Abstract
Background:
The value of a cancer screening programs is defined by its balance of benefits and harms; however, there are few data evaluating both attributes for hepatocellular carcinoma (HCC) surveillance. We aimed to characterize benefits and harms of HCC surveillance in a large prospective cohort of patients with cirrhosis.
Methods:
We conducted a secondary analysis of a clinical trial evaluating HCC surveillance among patients with cirrhosis at a safety-net health system enrolled between December 2014 and July 2015. We quantified surveillance-related benefits, defined as early HCC detection and curative treatment receipt, and physical harms, defined as diagnostic procedures for false positive or indeterminate results, over an 18-month period.
Results:
Of 614 cirrhosis patients with ≥1 surveillance exam, abnormal results were observed in 118 (19.2%) patients. Twenty-six patients developed HCC during follow-up, of whom 16 (61.5%) were detected by surveillance. The proportion of HCC detected at BCLC stage 0/A (62.5% vs 50%, p=0.69) and who underwent curative treatment (43.8% vs. 40.0%, p=1.0) did not significantly differ between surveillance-detected patients and those diagnosed incidentally/symptomatically. Physical harms were observed in 54 (8.8%) patients who underwent surveillance – most of mild severity with only one diagnostic CT or MRI and none undergoing invasive testing such as biopsy. Incidental findings on follow-up imaging were found in 40 (6.5%) patients —23 of low clinical importance and 17 medium clinical importance.
Conclusion:
In our cohort of patients with cirrhosis, HCC surveillance was associated with high early tumor detection and minimal physical harms.
Keywords: Surveillance, screening, liver cancer, cirrhosis, harms
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide and one of few cancers with an increasing mortality rate in the U.S.1 Over 90% of HCC in the Western world occurs in the setting of chronic liver disease and HCC is a leading cause of death in patients with cirrhosis.2, 3 However, prognosis depends on tumor stage, with 5-year survival exceeding 70% among those detected at an early stage. In contrast, patients found at more advanced stages have 5-year survival below 20%.
Professional societies including the AASLD and EASL recommend HCC surveillance using semi-annual ultrasound with or without alpha fetoprotein (AFP) in at-risk patients.4, 5 In the absence of randomized clinical trial data in cirrhosis, this practice is supported by cohort studies demonstrating an association between HCC surveillance and improved survival, after adjusting for lead-time and length-time biases.6–8 There is increasing recognition that the value of cancer screening programs must also account for potential screening-related harms.9, 10 These data are particularly relevant in light of experiences in prostate and breast cancer, where evolving data regarding harms led to changes in guideline recommendations and resultant confusion among patients and providers regarding best practices.11, 12 Unfortunately, there are limited data characterizing potential HCC surveillance harms, with only two retrospective studies to date.13, 14 Although ultrasound and AFP are non-invasive and safe, both studies found false positive or indeterminate screening results triggered diagnostic evaluation with CT, MRI, or biopsy in approximately 20% of patients – resulting in potential risks of radiation exposure, contrast injury, and biopsy-related complications.
To address this gap in the literature, we performed a secondary analysis of a prospective clinical trial evaluating mailed outreach invitations to increase HCC surveillance in patients with cirrhosis. Whereas the primary outcome for the trial was surveillance receipt, the aim of this study was to characterize surveillance benefits and harms among those who underwent surveillance.
METHODS
Study Population
As previously reported, we performed a pragmatic randomized clinical trial at Parkland Health and Hospital System, the safety-net health system for Dallas County, evaluating a mailed outreach strategy to increase HCC surveillance among patients with cirrhosis.15, 16 The clinical trial enrolled patients with documented or suspected cirrhosis between December 2014 and July 2015.17 HCC surveillance completion and results were captured for all patients over an 18-month period. This study was approved by the IRB of UT Southwestern Medical Center with a waiver of informed consent.
For this study, two authors (J.O. and S.P.) adjudicated the diagnosis of cirrhosis for all patients by manual chart review using criteria described in Supplemental Methods. All patients were required to have ≥1 outpatient clinic visit in year prior to enrollment to demonstrate Parkland was their medical home. Patients without known address or phone number or language other than Spanish or English were excluded from the clinical trial. Finally, patients with a history of Child C cirrhosis, HCC, or significant comorbid conditions were excluded given limited value of HCC surveillance in those subgroups.4, 5
Data Collection
HCC Surveillance Receipt
Dates of HCC surveillance tests were abstracted from the EMR. HCC surveillance at Parkland is typically performed using ultrasound, with or without AFP, per AASLD guidelines with low use of surveillance CT or MRI. Ultrasounds were classified as positive if there was a suspicious liver mass ≥1 cm and AFP results were considered positive if ≥20 ng/mL, the most common cut-off used for surveillance in clinical practice.18, 19
Benefits of HCC Surveillance
HCC surveillance benefits were defined for each surveillance test result as: 1) the proportion of HCC patients detected at an early stage and 2) proportion of HCC patients who underwent curative treatment. A secondary outcome for surveillance benefit was 1- and 2-year overall survival. We attributed HCC detection to abnormal/indeterminate ultrasound vs. AFP results based on listed CT or MRI indication and associated notes. All HCC cases were adjudicated to confirm they met diagnostic criteria per AASLD guidelines, i.e. LI-RADS 5 lesion or consistent histology.20 Tumor characteristics, including tumor nodules, maximum diameter, and presence of vascular invasion or distant metastases, were determined by imaging interpreted by radiologists as part of clinical care. HCC staging was done using Barcelona Clinic Liver Cancer (BCLC) system, with early HCC defined as BCLC 0-A21. HCC treatment was categorized as liver transplantation, surgical resection, local ablative therapy, transarterial chemoembolization (TACE) or transarterial radioembolization (TARE), systemic chemotherapy, or best supportive care. HCC treatment was considered curative if it included liver transplantation, surgical resection or local ablative therapy.
Physical Harms of HCC Surveillance
A binary outcome of “physical harm” was defined for each surveillance test result and included all follow-up tests (e.g. CT, MRI, liver biopsy) performed for false positive or indeterminate surveillance results. Two authors (J.O. and S.P.) abstracted CT and MRI imaging results and classified indication as surveillance, follow-up of abnormal surveillance results (liver lesion ≥1 cm or AFP >20 ng/mL), follow-up of indeterminate ultrasound results (liver lesion <1 cm), or diagnostic for other indications. To account for variation in degree of harm based on invasiveness and exposure to radiation or contrast injury, we categorized surveillance harms as an ordinal variable (no harm, mild harm, moderate harm, and severe harm).13 “No harm” was defined as patients without any follow-up CT, MRI, or biopsy for abnormal surveillance results; “mild harm” as those with a single diagnostic CT or MRI without complications; “moderate harm” as those with multiple CT and/or MRI exams without complications; and “severe harm” was defined as those who experienced acute kidney injury after CT imaging or underwent invasive procedures, e.g. liver biopsy, for surveillance results. Any patients diagnosed with HCC on diagnostic imaging were classified as no harm, regardless of number of imaging studies or need for biopsy. To minimize misclassification bias, we followed patients for an additional 6 months to confirm all false positive lesions were not subsequently diagnosed with HCC.
We examined potential correlates of surveillance-related harms including demographics (age, gender, race/ethnicity) and clinical characteristics of interest including liver disease etiology, presence of decompensation (ascites or hepatic encephalopathy), Charlson comorbidity score, primary care contact in year prior, and receipt of hepatology care in year prior to cohort entry. We classified etiology of liver disease as hepatitis C virus, hepatitis B virus, alcohol-related liver disease, nonalcoholic steatohepatitis (NASH), or other (Supplemental Methods). Degree of ascites and hepatic encephalopathy was categorized as none, mild or controlled on medications, or severe/uncontrolled.
Incidental Findings on Cross-Sectional Imaging
Two authors (J.O. and S.P.) manually abstracted any incidental findings from diagnostic imaging attributed to abnormal surveillance results. Findings were classified by type of abnormality (e.g. colonic, pancreatic, renal, or other) and degree of clinical importance (high, medium, or low). Findings of high importance included those requiring time-sensitive medical or surgical evaluation, e.g. solid organ masses; medium importance findings included conditions that would require non-urgent evaluation, e.g. pancreatic cysts; and findings of low importance were considered benign and unlikely to require further evaluation, e.g. cholelithiasis or diverticulosis.22 We recorded subsequent work-up for any incidental findings including repeat cross-sectional imaging, endoscopy, biopsy, or surgical evaluation completed during the study period.
Statistical Analysis
We described the proportions of patients with surveillance-related benefits and physical harms among those with ≥1 surveillance test during follow-up – overall and stratified by surveillance test (ultrasound vs. AFP). Differences in early stage detection and curative treatment receipt were assessed using Pearson chi square test, while 1- and 2-year survival were compared using log rank test. Differences in physical harms by surveillance test (ultrasound vs. AFP) was assessed using a multilevel generalized estimating equation. Univariable associations between patient-level factors and physical harms were assessed using Pearson chi square test. Covariates with p<0.20 on univariable analysis and those considered clinically important a priori (cirrhosis etiology, Child Pugh score, hepatology care in year prior) were included in the multivariable logistic regression model. Statistical significance was defined as p<0.05. All statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Characteristics of 803 eligible patients are described in Table 1. Median age of patients was 56.8 years and most (61.4%) were men. The most common cirrhosis etiologies were hepatitis C (71.0% active viremia), alcohol-related liver disease, and NASH. The majority of patients had Child Pugh A cirrhosis, with 42.8% having Child Pugh B cirrhosis. Most (n=552, 68.7%) patients had at least one abdominal imaging study (ultrasound, CT, or MRI) in the year prior to enrollment.
Table 1.
Patient Characteristics
| Total N=803 | |
|---|---|
|
| |
| Age (years) | |
| 21–50 | 192 (23.9) |
| 51–60 | 374 (46.6) |
| 61–90 | 237 (29.5) |
|
| |
| Male sex (%) | 493 (61.4) |
|
| |
| Race/Ethnicity (%) | |
| Non-Hispanic White | 240 (29.9) |
| Hispanic White | 203 (25.3) |
| Non-Hispanic Black | 350 (43.6) |
| Other/Unknown | 10 (1.2) |
|
| |
| Etiology of Liver Disease (%) | |
| Hepatitis C | 423 (52.7) |
| Alcohol-related | 203 (25.3) |
| Nonalcoholic steatohepatitis | 105 (13.1) |
| Hepatitis B | 21 (2.6) |
| Other/unknown | 51 (6.3) |
|
| |
| Hepatic decompensation (%) | 371 (46.2) |
|
| |
| Child Pugh Class (% Child A) | 459 (57.2) |
|
| |
| Charlson Comorbidity Index (%)* | |
| 0 | 60 (7.5) |
| 1 | 166 (20.7) |
| 2 | 135 (16.8) |
| ≥3 | 442 (55.0) |
|
| |
| Number of primary care visits* | |
| 0 – 1 | 142 (17.7) |
| 2 – 3 | 232 (28.9) |
| 4 – 7 | 250 (31.1) |
| ≥8 | 179 (22.3) |
|
| |
| Receipt of hepatology care* | 299 (37.2) |
Year prior to cohort entry
Most patients were followed for entire 18-month study period, although 50 (6.2%) of patients died during this time. Over the 18-month study period, 129 (16.1%) of patients had consistent semi-annual surveillance with ultrasound +/− AFP, 415 (51.7%) had inconsistent surveillance, and 259 (32.2%) had no surveillance or AFP testing alone. Overall, at least one surveillance ultrasound or AFP had been performed in 614 (76.5%) patients, with 544 (67.7%) having ≥1 ultrasound and 518 (64.5%) ≥1 serum AFP measurement. There were 448 (73.0%) patients with both ultrasound and AFP, 96 (15.6%) ultrasound alone, and 70 (11.4%) AFP alone. An abnormal result was observed in 118 (19.2%) patients— 63 (53.4%) with an abnormal AFP alone, 47 (39.8%) with an abnormal ultrasound alone, and 8 (6.8%) with both abnormal ultrasound and AFP.
HCC Surveillance Benefits
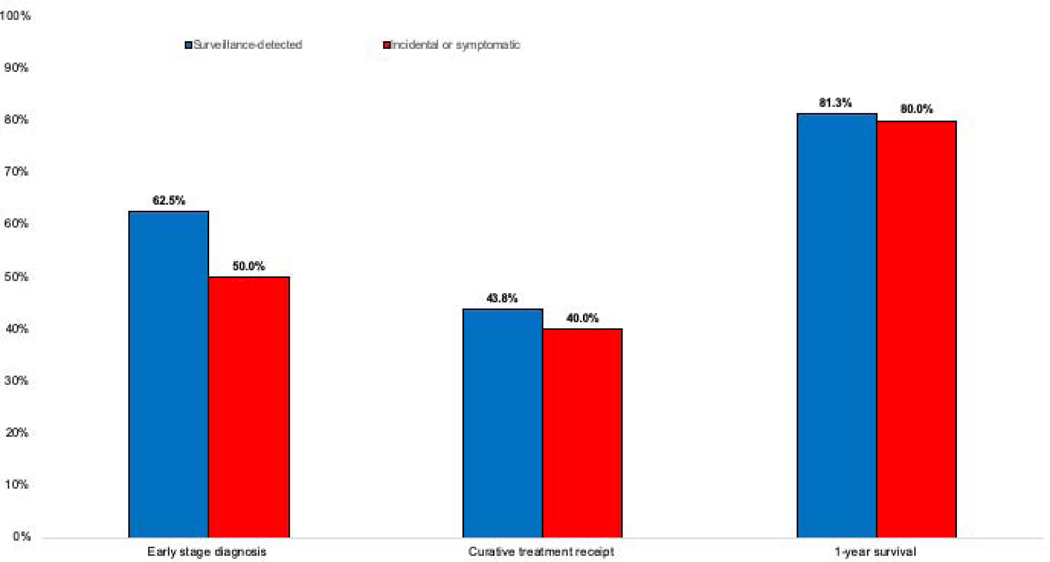
Twenty-six (3.2%) patients developed HCC during follow-up, of whom 15 (57.7%) were found at BCLC stage 0/A, 6 BCLC stage B, 2 BCLC stage C, and 3 BCLC stage D. HCC diagnosis was attributed to surveillance in 16 patients – ultrasound as the primary modality in 11 patients (9 abnormal results and 2 indeterminate results) and abnormal AFP in 5 patients. Three HCC patients whose diagnostic evaluation was triggered by ultrasound also had an elevated AFP, and one patient detected by AFP also subsequently had a liver mass observed on ultrasound. Of those detected by surveillance, 10 (62.5%) were detected at BCLC stage 0/A. Most HCC patients detected via surveillance (n=13) only required only one CT or MRI to establish HCC diagnosis; however, three required 2–4 imaging studies. No patients required biopsy for HCC diagnosis. The remaining 10 (38.5%) HCC patients were diagnosed outside of surveillance, of whom 5 (50.0%) were found incidentally at BCLC stage 0/A. The proportion of HCC detected at an early stage (62.5% vs 50%, p=0.69), curative treatment receipt (43.8% vs. 40.0%, p=1.0), and 1- and 2-year survival (81.3% vs.80.0%, p=0.91 and 75.0% v. 80.0%, p=0.83) did not significantly differ between those diagnosed via surveillance and those diagnosed incidentally/symptomatically, respectively (Supplemental Table, Figure 1).
Figure 1.
Proportion of HCC patients early HCC detection, curative treatment and 1-year survival, stratified by receipt of HCC surveillance
HCC Surveillance Harms
Physical harms related to false positive or indeterminate surveillance results are shown in Table 2 and Figure 2. Physical harms were observed in 54 (8.8%) patients who underwent surveillance – 45 with one CT or MRI, 6 with two studies, and 3 with ≥3 imaging studies.
Table 2.
Observed Benefits and Harms of HCC Surveillance
| Any surveillance (n=614) | Ultrasound (n=544) | Alpha fetoprotein (n=518) | |
|---|---|---|---|
|
| |||
| Screening Benefits* | |||
| HCC detection | 16 (2.6%) | 12 (2.2%) | 8 (1.5%) |
| Early detection | 10 | 8 | 5 |
| Curative treatment receipt | 7 | 5 | 4 |
|
| |||
| Abnormal surveillance result | 118 (19.2%) | 53 (9.7%) | 71 (13.7%) |
|
| |||
| Screening Harms | 54 (8.8%) | 43 (7.9%) | 11 (2.1%) |
| Mild harms | 45 | 34 | 11 |
| Moderate harms | 9 | 9 | ---- |
| Severe harms | ---- | ---- | ---- |
|
| |||
| Benefit-to-harm ratio** | |||
| Observed | 10:54 (0.19) | 12:43 (0.28) | 8:11 (0.72) |
| Theoreticalδ | 10:118 (0.08) | 12:53 (0.23) | 8:71 (0.11) |
Four patients were detected by both ultrasound and AFP (listed under both columns), of whom three were detected at an early stage and two underwent curative treatment
Benefits defined as HCC detection; harms defined as diagnostic evaluation for false positive or indeterminate results
Assuming 100% diagnostic evaluation for all abnormal surveillance results
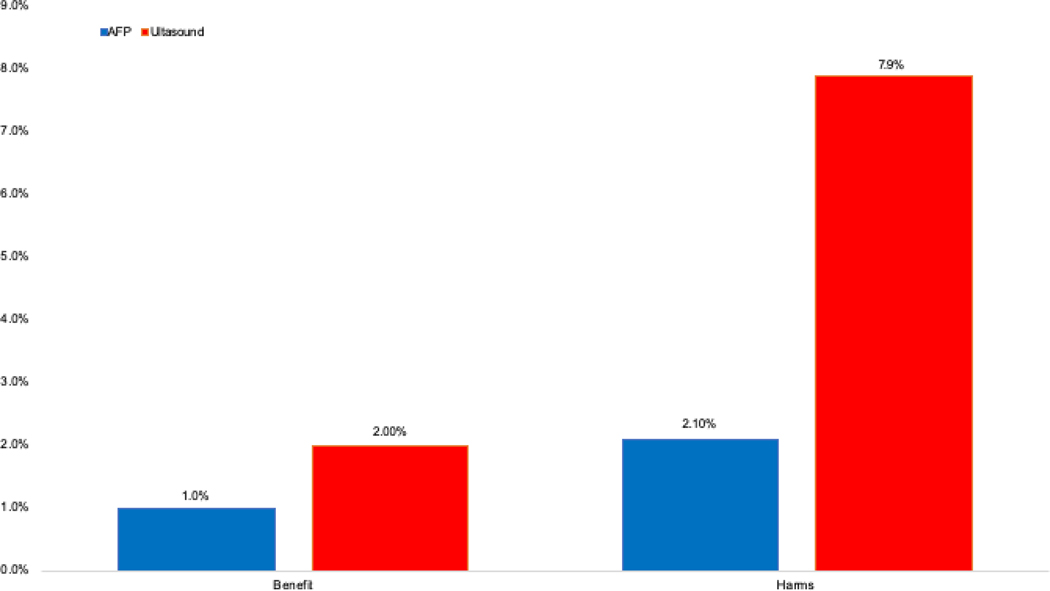
Figure 2.
Proportion of patients with screening-related benefits (early tumor detection) and physical harms, stratified by surveillance modality
Of 9 non-HCC patients with ≥2 diagnostic studies, 44.4% had multiple MRIs and 55.6% had a combination of CT and MRI scans. No patients underwent invasive testing such as biopsy or developed renal failure after CT imaging. The proportion of patients experiencing physical harm increased with the number of completed 6-month surveillance intervals, from 5.6% among those with 1 surveillance interval to 9.2% among those with 2 surveillance intervals and 11.0% among those with 3 surveillance intervals.
Despite ultrasound having fewer false positive results than AFP, it was associated with a higher odds of physical harms (OR 3.7, 95%CI 1.9 – 7.2). Although indeterminate ultrasound results (e.g. sub-centimeter lesions or suboptimal visualization) contributed to ultrasound-related harms, nearly three-fourths (71.1%) of ultrasound harms were due to abnormal results (i.e. liver lesion ≥1 cm). Of the 544 patients with ≥1 surveillance ultrasound, physical harms were observed in 43 (7.9%) patients – 34 with mild harm and 9 with moderate harm. In contrast, only 11 (2.1%) of 518 patients with ≥1 serum AFP measurement experienced AFP-related physical harms – all with mild harm (Table 2, Figure 2).
In univariable analyses, physical harm was associated with receipt of hepatology subspecialty care in year prior to cohort entry (p=0.003) and increased age (p=0.04). In multivariable analysis, the only correlate of physical harms was receipt of hepatology care (OR 2.4, 95%CI 1.3 – 4.4).
Incidental Findings
Of those with ≥1 surveillance test during the study period, 40 (6.5%) were found to have a total of 53 incidental findings on follow-up imaging – 23 (57.5%) with a worst finding of low clinical importance and 17 (42.5%) with a worst finding of medium clinical importance. No patients had an incidental finding of high clinical importance. While 29 patients were found to have only one incidental finding on follow-up imaging, 11 patients had multiple incidental findings—9 patients with 2 findings, and 2 patients with 3 findings. The most common incidental findings included renal cysts, gallstones, and pancreatic cysts (Table 3). Incidental findings indicated repeated imaging for 25 patients, with no patients requiring invasive work-up such as endoscopy, biopsy, or surgery.
Table 3.
Incidental findings related to HCC Surveillance (n=614)
| Clinical Importance | Incidental Finding* | N (%) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| low | adrenal adenoma | 3 (0.5) | |||||
| diverticulosis | 2 (0.3) | ||||||
| gallbladder cyst gallstones | 1 (0.2) | ||||||
| gallstones | 10 (1.6) | ||||||
| hernia | 2 (0.3) | ||||||
| renal cyst | 13 (2.1) | ||||||
| spine lesion | 2 (0.3) | ||||||
| spleen lesion | 2 (0.3) | ||||||
|
| |||||||
| medium | complex renal cyst | 1 (0.2) | |||||
| esophageal thickening | 1 (0.2) | ||||||
| kidney stones | 5 (0.8) | ||||||
| lung nodules | 2 (0.3) | ||||||
| pancreas cyst | 9 (1.5) | ||||||
Incidental findings (n=53) were found in 40 patients so some patients had >1 finding
DISCUSSION
In this prospective evaluation, HCC surveillance was associated with a high proportion of benefits and minimal surveillance harms. Surveillance was associated with early detection in nearly two-thirds of HCC patients and curative treatment receipt in nearly half of patients. Physical harms related to false positive findings were observed in less than 10% of patients, with most being mild in severity including a single diagnostic CT or MRI. Further studies over longer follow-up periods are needed to confirm high surveillance value in patients with cirrhosis.
Data evaluating surveillance benefits in contemporary cohorts are critical given lack of randomized data among patients with cirrhosis.6, 23 Although cohort studies illustrate an association between HCC surveillance and improved survival, a large case-control study in the VA system failed to demonstrate a survival benefit.24 These data are particularly important given a shift in cirrhosis epidemiology from viral-mediated to an increasing proportion related to NASH or post-SVR – subgroups in whom surveillance effectiveness may differ from prior cohorts.25, 26 In our study, surveillance resulted in most patients being detected at an early stage – congruent with data demonstrating surveillance can result in early detection in approximately 60% of patients.27 However, early detection and curative treatment receipt did not differ between surveillance-detected tumors and those found outside of surveillance. In contrast to a pooled early detection proportion of 30% from prior studies,6 half of HCC patients diagnosed outside of surveillance in this study were found at an early stage, potentially related to increased use of cross-sectional imaging in clinical practice over time.28 Larger studies, with longer follow-up times, are needed to confirm benefits of HCC surveillance in contemporary cohorts.
In parallel, there is a need for data evaluating potential harms of HCC surveillance. Most evaluations of cancer screening programs focus on benefits with a minority evaluating physical, psychological, or financial screening harms. For HCC surveillance, two retrospective studies have highlighted HCC surveillance may be associated with physical harms due to false positive or indeterminate results in approximately 20% of patients.13, 29 A subsequent modeling study reinforced the risk-benefit ratio of surveillance, finding a number needed to screen to prevent one HCC death of 77, compared to a number needed to harm of 7.30 In our study, we found a lower proportion of patients experiencing physical harms, observed in 8.8% of patients. Further, most harms were mild, including only a single CT or MRI, and no patients undergoing invasive testing such as a biopsy. Increased awareness of screening harms may have resulted in changes in management over time, such as avoidance of biopsy for indeterminate lesions and earlier return to ultrasound-based surveillance. Providers may be particularly aware of AFP being prone to false positive results, as many patients with elevated AFP levels were simply observed instead or proceeding to diagnostic evaluation. However, the lower proportion of screening harms may alternatively be related to the relatively short study duration. The proportion of patients experiencing screening harms doubled going from one surveillance exam to ≥3 exams, so it is possible physical harms may approach prior estimates with longer duration of follow-up. Further studies are needed to characterize HCC surveillance harms, particularly as alternative imaging- and biomarker-based surveillance strategies become available.
Although studies have evaluated the prevalence of incidental findings in other screening programs, our study is one of the first to do so for HCC surveillance. The prevalence of incidental findings varies widely, including 7.7% in coronary artery disease CT screening, 14.2% in lung cancer CT screening, and 10% of patients undergoing CT colonography for colon cancer screening.22, 31 In our study, we found incidental findings in ~7% of patients with most being of low to medium significance. These findings typically resulted in additional imaging and no patients underwent invasive testing. Incidental findings can result in either benefit (e.g. incidental detection of additional cancers) or compounded harm (e.g. additional diagnostic evaluation for findings of low clinical significance). In contrast to other cancer screening populations that are performed in generally healthy populations, HCC surveillance is restricted to patients with chronic liver disease and often cirrhosis. In light of this competing risk of mortality, any benefit of incidental findings may be lower than other cancer screening programs. Further studies in larger cohort should quantify the prevalence and significance of incidental findings in this patient population.
We acknowledge our study had limitations. First, our study was conducted in a single safety-net health system, so our results may not be generalized to other health systems, particularly given variation in HCC surveillance patterns and recall procedures.32 This may particularly impact the relative harms related to ultrasound and AFP, given differential use of diagnostic imaging for indeterminate ultrasounds or elevated AFP levels between providers and centers. However, safety-net health systems provide care to a racially diverse, socioeconomically disadvantaged patient population who are high risk for HCC and represent an important population to study.33 Second, most patients in our study underwent intermittent surveillance with ultrasound and/or AFP, so it is possible that benefits and harms may be increased if surveillance utilization were higher. Third, we cannot exclude ascertainment bias for surveillance or diagnostic imaging performed at outside institutions, although this is less likely because Parkland is an integrated system and most patients did not have insurance coverage for outside care. Fourth, our study focused on physical harms of HCC surveillance; however, screening can also result in potential financial and psychological harms.34 Finally, the study was conducted over an 18-month period and it possible the benefit-to-harm ratio may not be linear, resulting in changes over longer periods of time. However, we feel these limitations are outweighed by the study’s strengths including its large, contemporary patient population with confirmed cirrhosis from various etiologies and its clinical significance by addressing an understudied area for HCC surveillance.
In summary, our study suggests that HCC surveillance is associated with high early tumor detection, with a low proportion of patients experiencing screening-related harms, over an 18-month period. Further multi-center studies are needed to confirm this favorable risk-benefit ratio in larger cohorts over longer periods of follow-up.
Supplementary Material
Need to Know.
Background:
There are few prospective data characterizing both benefits and harms of hepatocellular carcinoma (HCC) surveillance in patients with cirrhosis.
Findings:
In a prospective cohort of 614 patients with cirrhosis undergoing surveillance over an 18-month period, most (62.5%) HCC were detected at an early stage. Surveillance-related physical harms from false positive or indeterminate results were observed in only 8.8% of patients, with most being mild in severity.
Implications for patient care:
HCC surveillance appears to have a favorable benefit-harm ratio and should be performed in patients with cirrhosis.
Acknowledgments
Financial Support: This work was conducted with support from AHRQ R24 HS022418, NIH R01CA12008, and U01CA230694. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AHRQ. Dr. Singal had full access to all data in the study and takes final responsibility for the decision to submit for publication.
Footnotes
Conflicts of Interest: Dr. Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Roche, Exact Sciences, Glycotest, Bayer, Eisai, BMS, Exelixis, Merck, Genentech, and TARGET Pharmasolutions. Dr. Parikh has served on advisory boards for Bayer, Eisai, Exelixis, Wako; Research Grants: TARGET Pharmasolutions, Bayer, Exact Sciences, Glycotest and a consultant for Exelixis, Bristol-Myers Squibb, Eli Lilly, Freenome. None of the author have any relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.White DL, Thrift AP, Kanwal F, et al. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017;152:812–820 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol 2019. doi: 10.1016/j.cgh.2019.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 6.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:976–987 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costentin CE, Layese R, Bourcier V, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology 2018;155:431–442 e10. [DOI] [PubMed] [Google Scholar]
- 9.Harris RP, Wilt TJ, Qaseem A, et al. A value framework for cancer screening: advice for high-value care from the American College of Physicians. Ann Intern Med 2015;162:712–7. [DOI] [PubMed] [Google Scholar]
- 10.Wilt TJ, Harris RP, Qaseem A, et al. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2015;162:718–25. [DOI] [PubMed] [Google Scholar]
- 11.Printz C. Mammogram debate flares up: Latest breast cancer screening study fuels controversy. Cancer 2014;120:1755–6. [DOI] [PubMed] [Google Scholar]
- 12.Gwede CK, Davis SN, Wilson S, et al. Perceptions of Prostate Cancer Screening Controversy and Informed Decision Making: Implications for Development of a Targeted Decision Aid for Unaffected Male First-Degree Relatives. Am J Health Promot 2015;29:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konerman MA, Verma A, Zhao B, et al. Frequency and Outcomes of Abnormal Imaging in Patients With Cirrhosis Enrolled in a Hepatocellular Carcinoma Surveillance Program. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singal AG, Tiro JA, Murphy CC, et al. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients With Cirrhosis: A Randomized Clinical Trial. Hepatology 2019;69:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singal AG, Tiro JA, Marrero JA, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology 2017;152:608–615.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehra MS, Ma Y, Clark C, et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol 2013;47:e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med 2003;139:46–50. [DOI] [PubMed] [Google Scholar]
- 19.Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Pol CB, Lim CS, Sirlin CB, et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology 2019;156:976–986. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329–38. [DOI] [PubMed] [Google Scholar]
- 22.Gluecker TM, Johnson CD, Wilson LA, et al. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology 2003;124:911–6. [DOI] [PubMed] [Google Scholar]
- 23.Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161:261–9. [DOI] [PubMed] [Google Scholar]
- 24.Moon AM, Weiss NS, Beste LA, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-related Mortality in Patients with Cirrhosis. Gastroenterology 2018; 155(4): 1128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Serag HB, Kanwal F, Feng Z, et al. Risk Factors for Cirrhosis in Contemporary Hepatology Practices-Findings from Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 2020; 159(1): 376–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706–1718.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith-Bindman R, Kwan ML, Marlow EC, et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. JAMA 2019;322:843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology 2019; 70(2): 487–95. [DOI] [PubMed] [Google Scholar]
- 30.Taylor EJ, Jones RL, Guthrie JA, et al. Modeling the benefits and harms of surveillance for hepatocellular carcinoma: Information to support informed choices. Hepatology 2017;66:1546–1555. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs PC, Mali WP, Grobbee DE, et al. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. J Comput Assist Tomogr 2008;32:214–21. [DOI] [PubMed] [Google Scholar]
- 32.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Utilization of hepatocellular carcinoma surveillance in patients with cirrhosis: A systematic review and meta-analysis. Hepatology (in press) doi: 10.1002/hep.31309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singal AG, Li X, Tiro J, et al. Racial, social, and clinical determinants of hepatocellular carcinoma surveillance. Am J Med 2015;128:90.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris R Sheridan S, Lewis C, Barclay C, Vu M, et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med 2014; 174(2): 281–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.