar between the two groups.
TABLE 1.
Baseline* Characteristics of the Population of Eyes with Uveitic Macular in the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study.
| Characteristic | Overall | Prevalent cases* | Incident cases* | P-value |
|---|---|---|---|---|
| Number eyes | 248 | 155 | 93 | |
| Demographic characteristics | ||||
| Age, years | 0.001 | |||
| Median (25th, 75th percentile) | 52 (38, 58) | 53 (42, 63) | 47 (35, 55) | |
| Gender, number (%) | 0.88 | |||
| Men | 67 (27) | 41 (26) | 26 (28) | |
| Women | 181 (73) | 114 (74) | 67 (72) | |
| Race/ethnicity, number (%) | 0.13 | |||
| White, non-Hispanic | 142 (57) | 96 (62) | 46 (49) | |
| Black, non-Hispanic | 64 (26) | 34 (22) | 30 (32) | |
| Hispanic/Other | 42 (17) | 25 (16) | 17 (18) | |
| Uveitic anatomic class, number (%) | 0.43 | |||
| Intermediate uveitides | 111 (45) | 66 (43) | 45 (48) | |
| Posterior/panuveitides | 137 (55) | 89 (57) | 48 (52) | |
| Inflammation activity, number (%) | <0.001 | |||
| Active | 171 (70) | 135 (87) | 36 (40) | |
| Inactive | 75 (30) | 20 (13) | 55 (60) | |
| Ocular characteristics at time of macular edema identification | ||||
| Uveitis duration, years | <0.001 | |||
| Median (25th, 75th percentile) | 5.2 (2.2, 9.8) | 4.2 (1.3, 8.3) | 6.3 (3.9, 10.2) | |
| OCT† center point macular thickness (μm) | <0.001 | |||
| Median (25th, 75th percentile) | 330 (266, 506) | 371 (283, 544) | 282 (258, 383) | |
| OCT† center point macular thickness (μm), number (%) | 0.001 | |||
| <370 | 143 (58) | 77 (50) | 66 (71) | |
| ≥370 | 105 (42) | 78 (50) | 27 (29) | |
| OCT cystoid spaces, number (%) | 0.54 | |||
| Present | 169 (68) | 107 (69) | 62 (67) | |
| Absent | 70 (28) | 41 (26) | 29 (31) | |
| Missing | 9 (4) | 7 (5) | 2 (2) | |
| Epiretinal membrane on OCT, number (%) | 0.18 | |||
| No | 128 (52) | 87 (56) | 41 (44) | |
| Yes | 115 (46) | 65 (42) | 50 (54) | |
| Missing | 5 (2) | 3 (2) | 2 (2) | |
| Lens status, number (%) | 0.007 | |||
| Phakic | 99 (40) | 72 (46) | 27 (29) | |
| Pseudophakic/aphakic | 149 (60) | 83 (54) | 66 (71) | |
| Glaucoma medications, number (%) | 0.014 | |||
| No | 196 (79) | 131 (85) | 65 (71) | |
| Yes | 51(21) | 24 (15) | 27 (29) | |
| Prior glaucoma surgery, number (%) | <0.001 | |||
| No | 215 (87) | 144 (93) | 71 (76) | |
| Yes | 33 (13) | 11 (7) | 22 (24) | |
| Visual acuity, letters | 0.11 | |||
| Median (25th, 75th percentile) | 65 (42, 73) | 62 (44, 71) | 66 (46, 76) | |
| VA‡ worse than 20/40, number (%) | 0.33 | |||
| Yes | 162 (66) | 105 (68) | 57 (62) | |
| No | 84 (34) | 49 (32) | 35 (38) | |
| VA 20/200 or worse, number (%) | >0.99 | |||
| Yes | 38 (15) | 24 (16) | 14 (15) | |
| No | 208 (85) | 130 (84) | 78 (85) | |
prevalent cases were present at enrollment; incident cases developed during follow-up. Baseline is defined as enrollment visit for prevalent cases and the diagnosis visit for incident cases.
OCT = optical coherence tomography; results from different machines normalized to Zeiss Stratus scale.
VA = visual acuity.
Six cases of incident macular edema (6%) were detected within 6 months of cataract surgery, of which only 2 (3%) occurred within 3 months of cataract surgery. Fourteen of the eyes included in this study (6.2%) underwent vitrectomy with epiretinal membrane removal. The majority of these membrane peels occurred in eyes with prevalent macular edema (10 eyes, 71%), as opposed to incident macular edema (4 eyes, 29%).
Resolution
The cumulative proportion of eyes with resolution of the macular edema is shown as Figure 1. Overall, the macular edema resolved in 94% of eyes within the 7-year time frame, although these eyes remained at risk for relapse of the macular edema. The median time to resolution was 1.09 years (95% confidence interval [CI]: 1.05,1.89). There were no differences in the time to resolution between eyes with prevalent macular edema at enrollment (median 1.09 years; 95% CI: 1.03, 1.95) and eyes with incident macular edema during follow-up (median 1.13 years; 95% CI: 1.03, 1.99; P=0.45). Because in general, there did not appear to be a difference in the behavior of prevalent and incident macular edema, subsequent analyses combined the two groups, unless noted otherwise.
Figure 1.
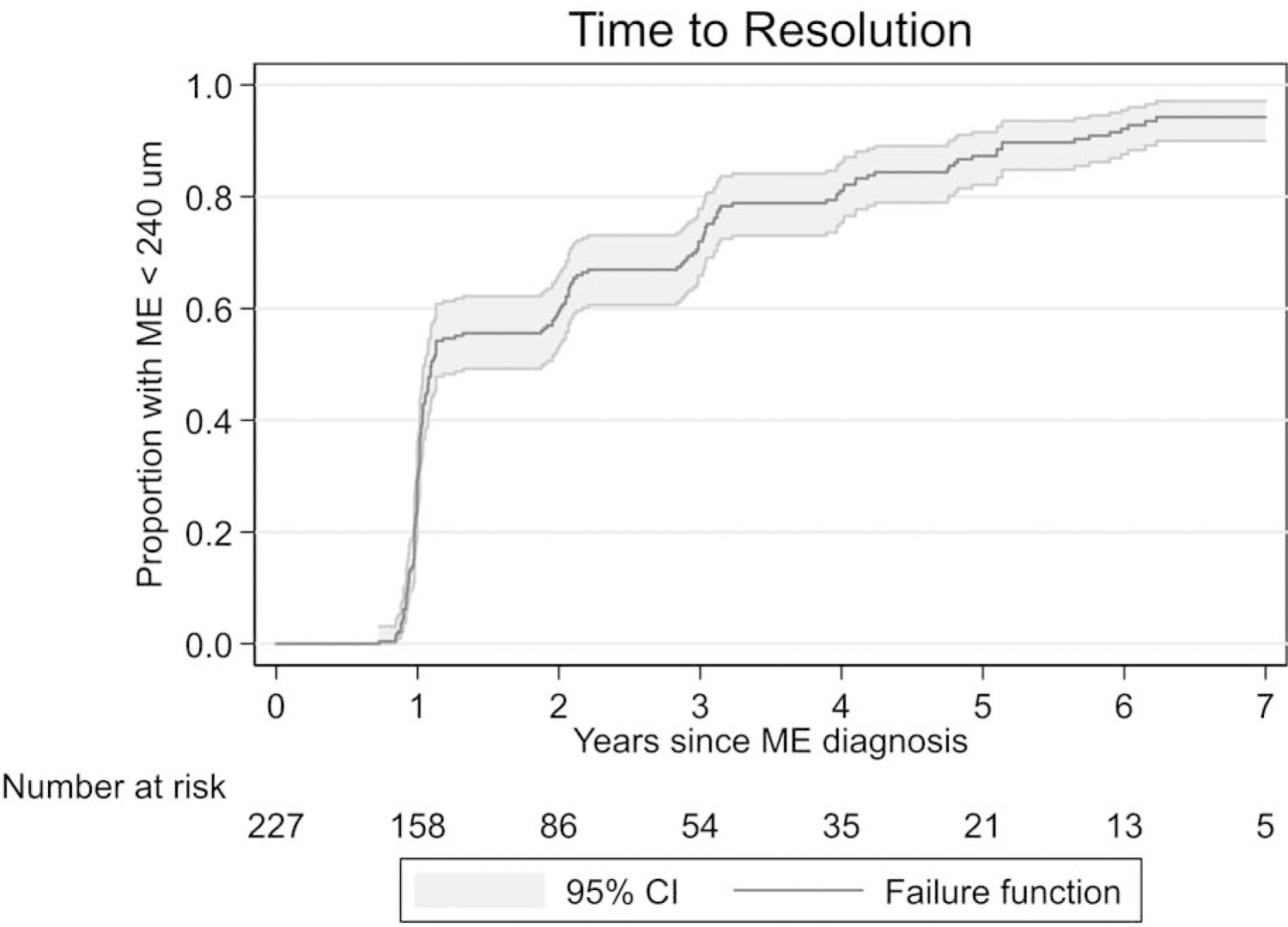
Time to resolution of macular edema for eyes with macular edema in the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study.
Associations between risk factors measured at the time of macular edema diagnosis and resolution of macular edema are shown in Table 2. With the exception of epiretinal membrane, there was no significant association between any risk factor and resolution. The effect of epiretinal membrane status at macular edema diagnosis appeared to differ based upon whether the macular edema was prevalent or incident (interaction P-value =0.03). The presence of epiretinal membranes decreased the likelihood of resolution of the macular edema in eyes with prevalent macular edema (HR = 0.58; 95% CI: 0.38, 0.88; P=0.01), but did not affect the likelihood of resolution for eyes with incident macular edema (HR = 1.15; 95% CI: 0.73, 1.80; P=0.55). The uveitis treatment received did not appear to affect the likelihood of resolution of the macular edema. The HR for resolution of the macular edema for fluocinolone acetonide implants ≤3 years since implantation vs. no implant was 1.15 (95% CI 0.84, 1.58, P=0.39).
Table 2.
Association between Characteristics at the Time of Macular Edema Diagnosis and Resolution of Macular Edema among Eyes with Uveitic Macular Edema in the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study.
| Characteristics | Number eyes | Percent resolved by 7 years (95% CI) | HR* | 95% CI* | P-value |
|---|---|---|---|---|---|
| Overall | 227 | 94 (89, 97) | |||
| Age, years | |||||
| <57 | 167 | 94 (87, 97) | 1.00 | ||
| ≥57 | 60 | 94 (85, 98) | 0.95 | 0.66, 1.51 | 0.77 |
| Gender, number | |||||
| Women | 166 | 95 (88, 98) | 1.00 | ||
| Men | 61 | 90 (79, 96) | 0.77 | 0.54, 1.09 | 0.14 |
| Race/ethnicity | |||||
| White, non-Hispanic | 131 | 93 (85, 96) | 1.00 | ||
| Black, non-Hispanic | 57 | 92 (81, 97) | 1.04 | 0.74, 1.47 | 0.81 |
| Hispanic/Other | 39 | 95 (83, 99) | 0.90 | 0.62, 1.30 | 0.58 |
| Uveitic anatomic class | |||||
| Intermediate uveitides | 105 | 92 (85, 96) | 1.00 | ||
| Posterior/panuveitides | 122 | 95 (86, 98) | 1.07 | 0.79, 1.46 | 0.64 |
| Macular edema diagnosis | |||||
| Prevalent (at baseline) | 143 | 93 (86, 97) | 1.00 | ||
| Incident (during follow-up) | 84 | 94 (85, 97) | 1.11 | 0.81, 1.51 | 0.64 |
| Uveitis duration, years | |||||
| ≤5 | 105 | 90 (71, 96) | 1.00 | ||
| >5 | 157 | 91 (81, 95) | 1.27 | 0.93, 1.75 | 0.14 |
| Inflammation activity at diagnosis macular edema | |||||
| Inactive | 69 | 92 (80, 96) | 1.00 | ||
| Active | 157 | 94 (88, 97) | 1.28 | 0.93, 1.75 | 0.13 |
| OCT† center point macular thickness (μm) | |||||
| <370 | 131 | 94 (88,97) | 1.00 | ||
| ≥370 | 95 | 93 (83, 97) | 0.99 | 0.93, 1,75 | 0.95 |
| OCT cystoid spaces | |||||
| Absent | 64 | 94 (85, 98) | 1.00 | ||
| Present | 156 | 93 (86, 96) | 1.20 | 0.91, 1.60 | 0.20 |
| Epiretinal membrane on OCT Overall comparison | |||||
| No | 120 | 97 (92, 99) | 1.00 | ||
| Yes | 102 | 89 (79, 94) | 0.74 | 0.55, 1.01 | 0.05 |
| Stratified by type ERM§ | |||||
| Prevalent | 0.58 | 0.38, 0.88 | 0.01 | ||
| Incident | 1.15 | 0.73, 1.80 | 0.55 | ||
| Lens status | |||||
| Phakic | 93 | 90 (80, 96) | 1.00 | ||
| Pseudophakic/aphakic | 134 | 96 (90, 98) | 1.19 | 0.86, 1.64 | 0.29 |
| Glaucoma medications | |||||
| No | 181 | 94 (88, 97) | 1.00 | ||
| Yes | 45 | 91 (75, 97) | 0.84 | 0.55, 1.27 | 0.41 |
| Prior glaucoma surgery | |||||
| No | 196 | 94 (88, 97) | 1.00 | ||
| Yes | 31 | 90 (72, 97) | 1.05 | 0.66, 1.66 | 0.84 |
| VA‡ worse than 20/40 | |||||
| Yes | 78 | 93 (82, 97) | 1.00 | ||
| No | 147 | 94 (88, 97) | 0.88 | 0.64, 1.19 | 0.40 |
| VA 20/200 or worse | |||||
| No | 194 | 93 (88, 96) | 1.00 | ||
| Yes | 31 | 96 (87, 99) | 0.83 | 0.57, 1.21 | 0.33 |
HR = unadjusted hazard ratio; HR’s >1.00 associated with faster resolution of macular edema; HR’s <1.00 associated with slower resolution of macular edema.
OCT = optical coherence tomography; results from different machines normalized to Zeiss Stratus scale.
VA = visual acuity.
ERM = epiretinal membrane. Interaction p-value = 0.03.
Relapse
One hundred seventy-seven eyes had resolution of the macular edema during the 7 years of follow-up and continued to have scheduled OCT’s performed for evaluation of relapse. The cumulative proportion of eyes with relapsed macular edema is shown as Figure 2. Of the 177 eyes with resolution of the macular edema, the cumulative proportion with relapsed macular edema by 7 years after resolution was 43% (95% CI: 32%, 51%). The associations between risk factors at the time of resolution and relapse of macular edema are shown in Table 3. Having thicker but still normal (200–239mm) center point macular thickness at the time of resolution (HR 2.02; 95% CI: 1.21, 3.36; P=0.007) and Black race (HR 1.80; 95% CI 1.03, 3.16; P=0.039) were associated with increased risk of macular edema relapse. There were no significant interactions between the type of macular edema (prevalent vs. incident) and other risk factors for relapse. Compared to eyes treated with systemic therapy, the HR for relapse of macular edema for eyes treated with the fluocinolone acetonide implant within 3 years of implantation (i.e. while the implant was releasing drug) was 0.83 (95% CI 0.47, 1.46; P=0.51), whereas the HR for relapse for implanted eyes 3 years after implantation was 1.99 (95% CI 1.01, 3.92; P=0.046).
Figure 2.
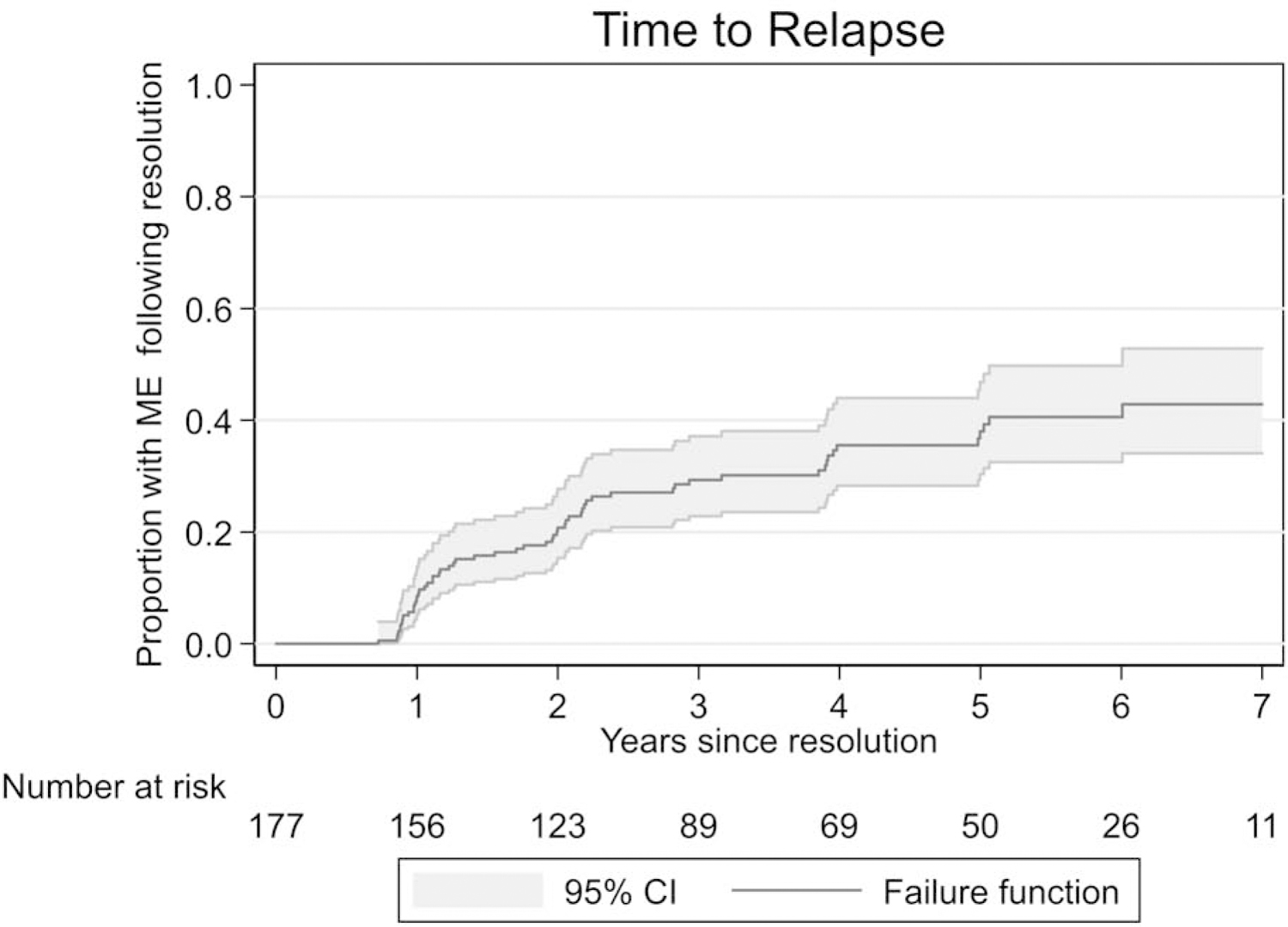
Time to relapse of macular edema for eyes with macular edema that achieved resolution in the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study.
Table 3.
Association between Characteristics at the Time of Resolution and Relapse of Macular Edema among Eyes with Resolution of Uveitic Macular Edema in the Multicenter Uveitis Steroid Treatment Trial and Follow-up Study.
| Characteristics | Number events/number eyes at risk | Cumulative percent relapsed by 7 years (95% CI*) | HR† | 95% CI* | P-value |
|---|---|---|---|---|---|
| Overall | 59/177 | 43(32,51) | |||
| Age, years | |||||
| <57 | 43/117 | 48 (34,58) | 1.00 | ||
| ≥57 | 16/60 | 34 (17,47) | 0.64 | 0.35, 1.17 | 0.15 |
| Gender, number | |||||
| Women | 40/127 | 42 (30,53) | 1.00 | ||
| Men | 19/50 | 42 (25,55) | 1.31 | 0.73, 2.33 | 0.36 |
| Race/ethnicity | |||||
| White, non-Hispanic | 31/107 | 35 (22,46) | 1.00 | ||
| Black, non-Hispanic | 17/39 | 58 (35,73) | 1.80 | 1.03, 3.16 | 0.039 |
| Hispanic/Other | 11/31 | 45 (23,61) | 1.45 | 0.74, 2.83 | 0.28 |
| Uveitic anatomic class | |||||
| Intermediate uveitides | 30/87 | 43 (28,55) | 1.00 | ||
| Posterior/panuveitides | 29/90 | 41 (27,52) | 0.98 | 0.58, 1.64 | 0.92 |
| Macular edema diagnosis | |||||
| Prevalent (at baseline) | 40/116 | 40 (29,50) | 1.00 | ||
| Incident (during follow-up) | 19/61 | 51 (23,69) | 1.13 | 0.65, 1.99 | 0.66 |
| Uveitis duration, years | |||||
| ≤5 | 15/51 | 33 (17,46) | 1.00 | ||
| >5 | 42/122 | 47 (33,58) | 1.49 | 0.81, 2.72 | 0.20 |
| Uveitis activity | |||||
| Inactive | 43/138 | 40 (28,50) | 1.00 | ||
| Active | 15/38 | 49 (28,64) | 1.32 | 0.75, 2.31 | 0.33 |
| Central midpoint thickness on OCT‡, μm | |||||
| <200 | 36/127 | 38 (26,49) | 1.00 | ||
| ≥200 | 23/50 | 53 (34,66) | 2.02 | 1.21, 3.36 | 0.007 |
| OCT cystoid spaces | |||||
| Absent | 39/120 | 43 (29,54) | 1.00 | ||
| Present | 20/56 | 41 (25,54) | 1.30 | 0.75, 2.25 | 0.35 |
| Epiretinal membrane on OCT | |||||
| No | 25/78 | 39 (24,51) | 1.00 | ||
| Yes | 34/98 | 46 (31,58) | 1.25 | 0.73, 2.13 | 0.42 |
| Lens status | |||||
| Phakic | 12/43 | 29 (14,42) | 1.00 | ||
| Pseudophakic/aphakic | 47/134 | 48 (35,58) | 1.43 | 0.73, 2.80 | 0.30 |
| VA§ worse than 20/40 | |||||
| No | 34/100 | 43 (29,54) | 1.00 | ||
| Yes | 25/77 | 41 (26,53) | 0.99 | 0.60, 1.64 | 0.97 |
| VA 20/200 or worse | |||||
| No | 56/162 | 44 (33,54) | 1.00 | ||
| Yes | 3/15 | 22 (0,42) | 0.54 | 0.16, 1.82 | 0.32 |
95% CI = 95% confidence interval.
HR = hazard ratio. HR’s > 1.00 associated with faster relapse.
OCT = optical coherence tomogram. Numbers from different machines normalized to Zeiss Stratus scale.
VA = visual acuity.
Ancillary regional corticosteroid injection therapy
The protocol permitted the use of short-acting regional corticosteroid injections for the treatment of macular edema which did not resolve with control of the inflammation with the assigned treatment for uveitis. Short-acting regional corticosteroid injections included periocular triamcinolone acetonide (Kenelog®), intravitreal triamcinolone acetonide (Triescence®), and intravitreal dexamethasone implant (Ozurdex®). Information on short-acting regional corticosteroid injections was available for 227 eyes. Forty percent of these 227 eyes (91 eyes) received at least one regional corticosteroid injection. Overall the rate of regional corticosteroid injections was 0.53/eye-year (EY). The rate of regional corticosteroid injections was lower among eyes with a fluocinolone acetonide implant (0.36/EY) than among eyes managed with systemic therapy (0.71/EY; rate ratio 2.0; 95% CI: 1.5, 2.6; P<0.001).
Visual acuity
Visual acuity over time is shown as Figure 3 and the time-updated change in acuity as Figure 4. Eyes without resolved macular edema could have either persistent or relapsed macular edema at the time of the visit. At one year of follow-up of the macular edema, eyes with resolved macular edema had significantly greater improvements in visual acuity than did eyes without resolution (6.8 vs 2.5 letters; Difference: −4.31, 95% CI: −6.65, −1.96, P<0.001). Thereafter, the visual acuity declined at each visit regardless of the macular edema status; however, the loss at each visit was unrelated to disease activity status but was significantly greater in eyes that had macular edema at that visit as compared to those that did not (−1.82 vs −0.72 letters with each additional year of follow-up, Difference: −1.11; 95% CI: −1.83, −0.38, P = 0.003). The rate of decline was related to the presence of macular edema at each visit and there was no significant difference between those that had persistent macular edema and those that recently-relapsed macular edema (P = 0.77).
Figure 3.
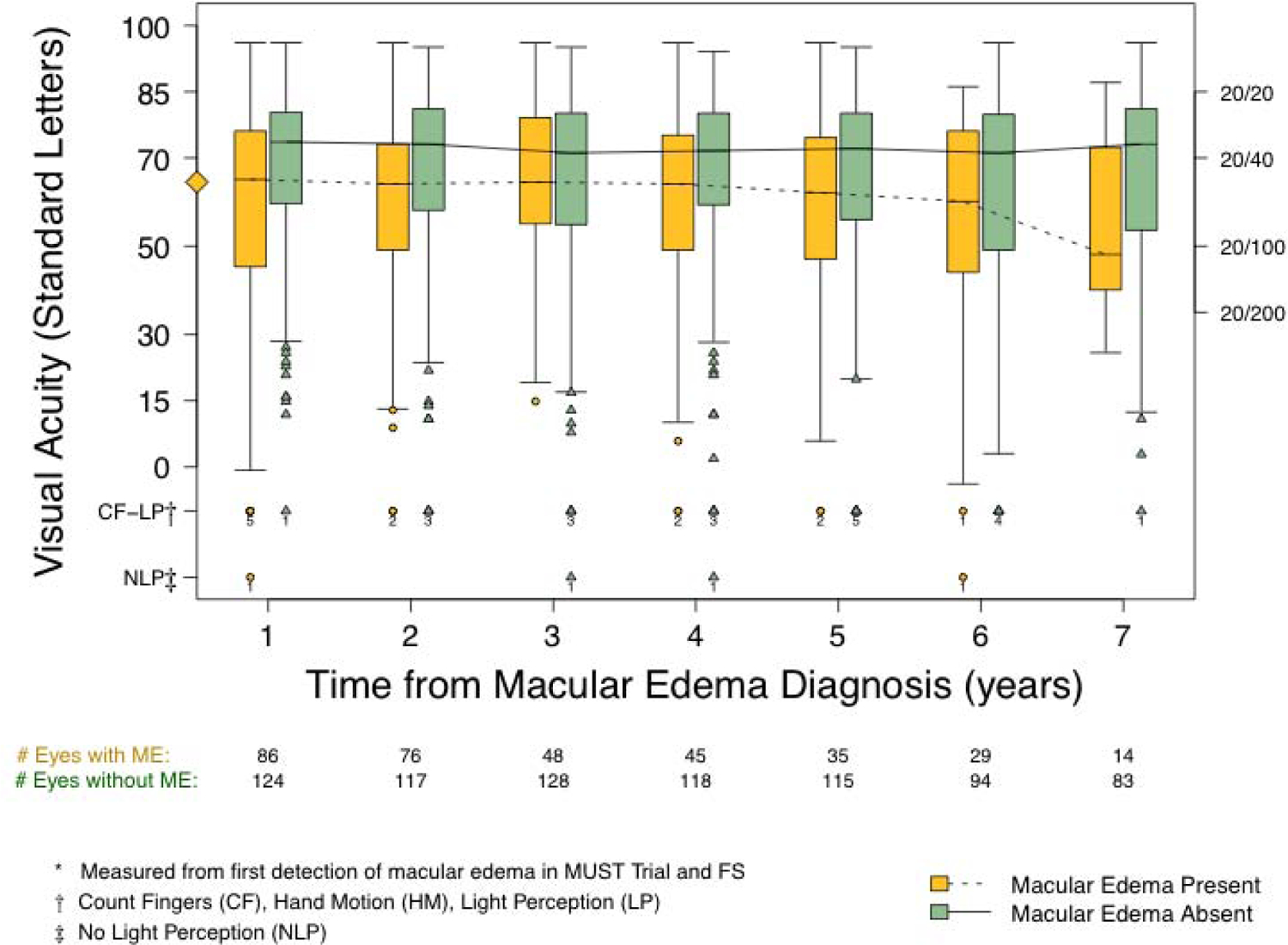
Visual acuity over time among participants with macular edema (ME) stratified by current (time-varying) macular edema status as either absent (green, solid line) or present (orange, dotted line). Macular edema after the initial detection could be either persistent or relapsed. The diamond on the Y-axis denotes median visual acuity at the time of macular edema detection for the entire cohort with macular edema (65 letters; Snellen equivalent 20/50).
Figure 4.
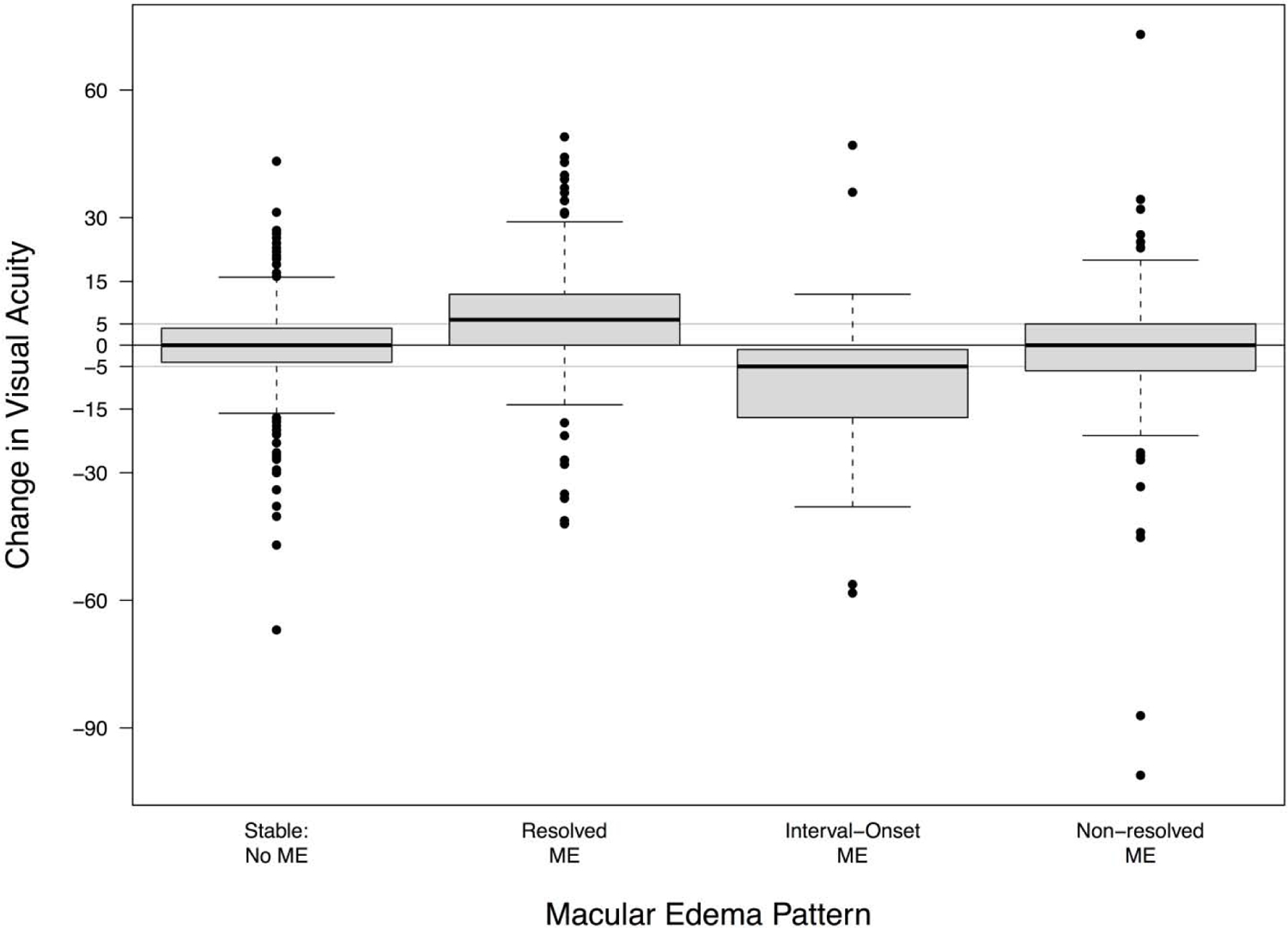
Change in visual acuity (in logarithmic acuity chart letters) stratified by the pattern of macular edema (ME) status during the 1-year follow-up intervals over the course of 7 years. The pattern of macular edema status for each eye during each interval was classified as stable without macular edema (no macular edema at the beginning or end of the interval), resolved (macular edema resolved during the interval), new-onset (eye developed macular edema during the interval, either as incident or relapsed), and non-resolved (macular edema present at the beginning and end of the interval).
The pattern of macular edema during the 1-year intervals between visits also was associated with changes in visual acuity during the same interval (Figure 4). In comparison to eyes that remained free from macular edema during the interval, eyes where macular edema resolved gained on average an additional 6.24 letters (95% CI: 4.40, 8.09, P < 0.001), and eyes that developed macular edema (“interval-onset” edema, either incident macular edema or relapsed macular edema) experienced a loss on average of an additional −8.65 letters (95% CI: −11.5, −5.84, P <0.001). There was a suggestion of an association between non-resolving macular edema and a loss of visual acuity (change: −1.30 letters; 95% CI: −2.70, 0.09, P=0.065); however, the difference did not achieve traditional statistical significance.
Discussion
Macular edema is a major cause of visual impairment in patients with uveitis. At presentation, participants in the MUST Trial without macular edema had a mean visual acuity of ~20/25, whereas those with cystoid macular edema had a mean visual acuity of 20/64.5 Retinal thickening (i.e. either diffuse or cystoid macular edema) alone was associated with, on average, ~9-letter (nearly 2 lines) worse vision.5 Although the MUST Trial and Follow-up Study were not designed as a trial of macular edema treatments, macular edema was an important secondary outcome in both the Trial and Follow-up Study. As such, the Trial and Follow-up Study provide an opportunity to describe the long-term outcomes of uveitic macular edema using prospectively collected data. The analysis of macular edema in the 7-year results paper evaluated macular edema cross-sectionally in the 2 treatment groups as present or absent, regardless of whether the edema was persistent or recurrent.13 In the current analyses, participants’ eyes were evaluated longitudinally to provide a clinically relevant description of the course of patients’ macular edema over a 7-year time frame.
Our data demonstrate that with treatment of the uveitis and the selective use of ancillary regional corticosteroid injections over 90% of eyes with uveitic macular edema can achieve resolution of the macular edema given sufficient time, but that these eyes remain at risk for relapse. Among patients whose uveitis is treated with systemic medications, resolution of the macular edema frequently requires adjunctive short-acting regional corticosteroid injections. In the analysis of macular edema in the original MUST Trial portion of this study, 62% of eyes with macular edema treated with systemic medication required at least one regional corticosteroid injection during the first 2 years of follow-up and that the median number of injections for patients receiving systemic therapy was 1.7 The data from the MUST Trial and Follow-up Study demonstrate that during long term-follow-up the rate of injections among patients treated with systemic medications was nearly 1 per year (mean 0.71/EY), suggesting an ongoing need for ancillary therapy in these patients.
The only risk factor identified that decreased the likelihood of macular edema resolution was the presence of an epiretinal membrane at baseline. The effect was present among eyes with prevalent macular edema but not incident macular edema. Previous work using spectral-domain OCT has demonstrated that mild epiretinal membranes that do not distort the retinal surface do not affect the resolution of macular edema, whereas more substantial epiretinal membranes that distort the retinal surface decrease the likelihood of improvement in the macular edema and improvement in vision.17 Given that the study was begun when only the less sensitive time-domain OCT’s were available but that spectral-domain OCT was permitted during the follow-up study, it is possible that the epiretinal membranes detected at enrollment in prevalent macular edema eyes may have been more substantial than those detected in eyes with incident macular edema, and hence more likely to decrease the rate of resolution of the macular edema. Our grading scheme for epiretinal membranes did not specify severity, information that would be necessary to confirm this possible explanation. It also is possible that prevalent epiretinal membranes (i.e. present at baseline) were associated with more long-standing macular edema than were those identified with incident macular edema, and as such prevalent macular edema with epiretinal membranes could be slower to resolve.
Despite the high success rate for achieving resolution of the macular edema, our data demonstrate a substantial rate of relapse after resolution, 43% have at least one episode of relapse within 7 years, which suggests that ongoing monitoring and management are required for eyes with a history of uveitic macular edema. Given the superiority of OCT to clinical examination at detecting macular edema15 these data suggest the need for regular monitoring using OCT even among those with a history of resolved macular edema. The use of multiple adjunctive regional corticosteroid injections over time suggests that treatment following relapse is needed to effect re-resolution of the macular edema and preserve vision.
The visual acuity results suggest that striving for resolution of the macular edema may be beneficial. As has been previously demonstrated, eyes with macular edema at enrollment had significantly worse visual acuity than those without.5 In this study, participants’ eyes with incident, persistent, or relapsed macular edema had consistently worse visual acuity over time than eyes with resolved macular edema. Eyes whose macular edema resolved experienced a significant improvement in acuity, whereas those whose edema did not resolve experienced no gains in acuity. New-onset of macular edema during follow-up (either newly-diagnosed, “incident” macular edema or relapsed macular edema) was associated with a significant decrease in acuity. The association of macular edema with worse visual acuity does not prove causality, and uveitic macular edema may be a marker for active or for more severe uveitis, However, the treatment protocol strove for uveitis control (inactive disease) with the assigned therapies (fluocinolone acetonide implant or systemic therapy), which was achieved in the large majority of patients.13 Regional adjunctive corticosteroid injections were permitted for persistent macular edema, not responsive to the assigned therapy despite control of the inflammation11,13 and were used about once every 2 years on average, suggesting that macular edema may have been the cause of the decreased vision. Hence these data suggest that a reasonable treatment goal might be resolution of uveitic macular edema whenever feasible, including the use of adjunctive regional corticosteroid injections, particularly among those patients whose uveitis is treated with systemic medications.7,18,19
Strengths of the study include its prospective nature, regular OCT measurements, and the multi-year follow-up, providing long-term data not typically available. Limitations of the study include the use of time-domain OCT during the initial MUST Trial portion of the study and the absence of a grading scheme for the severity of epiretinal membranes. Spectral-domain OCT was in development at the inception of the MUST Trial and was not readily available. During the Follow-up Study, spectral-domain OCT replaced time-domain OCT and was introduced into the study. Although we were able to normalize OCT measurements to the original OCT scale and thereby provide more easily comparable dataset, differences in the ability to detect subtle changes remain, and baseline OCT features related to resolution of the macular edema detectable on spectral-domain OCT but not time-domain OCT20 could have been missed. Other limitations include the limited frequency of follow-up during the study, restricting the ability to detect transient changes in macular edema and features related with relapse. Finally, routine fluorescein angiograms, although obtained in the MUST Trial, were not obtained during the MUST Follow-up Study; therefore, angiographic data could not be used in these analyses, so we do not have information on angiographic resolution or persistence of macular leakage. Nevertheless, visual acuity is more strongly associated with retinal thickness than with the amount of leakage on fluorescein angiography, and macular edema typically is followed with OCT,15,21,22 so our results are relevant to clinical management.
In conclusion, our data demonstrate the ability to resolve uveitic macular edema in the large majority of patients given sufficient time. Nevertheless, given the relatively high rate of relapse of resolved macular edema, ongoing monitoring and management are needed. Our treatment data suggest an ongoing need for ancillary regional corticosteroid therapy among patients treated with systemic therapy. Given the better visual acuity outcomes with resolved macular edema, these data suggest that, in addition to controlling the inflammation in patients with uveitis, it may be appropriate to direct treatment to resolving the macular edema.
Acknowledgments
Grant support: Supported by cooperative agreements U10 EY014655 (Dr. Jabs), U10 EY014660 (Dr. Holbrook), and U10 EY14654 (Dr. Altaweel) from the National Eye Institute, National Institutes of Health, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Oren Tomkins-Netzer: none; Susan L. Lightman: none; Alyce Burke: none; Elizabeth Sugar: none; Lyndell L. Lim: none; Glenn J. Jaffe: EyePoint Pharmaceuticals (consultant); Michael Altaweel: none; John Kempen: Gilead Sciences (data and safety monitoring committee); Douglas A. Jabs: none.
ClinicalTrials.gov registration number: 00132691
REFERENCES
- 1.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol 1990;14:303–8. [DOI] [PubMed] [Google Scholar]
- 4.Tomkins-Netzer O, Talat L, Bar A, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology 2014;121:2387–92. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SR, Lightman SL, Sugar EA, et al. The impact of macular edema on visual function in intermediate, posterior, and panuveitis. Ocul Immunol Inflamm 2012;20:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin MH, Pistilli M, Daniel E, et al. Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology 2014;121:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomkins-Netzer O, Lightman S, Drye L, et al. for the Multicenter Uveitis Steroid Treatment Trial Research Group. Outcome of treatment of uveitic macular edema: the Multicenter Uveitis Steroid Treatment (MUST) Trial 2-year results. Ophthalmology 2015;122:2351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habot-Wilner Z, Sallam A, Pacheco PA, et al. Intravitreal triamcinolone acetonide as adjunctive treatment with systemic therapy for uveitic macular edema. Eur J Ophthalmol 2011;21(Suppl6):S56–61. [DOI] [PubMed] [Google Scholar]
- 9.Markomichelakis NN, Halkiadakis I, Pantelia E, et al. Course of macular edema in uveitis under medical treatment. Ocul Immunol Inflamm 2007;15:71–9. [DOI] [PubMed] [Google Scholar]
- 10.Sugar EA, Jabs DA, Altaweel MM, et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol 2011;152:1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Multicenter Uveitis Steroid Treatment Trial Research Group. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol 2010;149:550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Multicenter Uveitis Steroid Treatment Trial Research. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology 2011;118:1916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Association between long-lasting intravitreous fluocinolone acetonide implant vs systemic ianti-inflammatroy therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA 2017;317:1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris FL 3rd, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: Guidelines from the Eye Care Technology Forum. Ophthalmology 1996;103:181–2. [DOI] [PubMed] [Google Scholar]
- 15.Kempen JH, Sugar EA, Jaffe GJ, et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology 2013;120:1852–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domalpally A, Altaweel MM, Kempen JH, et al. Optical coherence tomography evaluation in the Multicenter Uveitis Steroid Treatment (MUST) trial. Ocul Immunol Inflamm 2012;20:443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehpamer B, Moshier E, Pahk P, et al. Epiretinal membranes in uveitic macular edema: effect on vision and response to therapy. Am J Ophthalmol 2014;157:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000;130:492–513. [DOI] [PubMed] [Google Scholar]
- 19.Jabs DA. Immunosuppression for the uveitides. Ophthalmology 2018;125:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehpamer B, Moshier E, Goldberg N, Ackert J, Godbold J, Jabs DA. Subretinal fluid in uveitic macular edema: effect on vision and response to therapy. Am J Ophthalmol 2013;155:143–9. [DOI] [PubMed] [Google Scholar]
- 21.Sivaprasad S, Ikeji F, Xing W, Lightman S. Tomographic assessment of therapeutic response to uveitic macular oedema. Clin Experiment Ophthalmol 2007;35:719–23. [DOI] [PubMed] [Google Scholar]
- 22.Nussenblatt RB, Kaufman SC, Palestine AG, et al. Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology 1987;94:1134–9. [DOI] [PubMed] [Google Scholar]


