Abstract
Patients with decompensated cirrhosis are prescribed numerous medications. Data are limited as to whether patients are receiving medications they need and avoiding those they do not. We examined a large national claims database (2010–2015) to characterize the complete medication profile as well as the factors associated with appropriate and potentially inappropriate medication use in 12,621 patients with decompensated cirrhosis. Clinical guidelines and existing literature were used to determine appropriate and potentially inappropriate medications in decompensated cirrhosis. The total medication days’ supply was calculated from pharmacy data and divided by the follow up period for each decompensation. Ascites was the most common (86.5%), followed by hepatic encephalopathy (HE) (37.8%), variceal bleeding (VB) (17.5%), hepatorenal syndrome (HRS) (6.3%), and spontaneous bacterial peritonitis (SBP) (6.1%). 55.8% of patients with ascites filled a diuretic. 32.4% and 63.3% of patients with HE filled rifaximin and lactulose, respectively. 60.3% of patients filled a non-selective beta blocker after VB, and 48.0% of patients filled an antibiotic for prophylaxis after an episode of SBP. The minority (4.5–17.3%) had enough medication to cover >50% follow up days. Potentially inappropriate medication use was common, 53.2% filled an opiate, 46.0% proton pump inhibitors, 14.2% benzodiazepines, and 10.1% non-steroidal anti-inflammatory drugs. Disease severity markers were associated with more appropriate mediation use but not consistently associated with less inappropriate medication use.
Conclusion:
Patients with decompensated cirrhosis are not filling indicated medications as often or as long as is recommended, and are also filling medications that are potentially harmful. Future steps include integrating pharmacy records with medical records to obtain a complete medication list and counseling on medication use with patients at each visit.
Keywords: End Stage Liver Disease, Prescription, Pharmacy, Medication Misuse
Introduction:
Patients with decompensated cirrhosis see multiple providers and are prescribed many medications. Several factors impact optimal medication management including fractured care and inadequate medication reconciliation during clinic visits (1). Even when used appropriately, the risk of adverse effects from indicated medications is real in this population where the therapeutic window for some medications is narrow (2, 3). Medications indicated for decompensated cirrhosis include diuretics for ascites, lactulose and/or rifaximin for hepatic encephalopathy (HE), non-selective beta blockers (NSBBs) after an episode of variceal bleeding or for large nonbleeding varices, and antibiotics for secondary prophylaxis of spontaneous bacterial peritonitis (SBP) (4–7). Despite strong evidence in support of use of these medications, there are instances where there are good reasons for a patient to not take one of these drugs. For example, a patient with severe chronic obstructive pulmonary disease could clinically deteriorate after starting a NSBB. Conversely, there are classes of medications that are more likely to cause harm among those with decompensated cirrhosis. These include non-steroidal anti-inflammatory drugs (NSAIDs), opiates, benzodiazepines, and proton pump inhibitors (PPIs)(8–15).
Studies of medication use in cirrhosis have largely been limited to studying one drug class with a specific type of decompensation (16, 17). Patients with decompensated cirrhosis, however, often have more than one cirrhosis complication and they frequently have comorbidities whose medical management is challenged by their cirrhosis. Accordingly, we evaluated complete medication profiles using pharmacy claims data to analyze appropriate and potentially inappropriate medication use in decompensated cirrhosis, and identity factors associated with use.
Methods:
Database
This is a retrospective cohort analysis of patients with decompensated cirrhosis and at least one outpatient pharmacy claim in the Optum Clinformatics Data Mart (Eden Prairie, Minnesota). The Optum database consists of administrative health claims for a large national managed care organization covering 15–18 million lives in the United States. Commercial health plans with medication and prescription coverage, as well as Medicare Advantage Part D plans are included. The database includes inpatient and outpatient International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes, Current Procedural Terminology (CPT) codes (Supplementary Table 1), provider type, and outpatient pharmacy claims information. Outpatient pharmacy claims information includes American Hospital Formulary System (AHFS) classification for dispensed medications, generic medication names, as well as details of the quantity of drug dispensed, number of days’ supply, and cost. Pharmacy claims that were denied by insurance are not included in the database. Over the counter medications patients take without a prescription are not included. Data is linked at the individual level, i.e., all outpatient visits, inpatient admissions, prescriptions, tests and procedures that incurred an insurance claim are linked. However, the database does not include information found in the medical record, such as progress notes.
Population Sample
Decompensated cirrhosis was identified by the combination of an ICD-9 code for at least one cirrhosis complication (ascites, hepatic encephalopathy, variceal bleeding, hepatorenal syndrome, and spontaneous bacterial peritonitis, listed in Supplementary Table 2) and an ICD-9 code for an etiology of cirrhosis (Supplementary Table 3). The requirement that patients had an ICD-9 code for etiology of cirrhosis had a greater effect on decreasing the number of patients with coding for “Other Ascites” than other decompensation events, and was done to increase the specificity for decompensation from liver disease. As patients can have coexisting etiologies, priority was given to alcohol related liver disease (ALD) or chronic hepatitis C (CHC) related liver disease. Patients were considered to have ALD if they had at least one ICD-9 code for ALD or alcohol related end-organ damage, CHC related cirrhosis if they had at least one ICD-9 code for CHC, or ALD/CHC if they had coding for both. Patients in these categories could also have coding for other etiologies of cirrhosis, such as nonalcoholic fatty liver disease (NAFLD). A non-alcohol related liver disease/non chronic hepatitis C (non ALD/non CHC) category captured the remaining patients without any coding for ALD and/or CHC. Patients in this category could have more than one etiology (e.g. NAFLD and chronic hepatitis B).
Follow up periods started at each patient’s first episode of decompensation in the database (at or after 1/1/2010) until the end of continuous enrollment, liver transplant, death, or 9/30/2015, whichever occurred first. The date 9/30/2015 was chosen as the end of the follow up period as this was when ICD-9 transitioned to ICD-10. Patients had at least one year of continuous enrollment prior to as well as after the date of their first liver decompensation. Those with a history of liver transplant prior to their first decompensation event, or who underwent liver transplant or died within 90 days of their first decompensation were excluded. Finally, patients were required to have ICD-9 coding for an etiology of cirrhosis (Supplementary Table 3) and have filled at least one medication during their follow up period (Supplementary Figure 1).
Appropriate medication use
Appropriate medication use in decompensated cirrhosis was based largely on American Association for the Study of Liver Diseases (AASLD) guidelines and quality measures (Supplementary Table 4).
Inappropriate medication use in decompensated cirrhosis can be more variable based on patient factors, and was determined by clinical experience and reinforced by the existing literature (Supplementary Table 5).
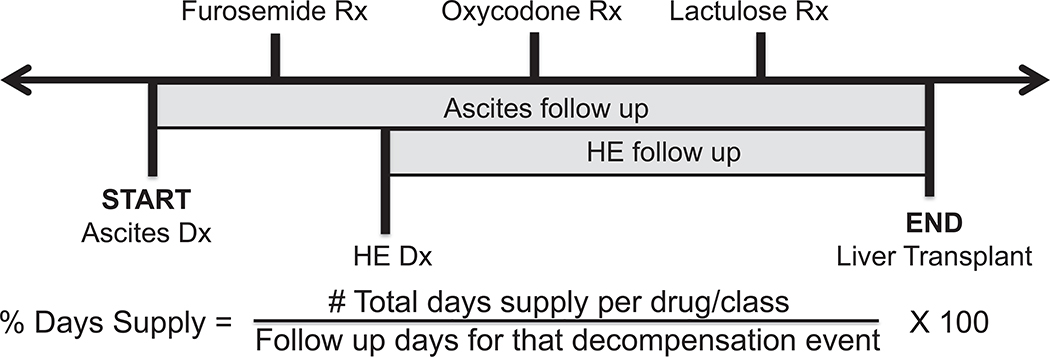
The proportion of patients with a specific cirrhosis complication who filled each class of medication was calculated. To calculate the proportion of follow up time the patient had a medication, the total days’ supply of a specific medication or medication class was calculated and divided by the follow up period, starting with the first day of a specific complication (e.g. follow up for lactulose period starting with first diagnosis code for HE, Figure 1). Generic medication names or medication classes, sorted by appropriate or potentially inappropriate use, are listed in Supplementary Tables 4 and 5. Consistent use was defined as having a supply of the medication for >50% of follow up days for appropriate medications, and ≥30 days’ supply per year for potentially inappropriate medications. Ascites and HE can be managed in the outpatient setting without requiring hospital admission. As such, diagnosis codes for ascites and HE were subdivided into those with only outpatient diagnosis codes (no inpatient codes), and those who had at least one inpatient diagnosis code. Of note, patients who had inpatient diagnosis codes could also have outpatient diagnoses of the same condition. ICD-9 codes were used to identify common co morbidities, and the codes used are listed in Supplementary Table 6.
Figure 1:
Medication Use Calculation During Follow Up Period
Statistical analyses:
Descriptive statistics were presented as percentages for categorical variables and medians with interquartile ranges for continuous variables. Fisher exact tests were used to compare categorical variables and Wilcoxon rank sum test to determine differences in medication days filled between two groups. Bivariate logistic regression was used to determine associations between patient factors/co morbidities and medication use. The results were reported as odds ratios. STATA was used for statistical analysis (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). This study was exempted from review by the University of Michigan Institutional Review Board (HUM00141255).
Results:
Patient Population
The final cohort included 12,621 patients with decompensated cirrhosis (Figure 2). Just over half of the cohort was male (56.3%) and the median age was 61 years. Almost two thirds (65.5%) were Caucasian and 14.8% were Hispanic. The patients were distributed through all four census regions, with the largest proportion (42.6%) coming from the south. Patients were followed for a median of 2.3 years (Table 1). Seventy-eight percent (77.9%) of patients saw a provider who specialized in Gastroenterology or Hepatology at least once during the follow up period.
Figure 2:
Appropriate Medication Use in Decompensated Cirrhosis: Any and Consistent Use
Consistent use was defined as having a supply of the medication for >50% of follow up days for appropriate medications
*p-value < 0.05
HE: Hepatic Encephalopathy
IP Dx: Inpatient Diagnosis
OP Dx: Outpatient Diagnosis
Rx: Prescription
SBP: Spontaneous Bacterial Peritonitis
VB: Variceal Bleeding
Table 1:
Demographics and Clinical Characteristics of the Decompensated Cirrhosis Cohort
| N | 12,621 (%) | |
| Gender (Male) | 7,106 (56.3%) | |
| Median Age at first liver decompensation | 61 years (53–69) | |
| Race | ||
| Caucasian | 8,266 (65.5%) | |
| African American | 1,150 (9.1%) | |
| Asian | 342 (2.7%) | |
| Unknown | 993 (7.9%) | |
| Ethnicity | ||
| Hispanic | 1,870 (14.8%) | |
| Census Region | ||
| South | 5,373 (42.6%) | |
| West | 3,359 (26.6%) | |
| Midwest | 2,272 (18.0%) | |
| Northeast | 1,447 (11.5%) | |
| Etiology of Chronic Liver Disease | ||
| Alcohol Related Liver Disease (ALD) only | 4,842 (38.4%) | |
| ALD and Chronic Hepatitis C (CHC) | 1,884 (14.9%) | |
| CHC only | 1,368 (10.8%) | |
| Non ALD/Non CHC* | 4,527 (35.9%) | |
| NAFLD | 4,185 (92.5%) | |
| Chronic Hepatitis | 357 (7.9%) | |
| Biliary Cirrhosis | 307 (6.8%) | |
| Hepatitis B | 166 (3.7%) | |
| Presence of Decompensation (≥ 1 may be present) | ||
| Ascites | 10, 914 (86.5%) | |
| Hepatic Encephalopathy (HE) | 4,776 (37.8%) | |
| Variceal Bleeding (VB) | 2,214 (17.5%) | |
| Hepatorenal syndrome (HRS) | 790 (6.3%) | |
| Spontaneous bacterial peritonitis (SBP) | 768 (6.1%) | |
| Unique Medications during follow up | 9 (5–13) | |
| Unique Medications per year follow up | 3.6 (1.8–6.8) | |
| Median Follow up | 2.3 years (1.5–3.6 years) | |
| Gastroenterology/Hepatology Consultation | 9,837 (77.9%) | |
| Reason for ending follow up | ||
| Enrollment Ended | 9,390 (74.4%) | |
| Died | 2,603 (20.6%) | |
| Underwent Liver Transplant | 628 (5.0%) | |
Data presented as number (%) or median (IQR)
Patient can have more than one etiology in the “Non ALD/Non CHC” Category breakdown
NAFLD: Non-alcoholic fatty liver disease
Etiology of Liver Disease and Type of Decompensation
Alcohol related liver disease was the most prevalent etiology (38.4%), followed by non ALD/non CHC at 35.9% (which largely consisted of patients with NAFLD), ALD with CHC (14.9%), and finally CHC only (10.8%). Ascites was the most common cirrhosis complication (86.5%), followed by hepatic encephalopathy (37.8%), variceal bleeding (17.5%), hepatorenal syndrome (6.3%) and spontaneous bacterial peritonitis (6.1%). Just over a third (36.4%) of patients had more than one type of decompensation. Patients took a median of 9 (IQR 5–13) unique medications during the entire follow up period, and a median of 3.6 (IQR 1.8–6.8) unique medications per year of follow up (Table 1).
Appropriate medication use: Diuretics
Diuretics are recommended once a patient develops ascites (Supplementary Table 4) (5, 19). Of the 10,139 patients with ICD-9 coding for ascites, 55.8% filled a prescription for a diuretic at least once during the follow up period, but only 17.3% filled a prescription for diuretics consistently (>50% of follow up period) (Figure 2–3). One third (32.8%) of patients with ascites had at least one inpatient diagnosis code for ascites, and they had significantly higher rates of any diuretic use (74.5% v. 46.7%, P < 0.001) and consistent diuretic use during follow up (27.1% v. 12.5%, P < 0.001) compared to those with only outpatient diagnoses of ascites (Figure 2).
Figure 3:
Appropriate Diuretic Use in Ascites: Any and Consistent Use by Inpatient and Outpatient Diagnosis Codes
Consistent use was defined as having a supply of the medication for >50% of follow up days for appropriate medications
*p-value < 0.05
HE: Hepatic Encephalopathy
IP Dx: Inpatient Diagnosis
OP Dx: Outpatient Diagnosis
Rx: Prescription
SBP: Spontaneous Bacterial Peritonitis
VB: Variceal Bleeding
The presence of an inpatient diagnosis code for ascites (OR 3.3, 95% CI 3.0–3.6, P < 0.001) was found to be associated with any diuretic use. Subsequent requirement of a Transjugular Intrahepatic Portosystemic Shunt (TIPS) (OR 2.7, 95% CI 1.9–3.6, P < 0.001), a history of paracentesis (OR 2.7, 95% CI 2.4–3.0, P < 0.001), and the number of paracenteses completed during follow up (OR 1.2, 95% CI 1.2–1.3, P < 0.001) were also associated with any diuretic use, as was male gender (OR 1.4, 95% CI 1.3–1.5, P < 0.001) and older age at time of first decompensation (OR per year 1.03, 95% CI 1.03–1.03, P < 0.001). Acute kidney injury (AKI) (OR 2.4, 95% CI 2.2–2.6, P <0.001), chronic kidney disease (CKD) (OR 2.3, 95% CI 2.1–2.5, P <0.001) and end stage renal disease (ESRD) (OR 1.9, 95% CI 1.7–2.2, P < 0.001) were all associated with any diuretic use. Seeing a gastroenterology or hepatology provider was also associated with increased use of any diuretic (OR 1.8, 95% CI 1.6–2.0, P < 0.001) (Table 2).
Table 2:
Factors Associated with Any Appropriate Medication Use
| Ascites n = 10,139 | Hepatic Encephalopathy n = 3,365 | Variceal Bleeding n = 1,582 | Spontaneous Bacterial Peritonitis n = 296 | ||
|---|---|---|---|---|---|
| Diuretic Use | Lactulose use | Lactulose & Rifaximin Use | NSBB use | Abx use for secondary ppx | |
| OR (95% CI) | |||||
| Gender (Male) |
1.4 (1.3–1.5) p<0.001 |
1.0 (0.9–1.2) p=0.989 |
0.9 (0.8–1.1) p=0.364 |
1.1 (0.9–1.4) p=0.308 |
1.3 (0.8–2.1) p=0.223 |
| Median Age at first liver decompensation (year) |
1.03 (1.03-1.03) p<0.001 |
1.02 (1.01-1.02) p<0.001 |
1.0 (0.99-1.01) p=0.791 |
0.99 (0.98-0.995) p=0.003 |
1.0 (0.99-1.03) p=0.422 |
| Race/Ethnicity | |||||
| White | 1.2 (0.9–1.4) p=0.156 |
0.8 (0.5–1.1) p=0.210 |
1.1 (0.7–1.7) p=0.575 |
0.9 (0.5–1.5) p=0.672 |
0.8 (0.3–2.3) p=0.659 |
| Hispanic | 1.1 (0.9–1.4) p=0.277 |
1.1 (0.7–1.7) p=0.603 |
1.3 (0.8–2.0) p=0.267 |
0.9 (0.5–1.6) p=0.712 |
0.7 (0.2–2.2) p=0.526 |
| African American |
0.9 (0.7–1.1) p=0.349 |
0.8 (0.5–1.2) p=0.301 |
1.0 (0.6–1.7) p=0.874 |
1.0 (0.6–1.8) p=0.963 |
1.1 (0.3–4.0) p=0.870 |
| Asian | 0.8 (0.6–1.0) p=0.095 |
1.1 (0.6–2.0) p=0.773 |
1.4 (0.7–2.7) p=0.324 |
1.0 (0.5–2.3) p=0.939 |
0.6 (0.1–4.6) p=0.608 |
| Inpatient Ascites Diagnosis |
3.3 (3.0–3.6) p<0.001 |
2.1 (1.3–3.6) p=0.004 |
|||
| Inpatient HE Diagnosis |
2.4 (2.1–2.7) p<0.001 |
2.1 (1.8–2.5) p<0.001 |
|||
| Rx before TIPS* |
2.7 (1.9–3.6) p<0.001 |
||||
| Rx after TIPS** |
3.6 (1.8–7.2) p<0.001 |
3.9 (2.4–6.4) p<0.001 |
|||
| Rx after Paracentesis*** |
2.7 (2.4–3.0) p<0.001 |
1.5 (0.9–2.5) p=0.095 | |||
| Paracentesis # During Follow up |
1.2 (1.2–1.3) p<0.001 |
1.0 (0.9951.1) p=0.074 | |||
| Rx after EGD**** | 1.2 (0.9–1.6) p=0.177 | ||||
| Acute Kidney Injury (AKI) |
2.4 (2.2–2.6) p<0.001 |
1.0 (0.8–1.2) P=0.807 |
1.7 (1.1–2.7) P=0.029 |
||
| Chronic Kidney Disease (CKD) |
2.3 (2.1–2.5) p<0.001 |
0.8 (0.7–1.1) P=0.170 | 1.3 (0.8–2.1) P=0.312 | ||
| End Stage Renal Disease (ESRD) |
1.9 (1.7–2.2) p<0.001 |
0.8 (0.6–1.1) P=0.168 | 1.9 (1.1–3.2) P=0.023 | ||
| Bradycardia | 1.2 (0.9615) p=0.115 |
||||
| Chronic Obstructive Pulmonary Disease/Asthma | 0.7 (0.5–0.8) P<0.001 | ||||
| Diabetes Mellitus | 1.0 (0.8–1.2) p=0.777 | ||||
| Gastroenterology or Hepatology Provider Consultation | 1.8 (1.6–2.0) P<0.001 | 1.4 (1.2–1.7) P<0.001 | 2.4 (1.9–3.1) P<0.001 | 1.0 (0.8–1.4) P=0.852 | 2.2 (0.9–5.6) P=0.086 |
Medication use prior to TIPS date for those who underwent TIPS compared to medication use for those who did not undergo TIPS
Medication use after TIPS for those who underwent TIPS compared to medication use for those who did not undergo TIPS
Medication use after paracentesis compared to medication use for those who did not undergo paracentesis
Medication use after EGD compared to medication use for those who did not undergo EGD
Abx: Antibiotic
EGD: Esophagogastroduodenoscopy
HE: Hepatic Encephalopathy
NSBB: Non-selective Beta Blocker
PPx: Prophylaxis
Rx: Prescription
TIPS: Transjugular Intrahepatic Portosystemic Shunt
Lactulose and/or Rifaximin
Lactulose is a first line treatment after a patient develops overt HE (6), while rifaximin is recommended after re-hospitalization for HE (Supplementary Table 4) (19). Of the 3,365 patients with HE, 69.7% were prescribed either lactulose or rifaximin, 63.3% lactulose, 32.4% rifaximin, and 25.9% both (Figure 4). When each drug was evaluated individually, less than 5% of patients had enough supply of lactulose or rifaximin for consistent use (>50% of the follow up period (Figure 2)). Half (50.0%) of the patients with HE diagnoses had inpatient diagnosis codes for HE. An inpatient HE diagnosis was associated with higher rates of any lactulose use (73.1% v. 53.4%, P < 0.001), any rifaximin use (38.2% v. 26.5%, P < 0.001), and consistent rifaximin use (5.5% v. 3.6%, P = 0.01).
Figure 4:
Appropriate Medication Use in Hepatic Encephalopathy: By Medication Type and Inpatient and Outpatient Diagnosis Codes
Consistent use was defined as having a supply of the medication for >50% of follow up days for appropriate medications
*p-value < 0.05
HE: Hepatic Encephalopathy
IP Dx: Inpatient Diagnosis
OP Dx: Outpatient Diagnosis
Rx: Prescription
SBP: Spontaneous Bacterial Peritonitis
VB: Variceal Bleeding
Any lactulose use was associated with a history of TIPS (OR 3.6, 95% CI 1.8–7.2, P < 0.001), an inpatient diagnosis of HE (OR 2.4, 95% CI 2.1–2.7, P < 0.001), and older age at time of first decompensation (OR per year 1.02, 95% CI 1.01–1.02, P < 0.001). Dual lactulose and rifaximin use was associated with a history of a TIPS (OR 3.9, 95% CI 2.4–6.4, P < 0.001) and an inpatient diagnosis code of HE (OR 2.1, 95% CI 1.8–2.5, P < 0.001). Seeing a gastroenterology or hepatology provider was also associated with increased use of lactulose (OR 1.4, 95% CI 1.2–1.7, P < 0.001) and concomitant lactulose and rifaximin use (OR 2.4, 95% CI 1.9–3.1, P < 0.001) (Table 2).
Variceal Bleeding
Non selective beta blockers are recommended after an episode of variceal bleeding (Supplementary Table 4) (7). Among 1,582 patients with an episode of variceal bleeding, just over half (60.3%) filled a prescription for a NSBB after the first episode, and only 8.3% filled this prescription consistently (Figure 2). Having a diagnosis of chronic obstructive pulmonary disease or asthma (OR 0.7, 95% CI 0.5–0.8, P <0.001) and older age at the time of first decompensation was associated with lower likelihood of any NSBB use (OR per year 0.99, 95% CI 0.98–0.995, P = 0.003) (Table 2).
Non selective beta blockers may be dangerous in patients with ascites, spontaneous bacterial peritonitis, or renal failure (18). Patients with a history of variceal bleeding and ascites were more likely to have filled a NSBB (OR 1.3, 95% CI 1.0–1.6, P = 0.02) compared to patients without concomitant ascites. No association with NSBB fill was seen in those with a history of variceal bleeding with SBP compared to those without SBP (OR 1.3, 95% CI 0.9–1.9, P = 0.178) or those with variceal bleeding with HRS compared to those without HRS (OR 0.9, 95% CI 0.6–1.2, P = 0.0424).
Spontaneous Bacterial Peritonitis
Patients with a history of SBP should be started on antibiotics to reduce risk of recurrence (Supplementary Table 4) (5). Recommended antibiotics for this indication include Ciprofloxacin, Sulfamethoxazole/Trimethoprim, and Norfloxacin. Half (48.0%) of the 296 patients with SBP filled a prescription for one of these antibiotics after the SBP diagnosis, but only 8.8% filled this prescription consistently (Figure 2). An inpatient diagnosis of ascites (OR 2.1, 95% CI 1.3–3.6, P = 0.004) was associated with increased antibiotic use for secondary prophylaxis after SBP. AKI (OR 1.7, 95% CI 1.1–2.7, P = 0.029) and ESRD (OR 1.9, 95% CI 1.1–3.2, P = 0.023) were both associated with antibiotic use after SBP (Table 2).
Potentially Inappropriate Medication Use: NSAIDs
NSAIDs are generally felt to be unsafe in decompensated cirrhosis (Supplementary Table 5) (8, 15). Roughly 10% of patients with decompensated cirrhosis filled prescriptions for NSAIDs during the follow up period, and 2.8% of patients with decompensated cirrhosis filled these prescriptions consistently (≥ 30 days’ supply annually) (Figure 5). Male gender (OR 0.6, 0.5–0.8, P < 0.001), an inpatient ascites diagnosis (OR 0.7, 0.6–0.8, P = 0.009), and having more paracenteses (OR 0.9, 0.9–0.97, P = 0.008) during follow up were associated with less consistent NSAID use (Table 3).
Figure 5:
Potentially Inappropriate Medication Use in Decompensated Cirrhosis: Any and Consistent Use
Consistent use was defined as having a supply of the medication for ≥30 days’ supply per year follow up days for potentially inappropriate medications
NSAID: Non-steroidal anti-inflammatory drug
BDZ: Benzodiazepine
PPI: Proton Pump Inhibitor
Rx: Prescription
Table 3:
Factors Associated with consistent (≥ 30 days/year) Inappropriate and Potentially Inappropriate Medication Use
| NSAID Use | Opiate Use | Benzodiazepine Use | PPI Use | |
| OR (95% CI) | ||||
| Gender (Male) |
0.6 (0.5–0.8) p<0.001 |
0.9 (0.8–0.97) p=0.006 |
0.7 (0.6–0.8) p<0.001 |
1.0 (0.9–1.1) p=0.667 |
| Age at first liver decompensation | 1.00 (0.99–1.01) p=0.563 |
0.99 (0.98–0.99) p<0.001 |
0.97 (0.97–0.98) p<0.001 |
1.02 (1.01–1.02) p<0.001 |
| Race/Ethnicity | ||||
| White | 1.0 (0.6–1.7) p=0.972 |
1.0 (0.8–1.2) p=0.975 |
1.1 (0.8–1.5) p=0.696 |
1.0 (0.8–1.2) p=0.838 |
| Hispanic | 0.9 (0.5–1.6) p=0.629 |
0.8 (0.6–1.0) p=0.094 |
0.6 (0.4–0.9) p=0.006 |
1.1 (0.9–1.4) p=0.397 |
| African American |
1.2 (0.7–2.3) p=0.511 |
1.0 (0.8–1.3) p=0.876 |
0.8 (0.5–1.2) p=0.226 |
0.9 (0.7–1.1) p=0.289 |
| Asian | 0.8 (0.3–2.0) p=0.654 |
0.4 (0.3–0.6) p<0.001 |
0.5 (0.2–0.9) p=0.023 |
0.9 (0.6–1.2) p=0.317 |
| Co-morbid Conditions |
||||
| Peptic Ulcer Disease |
1.7 (1.6–1.9) p<0.001 |
|||
| Gastroesophageal Reflux |
2.3 (2.1–2.5) p<0.001 |
|||
| Dyspepsia |
1.2 (1.1–1.4) p=0.008 |
|||
| Inpatient Codes | ||||
| Inpatient Ascites Diagnosis |
0.7 (0.6–0.9) p=0.009 |
|||
| Inpatient HE | 1.3 (1.2–1.5) | 1.0 (0.8–1.1) | ||
| Diagnosis | p<0.001 | p=0.683 | ||
| Procedures | ||||
| Rx after TIPS* |
0.6 (0.3–0.98) p=0.045 |
1.9 (0.7–5.8) p=0.234 |
||
| Rx after Paracentesis** | 0.8 (0.5–1.1) p=0.141 |
|||
| Paracentesis # During Follow up |
0.9 (0.8–0.97) p=0.008 |
|||
| Gastroenterology or Hepatology Provider Consultation | 0.9 (0.7–1.1) p=0.318 |
1.1 (0.99–1.2) p=0.056 |
1.2 (0.99–1.4) p=0.073 |
1.7 (1.5–1.8) p<0.001 |
HE: Hepatic Encephalopathy
PPI: Proton Pump Inhibitor
NSAID: Non-Steroidal Anti-Inflammatory Drug
TIPS: Transjugular Intrahepatic Portosystemic Shunt
Medication use after TIPS for those who underwent TIPS compared to medication use for those who did not undergo TIPS
Medication use after paracentesis compared to medication use for those who did not undergo paracentesis
Opiates & Benzodiazepines
Opiates and benzodiazepines should be used with caution in decompensated cirrhosis (Table 2). Alarmingly, over half the patients with decompensated cirrhosis (53.2%) filled a prescription for an opiate during follow up, with almost a fifth filling them consistently (19.7%). Tramadol is classified as an opiate, still the vast majority (91.1%) who filled an opiate prescription filled at least one nontramadol opiate. A smaller but still substantial percentage (14.2%) of patients filled a prescription for a benzodiazepine during the study follow up, with 6.8% filling them consistently (Figure 5).
An inpatient diagnosis of HE was associated with consistent opiate use (OR 1.3, 95% CI 1.2–1.5, P < 0.001). Factors associated with less consistent opiate use included Asian race (OR 0.4, 95% CI, 0.3–0.6, P < 0.001), history of TIPS (OR 0.6, 95% CI 0.3–0.98, P = 0.045), male gender (OR 0.9, 95% CI 0.8–0.97, P = 0.006), and older age at first decompensation (OR per year 0.99, 95% CI 0.98–0.99, P < 0.001). No factors were associated with more consistent benzodiazepine use. Factors associated with less consistent benzodiazepine use included Asian race (OR 0.5, 95% CI 0.2–0.9, P = 0.023), Hispanic race (OR 0.6, 95% CI 0.4–0.9, P = 0.006), male gender (OR 0.7 95% CI 0.6–0.8, P < 0.001), and older age at first decompensation (OR per year 0.97, 95% CI 0.97–0.98, P < 0.001) (Table 3).
PPIs
Proton Pump Inhibitors are commonly used but have the potential for harm in decompensated cirrhosis (Supplementary Table 5). Not surprisingly, PPI use was prevalent in our cohort, with almost half (46.0%) of the patients taking PPI and nearly a third (31.1%) taking them consistently (Figure 5). A diagnosis of gastroesophageal reflux disease (OR 2.3, 2.1–2.5, P < 0.001), peptic ulcer disease (OR 1.7, 1.6–1.9, P < 0.001), and dyspepsia (OR 1.2, 1.1–1.4, P = 0.008) were associated with consistent PPI use, as was older age at the time of first decompensation (OR per year 1.02, 1.01–1.02, P < 0.001) (Table 3). Seeing a gastroenterology or hepatology provider was associated with more consistent PPI use (OR 1.7, 95% CI 1.5–1.8, P <0.001).
Discussion
Medications are the cornerstone of optimal care for persons with decompensated cirrhosis. Patterns of medication use therefore provide crucial data regarding the quality of care provided. We studied a large, national sample of patients with cirrhosis and found that patients with decompensated cirrhosis are not regularly filling indicated medications while many are routinely taking potentially dangerous medications. Furthermore, markers of cirrhosis severity were often, but not always associated with appropriate medication use.
Gaps in Appropriate Use
Society guidelines and quality measures (5–7, 19, 20) provide clear recommendations of medications that should be prescribed to patients with decompensated cirrhosis. Our study showed that few patients are consistently taking recommended medications for their decompensated cirrhosis. Although 48.0–63.3% of patients filled an indicated medication at least once, only 32.4% filled a prescription for rifaximin after an inpatient diagnosis of HE. Alarmingly, only 17.3% of patients with ascites had enough diuretic to cover at least 50% of follow up days, and even fewer (4.5–8.8%) had enough medication for consistent use for the other indications.
Markers of disease severity were often associated with appropriate medication use. Patients with ascites who had been hospitalized with this diagnosis were more likely to fill a prescription for diuretics for ascites and antibiotics for secondary prophylaxis of SBP. Patients with coding for renal impairment were more likely to be prescribed a diuretic; however, it is unclear if the renal impairment was secondary to diuretic use or a marker of the natural history of ascites progressing to refractory ascites. In addition, diuretics were more likely to be filled prior to TIPS placement in patients who eventually underwent this procedure compared to those who did not have TIPS, and patients who had a history of paracentesis were more likely to fill diuretics. Patients who had been hospitalized for HE were more likely to fill lactulose and rifaximin compared to those who only had been diagnosed with HE in the outpatient setting. Patients with HE and a history of TIPS were more likely to fill lactulose and rifaximin compared to those with HE who had not undergone TIPS. NSBB use after variceal bleeding was the only cohort that had consistent associations with medication non-use. This may be related to comorbid conditions that may increase the risk of adverse events with NSBB use. Indeed, we found that older patients and those with COPD/Asthma were less likely to fill a NSBB after variceal bleeding.
During the follow up period, evidence arose that NSBBs may be harmful in patients with refractory ascites, renal impairment, or SBP (18). We found patients with a history of ascites were more likely to have filled a NSBB than those with no ascites, after an episode of variceal bleeding. A limitation of this dataset is that we do not have data on blood pressure or renal function and are unable to confidently discern the severity of ascites (i.e. refractory ascites), to make firm conclusions whether NSBB use was appropriate or not. There was no association between NSBB fills after variceal bleeding for patients with a concomitant history of SBP or HRS. This finding may be related to concerns about NSBB use in these patients with more advanced cirrhosis.
The majority of patients in our cohort saw a gastroenterologist or hepatologist at least once. This consultation was associated with significantly more appropriate medication use in ascites and HE, and a trend towards appropriate antibiotic use after an episode of SBP.
Potentially Inappropriate Use
A substantial proportion of patients with decompensated cirrhosis were taking potentially harmful medications, despite agreement among hepatologists these are unsafe in decompensated cirrhosis (Supplementary Table 5). Opiates are associated with hospital readmissions (11) and HE (12) in patients with decompensated cirrhosis, but over half of patients with decompensated cirrhosis filled at least one prescription for an opiate, and one fifth filled an opiate prescription consistently (≥30 days/annually). PPI use followed closely with just under half filling a prescription for one of these medications. While the concerns about PPIs causing harms in cirrhosis are less direct (13, 14), they are amplified given how prevalent PPI use is in this population. Approximately 15% of patients with decompensated cirrhosis filled at least one prescription for a benzodiazepine, a class of medication associated with similar risks (9) as opiates. NSAIDs clearly have the ability to harm patients with decompensated cirrhosis by precipitating renal failure (15) and gastrointestinal bleeding (8), but still 10% of these patients filled a prescription for this class of medications. Of note, NSAID and PPI use are likely underestimated as this database did not capture over the counter medication use.
Male gender was largely associated with less frequent use of potentially inappropriate medications, including NSAIDs, benzodiazepines, and opiates. Markers of liver disease severity had inconsistent associations with inappropriate medication use. For example, patients with ascites who received an inpatient diagnosis of ascites or had a history of paracentesis were less likely to consistently fill an NSAID, but patients with HE who had been hospitalized for this were more likely to consistently fill an opiate.
There was no significant association between inappropriate medication use in decompensated cirrhosis and seeing a gastroenterologist or hepatologist, with the exception of increased use of PPIs. Patients with concomitant gastrointestinal problems may be more likely to see gastroenterologists and hepatologists, and this likely explains this finding.
Proposed Next Steps
Our data suggest that health care providers clearly do not know all the medications their patients take and how often they take these medications. Pharmacy records should be integrated with electronic health records to enable clinics and providers to have a list of not just medications prescribed but also all filled medications. This is key as even highly educated patients have significant medication discrepancies when they are asked to compile their medication list (21). Having not only a complete list of medications but also data on how often they are filled informs adherence and provides insight why some patients appear “not to respond” to appropriate treatment. Ideally, there is a multidisciplinary team that includes a pharmacist to assist with medication reconciliation in real-time during clinic visits (1). This information can then be used to counsel patients about the importance of consistently taking indicated medications and to discuss de-prescribing potentially harmful medications. However, complete pharmacy and health record data still won’t provide the full picture, as over the counter products, including NSAIDS, and health supplements will be missed.
In addition to gaps in our current healthcare system and our patients, provider knowledge deficiencies likely also contribute to suboptimal medication use. Future education efforts should include not just hepatologists and gastroenterologists but also other providers in primary care settings about recommended medications and potentially harmful medications in decompensated cirrhosis.
Contextual Factors
These data must be interpreted in the context of the study design. Our study included a large cohort (>12,000) of patients with decompensated cirrhosis, representative of the insured United States population spread across the country. Despite this, there are still populations excluded from the database (i.e. patients receiving all care at Veterans Affairs hospital or other private insurers not participating in Optum). The database includes all medications patients actually filled from the pharmacy and prescriptions from all providers caring for a patient, over several years. It also included all diagnosis and procedure codes billed to insurance during the study period allowing us to determine whether presence of co-morbidities such as asthma accounted for NSBB non-use, and whether inpatient diagnosis of HE was associated with more frequent rifaximin use.
However, these data have multiple limits. First, we are unable to verify accuracy and completeness of ICD-9 coding as patient level data was not available for review. Liver disease etiology was felt to be at highest risk for incomplete or inaccurate coding, so it was not used in analysis of factors associated with medication use. In fact, 64% of potentially eligible patients had to be excluded because of the lack of a diagnosis code for liver disease etiology as we wanted to be certain that the cohort included had decompensated cirrhosis. The database only included laboratory data for a small subset; thus, severity of liver disease was based on diagnosis codes and whether those codes occurred as inpatient or outpatient.
Second, while a patient filling a prescription from a pharmacy is a more accurate measure of medication use than data based on prescriptions, it is still not a direct measure of what medications patients take day to day. For patients who present in person to pharmacies to pick up their refills, each refill likely represents consumption of the previous supply; however, for patients who get their refills through automated mail delivery, refills may not represent actual consumption. In addition, for medications such as diuretics or lactulose where daily doses are frequently adjusted, the days’ supply may not reflect the amount needed.
Finally, as this database only reports medications patients filled from a pharmacy, we are unable to determine if a medication was not filled (at all or consistently) because of a patient factor, an insurance factor, or a provider factor. We assumed that all patients with decompensated cirrhosis should be on medications recommended for the cirrhosis complication(s) during the entire follow up period, but there are often legitimate and illegitimate reasons why this may not be the case. While we were able to examine some of these reasons and found explanations for not using recommended medications e.g. fewer patients with chronic obstructive pulmonary disease/Asthma taking NSBB after a variceal bleed, the lack of granular information in the database did not allow us to determine whether there were legitimate reasons such as resolution of cirrhosis complication, for not using or not consistently using medications in most patients. For example, a patient with alcohol related liver disease may have improvement in portal hypertension with sobriety, and no longer requires diuretics. We expect scenarios like this to be the minority and the majority of patients will require long term medications to control or to prevent recurrence of cirrhosis complications. Similarly, there may be legitimate reasons for use of some of the potentially inappropriate medications such as PPI or even opiates. While one could argue that a short course of opiates is appropriate after a mechanical injury, particularly to avoid NSAIDs, having more than half of the patients with decompensated cirrhosis taking this class of medication and one-fifth taking opiates consistently is concerning. We also could not determine if a medication was not filled because the patient chose to not fill it, or was unable to fill it as it was not covered by insurance. We expect rifaximin fills to be affected most by the latter, as this medication is often prohibitively expensive or not covered by insurance.
Conclusions:
In this large nationally representative cohort study, we found that patients with decompensated cirrhosis are not filling indicated medications as often or for as long as is recommended per our society guidelines, even among patients who had been hospitalized for decompensation. In addition, patients are filling medications that are potentially harmful. Future steps to improve appropriate medication use in decompensated cirrhosis include integrating pharmacy records with the electronic health record to obtain a complete list of medications, and dedicated time during each visit to counsel patients on the medications they should or should be not be taking, the purpose of each medication, their use and possible side effects.
Supplementary Material
Acknowledgments
Grant Support: This study was funded in part by an NIH Training Grant in Epidemiology and Health Services (T32 DK062708 - MJT), and AASLD Advanced Hepatology Award (MJT), and an NIH NIDDK (1K23DK117055-01A1- EBT).
Disclosures: The authors have no conflict of interests that pertain to this work. Elliot Tapper has served as a consultant to Norvartis, Axcella, Kaleido, and Allergan, has served on advisory boards for Mallinckrodt, Rebiotix, and Bausch Health, and has received unrestricted research grants from Gilead and Valeant. Valeant is the maker of Rifaximin, a medication approved for treatment of hepatic encephalopathy.
Abbreviations:
- AASLD
American Association for the Study of Liver Disease
- AHFS
American Hospital Formulary System
- AKI
Acute Kidney Injury
- ALD
Alcohol Related Liver Disease
- CHC
Chronic Hepatitis C
- CI
Confidence Interval
- CKD
Chronic Kidney Disease
- COPD
Chronic Obstructive Pulmonary Disease
- CPT
Current Procedural Terminology
- ESRD
End Stage Renal Disease
- HE
Hepatic Encephalopathy
- HRS
Hepatorenal Syndrome
- ICD-9
International Classification of Diseases, 9th Revision
- IQR
Interquartile Range
- NAFLD
Non-Alcoholic Fatty Liver Disease
- NSAID
Non-Steroidal Anti-inflammatory drug
- NSBB
Non-Selective Beta Blocker
- OR
Odds Ratio
- PPI
Proton Pump Inhibitor
- SBP
Spontaneous Bacterial Peritonitis
- TIPS
Transjugular Intrahepatic Portosystemic Shunt
References:
- 1.Thomson MJ, Lok AS, Tapper EB. Optimizing medication management for patients with cirrhosis: Evidence-based strategies and their outcomes. Liver Int 2018;38:1882–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weersink RA, Burger DM, Hayward KL, Taxis K, Drenth JPH, Borgsteede SD. Safe use of medication in patients with cirrhosis: pharmacokinetic and pharmacodynamic considerations. Expert Opin Drug Metab Toxicol 2020;16:45–57. [DOI] [PubMed] [Google Scholar]
- 3.Hayward KL, Patel PJ, Valery PC, Horsfall LU, Li CY, Wright PL, Tallis CJ, et al. Medication-Related Problems in Outpatients With Decompensated Cirrhosis: Opportunities for Harm Prevention. Hepatol Commun 2019;3:620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087–2107. [DOI] [PubMed] [Google Scholar]
- 5.Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology 2004;39:841–856. [DOI] [PubMed] [Google Scholar]
- 6.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310–335. [DOI] [PubMed] [Google Scholar]
- 8.Rakoski M, Goyal P, Spencer-Safier M, Weissman J, Mohr G, Volk M. Pain management in patients with cirrhosis. Clin Liver Dis (Hoboken) 2018;11:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and Risk Factors for Hepatic Encephalopathy in a Population-Based Cohort of Americans With Cirrhosis. Hepatol Commun 2019;3:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive Medications Increase the Risk of Falls and Fall-related Injuries in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol 2015;13:1670–1675. [DOI] [PubMed] [Google Scholar]
- 11.Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, et al. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:319–331. [DOI] [PubMed] [Google Scholar]
- 12.Zedler B, Xie L, Wang L, Joyce A, Vick C, Kariburyo F, Rajan P, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med 2014;15:1911–1929. [DOI] [PubMed] [Google Scholar]
- 13.Trikudanathan G, Israel J, Cappa J, O’Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract 2011;65:674–678. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj JS, Ananthakrishnan AN, Hafeezullah M, Zadvornova Y, Dye A, McGinley EL, Saeian K, et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol 2010;105:106–113. [DOI] [PubMed] [Google Scholar]
- 15.Tapper EB, Bonder A, Cardenas A. Preventing and Treating Acute Kidney Injury Among Hospitalized Patients with Cirrhosis and Ascites: A Narrative Review. Am J Med 2016;129:461–467. [DOI] [PubMed] [Google Scholar]
- 16.Pfisterer N, Dexheimer C, Fuchs EM, Bucsics T, Schwabl P, Mandorfer M, Gessl I, et al. Betablockers do not increase efficacy of band ligation in primary prophylaxis but they improve survival in secondary prophylaxis of variceal bleeding. Aliment Pharmacol Ther 2018;47:966–979. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, O’Leary JG, Tandon P, Wong F, Kamath PS, Biggins SW, Garcia-Tsao G, et al. Targets to improve quality of care for patients with hepatic encephalopathy: data from a multi-centre cohort. Aliment Pharmacol Ther 2019;49:1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014;146:1680–1690. [DOI] [PubMed] [Google Scholar]
- 19.Kanwal F, Tapper EB, Ho C, Asrani SK, Ovchinsky N, Poterucha J, Flores A, et al. Development of Quality Measures in Cirrhosis by the Practice Metrics Committee of the American Association for the Study of Liver Diseases. Hepatology 2019;69:1787–1797. [DOI] [PubMed] [Google Scholar]
- 20.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013;57:1651–1653. [DOI] [PubMed] [Google Scholar]
- 21.Hayward KL, Valery PC, Cottrell WN, Irvine KM, Horsfall LU, Tallis CJ, Chachay VS, et al. Prevalence of medication discrepancies in patients with cirrhosis: a pilot study. BMC Gastroenterol 2016;16:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.