Abstract
Exposure to loud noise can cause hearing loss and tinnitus in mice and humans. In mice, one major underlying mechanism of noise-induced tinnitus is hyperactivity of auditory brainstem neurons, due at least in part, to decreased Kv7.2/3 (KCNQ2/3) potassium channel activity. In our previous studies, we used a reflex-based mouse model of tinnitus and showed that administration of a non-specific KCNQ channel activator, immediately after noise trauma, prevented the development of noise-induced tinnitus, assessed 1 week after trauma. Subsequently, we developed RL-81, a very potent and highly specific activator of KCNQ2/3 channels. Here, to test the timing window within which RL-81 prevents tinnitus in mice, we modified and employed an operant animal model of tinnitus, where mice are trained to move in response to sound but not move in silence. Mice with behavioral evidence of tinnitus are expected to move in silence. We validated this mouse model by testing the effect of salicylate, which is known to induce tinnitus. We found that transient administration of RL-81 1 week after noise exposure did not affect hearing loss but reduced significantly the percentage of mice with behavioral evidence of tinnitus, assessed 2 weeks after noise exposure. Our results indicate that RL-81 is a promising drug candidate for further development for the treatment of noise-induced tinnitus.
Keywords: KCNQ potassium channel activators, tinnitus, hearing loss, auditory brainstem, cochlea, therapeutic window
Introduction
Tinnitus is the unintentional and irrepressible perception of internally generated simple non-verbal noises and tones that do not exist in the outside world. Tinnitus is experienced by up to 15 % of the general population (Adams et al. 1999). Of the 40–50 million people in the US affected by tinnitus, approximately 10 million seek medical attention (Seidman and Jacobson 1996), and 2.5 millions of these are considered disabled by tinnitus due to its persistence and intensity (Shargorodsky et al. 2010). Among veterans, tinnitus is the highest service-connected disability of all compensation and new compensation recipients. Namely, tinnitus impacts more than 2.17 million veteran overall (https://www.benefits.va.gov/REPORTS/abr/), and in fiscal year 2019 alone, 183,145 new veterans entered this pool (https://www.benefits.va.gov/REPORTS/abr/). Although progress has occurred in understanding the underlying neurobiological mechanisms, and developing treatment options to reduce the severity of symptoms of tinnitus, there is no evidenced-based treatment to permanently eliminate or reduce the long-term perception of tinnitus—as a result, currently there is no available cure for tinnitus (Tzounopoulos et al. 2019).
The detailed mechanisms that are necessary and sufficient for the triggering and maintenance of tinnitus are not fully understood. However, the research community generally agrees that tinnitus is usually generated in the brain in response to noise-induced and/or age-dependent reduction of auditory nerve input to the brain (Shore and Wu 2019). Reductions of the auditory nerve drive may be due to changes at the cellular and/or subcellular level (synapses). This loss of input may be compensated at several central brain regions. These compensations result in various forms of plasticity, including preserved, reduced or even enhanced spontaneous and sound-evoked activity (Schaette and Kempter 2006; Yang et al. 2011; Li et al. 2013; Shore et al. 2016).
The brain is very plastic and capable of continuous, small- and large-scale anatomical, neurochemical, and functional changes. These plastic changes can occur in either a positive or negative direction and across lifespan. Therefore, it has been hypothesized that the plasticity mechanisms that underlie the emergence of and that perpetuate and elaborate the deficits of tinnitus are likely the same mechanisms and processes that can be used to drive changes in a corrective direction. The validity of this statement is supported by recent studies, where corrective plasticity has been used successfully in mice or has shown promise in trials in humans. For example, combination of vagal nerve stimulation (VNS) with sound stimulation induces cortical plasticity to increase the representation of sounds outside the tinnitus region to normalize the noise-induced perturbed activity in primary auditory cortex (Engineer et al. 2011; Vanneste et al. 2017). Another approach targets noise-induced maladaptive plasticity in the auditory brainstem by using auditory and somatosensory (trigeminal nerve) stimulation to normalize maladaptive DCN plasticity linked to tinnitus (Marks et al. 2018).
One major path of tinnitus-related plasticity involves noise-induced reduction of KCNQ potassium channel activity, a family consisting of five different subunits (KCNQ1–5). Specifically, tinnitus-related reduction in KCNQ2/3 channel activity contributes significantly to tinnitus-related auditory brainstem hyperexcitability (Li et al. 2013). This reduction is due to a depolarizing shift in the voltage-dependence of KCNQ2/3 channel activation (Li et al. 2013; Li et al. 2015). Importantly, intraperitoneal (IP) administration of KCNQ channel activators, including retigabine, immediately after noise exposure eliminated tinnitus in mice (Li et al. 2013; Kalappa et al. 2015; Li et al. 2015). Retigabine, an FDA-approved antiepileptic drug that is an activator of KCNQ2-5 channels, was discontinued in 2017, due to slow sales as a consequence of severe side effects, including urinary retention, blue skin, and retinal discoloration (Eskioglou et al. 2017). These side effects were likely due in part to the poor selectivity of retigabine among KCNQ channels as well as binding of its metabolites in melanin-containing layers and their subsequent dimerization (Groseclose and Castellino 2019). To mitigate these undesirable effects, we modified retigabine. When we introduced a CF3–group at the 4-position of the benzylamine moiety, combined with a fluorine atom at the 3-position of the aniline ring, we generated RL648–81 (RL-81), a new KCNQ2/3-specific activator with increased chemical stability, as a function of its fluorinated backbone structure, that is 15 times more potent and also more selective than retigabine (Kumar et al. 2016).
However, the effects of RL-81 on tinnitus and the critical window for its delivery to treat noise-induced tinnitus remain unknown. To answer these questions, we modified and validated an operant animal model of tinnitus and tested the effect of transient RL-81 delivery 1 week after noise trauma on noise-induced tinnitus and hearing loss, assessed 2 and 3 weeks after trauma, respectively.
Materials and Methods
Animals
C57BL/6J (C57) mice (https://www.jax.org/strain/000664) were obtained from either Jackson Laboratory (Bar Harbor, ME) or bred within our animal facilities. C57 mice were chosen due to their good performance in behavioral tasks and because their hearing has been well studied (Henry and Chole 1980). Because C57 mice show age-dependent hearing loss after 6 months of age (Kikkawa et al. 2012), all experiments and hearing assays were completed by postnatal (P) day P83. Forty-two mice were used for the noise-exposed group, twenty-one for RL-81-treated group (RL-81: 8 males, 13 females; fourteen of these mice were successfully trained, see criteria in Training section, and used for tinnitus assessment) and twenty-one for vehicle-treated group (methylcellulose: 12 males, 9 females; fifteen of these mice were successfully trained and used for tinnitus assessment). An additional thirteen mice were used for the sham-exposed groups (methylcellulose: 2 males, 5 females and RL-81: 3 males, 3 females). Mice were housed in same sex and litter groups and kept in a 12 h light-dark cycle (lights on at 7:00 am), where temperature was maintained constant. All procedures and animal handling were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Apparatus
A shuttle box (14″ W × 7″ D × 12″ H) was used for the operant conditioning detection of tinnitus. Coulbourn designed the shuttle box, which consists of two equal size compartments, separated by a programmable door. Mouse movement was monitored with light emitting diode (LED) beams, which automatically tracked movement between the two halves. Programming of the behavioral protocols was done in the Graphic State software. The H02-01 Habitest Linc controlled the apparatus. Tones (8, 10, 12.5, 16, and 20 kHz, 69–77 dB SPL) were delivered randomly through a calibrated speaker (8 Ohm Speaker Module for Mouse, Coulbourn, Holliston, MA) placed at the right side of the shuttle box. The speaker was calibrated with a 1/4-in. microphone (4954-B, Bruel & Kjaer) and a reference 1 kHz, 94 dB SPL certified speaker (Type 4231, Bruel & Kjaer). More specifically, we placed the microphone at the center of the shuttle box in approximately the same position as the mouse. We delivered the pure tone stimuli (8, 10, 12.5, 16, and 20 kHz) at a specific voltage input (via the Graphic State software) and recorded output voltage using the 1/4-in. microphone. Then, we determined the voltage input needed to generate the desired dB output (69–77 dB SPL) using the Bruel & Kjaer 4231 1 kHz 94 dB speaker as the reference voltage. Electric shock was programmed via the Coulbourn Precision Animal Shocker (Model H113-17A) and delivered via the metal grid shock floor. Shock intensity ranged from 0.2 to 0.4 mA (see Training). We selected to shock using eight poles, so mice could not avoid the shock by cowering in corners. Mice had to cross to terminate the shock.
Drugs: Salicylate
To validate our animal model of tinnitus, we used the ototoxic drug salicylate to induce acute tinnitus in mice. For three consecutive days, each mouse received an intraperitoneal (IP) injection of either sodium salicylate (300 mg/kg) (Hwang et al. 2011a, b, 2013), suspended in saline (vehicle) or vehicle 2 h prior to testing for tinnitus. Mice were trained and tested at the same time during the day to avoid potential circadian rhythm effects.
Drugs: RL648_81 (RL-81)
RL-81 was synthesized as previously described (Kumar et al. 2016). RL-81 was dissolved in dimethyl sulfoxide (DMSO) in a 50 mM stock solution. It was then formulated as a suspension in 0.5 % methylcellulose and administered via intraperitoneal (IP) injections 1 week after noise or sham exposure twice per day, for 5 days every 12 h, and at a dose of 5 mg/kg. Vehicle-treated groups were injected with DMSO suspended in 0.5 % methylcellulose, using the same injection mode and schedule as those of RL-81-treated groups.
Noise and Sham Exposure
Mice were anesthetized with isoflurane (3 % induction/1.5 % maintenance, in oxygen) during a unilateral exposure, via insertion of a plastic pipette tip into the left ear. The pipette tip was connected to a calibrated speaker (FT17H, Fostex, Middleton, WI). Mice were maintained at a constant temperature (~ 37 °C) during anesthesia via a heating pad. Sham- and noise-exposed mice were treated in the same manner, but noise-exposed mice received an 8–16 kHz octave broadband noise at 116 dB SPL for 1 h. For the bandwidth limited white noise, we used the DS360 Ultra-Low Distortion Function Generator (Stanford Research Systems-SRS). The bandwidth limiting is accomplished using a 3-Pole Butterworth Filter, for both the high and low pass filters. The speaker was calibrated with a 1/4-in. microphone (4954-B, Bruel & Kjaer) using a 1 kHz, 94 dB SPL certified speaker (Type 4231, Bruel & Kjaer). We connected the microphone to the speaker, and used the same pipette employed for noise exposure with a rubber adapter that mimics the mouse ear canal. We delivered noise stimuli across a range of voltage inputs and recorded output voltage with a 1/4-in. microphone. From this, we generated an input/output curve, and determined the voltage input needed to generate the desired dB output. We used the 1 kHz 94 dB as the voltage standard.
Functional Assays
Auditory Brainstem Responses
Auditory Brainstem Response (ABR) measurements were taken from the left (exposed) and right (unexposed) ear and conducted in a sound-attenuating chamber (ENV-022SD; Med Associates). Mice were maintained at a stable temperature using a heating pad (~ 37 °C), and were anesthetized under isoflurane (3 % Induction/ 1.5 % Maintenance, in oxygen). To collect ABR measurements, subdermal electrodes were placed at the vertex (active), ventral to the right pinna (ground), and ventral to the left pinna (reference). To acquire ABR measurements from the exposed ear, we used a calibrated speaker (CF-1; Tucker Davis Technologies) to deliver acoustic stimuli through a pipette tip fixed to a plastic tube set up in the left ear. Electrodes and speaker (pipette tip) were adjusted accordingly to acquire ABR measurements from the unexposed, right ear. ABR measurements were obtained for 1-ms tone bursts of 10, 12, 16, 20, 24, and 32 kHz, which were delivered at a rate of 18.56/s, and in a descending order from 80 to 0 dB (or until 10 dB below threshold). The rise-fall time of the tones was 1.5 ms (Cos2 gate). ABR measurements were collected with BioSigRX software, filtered with a 300–3000 Hz bandpass filter, and averaged over 512 times. Calibration for ABR tone delivery was conducted via a 1/8″ microphone amplifier (4954-B, Bruel & Kjaer, Narum, Denmark) affixed to the end of the pipette tip used for tone delivery. The microphone amplifier was fed into the TDT RX-1 system, and the TDT program SigCal was used to calibrate the amplitude for each tone used. Threshold was defined as the lowest stimulus intensity that produced a Wave 1 response. A Wave 1 response waveform is identified, and distinguished from non-response (noise), as the first consistent wave generated with decreased amplitude and increased latency as the intensity decreases.
Operant Conditioning Animal Model for Tinnitus Detection
Training
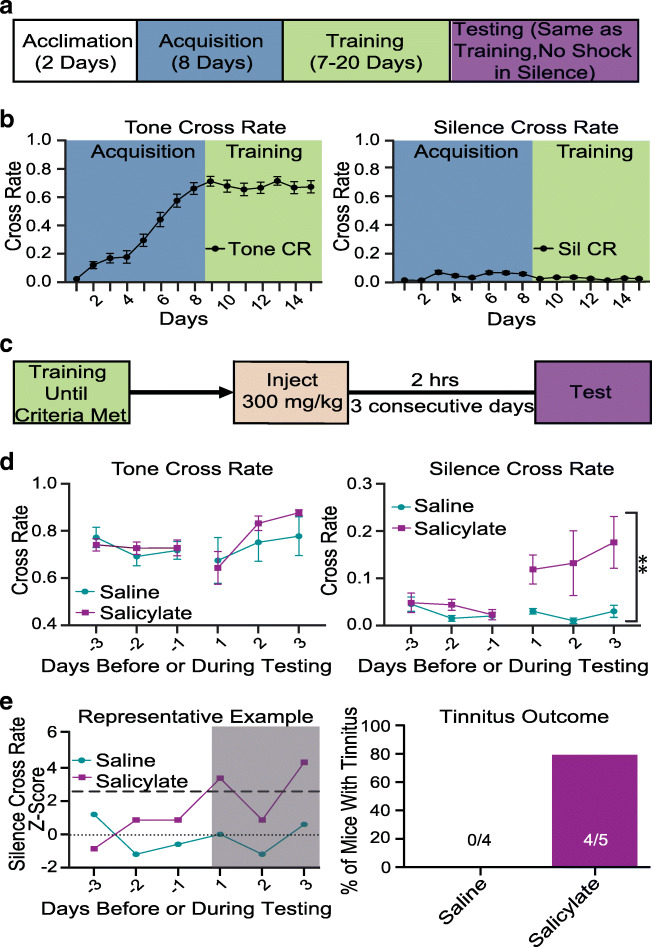
The training for the operant conditioning model was based on the published sound-based avoidance detection (SBAD) model (Zuo et al. 2017). Namely, we trained the mice to cross from one compartment of the chamber to the other in tone trials and to not cross (stay in the same compartment) in silence trials. We calculated the cross rate (CR), which is the number of trials the mouse crossed over the total number of trials for each task (i.e., if a mouse crossed 40/50 times in tone, tone CR = 0.8). To guarantee low variability and consistency, we established the following criteria for successful training: stable baseline difference in cross rate of > 0.6, variance of < 0.0025; and an average silence cross rate of < 0.05 for four consecutive days. The difference in cross rate is the calculated cross rate when we subtracted cross rate in silence from cross rate in tones. The training process (Fig. 1a) began with a two-day acclimation period where mice (P30-P38) were given 20 min each day to explore the shuttle box and to adjust to the experimenter. After these 2 days, the acquisition phase began. During the acquisition, training and testing phases, mice received 100 trials per day (session), which consisted of 50 random tone trials (8, 10, 12.5, 16, 20 kHz 69–77 dB SPL) and 50 silent trials. Tone and silent trials were initiated by opening the door of the chamber. For the tone trials, since mice instinctively prefer to not cross, a gradual association between the tone and the crossing behavior was implemented during the acquisition phase. This association was created by gradually increasing the duration of the presented tone prior to delivering the electric shock (if the mouse did not cross in the tone trial). On the first day of acquisition, the tone duration was 1.5 s. Then, the tone duration increased by 0.5 s per day until the eighth day, when the mice were given 5 s tone before receiving any electric shock. To further consolidate the link between the tone and the crossing behavior, the shock intensity was also gradually increased. The intensity of the electric shock was 0.2 mA for the first 4 days. Then, the shock intensity increased daily until it reached a max of 0.4 mA. The silent trials lasted 10 s after initiation of the trial. If the mouse did not cross during the trial, then the door would close and nothing would happen, but if the mouse crossed, the mouse received a shock (and immediately crossed back to the original side). After completing the acquisition phase, mice were subsequently trained until they reached the criteria for successful training. The whole behavioral protocol took on average 3 weeks. Following the completion of successful training, mice were tested for three consecutive days for salicylate-induced (see Salicylate Experiments) or noise-induced tinnitus (see RL-81 Administration and Tinnitus and Hearing Loss Assessment). During the testing period, testing was conducted in the same manner as training, but the shock was removed in silent trials (Zuo et al. 2017).
Fig. 1.
Establishment and validation of tinnitus operant mouse model. a Timeline of the experimental approach. b Tone (left) and silence (right) cross rates during training (N = 16). To quantify this behavior, we used the cross rate (CR), which is the number of trials the mouse crossed over the total number of trials for each task (i.e., if a mouse crosses 40/50 times in tone, tone CR = 0.8). c Timeline of salicylate/saline administration. d Tone (left) and silence (right) in saline vs. salicylate cross rates during training and testing. We used 2-way ANOVA to compare silence CR in saline vs. salicylate: Saline N = 4; salicylate N = 5; F (1,21) = 11.28, P = 0.003. e Example of how tinnitus was assessed using Z-score in individual mice (left) and percentage of mice with tinnitus (right)
Salicylate Experiments
To verify that our model is detecting tinnitus, we used the ototoxic drug salicylate, which is known to reliably induce tinnitus. Once the training criteria were achieved, either 300 mg/kg sodium salicylate or saline (vehicle) were given 2 h before testing. Salicylate and vehicle were administered via an IP injection each day. The previously described protocol was used for tinnitus assessment (the shock was removed in silent trials). To assess whether a mouse showed behavioral evidence of tinnitus, we used the Z-score. Namely, we used the average silence cross rate of the last 4 days of successful training. We considered a mouse with behavioral evidence of tinnitus when at least one of the 3 days of testing had a silence cross rate that was two and a half standard deviations greater than the mean baseline silence cross rate (Z-score ≧ 2.5). During the acquisition, training and testing phases, mice received 100 trials per day (session), which consisted of 50 random tone trials (8, 10, 12.5, 16, 20 kHz 69–77 dB SPL) and 50 silent trials per session.
RL-81 Administration and Tinnitus and Hearing Loss Assessment
To test for noise-induced tinnitus, we noise exposed the mice and then we tested for behavioral evidence of tinnitus 2 weeks following the exposure. After mice reached the four-day baseline training criteria (see Training), hearing was assessed using ABR measurements. All mice were assessed before noise or sham exposure (P50–62). ABR thresholds were taken from the left (exposed) and right (unexposed) ear. The unilateral sham or noise (8–16 kHz octave broadband, 116 dB SPL noise for 1 h) exposure was given immediately following ABR measurements. After 1 week, mice were given 5 mg/kg (IP injection, 5 mg/kg) of RL-81 or 0.5 % methylcellulose (vehicle), every 12 h, for 5 days. Tinnitus assessment was conducted as described earlier (see Salicylate Experiment, with the shock off in silence errors and with using Z-score criterion). Testing (tinnitus assessment) was conducted 2 weeks following the noise exposure. To assess hearing, ABR measurements were collected 3 weeks post noise or sham exposure.
Cochlear Histology
Cochlear Extraction and Processing
Within 1 week of the final ABR measurements, which were conducted 3 weeks after the noise exposure, mice were deeply anesthetized with isoflurane and sacrificed by decapitation. Cochleae were extracted and perfused intralabyrinthly (through the round and oval windows via a small needle) with 4 % paraformaldehyde in 0.1 M phosphate buffer. Left (noise-exposed) cochleae were used for immunostaining. Cochleae were post-fixed for 2 h at room temperature and decalcified in 120 mM EDTA for 2–3 days at room temperature on a rocker. Decalcified cochleae were then microdissected under a stereomicroscope. Cochlear sections were blocked in 5 % normal goat serum with 0.3 % Triton X-100 in phosphate-buffered saline (PBS) for 1 h at room temperature. Sections were then incubated in primary antibodies diluted in blocking buffer overnight (18–24 h) at room temperature. The primary antibodies used were: anti-myosin VIIa (rabbit anti-MyoVIIa; Proteus Biosciences; 1:500), anti-C-terminal binding protein 2 (mouse anti-CtBP2 IgG1; BD Biosciences; 1:200), and anti-glutamate receptor 2 (mouse anti-GluR2 IgG2a; Millipore; 1:2000). Sections were then washed with PBS and incubated in Alexa Fluor-conjugated fluorescent secondary antibodies (Invitrogen; 1:500) for 2 h at room temperature. Sections were again washed in PBS and finally mounted on microscope slides using Prolong Diamond Antifade Mountant (Invitrogen).
Imaging and Quantification
Cochlear sections were imaged in their entirety at low magnification to reconstruct the cochlear frequency map using an ImageJ plugin provided by Eaton Peabody Laboratories (http://www.masseyeandear.org/research/otolaryngology/investigators/laboratories/eaton-peabody-laboratories/epl-histology-resources/imagej-plugin-for-cochlear-frequency-mapping-in-whole-mounts). This preparation allowed us to trace the organ of Corti in its entirety from base to apex; the plugin superimposes the frequency map on the traced sections. Confocal z-stacks (0.25 μm step size) of the 8, 12, 16, 24, and 32 kHz regions from each cochlea were captured using a Nikon A1 microscope under a 60× oil immersion lens. Images were imported to ImageJ imaging software for quantification, where maximum projections were rendered from the z-stacks. CtBP2 and GluR2 puncta were counted to identify intact ribbon synapses. Synapses were only considered intact if CtBP2 and GluR2 puncta were juxtaposed. Orphan synapses were defined as CtBP2 puncta that lacked GluR2 puncta. To assess inner hair cell (IHC) and outer hair cell (OHC) loss, 200 μm lengths of the organ of Corti were used. Hair cells were equally sampled from all frequency areas: 14–18 inner hair cells were sampled in each frequency region for synapse quantification. MyoVIIa staining was used to identify missing IHCs and OHCs from each frequency region and calculated as fraction lost.
Statistical Analysis
All data analysis was conducted in GraphPad Prism 8.4.0 (455). Group data are presented as mean ± SEM. The statistical test used for each comparison is described in the figure legend, including N, F (degrees of freedom), the statistical value, and the P value. In group comparisons of cross rates in tone and silence, we used 2-way ANOVA with Bonferroni corrections for multiple comparisons (Figs. 1 and 2). Binomial test was used for comparing percentages of tinnitus mice in response to different manipulations (Fig. 2). We used 3-way ANOVA analysis to determine how ABR threshold shifts are affected by frequency, exposure condition (sham SE vs. noise exposure NE), and drug condition (vehicle vs. RL-81) (Fig. 3, Table 1). To probe the drug effect, because frequency had no impact, we averaged the threshold shifts across frequencies within animals, and we used unpaired t test (single comparisons) to examine whether RL-81 affects ABR threshold shifts in either sham- or noise-exposed mice (Fig. 3c). We used 3-way ANOVA to assess input (sound intensity)–output (wave I amplitude) functions between vehicle and RL-81 treated mice (Fig. 4a–f, Table 2). Cochlear histology in noise-exposed mice was assessed using 2-way ANOVA (Fig. 4g–o, Table 3).
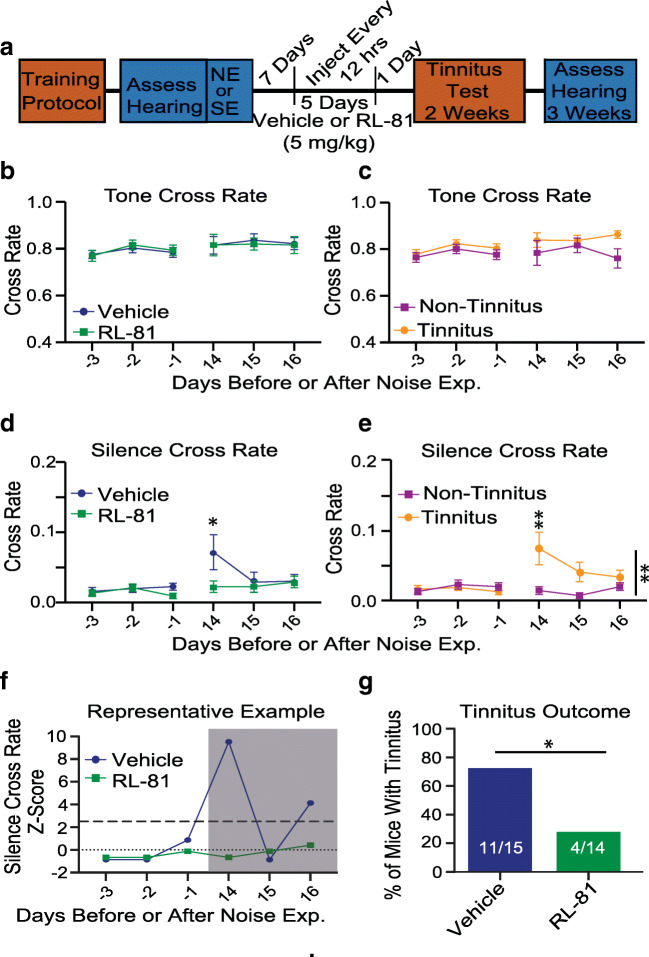
Fig. 2.
Transient RL-81 IP application 1 week after noise exposure reduced the percentage of mice with tinnitus, assessed 2 weeks post noise exposure. a Timeline of the experimental procedure. b Tone cross rate in vehicle- and RL-81-treated mice (N = 15 vehicle, 14 RL-81). c Tone cross rate in tinnitus and non-tinnitus mice (N = 15 vehicle, 14 RL-81). d Silence cross rate in vehicle- and RL-81-treated mice was not significantly different after noise exposure across days 14–16; an increase found exclusively in the first day of testing, day 14 (N = 15 vehicle, 14 RL-81; days 14–16: F (1,84) = 2.886, P = 0.093; day 14: t (84) = 2.55, P = 0.038). e Silence cross rate in tinnitus and non-tinnitus mice were significantly different across days 14–16, with the first day of testing, day 14, showing a significant increase in tinnitus vs. non-tinnitus mice (N = 15 tinnitus, 14 non-tinnitus; day 14–16: F (1,84) = 11.34, P = 0.0011; day 14: t (84) = 3.268, P = 0.0047). f Representative example of the Z-score metric to determine tinnitus (vehicle) vs. non-tinnitus (RL-81) in individual mice. g Percentage of mice with tinnitus (binomial test: P = 0.016)
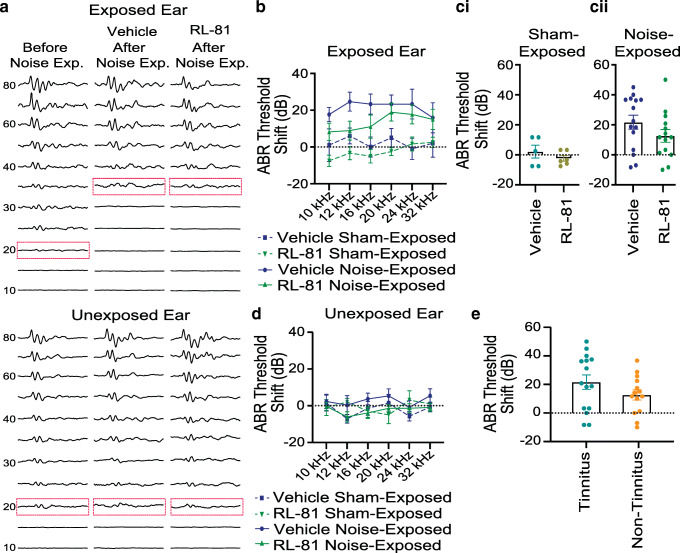
Fig. 3.
Transient RL-81 IP application 1 week after noise exposure had no effect on noise-induced hearing loss, assessed 3 weeks post noise exposure. a Representative traces of ABR recordings (in response to 12 kHz tones) with thresholds highlighted (dotted rectangle) before and 3 weeks post noise exposure in vehicle- and RL-81-treated mice for exposed (top) and unexposed (bottom) ear. b ABR threshold shifts in the exposed ear in vehicle vs. RL-81 in noise- and sham-exposed mice (NE: N = 15 vehicle; 14 RL-81; SE: N = 5 vehicle; 6 RL-81; 3-way ANOVA exposure: F (1,203) = 41.25, P < 0.0001; drug condition: F (1,203) = 5.375, P = 0.021). c Effect of RL-81 on ABR threshold shifts from sham- (ci) and noise-exposed (cii) mice (sham-exposed: N = 5 vehicle, 6 RL-81; unpaired t test: t (9) = 1.029, P = 0.33; noise-exposed: N = 15 vehicle, 14 RL-81; unpaired t test: t (27) = 1.448, P = 0.16). d ABR threshold shifts in the unexposed ear in vehicle vs. RL-81 in noise- and sham-exposed mice (NE: N = 15 vehicle; 14 RL-81; SE: N = 7 vehicle; 5 RL-81; 3-way ANOVA exposure: F (1,210) = 0.75, P = 0.39; drug condition: F (1,210) = 1.123, P = 0.29). e Average ABR threshold shifts across frequencies in tinnitus assessed mice (N = 14 non-tinnitus, 15 tinnitus: t (27) = 1.416, P = 0.17)
Table 1.
Statistical assessment of ABR threshold shifts
| Exposed ear | Unexposed ear | |||||||
|---|---|---|---|---|---|---|---|---|
| Three-way ANOVA | P value | Sig | Unpaired t test | P value | Sig | Three-way ANOVA | P value | Sig |
| Frequency | 0.79 | ns | Frequency | 0.77 | ns | |||
| Exposure | < 0.0001 | **** | Sham-exposed: vehicle vs. RL-81 | 0.33 | ns | Exposure | 0.39 | ns |
| Drug condition | 0.021 | * | Drug condition | 0.29 | ns | |||
| Frequency × exposure | 0.97 | ns | Noise-exposed: vehicle vs. RL-81 | 0.16 | ns | Frequency × exposure | 0.98 | ns |
| Frequency × drug condition | 0.78 | ns | Frequency × drug condition | 0.47 | ns | |||
| Exposure × drug condition | 0.52 | ns | Exposure × drug condition | 0.05 | ns | |||
| Frequency × exposure × drug condition | 0.99 | ns | Tinnitus vs. non-tinnitus | 0.17 | ns | Frequency × exposure × drug condition | 0.76 | ns |
*P < 0.05
****P < 0.0001
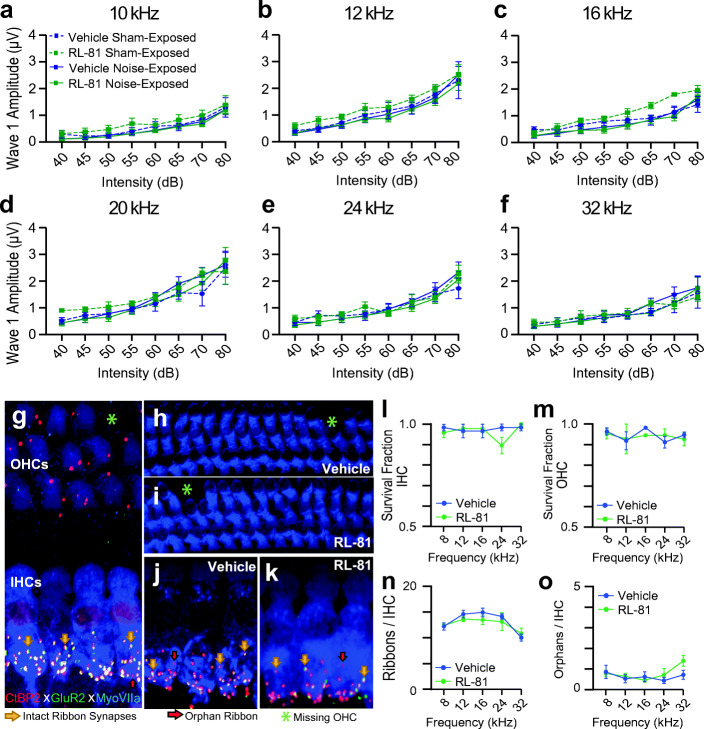
Fig. 4.
Transient RL-81 IP application 1 week after noise exposure did not alter either wave I amplitude across frequencies or cochlear structure, both assessed 3 weeks post noise exposure. a–f Wave I amplitudes in vehicle vs. RL-81 between sham- and noise-exposed mice for a 10 kHz, b 12 kHz, c 16 kHz, d 20 kHz, e 24 kHz, and f 32 kHz (no significant differences found; see Table 2). g Cochlear whole mount stained with antibodies for MyoVIIa (blue), CtBP2 (red), and GluR2 (green) shows inner hair cells (blue cell bodies with red nucleus), outer hair cells (blue cell bodies only), and ribbon synapses (red presynaptic staining adjacent to green postsynaptic staining). Intact synapses (yellow arrows) are counted when red presynaptic staining is closely opposed by green postsynaptic staining. Orphan ribbons (red arrows) consist of presynaptic puncta that are not opposed by postsynaptic puncta. h, i Representative images of OHCs from the 16 kHz region of vehicle- (h) and RL-81-treated i cochleae. (j), (k) Representative images of the 16 kHz area IHCs with ribbon synapses in vehicle- j and RL-81-treated (k) cochleae. l, m Quantification of IHC l and OHC m survival from vehicle- and RL-81-treated mice. n, o Quantification of intact ribbon n and orphan ribbon synapses o per IHC of vehicle- and RL-81-treated mice. Vehicle N = 5, RL-81 N = 4; 2-way ANOVA (no significant differences were found)
Table 2.
Statistical assessment of input (sound intensity)–output (wave I amplitude)
| 10–16 kHz | 10 kHz | 12 kHz | 16 kHz | |||
| Source of variation | P value | Sig | P value | Sig | P value | Sig |
| Intensity | < 0.0001 | **** | < 0.0001 | **** | < 0.0001 | **** |
| Exposure | 0.001 | *** | 0.02 | * | 0.002 | ** |
| Drug condition | 0.25 | ns | 0.42 | ns | 0.11 | ns |
| Intensity × exposure | 0.99 | ns | 0.99 | ns | 0.96 | ns |
| Intensity × drug condition | 0.99 | ns | 0.99 | ns | 0.93 | ns |
| Exposure × drug condition | 0.08 | ns | 0.10 | ns | 0.07 | ns |
| Intensity × exposure × drug condition | 0.99 | ns | 0.99 | ns | 0.90 | ns |
| 20–32 kHz | 20 kHz | 24 kHz | 32 kHz | |||
| Source of variation | P value | Sig | P value | Sig | P value | Sig |
| Intensity | < 0.0001 | **** | < 0.0001 | **** | < 0.0001 | **** |
| Exposure | 0.67 | ns | 0.54 | ns | 0.94 | ns |
| Drug condition | 0.46 | ns | 0.88 | ns | 0.77 | ns |
| Intensity × exposure | 0.94 | ns | 0.96 | ns | 0.96 | ns |
| Intensity × drug condition | 0.99 | ns | 0.99 | ns | 0.99 | ns |
| Exposure × drug condition | 0.09 | ns | 0.29 | ns | 0.33 | ns |
| Intensity × exposure × drug condition | 0.95 | ns | 0.98 | ns | 0.96 | ns |
Table 3.
Statistical assessment of cochlear histology
| IHC survival | OHC survival | Ribbons/IHC | Orphans/IHC | |||||
|---|---|---|---|---|---|---|---|---|
| P value | Sig | P value | Sig | P value | Sig | P value | Sig | |
| Interaction | 0.20 | ns | 0.77 | ns | 0.65 | ns | 0.38 | ns |
| Frequency | 0.36 | ns | 0.59 | ns | 0.003 | *** | 0.13 | ns |
| Drug condition | 0.38 | ns | 0.79 | ns | 0.37 | ns | 0.27 | ns |
Rigor and Transparency
All experiments in the manuscript were reanalyzed by a blinded independent referee (Dr. Kouvaros). For 5 out of 15 and 5 out of 14 mice per group (10 out of 29 total, tinnitus and hearing assessment, Figs. 2, 3, and 4a–f), the primary experimenter Laura Marinos was blinded both during the data acquisition and analysis. Regarding the 10 out of 15 and 9 out of 14 of the experiments (vehicle = 10, RL-81 = 9; 19 out of 29 total, tinnitus and hearing assessment, Figs. 2, 3, and 4a–f), although the experimenter (Laura Marinos) was not “blind” during acquisition, those experiments involved automated movement detection and acquisition/recording of mice movement between the two compartments of the chamber. Thus, the experimenter did not have any influence over the experiment. Yet, blinded and non-blinded sets of experiments performed by Laura Marinos were subsequently reanalyzed in a blind fashion by Laura Marinos and Dr. Kouvaros. Laura Marinos and Dr. Kouvaros reached similar conclusions after their independent analysis. All other experiments and analyses were carried out in a blind mode. Thus, between the data acquisition and analyses, all experiments and analyses are transparent, rigorous and reproducible.
Results
Previous studies used a reflex-based animal model of tinnitus to report that application of retigabine or SF0034, a potent KCNQ2/3-specific activator, prevented the development of tinnitus when applied 30 min and up to 4 days after the noise trauma (Li et al. 2013; Kalappa et al. 2015; Li et al. 2015). In these studies, behavioral evidence of tinnitus was evaluated based on the reduced ability of tinnitus mice to detect a silent sound gap in a continuous background sound. However, significant skepticism has been generated regarding the appropriateness of this reflex-based test in detecting tinnitus (Campolo et al. 2013; Hickox and Liberman 2014; Boyen et al. 2015). Thus, to assess the behavioral evidence of tinnitus more accurately, we modified and then employed an operant animal model of tinnitus (Yang et al. 2011; Zuo et al. 2017). Briefly, mice are conditioned to cross from one side of the shuttle box to the other when they hear an external tone and to not cross during silence (Fig. 1a–c). However, after noise exposure (unilateral left ear, 8–16 kHz octave broadband noise at 116 dB, for 1 h), mice that experience tinnitus are expected to cross more in silence, presumably because they hear the phantom sound. Thus, increased crossings during silence after noise exposure, in combination with intact crossing in the presence of external sound, could reflect behavioral evidence of tinnitus. To validate this animal model, we tested the effect of salicylate, which is known to reliably induce tinnitus. Salicylate, but not saline (vehicle), application led to increased crossings in silence (Fig. 1d right; 2-way ANOVA: after testing saline vs. salicylate: (F = 1,21) = 11.28, P = 0.003) without affecting crossings in sound (Fig. 1d left), thus validating that our protocol likely assesses tinnitus. In individual mice, salicylate led to a significant increase in silence cross rates. This increase was at least 2.5 standard deviations greater than the average baseline in 80 % of salicylate-treated mice, at least in one of the 3 days of testing (Fig. 1e, see Methods). Application of this increase in silent cross rate as a criterion (threshold) for behavioral evidence of tinnitus revealed no evidence of tinnitus in saline-treated mice (Fig. 1e right). We therefore used this criterion for assessing the behavioral evidence of noise-induced tinnitus in individual mice.
Next, we tested the effect of intraperitoneal (IP, 5 mg/kg) administration of RL-81 on noise-induced tinnitus. To determine the critical timing window within which RL-81 can potentially treat tinnitus, we administered RL-81 1 week after the noise exposure twice per day for 5 days every 12 h. Tinnitus was assessed 2 weeks after noise exposure. Intraperitoneal injections of RL-81 did not affect crossings in tones (Fig. 2b, c) but reduced the crossings in silence (Fig. 2d, e). The percentage of mice that showed tinnitus, as assessed by increased crossings in silence, was significantly reduced (Fig. 2e–g). This increase was not caused by inability of mice to hear the tone, because the crossing rates in tone were not affected by RL-81 (Fig. 2b). Moreover, the lack of changes in crossing rates in tone suggests that RL-81 does not cause any general behavioral or motor problems.
Together, these results suggest that RL-81 affects the processing of sound in silence, which is the major symptom of tinnitus. Remarkably, these results suggest that RL-81 has a therapeutic window of at least 7 days long and mitigates tinnitus, assessed 2 weeks after noise trauma. This is a key finding for further development of the compound, highlighting the crucial therapeutic potential of RL-81 for tinnitus.
We next tested the effect of RL-81 on hearing. To assess hearing thresholds, we used auditory brainstem responses (ABRs), which reflect the synchronous activity of auditory brainstem nuclei in response to sound arriving from the auditory nerve (wave I) to the inferior colliculus (wave V) (Fig. 3a). RL-81 did not affect ABR thresholds in either sham- or noise-exposed mice (Fig. 3b, c, Table 1). We used 3-way ANOVA analysis to determine how ABR threshold shifts are affected at different sound frequencies (10–32 kHz), exposure condition (sham vs. noise exposure) and drug condition (Vehicle vs. RL-81). We found that exposure and drug condition had an effect (Fig. 3b, Table 1: NE: N = 15 vehicle; 14 RL-81; SE: N = 5 vehicle; 6 RL-81; 3-way ANOVA; exposure: F (1,203) = 41.25, P < 0.0001; drug condition: F (1,203) = 5.375, P = 0.021). The exposure condition effect was robust and expected, but the drug effect was weak and unclear. To probe the drug effect, because frequency had no impact, we averaged the threshold shifts across frequencies within animals, and we used unpaired t test (single comparisons) to examine whether RL-81 affects ABR threshold shifts in either sham- or noise-exposed mice (Fig. 3c). We found that RL-81 did not affect ABR threshold shifts in either sham- or noise-exposed mice (Fig. 3c, Table 1: SE: N = 5 vehicle; 6 RL-81; unpaired t test: t (9) = 1.029, P = 0.33; NE: N = 15 vehicle; 14 RL-81; unpaired t test: t (27) = 1.448, P = 0.16). Next, we measured ABR thresholds in the unexposed ear. We used the same statistical analysis and found that noise exposure had no effect on hearing thresholds on the unexposed ear (Fig. 3d, Table 1: NE: N = 15 vehicle; 14 RL-81; SE: N = 7 vehicle; 5 RL-81; exposure: F (1,210) = 0.75, P = 0.39; drug condition: F (1,210) = 1.123, P = 0.29). Together, these results suggest that RL-81 does not affect hearing in either sham- or noise-exposed mice.
Finally, consistent with previous studies (Li et al. 2015; Shore and Wu 2019), ABR threshold shifts were not different between tinnitus and non-tinnitus mice (Fig. 3e, Table 1: N = 14 non-tinnitus, 15 tinnitus; unpaired t test: t (27) = 1.416, P = 0.17). Together, these results suggest that the effects of RL-81 in mitigating tinnitus are not due to potential protective actions on hearing.
To further explore any potential effects of RL-81 on hearing, we analyzed input (sound intensity)–output (wave I amplitude) functions between vehicle- and RL-81-treated mice. These functions revealed no differences (Fig. 4a–f, Table 2). Additionally, we performed immunohistochemical analysis of the noise-exposed cochleae from RL-81- and vehicle-treated mice to analyze hair cell and synapse density. We analyzed cochleae from the exposed ear immediately following the conclusion of the electrophysiological experiments. Cochlear sections were imaged in their entirety at low magnification, thus allowing us to evaluate the health of the cochlea along its tonotopic axis (Fig. 4g–k). OHC survival, IHC survival, and survival of synapses between IHCs and spiral ganglion neurons (ribbon synapses) were quantified at the frequency regions that were also assessed by ABR measurements. We did not observe any differences between RL-81- and vehicle-treated cochleae in either IHC/OHC numbers (Fig. 4l–m, Table 3) or synapse density (Fig. 4n–o, Table 3). These results are consistent with our electrophysiological data (Figs. 3 and 4) and support that RL-81 does not affect either cochlea structure or function.
Discussion
The most important finding of our study is the wide time-window after noise exposure within which RL-81 can reduce the percentage of mice that show tinnitus. In previous reports, retigabine was administered 30 min and up to 4 days after the acoustic trauma/hearing loss, and tinnitus was assessed by deficiencies in the gap detection reflex 7 days after noise exposure (Li et al. 2013, 2015). Patients, on the other hand, may not seek treatment immediately after the sudden onset of tinnitus. Therefore, it is crucial that the critical window during which KCNQ potentiation is effective against tinnitus is delineated in a validated animal model. Here, we validated and used an operant animal model of tinnitus, and showed that application of RL-81 even 7 days after noise exposure reduced the percentage of mice that showed tinnitus. Whereas 7 days is the minimum therapeutic window, it is optimistic for future clinical trials. However, more studies are needed to evaluate the full extent of this therapeutic window. Moreover, the duration of the therapeutic benefit following transient administration of RL-81, and whether RL-81 can mitigate tinnitus when assessed months after noise trauma remain to be determined. Finally, given that this potential treatment strategy might be feasible for military personnel exposed to extremely loud sounds, such as blast exposure, assessment of RL-81 in a blast-induced animal model of tinnitus could be beneficial. Evaluation of these parameters will be useful for selecting the most appropriate target population for subsequent clinical trials to assess RL-81 as a tinnitus treatment strategy.
We found that RL-81 does not affect hearing (Figs. 3a–d and 4) and that hearing is not different between tinnitus and non-tinnitus mice (Fig. 3e). These results suggest that the tinnitus behavioral data are not confounded by potential greater hearing loss. Moreover, the hearing thresholds of the unexposed ear are not affected after noise exposure (Fig. 3d), suggesting that mice can reliably hear tones during the tinnitus assessment protocol. Additionally, input (sound intensity)–output (wave I amplitude) functions revealed no differences between vehicle and RL-81 treated mice (Fig. 4a–f, Table 2). These electrophysiological results along with our immunohistochemical results (Fig. 4g–o) suggest that RL-81 does not affect hearing either after noise exposure or in baseline conditions. Together, these results suggest that peripheral auditory function was not worse in tinnitus-positive animals, and thus the behavioral data are not confounded by differential peripheral function. Finally, tinnitus and non-tinnitus mice performed equally well in tones (Fig. 2c), but tinnitus and non-tinnitus mice showed differences in silence (Fig. 2e). Together, all these results suggest that the behavioral assessment of tinnitus is not confounded by hearing loss, and that RL-81 acts centrally in mitigating tinnitus, without affecting the peripheral auditory system.
The proposed mechanism of action of RL-81 is consistent with the known mechanisms underlying effects of KCNQ2/3 channel activators on central auditory neurons. These mechanisms involve the classic effects of KCNQ channel openers on inhibition of subthreshold and suprathreshold neuronal excitability (Delmas and Brown 2005; Soldovieri et al. 2011). Because KCNQ channel activators are very efficient at inhibiting neuronal excitability, they have been used and considered potential treatments for mitigating the symptoms of hyperexcitability-related disorders such as epilepsy, tinnitus, neuropathic pain, amyotrophic lateral sclerosis, and traumatic brain injury (Jentsch et al. 2000; Delmas and Brown 2005; Korsgaard et al. 2005; Munro and Dalby-Brown 2007; Li et al. 2013; Wainger et al. 2014; Vigil et al. 2019). As such, and given the recent removal from the market of the FDA-approved KCNQ2-5 activator retigabine, development of novel KCNQ2/3-specific channel activators with drug-like properties is an unmet need and a highly active research topic in medicinal chemistry (Ostacolo et al. 2020; Liu et al. 2019). As demonstrated by previous studies, RL-81 is more selective and potent than retigabine (Kumar et al. 2016). Combined with the increased chemical stability as a function of the fluorinated backbone structure in RL-81, RL-81 is less likely to exhibit the clinical pathology characteristic of retigabine. Further toxicology assessments and a transition to early clinical evaluation are therefore in progress.
Acknowledgments
We thank Dr. Wai Lok Chang for help with initiating the behavioral paradigm of tinnitus, Patrick Cody for help with programming, and Dr. Thathiah for help with histology and imaging.
Author Contributions
L.M, P.W, and T.T designed experiments; L.M, S.K, B.B, and B.H conducted experiments and performed data analysis; L.M, S.K, P.W, and T.T wrote the manuscript.
Funding
This work was supported by US Department of Defense grant W81XWH-14-1-0117 (TT and PW) and NIH grant DC007905 (TT).
Compliance with Ethical Standards
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Marinos, Email: lam284@pitt.edu.
Stylianos Kouvaros, Email: stk@pitt.edu.
Brandon Bizup, Email: bbizup@pitt.edu.
Bryce Hambach, Email: bhh17@pitt.edu.
Peter Wipf, Email: pwipf@pitt.edu.
Thanos Tzounopoulos, Email: thanos@pitt.edu.
References
- Adams PF, Hendershot GE, Marano MA, Centers for Disease C, Prevention/National Center for Health S Current estimates from the National Health Interview Survey, 1996. Vital Health Stat. 1999;10:1–203. [PubMed] [Google Scholar]
- Boyen K, Baskent D, van Dijk P. The gap detection test: can it be used to diagnose tinnitus? Ear Hear. 2015;36:e138–e145. doi: 10.1097/AUD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolo J, Lobarinas E, Salvi R. Does tinnitus “fill in” the silent gaps? Noise Health. 2013;15:398–405. doi: 10.4103/1463-1741.121232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskioglou E, Perrenoud MP, Ryvlin P, Novy J. Novel treatment and new drugs in epilepsy treatment. Curr Pharm Des. 2017;23:6389–6398. doi: 10.1038/nature09656. [DOI] [PubMed] [Google Scholar]
- Groseclose MR, Castellino S. An investigation into retigabine (ezogabine) associated dyspigmentation in rat eyes by MALDI imaging mass spectrometry. Chem Res Toxicol. 2019;32:294–303. doi: 10.1021/acs.chemrestox.8b00313. [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss in the laboratory mouse. Audiology. 1980;19:369–383. doi: 10.3109/00206098009070071. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111:552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Chen J, Yang S, et al. Expression of tumor necrosis factor-α and interleukin-1β genes in the cochlea and inferior colliculus in salicylate-induced tinnitus. J Neuroinflammation. 2011;8:30. doi: 10.1186/1742-2094-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Chen JC, Yang SY, Wang MF, Liu TC, Chan YC. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope. 2011;121:361–364. doi: 10.1002/lary.21283. [DOI] [PubMed] [Google Scholar]
- Hwang J-H, Chen J-C, Chan Y-C. Effects of C-phycocyanin and Spirulina on salicylate-induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS ONE. 2013;8(3):e58215. doi: 10.1371/journal.pone.0058215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Schroeder BC, Kubisch C, Friedrich T, Stein V. Pathophysiology of KCNQ channels: neonatal epilepsy and progressive deafness. Epilepsia. 2000;41:1068–1069. doi: 10.1113/jphysiol.2008.150656. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Soh H, Duignan KM, Furuya T, Edwards S, Tzingounis AV, Tzounopoulos T. Potent KCNQ2/3-specific channel activator suppresses in vivo epileptic activity and prevents the development of tinnitus. J Neurosci. 2015;35:8829–8842. doi: 10.1523/JNEUROSCI.5176-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y, Seki Y, Okumura K, Ohshiba Y, Miyasaka Y, Suzuki S, Ozaki M, Matsuoka K, Noguchi Y, Yonekawa H. Advantages of a mouse model for human hearing impairment. Exp Anim. 2012;61:85–98. doi: 10.1538/expanim.61.85. [DOI] [PubMed] [Google Scholar]
- Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strobaek D, Mirza NR. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther. 2005;314:282–292. doi: 10.1124/jpet.105.083923. [DOI] [PubMed] [Google Scholar]
- Kumar M, Reed N, Liu R, Aizenman E, Wipf P, Tzounopoulos T. Synthesis and evaluation of potent KCNQ2/3-specific channel activators. Mol Pharmacol. 2016;89:667–677. doi: 10.1124/mol.115.103200. [DOI] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A. 2013;110:9980–9985. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kalappa BI, Tzounopoulos T. Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. Elife. 2015;4:e07242. doi: 10.1523/JNEUROSCI.5176-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Tzounopoulos T, Wipf P. Synthesis and optimization of Kv7 (KCNQ) potassium channel agonists: the role of fluorines in potency and selectivity. ACS Med Chem Lett. 2019;10:929–935. doi: 10.1021/acsmedchemlett.9b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, Shore SE. Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med. 2018;10(422):eaal3175. doi: 10.1126/scitranslmed.aal3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Dalby-Brown W. Kv7 (KCNQ) channel modulators and neuropathic pain. J Med Chem. 2007;50:2576–2582. doi: 10.1021/jm060989l. [DOI] [PubMed] [Google Scholar]
- Ostacolo C, Miceli F, Di Sarno V, Nappi P, Iraci N, Soldovieri MV, Ciaglia T, Ambrosino P, Vestuto V, Lauritano A, Musella S, Pepe G, Basilicata MG, Manfra M, Perinelli DR, Novellino E, Bertamino A, Gomez-Monterrey IM, Campiglia P, Taglialatela M. Synthesis and pharmacological characterization of conformationally restricted retigabine analogues as novel neuronal Kv7 channel activators. J Med Chem. 2020;63:163–185. doi: 10.1021/acs.jmedchem.9b00796. [DOI] [PubMed] [Google Scholar]
- Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur J Neurosci. 2006;23:3124–3138. doi: 10.1111/j.1460-9568.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Jacobson GP. Update on tinnitus. Otolaryngol Clin N Am. 1996;29:455–465. doi: 10.1016/S0030-6665(20)30367-4. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Shore SE, Wu C. Mechanisms of noise-induced tinnitus: insights from cellular studies. Neuron. 2019;103:8–20. doi: 10.1016/j.neuron.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus-triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150–160. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 2011;26:365–376. doi: 10.1038/nrneurol.2016.12. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Balaban C, Zitelli L, Palmer C. Towards a mechanistic-driven precision medicine approach for tinnitus. J Assoc Res Otolaryngol. 2019;20:115–131. doi: 10.1007/s10162-018-00709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Martin J, Rennaker RL, 2nd, Kilgard MP. Pairing sound with vagus nerve stimulation modulates cortical synchrony and phase coherence in tinnitus: an exploratory retrospective study. Sci Rep. 2017;7:17345. doi: 10.1038/s41598-017-17750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil FA, Bozdemir E, Bugay V, Chun SH, Hobbs M, Sanchez I, Hastings SD, Veraza RJ, Holstein DM, Sprague SM, Carver MC, Cavazos JE, Brenner R, Lechleiter JD, Shapiro MS. Prevention of brain damage after traumatic brain injury by pharmacological enhancement of KCNQ (Kv7, “M-type”) K(+) currents in neurons. J Cereb Blood Flow Metab. 2019;6:1256–1273. doi: 10.1177/0271678X19857818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SS, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, Berry JD, Brown RH, Jr, Cudkowicz ME, Bean BP, Eggan K, Woolf CJ. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A. 2011;108:14974–14979. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo H, Lei D, Sivaramakrishnan S, Howie B, Mulvany J, Bao J. An operant-based detection method for inferring tinnitus in mice. J Neurosci Methods. 2017;291:227–237. doi: 10.1016/j.jneumeth.2017.08.029. [DOI] [PubMed] [Google Scholar]