Abstract
Background and objectives:
Thyroid cancer therapy is increasingly tailored to patients’ risk of recurrence and death, placing renewed importance on pathologic parameters. The International Collaboration on Cancer Reporting (ICCR), an organization promoting evidence-based, internationally agreed upon standardized pathology data sets, is the ideal conduit for the development of a pathology reporting protocol aimed at improving the care of patients with thyroid carcinomas.
Methods:
An international expert panel reviewed each element of thyroid pathology reporting. Recommendations were made based on the most recent literature and expert opinion.
Results:
The data set uses the most recent World Health Organization (WHO) classification for the purpose of a more clinically and prognostically relevant nomenclature. One example is the restriction of the term minimally invasive follicular carcinoma to tumors with capsular invasion only. It reinforces the already established criteria for blood vessel invasion adopted by the most recent WHO classification and Armed Forces Institute of Pathology fascicle. It emphasizes the importance of the extent of blood vessel invasion and extra-thyroid extension to better stratify patients for appropriate therapy. It is the first data set that requires pathologists to use the more recently recognized prognostically powerful parameters of mitotic activity and tumor necrosis. It highlights the importance of assessing nodal disease volume in predicting the risk of recurrence.
Conclusions:
The ICCR thyroid data set provides the tools to generate a report that will guide patient treatment in a more rational manner aiming to prevent the undertreatment of threatening malignancies and spare patients with indolent tumors the morbidity of unnecessary therapy. We recommend its routine use internationally for reporting thyroid carcinoma histology.
Keywords: data set, protocol, structured report, synoptic report, ICCR, thyroid carcinoma
Introduction
Over the last two decades, there have been significant advances in the treatment of thyroid carcinoma. At the end of the 20th century, the established consensus approach was that the treatment of most thyroid carcinomas should consist of sub or total thyroidectomy followed by post-operative radioactive iodine (RAI) remnant ablation (1). This “one size fits all” approach is no longer valid and the current recommendations from the American Thyroid Association (ATA) and the National Comprehensive Cancer Network (NCCN) advocate therapies tailored more closely to the individual patient’s risk of recurrence and death (2–4). This paradigm shift is based on studies that were able to stratify patients into various prognostic groups using clinical and histopathologic parameters (5–8).
Many of the key elements forming the basis of risk stratification schemes used to guide therapy are identified only by pathological assessment of thyroidectomy specimens including the presence and extent of blood vessel invasion and extrathyroidal extension as well as the volume of metastatic regional nodal disease (5–9). A detailed surgical pathology report is therefore the cornerstone of thyroid cancer treatment.
The International Collaboration on Cancer Reporting (ICCR) is a not-for-profit organisation founded in 2010 with the goal of producing standardized internationally agreed and evidence-based data sets for pathology reporting. The ICCR is sponsored by major pathology organizations including the Royal Colleges of Pathologists of Australasia and the United Kingdom, the College of American Pathologists, the Canadian Association of Pathologists in association with the Canadian Partnership Against Cancer, the European Society of Pathology, the American Society of Clinical Pathology and the Faculty of Pathology, Royal College of Physicians of Ireland. With input from pathologists worldwide, this thyroid data set is intended to include all items necessary for an objective and accurate pathology report which will enable clinicians to risk stratify their patients and apply the most appropriate therapy.
Methods
The ICCR has developed standard operating procedures for the process of data set development overseen by a Dataset Steering Committee (DSC), and these have been described in earlier publications (http://www.iccr-cancer.org/articles/publications). Under the leadership of Dr Ronald Ghossein, and utilizing the framework established by the ICCR, the Dataset Authoring Committee (DAC) comprised 12 pathologists with special expertise in thyroid neoplasia. They were joined by an endocrinologist (RMT) who is one of the authors of the most recent ATA guidelines for the management of thyroid carcinomas (3) and the principal author of the 8th edition of the American Joint Committee on Cancer (AJCC) staging system for follicular cell derived thyroid carcinomas (10). This global group of experts emanated from several regions and continents (North America, Europe, Asia and Australia).
A commentary for each data item was formulated on the basis of a review of the current literature, pathology reporting protocols and treatment recommendations for carcinoma of the thyroid and formed the basis of discussion among the experts. A series of teleconferences were held to review and discuss each of the elements. After the DAC reached general agreement and after approval by the ICCR DSC, the data set was posted to the ICCR website for a period of two months for public comment. After collecting all feedback, the data set was once again reviewed, approved and final changes were made by the DAC.
In this review, we describe and discuss the most important core (essential) elements of this data set with greater emphasis on the features that are crucial for prognosis and management. The data set reporting guide and synoptic pathology report are available online at http://www.iccr-cancer.org/datasets/published-datasets/endocrine-organs/carcinoma-of-the-thyroid-tnm8
Results and Discussion
Scope
The data set was developed for the pathology reporting of thyroid resection specimens for primary thyroid carcinomas. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), tumors of uncertain malignant potential (UMP), thyroid carcinomas arising from struma ovarii, thyroid carcinomas arising in thyroglossal duct cysts, sarcomas, lymphomas and metastases to the thyroid gland are not covered in the data set.
Core elements
The data set includes 19 ‘core elements’ and 8 ‘non-core elements’. Core elements are defined as those that are essential for the clinical management, staging or prognosis of the cancer. Core elements have either level III-2 or above evidentiary support (11) or were viewed by the committee to be critical to the described disease and comprised the minimum reporting standard. Non-core data elements may not be readily available to all users of the data set and/or may fail to meet the above evidentiary criteria. A summary of the core and non-core elements for carcinoma of the thyroid is provided in Table 1 and the crucial core items described below:
Table 1.
Core and noncure elements for the pathology reporting of carcinoma of the thyroid.
| Core | Noncore |
|---|---|
| Operative procedure | Clinical information |
| Operative findings | Tumor focality |
| • Number of tumors in specimen | |
| Specimen(s) submitted | Tumor dimensions |
| • Additional dimensions (largest tumor) | |
| Tumor focality | Histological tumor grade |
| Tumor site | Lymphatic or blood vessel invasion |
| • Extrathyroid blood vessel invasion | |
| Tumor dimensions | Margin status |
| • Distance of the tumor to the closest margin | |
| • Involvement of anterior or posterior | |
| Histological tumor type | C-cell hyperplasia |
| Mitotic activity | Ancillary studies |
| Tumor encapsulation/circumscription | |
| Capsular invasion | |
| Lymphatic or blood vessel invasion | |
| Necrosis | |
| Extrathyroidal extension | |
| Margin status | |
| Lymph node status | |
| Coexistent pathology | |
| Parathyroid gland status | |
| Histologically confirmed distant metastases | |
| Pathological staging |
Surgical procedures, operative findings and specimen submitted.
It is expected that the surgeon reports the type of procedure performed, the presence or absence of gross extrathyroidal extension (ETE), and the completeness of excision. Gross ETE is a crucial element in the staging systems of thyroid carcinomas (10, 12). The pathologist should indicate if the intra-operative data on gross ETE or margin completeness is lacking at the time of pathology reporting.
Tumor site and focality.
Thyroid carcinoma, particularly papillary thyroid carcinoma, is commonly multifocal. Indeed, the combined results of several studies have estimated that as many as 39% of all clinically apparent papillary carcinoma are multifocal (13, 14). Whether the tumor is unifocal or multifocal is considered a core item. When multifocal, the number of tumors should be stated. However, if greater than 5 tumors are present, this may simply be stated rather than counting the exact number of tumors. For the purposes of reporting all other pathological data, the full data set need only be applied to the ‘dominant’ resected carcinoma of a particular lineage. The dominant tumor is defined as the most clinically relevant carcinoma because of its aggressiveness and/or its higher T category. The dominant tumor is often, but not always, the largest carcinoma. For example, in the case of a papillary thyroid carcinoma with gross extension into muscle associated with a separate papillary carcinoma without adverse histologic features, the data set should be filled for the tumor with gross extra-thyroid extension. If tumors of different lineage occur in the same specimen, then a data set should be completed for each of these carcinomas. For example, if a lobectomy contains separate medullary and papillary thyroid carcinoma, a data set should be completed for each of these carcinoma types. However if two separate papillary carcinomas of different subtypes (for example a follicular variant and a classic type) are identified, only the dominant tumor need be annotated. For non-dominant tumors that do not alter management, basic pathological features (such as size and location) may be reported at the pathologist’s discretion (non-core element).
Tumor dimensions
The dimensions are those of the dominant tumor, based upon a reconciliation of the imaging, macroscopic and microscopic findings. The largest dimension of the tumor is a core element since it has an impact on prognosis and is a component of TNM staging (15).
Histological tumor type
All tumors of the thyroid should be given a type based on the most recent edition of the World Health Organization (WHO) Classification of Tumors of Endocrine Organs (16).
Papillary carcinomas
Papillary carcinoma is the most common carcinoma type and consists of numerous named variants, although only some of these currently have sufficient evidence to be considered clinically and biologically relevant. Thus efforts should be made to flag or document the following types when present:
Classic papillary thyroid carcinoma (PTC), tall cell and microcarcinoma variants
Classic (usual, conventional) papillary carcinoma is the “default” variant of papillary carcinoma. Tall cell variant of papillary carcinoma is a more aggressive variant as a whole than classic PTC. It has a higher prevalence of BRAF mutations and is more frequently refractory to radioactive iodine therapy (17–19). It is defined by the presence of >30% tall cells with their height at least twice their width (16). Papillary micro carcinomas are defined by their small size (≤1 cm) and are extremely indolent and often incidental (16).
Follicular variants and related lesions
Follicular variant of papillary carcinoma should be documented. It has recently been subdivided based on outcome into completely encapsulated/well demarcated and infiltrative follicular variants which completely or partially lack a capsule (20). Infiltrative follicular variants behaves similarly to classic papillary carcinoma, particularly in terms of their tendency to spread to regional lymph nodes, while the behaviour of encapsulated/well circumscribed follicular variant is more indolent, especially if non-invasive (21–23). Many non-invasive encapsulated/well circumscribed follicular variants of papillary thyroid carcinoma can now be reclassified under the novel designation non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). This shift in nomenclature arose as an effort to encourage conservative management of these lesions given their extremely low risk of recurrence (24). As NIFTP is not overtly malignant, it is reported under coexistent pathology.
Cribriform-morular, Diffuse sclerosing, and hobnail variants:
The cribriform morular variant is a biologically distinct variant characterized by APC or beta-catenin mutations and shows an association with familial adenomatous polyposis coli (FAP), in some cases preceding recognition of colorectal neoplasia or other extracolonic manifestations (25). The recognition of this rare variant can therefore be life-saving if it suggests the diagnosis of FAP before the occurrence of colorectal adenocarcinoma (25). Diffuse sclerosing variant is an aggressive variant with a high rate of nodal metastasis and locoregional recurrence, although overall survival is good possibly because of the young age of the patients. Nonetheless, this variant appears to necessitate more aggressive initial surgical management including more extensive node dissection (26).
The hobnail variant is a rare aggressive form of PTC characterized by the presence of at least 30% of hobnail cells in the tumor, frequent distant metastases, a lack of radioactive iodine uptake and death from disease in a significant proportion of patients (16, 27, 28). The hobnail term refers to tumor cells with apically placed nuclei that produce a surface bulge, cellular discohesion and frequently a high mitotic rate (27).
Other variants that may have prognostic and therapeutic value but are rare and not well validated include the oncocytic and solid variant.
Follicular and Hürthle (oncocytic) cell carcinomas
The diagnosis of follicular carcinoma and its distinction from follicular adenoma rests primarily on the identification of invasion of the tumor capsule and/or vascular spaces. The most recent WHO classification, which is endorsed by the ICCR, subdivides these carcinomas into a three tiered system: minimally invasive - capsular invasion (CI) only - encapsulated angioinvasive (any focus of vascular invasion) and widely invasive (grossly apparent extensive invasion of the thyroid and/or extra-thyroid tissue frequently with prominent vascular invasion). In keeping with the latest WHO classification, Hürthle (oncocytic) cell carcinoma is defined as a tumor composed of at least 75% oncocytes and lacking the nuclear features of papillary carcinoma, and demonstrating capsular and/or vascular invasion (16). Although it is no longer considered a variant of follicular carcinoma on the basis of its overall more aggressive behaviour, different molecular profile and much lower propensity to concentrate radioactive iodine (16), the definitions of minimally invasive, angioinvasive and widely invasive Hürthle (oncocytic) cell carcinoma mirror those of follicular carcinoma.
Poorly differentiated thyroid carcinomas (PDTC)
Poorly differentiated thyroid carcinomas (PDTC) have a prognosis intermediate between the indolent well differentiated papillary thyroid carcinoma and the frequently fatal anaplastic thyroid carcinoma. According to the most recent WHO classification, poorly differentiated carcinomas are tumors that display a solid, trabecular, and/or insular growth pattern, lack nuclear features of papillary carcinoma and show 1 or more of the following: 3 or more mitoses per 10 high power fields (HPF), tumor necrosis, and nuclear convolution (16, 29). The recognition of this aggressive subtype is important for planning treatment and follow up (3, 17, 30). As defined, PDTC is a tumor type that has a prognosis intermediate between well-differentiated and undifferentiated (anaplastic) thyroid carcinoma (16). Grading (based on high mitotic count and necrosis as used at Memorial Sloan-Kettering Cancer Center, New York) also identifies aggressive thyroid tumors of intermediate prognosis, regardless of their cytoarchitectural features (16, 31).
Anaplastic (undifferentiated) thyroid carcinomas
Undifferentiated carcinoma represents the most extreme form of tumor progression and consists of a high-grade malignancy with spindled, pleomorphic, squamoid, giant cell or rhabdoid morphology (32). Anaplastic carcinoma is very often rapidly lethal. A better differentiated component such as PTC or Hürthle cell carcinoma may be found and its presence should be reported. Although the percentage and size of the anaplastic component in a tumor have prognostic impact by univariate analysis, they are not independent predictors of outcome (33). In a recent study of 163 patients with anaplastic carcinoma who underwent resection of their primary tumors, the presence of gross residual disease remained the only independent prognostic factor predicting overall survival on multivariate analysis.(33).
Mitotic activity
In the last 20 years, several publications have recognized the role of mitotic count in prognosticating and diagnosing thyroid carcinomas (29, 31, 34). However many pathologists and current College of American Pathologist reporting guidelines consider mitotic activity an optional item (35). It is the opinion of this expert panel that mitotic status should be reported in every thyroid carcinoma on the basis that it is an essential defining criterion for PDTC regardless of the definition used for this entity (29, 31, 36). Furthermore, two recent large studies have shown that high mitotic activity particularly correlates with poorer outcome in medullary thyroid carcinoma (37, 38). In recognition of the fact that the vast majority of thyroid carcinomas have a very low mitotic rate, and mindful of the need to maximize pathologist efficiency, a numerical mitotic count is required only in those cases with elevated mitotic activity (≥3 mitoses/2 mm2), otherwise it can merely be stated as <3 mitoses/2 mm2. Mitotic count should be performed in the area of highest mitotic activity (hotspots) in consecutive HPFs (39).
Tumor encapsulation/circumscription
The presence of a fibrous capsule or a well demarcated tumor border (ie, well circumscribed tumor edge directly adjacent to benign thyroid parenchyma with no intervening capsule) is a crucial element in thyroid carcinomas (16). Even in high grade tumors such as poorly differentiated carcinoma, the presence of a capsule has been shown to convey a better outcome (31, 39, 40).
Capsular invasion
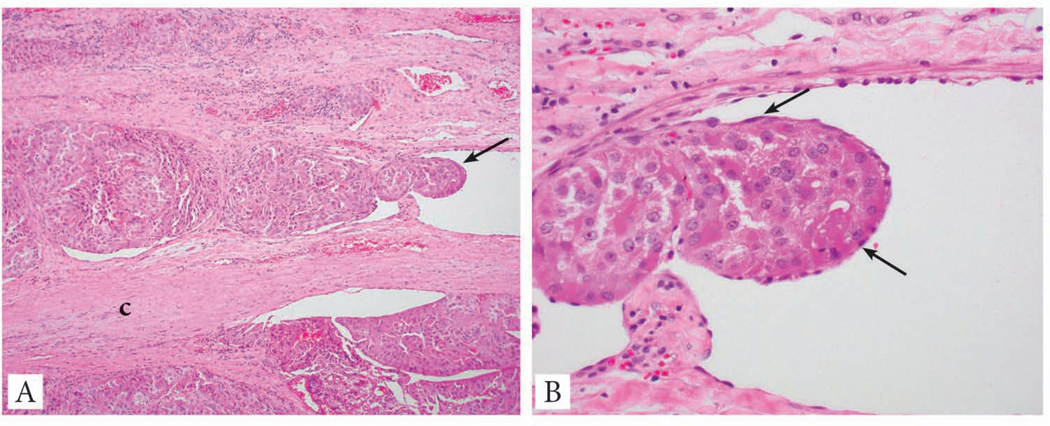
There are still some differences in opinion regarding the pathological definition of capsular invasion (CI). While there is universal agreement that complete penetration of the capsule constitutes CI (41), some endocrine pathologists do not require complete transgression of the capsule (42). Currently the majority of pathologists have adopted the criteria endorsed by Chan (2007) (41) requiring full thickness capsular penetration, including the College of American Pathologists, and this approach is endorsed by the ICCR panel (Fig. 1). There is no need to report on the extent (number of foci) of CI since it has not yet been shown to have clinical value.
Fig. 1:
Capsular invasion in the form of a mushrooming bud of tumor completely penetrating the capsule.
Lymphatic and blood vessel invasion
The issue of whether to separately identify blood vessel invasion from lymphatic invasion was difficult and the committee clarified its position after feedback from the endocrine pathology community upon publication of the initial document. The final approach is that the presence of lymphatic or blood vessel invasion should be noted and then it should be recorded if the invasion is in blood vessels or lymphatics. Based on the type of carcinoma and the morphologic appearance of the vessel, the pathologist can in most instances indicate the type of vessel involved by tumor. All follicular carcinomas and the vast majority of Hürthle cell carcinomas spread via the bloodstream to distant sites bypassing lymph nodes while most papillary carcinomas (with the notable exception of invasive encapsulated follicular variant papillary carcinoma) preferentially spread to lymph nodes. It is therefore assumed that small thin walled vessels invaded by follicular carcinoma and Hürthle (oncocytic) cell carcinoma are almost always blood vessels while those invaded by papillary carcinoma (except for the invasive encapsulated follicular variant) are usually lymphatic spaces. Invasion of lymphatic vessels is however difficult to identify except in the rare diffuse sclerosing variant of papillary thyroid carcinoma (16). Lymphatic invasion is undetected by routine histology in many primary papillary carcinomas despite the patients having a large volume of nodal metastasis. Therefore, in contrast to blood vessel invasion, the presence of lymphatic space permeation has not been shown yet to have any prognostic value. Of note, blood vessel invasion can occur in papillary carcinomas (including classic) and the vessels involved are often easily identified as blood vessels because of their larger size and the presence of smooth muscle in their walls. There are, however, instances where determining the type of thin walled vessel involved is not possible. In these cases, the term “small vessel, not otherwise classifiable” is used. As blood vessel invasion (BVI) is a crucial diagnostic and prognostic feature, the criteria for its identification should be well defined. All authors agree that BVI should involve capsular or extra-capsular vessels in encapsulated tumors. In infiltrative tumors partially encapsulated or totally lacking a capsule, BVI can be present within the tumor nodule. The ICCR panel endorses the criteria proposed by Chan (2007) to diagnose BVI (41).
BVI should be diagnosed if the tumor thrombus is attached to the vessel wall hanging in the lumen, covered by endothelium or associated with fibrin (Fig. 2). If the tumor is encapsulated, intra-tumoral or subcapsular vessels do not qualify for BVI and should not be interpreted as such.
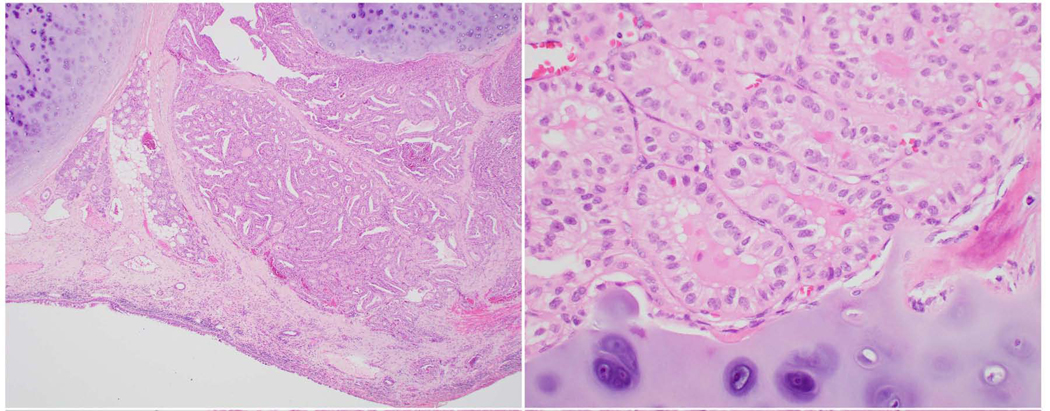
Fig. 2:
42 year old male with a 5 cm encapsulated angioinvasive Hürthle (oncocytic) cell carcinoma with extensive blood vessel invasion. The patient developed lung and bone metastasis. A: Medium power view (200X) of the tumor capsule (c). A tumor thrombus (arrow) is hanging in the lumen of an intra-capsular vessel. B: High power view (400X) of the involved vessel present in A shows the tumor thrombus covered by endothelial cells (arrows).
Several publications have shown that the presence of 4–5 foci of BVI in encapsulated follicular/Hürthle (oncocytic) cell carcinoma confers a much worse outcome than lower number of BVI foci (9, 43, 44). The most recent American Thyroid Association (ATA) guidelines classify a patient in a high risk category, if having 4 foci or more of BVI, while focal BVI (<4 foci) in an intrathyroidal follicular carcinoma will put the patient in a low risk category (3). Following the same conceptual approach, the National Comprehensive Cancer Network (NCCN) 2019 guidelines have defined minimal vascular invasion as a few foci of vascular invasion, and does not mandate radioiodine (RAI) administration in an intrathyroidal, well defined, follicular or Hürthle cell carcinoma, with minimal vascular invasion (4). Therefore, the ICCR DAC concluded that for encapsulated lesions it is important to indicate whether vascular space invasion was focal (1–3 foci) or extensive (≥4 foci). There is no evidence however that the number of BVI foci impacts prognosis in non-encapsulated PTC. Counting the BVI foci in non-encapsulated PTC is therefore not a required item in this data set. In the opinion of this expert panel, it is however a core item in those PTC that are completely encapsulated. This is because the latter category is composed in its vast majority of encapsulated FVPTC with vascular invasion that have a tendency to spread hematogenously.
Necrosis
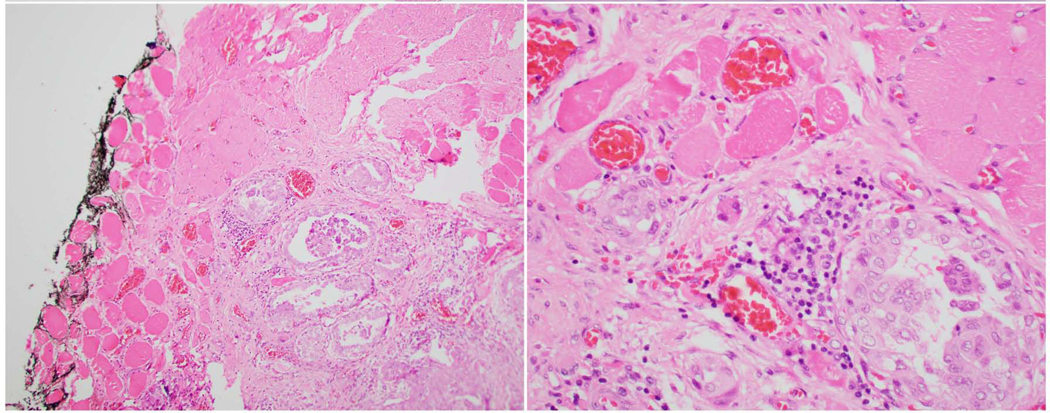
Since the year 2000, several studies have recognized the role of tumor necrosis in prognosticating and diagnosing differentiated thyroid carcinomas (17, 29, 31, 34, 36) and medullary thyroid carcinoma (37, 38). In the opinion of this expert panel, tumor necrosis should be reported in every thyroid carcinoma since it is an essential defining criterion for PDTC regardless of the definition used for this entity (29, 31). Tumor necrosis is defined as coagulative or comedo-necrosis and should be differentiated from infarct-like necrosis and reactive change related to previous FNA or ischemic changes within the tumor (Fig. 3).
Fig. 3:
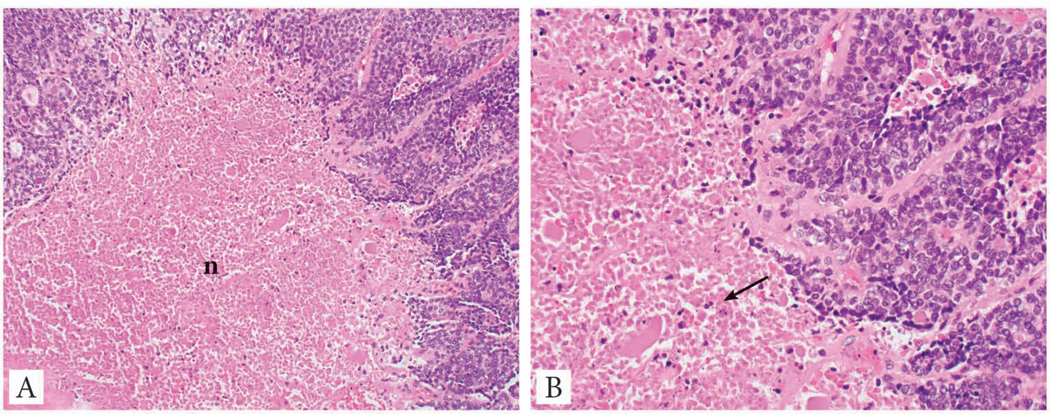
Tumor necrosis in a poorly differentiated thyroid carcinoma. A: Medium power view of the tumor necrosis (n) which has a comedo-like appearance. It lacks the fibrosis, vascular proliferation and hemosiderin deposition seen in infarct type necrosis due to fine needle aspiration. B: High power view of the tumor necrosis showing nuclear debris, a typical feature of tumor necrosis (arrow).
Extrathyroidal extension
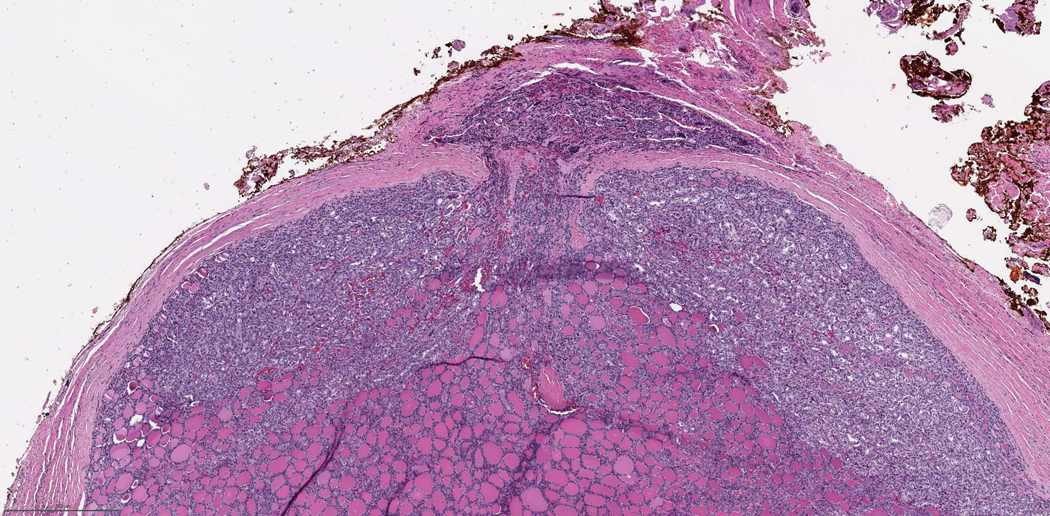
Extrathyroidal extension (ETE), defined as tumor extension beyond the thyroid capsule into the adjacent perithyroid tissue, is a common pathologic finding being detected in up to 23.5% of all papillary thyroid carcinomas (45). ETE has long been considered an adverse prognostic factor associated with an increased risk of recurrence and mortality (45–48). The risk of recurrence associated with microscopic extrathyroidal extension is approximately 3 to 9% (5, 49–54), compared with a 23 to 40% risk of recurrence in patients with gross ETE (Fig. 4, 5) (5, 49, 51–53, 55, 56). The assessment of microscopic ETE shows poor interobserver concordance (10, 12, 57, 58) and several studies have demonstrated that microscopic ETE is not an independent predictor for persistent disease, recurrence free survival and disease specific survival (50, 51, 54, 56, 59, 60). As a result of this data, microscopic ETE no longer upstages tumors under the AJCC and Union for International Cancer Control (UICC) 8th editions (10, 12). Therefore, the ICCR DAC emphasized that the pathologists’ role is now to 1) comment on the presence or absence of ETE status and 2) communicate with the surgeon in regard to staging since the determination of gross ETE is very often determined intra-operatively.
Fig. 4:
65 year old male with recurrent papillary carcinoma, tall cell variant grossly extending into trachea. The patient died of disease 10 years after this tracheal resection. Left: Medium power view showing the carcinoma invading in between tracheal cartilage extending into respiratory mucosa. Right: High power view showing papillary carcinoma, tall cell variant invading tracheal cartilage.
Fig. 5:
19 year old male with papillary carcinoma, classic with microscopic extrathyroid extension. Patient is alive without evidence of disease 23 year later. Left: Medium power view showing tumor invading perithyroid skeletal muscle. Right: High power view of the tumor with typical papillary carcinoma nuclei infiltrating in between skeletal muscle fibers.
Margin status
The margin status of a surgical resection for thyroid carcinoma is a required element and can be divided into three categories: a R0 resection (microscopically negative margin), a R1 resection (grossly complete resection with microscopically positive margin), and a R2 resection (grossly positive margin or incomplete resection) (10). The macroscopic impression of the margin status should be conveyed to the pathologist by the operating surgeon. Histologically, a positive margin is defined by the presence of tumor cells at the inked tissue border and/or the outer aspect of the thyroid gland (61–64). In contrast to carcinomas in many other organ systems, several studies have shown that a microscopically positive margin is not an independent predictor for recurrence and disease free survival, especially after adjusting for tumor stage and ETE (62–64). In view of these findings, the current ATA guidelines have only included grossly incomplete (R2) resection as a feature of high risk lesions (3). In contrast, the NCCN 2019 guideline has included any positive resection margin as one of the criteria to recommend completion thyroidectomy (4). Although margin status was considered a core element, the ICCR DAC recognizes that there are conflicting data on the significance of the location of the positive margin particularly at the posterior surface with emerging evidence suggesting it may not affect prognosis (62, 65). Reporting the location of an involved margin is therefore considered a non-core element.
Lymph node status
Until 2012, the accepted paradigm assigned the same magnitude of risk for recurrence to all patients with regional nodal disease (66). For example, in the 2009 ATA guidelines, any nodal disease was categorized as intermediate risk independent of the volume of nodal metastasis (2). There is now mounting evidence that various characteristics of regional nodal metastases such as number, size, and extranodal extension (ENE), may provide additional prognostic information. Thus, detailed features of nodal disease should be included in the pathology report, and be considered in risk grouping and staging systems (7, 59, 66–72). Taking this data into consideration, the NCCN 2019 guidelines no longer advocate completion thyroidectomy and post-operative RAI in small volume pN1a disease, ie, <5 involved nodes with metastasis <2 mm in largest dimension (4). Similarly, the ATA guidelines low risk category includes small volume nodal disease defined as “clinical N0 or ≤5 pathologic N1 micrometastases, <0.2 cm in largest dimension)” (3).In regard to higher risk groups, the ATA now considers the presence of more than 5 nodes with metastasis as intermediate risk and a positive lymph node that is ≥3 cm in size as high risk. The greatest dimension of the largest metastatic deposit in a lymph node should also be measured. The presence of psammoma bodies alone (without associated viable tumor cells) in a node is subject to controversy. While some practicing pathologists do not interpret these as metastasis, the ICCR is in agreement with the College of American Pathologists and the current AJCC staging manual in considering these lymph nodes as positive for micrometastases (10, 35).
ENE is not an infrequent finding, being reported in up to 12% of PTC overall and 33% of nodal metastatic PTC (59, 70). Similar to ETE, there are no well-defined, consensus histologic criteria for ENE in thyroid carcinomas and there is poor interobserver concordance (57). Nonetheless, many studies have demonstrated an association between ENE and persistent and/or recurrence disease (59, 66–72). Therefore, it is important to document ENE in the pathology report of a differentiated thyroid carcinoma.
Coexistent pathology
The presence of chronic lymphocytic thyroiditis, follicular adenoma, Hürthle (oncocytic) cell adenoma, NIFTP and nodular hyperplasia for example can help explain the clinical/imaging/cytologic findings and therefore should be reported.
Pathological staging
The pathological staging applies to all carcinoma types, including anaplastic thyroid carcinoma, which hitherto had automatically been staged as stage 4 irrespective of all other details (10, 12).
Non-core elements
A summary of the non-core elements are outlined in Table 1 and their description is available at http://www.iccr-cancer.org/datasets/published-datasets/endocrine-organs/carcinoma-of-the-thyroid-tnm8.
Conclusions
The aim of this data set is to better diagnose and prognosticate thyroid carcinoma patients through meticulous pathologic examination. Via its global reach, we hope this data set will provide pathologists worldwide the tools to generate a report that will guide patient treatment in a more rational manner. This will prevent the undertreatment of threatening malignancies and spare patients with indolent tumors the side effects of unnecessary therapy.
Acknowledgement:
We are very thankful to Ms Fleur Webster for her editorial and organizational contributions.
Source of Funding: Supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748
Footnotes
Declarations of conflicts of interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Transactions of the American Clinical and Climatological Association. 2002;113:241–60. [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009;19(11):1167–214. [DOI] [PubMed] [Google Scholar]
- 3.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Thyroid Cancer (Version 2.2019). Available from: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (Accessed 1st November 2019).
- 5.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World journal of surgery. 2006;30(5):780–6. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Miyauchi A, Kihara M, Kobayashi K, Miya A. Prognostic values of clinical lymph node metastasis and macroscopic extrathyroid extension in papillary thyroid carcinoma. Endocrine journal. 2014;61(8):745–50. [DOI] [PubMed] [Google Scholar]
- 7.Ricarte-Filho J, Ganly I, Rivera M, Katabi N, Fu W, Shaha A, et al. Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, the number of nodal metastases, and extranodal extension. Thyroid : official journal of the American Thyroid Association. 2012;22(6):575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu B, Wang L, Tuttle RM, Ganly I, Ghossein R. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell-derived thyroid carcinomas: a clinicopathologic study of 276 cases. Human pathology. 2015;46(12):1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghossein RA, Hiltzik DH, Carlson DL, Patel S, Shaha A, Shah JP, et al. Prognostic factors of recurrence in encapsulated Hurthle cell carcinoma of the thyroid gland: a clinicopathologic study of 50 cases. Cancer. 2006;106(8):1669–76. [DOI] [PubMed] [Google Scholar]
- 10.Amin M, Edge S, Greene F, Byrd D, Brookland R, Washington M, et al. (eds) (2017). AJCC Cancer Staging Manual. 8th ed. Springer., New York. [Google Scholar]
- 11.Merlin T, Weston A, Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian ‘levels of evidence’. BMC medical research methodology. 2009;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Union against Cancer (2017). TNM Classification of Malignant Tumours (8th Edition). Brierley JD, Gospodarowicz MK and Wittekind C (eds). Wiley-Blackwell., New York. [Google Scholar]
- 13.Wang F, Yu X, Shen X, Zhu G, Huang Y, Liu R, et al. The Prognostic Value of Tumor Multifocality in Clinical Outcomes of Papillary Thyroid Cancer. The Journal of clinical endocrinology and metabolism. 2017;102(9):3241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, et al. Differential Clinicopathological Risk and Prognosis of Major Papillary Thyroid Cancer Variants. The Journal of clinical endocrinology and metabolism. 2016;101(1):264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–73. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd R, Osamura R, Klöppel G and Rosai J (eds) (2017). WHO Classification of Tumours of Endocrine Organs, 4th ed. IARC Press, Lyon. [Google Scholar]
- 17.Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008;113(1):48–56. [DOI] [PubMed] [Google Scholar]
- 18.Morris LG, Shaha AR, Tuttle RM, Sikora AG, Ganly I. Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid : official journal of the American Thyroid Association. 2010;20(2):153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–80. [DOI] [PubMed] [Google Scholar]
- 20.Tallini G, Tuttle RM, Ghossein RA. The History of the Follicular Variant of Papillary Thyroid Carcinoma. The Journal of clinical endocrinology and metabolism. 2017;102(1):15–22. [DOI] [PubMed] [Google Scholar]
- 21.Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern). Thyroid : official journal of the American Thyroid Association. 2009;19(2):119–27. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–64. [DOI] [PubMed] [Google Scholar]
- 23.Vivero M, Kraft S, Barletta JA. Risk stratification of follicular variant of papillary thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2013;23(3):273–9. [DOI] [PubMed] [Google Scholar]
- 24.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016;2(8):1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameselle-Teijeiro J, Chan JK. Cribriform-morular variant of papillary carcinoma: a distinctive variant representing the sporadic counterpart of familial adenomatous polyposis-associated thyroid carcinoma? Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1999;12(4):400–11. [PubMed] [Google Scholar]
- 26.Regalbuto C, Malandrino P, Tumminia A, Le Moli R, Vigneri R, Pezzino V. A diffuse sclerosing variant of papillary thyroid carcinoma: clinical and pathologic features and outcomes of 34 consecutive cases. Thyroid : official journal of the American Thyroid Association. 2011;21(4):383–9. [DOI] [PubMed] [Google Scholar]
- 27.Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, et al. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of eight cases. The American journal of surgical pathology. 2010;34(1):44–52. [DOI] [PubMed] [Google Scholar]
- 28.Asioli S, Erickson LA, Righi A, Lloyd RV. Papillary thyroid carcinoma with hobnail features: histopathologic criteria to predict aggressive behavior. Human pathology. 2013;44(3):320–8. [DOI] [PubMed] [Google Scholar]
- 29.Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. The American journal of surgical pathology. 2007;31(8):1256–64. [DOI] [PubMed] [Google Scholar]
- 30.Grabellus F, Nagarajah J, Bockisch A, Schmid KW, Sheu SY. Glucose transporter 1 expression, tumor proliferation, and iodine/glucose uptake in thyroid cancer with emphasis on poorly differentiated thyroid carcinoma. Clinical nuclear medicine. 2012;37(2):121–7. [DOI] [PubMed] [Google Scholar]
- 31.Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106(6):1286–95. [DOI] [PubMed] [Google Scholar]
- 32.Nikiforov YE, Seethala RR. Anaplastic (undifferentiated) carcinoma. In Diagnostic Pathology and Molecular Genetics of the Thyroid: Second Edition. Nikiforov YE, Biddinger PW, Thompson LDR, editors. Philadelphia.: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- 33.Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid : official journal of the American Thyroid Association. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akslen LA, LiVolsi VA. Prognostic significance of histologic grading compared with subclassification of papillary thyroid carcinoma. Cancer. 2000;88(8):1902–8. [PubMed] [Google Scholar]
- 35.College of American Pathologists (2019). Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland. Available from: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates (Accessed 1st May 2019).
- 36.Tallini G. Poorly differentiated thyroid carcinoma. Are we there yet? Endocrine pathology. 2011;22(4):190–4. [DOI] [PubMed] [Google Scholar]
- 37.Alzumaili B, Xu B, Spanheimer PM, Tuttle RM, Sherman E, Katabi N, et al. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs TL, Nassour AJ, Glover A, Sywak MS, Sidhu SB, Delbridge LW, et al. A Proposed Grading Scheme for Medullary Thyroid Carcinoma Based on Proliferative Activity (Ki-67 and Mitotic Count) and Coagulative Necrosis. The American journal of surgical pathology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera M, Ricarte-Filho J, Patel S, Tuttle M, Shaha A, Shah JP, et al. Encapsulated thyroid tumors of follicular cell origin with high grade features (high mitotic rate/tumor necrosis): a clinicopathologic and molecular study. Human pathology. 2010;41(2):172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong KS, Lorch JH, Alexander EK, Marqusee E, Cho NL, Nehs MA, et al. Prognostic Significance of Extent of Invasion in Poorly Differentiated Thyroid Carcinoma. Thyroid : official journal of the American Thyroid Association. 2019;29(9):1255–61. [DOI] [PubMed] [Google Scholar]
- 41.Chan J. Tumours of the thyroid and parathyroid glands. In: Diagnostic Histopathology of tumours. Fletcher CDM (ed). Philadelphia: Churchill Livingstone Elsevier; 2007. [Google Scholar]
- 42.Thompson LD, Wieneke JA, Paal E, Frommelt RA, Adair CF, Heffess CS. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer. 2001;91(3):505–24. [DOI] [PubMed] [Google Scholar]
- 43.Collini P, Sampietro G, Pilotti S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non-Hurthle cell follicular carcinoma of the thyroid gland: a clinicopathological study of 18 consecutive cases from a single institution with a 11-year median follow-up. Histopathology. 2004;44(1):35–9. [DOI] [PubMed] [Google Scholar]
- 44.Lang W, Choritz H, Hundeshagen H. Risk factors in follicular thyroid carcinomas. A retrospective follow-up study covering a 14-year period with emphasis on morphological findings. The American journal of surgical pathology. 1986;10(4):246–55. [PubMed] [Google Scholar]
- 45.Ortiz S, Rodriguez JM, Soria T, Perez-Flores D, Pinero A, Moreno J, et al. Extrathyroid spread in papillary carcinoma of the thyroid: clinicopathological and prognostic study. Otolaryngol Head Neck Surg. 2001;124(3):261–5. [DOI] [PubMed] [Google Scholar]
- 46.Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg. 1995;170(5):467–70. [DOI] [PubMed] [Google Scholar]
- 47.Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer. 1985;55(4):805–28. [DOI] [PubMed] [Google Scholar]
- 48.McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1986;61(12):978–96. [DOI] [PubMed] [Google Scholar]
- 49.Jukkola A, Bloigu R, Ebeling T, Salmela P, Blanco G. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocrine-related cancer. 2004;11(3):571–9. [DOI] [PubMed] [Google Scholar]
- 50.Nixon IJ, Ganly I, Patel S, Palmer FL, Whitcher MM, Tuttle RM, et al. The impact of microscopic extrathyroid extension on outcome in patients with clinical T1 and T2 well-differentiated thyroid cancer. Surgery. 2011;150(6):1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid : official journal of the American Thyroid Association. 2014;24(2):241–4.23713855 [Google Scholar]
- 52.Riemann B, Kramer JA, Schmid KW, Dralle H, Dietlein M, Schicha H, et al. Risk stratification of patients with locally aggressive differentiated thyroid cancer. Results of the MSDS trial. Nuklearmedizin. 2010;49(3):79–84. [DOI] [PubMed] [Google Scholar]
- 53.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Minimal extrathyroid extension does not affect the relapse-free survival of patients with papillary thyroid carcinoma measuring 4 cm or less over the age of 45 years. Surg Today. 2006;36(1):12–8. [DOI] [PubMed] [Google Scholar]
- 54.Shin JH, Ha TK, Park HK, Ahn MS, Kim KH, Bae KB, et al. Implication of minimal extrathyroidal extension as a prognostic factor in papillary thyroid carcinoma. Int J Surg. 2013;11(9):944–7. [DOI] [PubMed] [Google Scholar]
- 55.Fukushima M, Ito Y, Hirokawa M, Miya A, Shimizu K, Miyauchi A. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World journal of surgery. 2010;34(12):3007–14. [DOI] [PubMed] [Google Scholar]
- 56.Xu B, Ibrahimpasic T, Wang L, Sabra MM, Migliacci JC, Tuttle RM, et al. Clinicopathologic Features of Fatal Non-Anaplastic Follicular Cell-Derived Thyroid Carcinomas. Thyroid : official journal of the American Thyroid Association. 2016;26(11):1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su HK, Wenig BM, Haser GC, Rowe ME, Asa SL, Baloch Z, et al. Inter-Observer Variation in the Pathologic Identification of Minimal Extrathyroidal Extension in Papillary Thyroid Carcinoma. Thyroid : official journal of the American Thyroid Association. 2016;26(4):512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komorowski RA, Hanson GA. Occult thyroid pathology in the young adult: an autopsy study of 138 patients without clinical thyroid disease. Human pathology. 1988;19(6):689–96. [DOI] [PubMed] [Google Scholar]
- 59.Kim JW, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Extent of Extrathyroidal Extension as a Significant Predictor of Nodal Metastasis and Extranodal Extension in Patients with Papillary Thyroid Carcinoma. Ann Surg Oncol. 2017;24(2):460–8. [DOI] [PubMed] [Google Scholar]
- 60.Rivera M, Ricarte-Filho J, Tuttle RM, Ganly I, Shaha A, Knauf J, et al. Molecular, morphologic, and outcome analysis of thyroid carcinomas according to degree of extrathyroid extension. Thyroid : official journal of the American Thyroid Association. 2010;20(10):1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong CM, Ahn BC, Park JY, Jeong SY, Lee SW, Lee J. Prognostic implications of microscopic involvement of surgical resection margin in patients with differentiated papillary thyroid cancer after high-dose radioactive iodine ablation. Ann Nucl Med. 2012;26(4):311–8. [DOI] [PubMed] [Google Scholar]
- 62.Lang BH, Shek TW, Wan KY. Does microscopically involved margin increase disease recurrence after curative surgery in papillary thyroid carcinoma? J Surg Oncol. 2016;113(6):635–9. [DOI] [PubMed] [Google Scholar]
- 63.Kluijfhout WP, Pasternak JD, Kwon JS, Lim J, Shen WT, Gosnell JE, et al. Microscopic Positive Tumor Margin Does Not Increase the Risk of Recurrence in Patients with T1-T2 Well-Differentiated Thyroid Cancer. Ann Surg Oncol. 2016;23(5):1446–51. [DOI] [PubMed] [Google Scholar]
- 64.Wang LY, Ghossein R, Palmer FL, Nixon IJ, Tuttle RM, Shaha AR, et al. Microscopic Positive Margins in Differentiated Thyroid Cancer Is Not an Independent Predictor of Local Failure. Thyroid : official journal of the American Thyroid Association. 2015;25(9):993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Back K, Kim SK, Chai YJ, Kim JH, Choe JH, Kim JS. Does microscopic positive tumor margin in papillary thyroid cancer really matter? Surgery. 2019;166(6):1160–7. [DOI] [PubMed] [Google Scholar]
- 66.Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid : official journal of the American Thyroid Association. 2012;22(11):1144–52. [DOI] [PubMed] [Google Scholar]
- 67.Wu MH, Shen WT, Gosnell J, Duh QY. Prognostic significance of extranodal extension of regional lymph node metastasis in papillary thyroid cancer. Head Neck. 2015;37(9):1336–43. [DOI] [PubMed] [Google Scholar]
- 68.Alpert EH, Wenig BM, Dewey EH, Su HK, Dos Reis L, Urken ML. Size distribution of metastatic lymph nodes with extranodal extension in patients with papillary thyroid cancer: a pilot study. Thyroid : official journal of the American Thyroid Association. 2015;25(2):238–41. [DOI] [PubMed] [Google Scholar]
- 69.Moritani S. Impact of invasive extranodal extension on the prognosis of patients with papillary thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2014;24(12):1779–83. [DOI] [PubMed] [Google Scholar]
- 70.Lango M, Flieder D, Arrangoiz R, Veloski C, Yu JQ, Li T, et al. Extranodal extension of metastatic papillary thyroid carcinoma: correlation with biochemical endpoints, nodal persistence, and systemic disease progression. Thyroid : official journal of the American Thyroid Association. 2013;23(9):1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito Y, Hirokawa M, Jikuzono T, Higashiyama T, Takamura Y, Miya A, et al. Extranodal tumor extension to adjacent organs predicts a worse cause-specific survival in patients with papillary thyroid carcinoma. World journal of surgery. 2007;31(6):1194–201. [DOI] [PubMed] [Google Scholar]
- 72.Asanuma K, Kusama R, Maruyama M, Fujimori M, Amano J. Macroscopic extranodal invasion is a risk factor for tumor recurrence in papillary thyroid cancer. Cancer Lett. 2001;164(1):85–9. [DOI] [PubMed] [Google Scholar]