Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors often increase the hematocrit. It remains unclear whether this increase would be observed in all patients administered SGLT2 inhibitors, however. We therefore used the data from the previous study and investigated time-dependent alterations of various outcomes related to erythrocytes, erythropoiesis, and clinical outcome in type 2 diabetes subjects (n = 89) treated with ipragliflozin for 16 weeks. Among a total of 89 participants, 71 subjects (80.0% of total participants) showed the elevation of the hematocrit and 18 subjects (20.0% of total participants) did not at 16 weeks. Although the hematocrit levels at baseline were significantly lower in hematocrit-elevated group than non-elevated group, they reached the same levels 4 weeks after the onset of treatment. Binomial logistic regression analysis demonstrated that a lower baseline hematocrit level was related to the elevation of hematocrit at 16 weeks. Optimal cutoff hematocrit levels at baseline to predict hematocrit elevation were 46.9% (male) and 41.7% (female) in ROC analysis. Random intercept model analysis revealed the serum erythropoietin level increased in both hematocrit-elevated and non-elevated groups, whereas only the former group showed an increase in the percentage of reticulocytes during the first 4 weeks. These results suggest that the ipragliflozin-induced increase in hematocrit which is affected by the baseline hematocrit level is attributable to the responsiveness to, but not to the production of, erythropoietin. Collectively, Ht elevation observed in administration of SGLT2 inhibitors can result from erythropoietin-induced erythropoiesis, which is determined by the pre-treatment Ht level.
Trial registration: This trial has been registered with University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR no. 000015478).
Keywords: Ipragliflozin, Erythropoiesis, Erythropoietin, Reticulocyte, Random intercept model analysis
Introduction
Treatment with SGLT2 inhibitors is often associated with an increased hematocrit (Ht) [1–8]. In post hoc mediation analysis of the risk of cardiovascular death in a large-scale SGLT2 inhibitor trial (EMPA-REG Outcome trial) [1], the changes in Ht and hemoglobin (Hb) were the most prominently contributing factors to the favorable effects of the drug [9]. It has not been clarified how the Ht elevation would be caused after the administration of SGLT2 inhibitor, however.
Evidence suggests that serum concentration of erythropoietin, an important stimulator of erythropoiesis [3, 10, 11], as well as parameters of erythropoiesis including the number or percentage of reticulocytes [3] elevate during treatment with SGLT2 inhibitors. These observations suggest that SGLT2 inhibitors increase the Ht by stimulating erythropoiesis, and the elevation of serum erythropoietin levels by SGLT2 inhibitors has been demonstrated in several reports [3, 11, 12]. However, the time-dependent alterations of both serum erythropoietin and reticulocyte have been shown in only 1 small-scale study [3]. Furthermore, other studies found that the circulating level of erythropoietin was unaltered during the treatment with SGLT2 inhibitors [8, 13].
It thus remains to be elucidated whether erythropoietin-dependent erythropoiesis contributes to the SGLT2 inhibitor-induced elevation of the Ht with the increase in reticulocyte in a relatively large-sized study. Additionally, given that the Ht elevation was the most prominently contributing factor to the favorable effects of the drug [9], it was important to identify factors predicting the drug-induced elevation of the Ht before the initiation of SGLT2 inhibitors.
The aim of this study was to investigate the time-dependent changes in various parameters related to erythrocytes, including those of erythropoiesis and RBC-related indices and to characterize differences in individuals who showed and did not show the elevation of the Ht during the treatment with the SGLT2 inhibitor.
Materials and methods
Study subjects
This study was conducted using data of the previously published study (SORE-KOBE study) [14]. Study participants administered ipragliflozin at a dose of 50 mg/day after breakfast for 16 weeks, while continuing their previously prescribed medications. They were individuals with type 2 diabetes aged 20 to 75 years with a body mass index of ≥ 22 kg/m2 and a glycated hemoglobin (HbA1c) level of ≥ 6.5% and < 9.5%, and they were treated at nine medical facilities in Japan. Details of the inclusion and exclusion criteria for the study were described previously [14]. The study was conducted in accordance with the Declaration of Helsinki and its amendments and approved by the institutional review boards of the participating facilities. Written informed consent was obtained from all participants. The study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) as UMIN000015478, performed with funding by Astellas Pharma Inc. (Tokyo, Japan), and monitored by a third party (DOT WORLD, Tokyo, Japan) to ensure compliance with ethical guidelines for medical research involving human subjects and with the study protocol as well as that the data were collected accurately and completely.
Study groups
Based on the Ht increase ratio {[(Ht at 16 week)-(Ht at baseline)] × 100/Ht at baseline (%)}, the study subjects were classified into two groups of Ht-elevated group (Ht increase ratio > 0%) and Ht non-elevated group (Ht increase rate ≤ 0%).
Outcomes
The primary outcome of this study was to examine time-dependent alteration (0, 4, 8, 12, and 16 weeks) of Ht after ipragliflozin administration. To examine the factors related to the change of Ht, we collected parameters shown below.RBC count, blood level of Hb, and eGFR were measured at 0, 4, 8, 12, and 16 weeks. The serum levels of erythropoietin and NT-proBNP as well as Ret% were measured at 0, 2, 4, 8, and 16 weeks. The reticulocyte count was measured at 0, 4, 8, and 16 weeks. Blood samples were collected at baseline and at the predetermined time-points after overnight fasting. The RBC, Hb, and Ht were measured by Sheath Flow Direct Current Detection method. MCV, MCH, and MCHC were calculated as Ht (%)/RBC (106/μL) × 10, Hb (g/dL)/RBC (106/μL) × 10, and Hb (g/dL)/Ht (%) × 100, respectively. The eGFR was calculated with serum creatinine level (sCr), age, and sex (male eGFR = 194 × sCr−1.094 × age (years)−0.287, female eGFR = (194 × sCr−1.094 × age (years)−0.287) × 0.739). The plasma erythropoietin levels were analyzed by measuring using chemiluminescent enzyme immunoassay (CLEIA) with Access EPO kit and Access2 PRO (Beckman Coulter. Tokyo, Japan) in LSI Medience Corporation (Tokyo, Japan). Reticulocytes were measured by flow cytometric analysis. Clinical data including age, sex, height, body weight, body mass index, waist circumference, disease duration, antidiabetic medications, and antihypertensive medications were collected at baseline.
Data analysis
The baseline characteristics and clinical parameters were summarized by means ± SD for all patients and by groups. Student’s t or Mann–Whitney U test was performed in comparison with two groups. Categorical variables were analyzed with the use of χ2 test. We used binomial logistic regression analysis to assess the contributing factors for Ht elevation. In this analysis, baseline Ht level, sex, the maximum level of serum erythropoietin, as well as the change in eGFR and body weight during 16 weeks were applied as explanatory variables. Odds ratio and 95% confidence interval were shown. ROC analysis was performed to determine the optimal cutoff level of Ht at baseline to predict hematocrit elevation.
For the time-dependent changes in various parameters related to erythrocytes including those of erythropoiesis, Ht, RBC count, blood Hb level, the serum levels of erythropoietin, Ret%, reticulocyte count, MCV, MCH, MCHC, eGFR, and NT-proBNP were analyzed with repeated-measures one-way analysis of variance (ANOVA) and post hoc test based on t statistics adjusting Bonferroni correction.
For the comparison of time-dependent changes between Ht-elevated and non-elevated groups, the changes in the HbA1c, eGFR, and body weight (from 0 to 16 weeks), and the change in the serum levels of erythropoietin, Ret%, reticulocyte count, MCV, MCH, and MCHC (from 0 to 2 or 4 weeks) were analyzed with random intercept model, in which group variable, time variable, and these interaction terms were included as independent variables, and subjects were treated as random variable for dealing with repeated-measure data. P values for interaction term were calculated.
A P value of < 0.05 was considered statistically significant. All statistical analysis was performed with SPSS software version 22.0.
Results
Patient recruitment and baseline characteristics
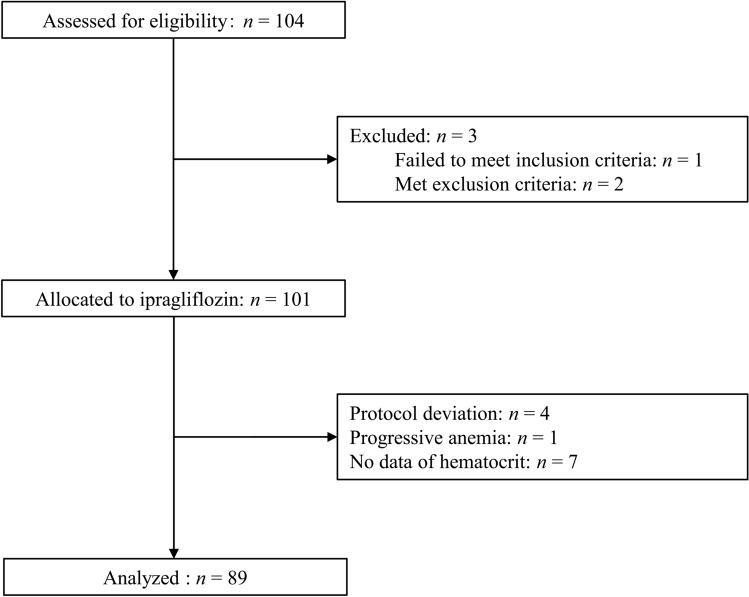
Among a total of 104 potential participants, one failed to satisfy the inclusion criteria, two met the exclusion criteria, four deviated from the study protocol, and one was excluded from the analysis because of the progression of anemia, as previously described [14]. Of the remaining 96 individuals, data on Ht were not collected in seven subjects. A total of 89 subjects who completed the study were therefore included in the current analysis (Fig. 1). The baseline characteristics of all individuals (n = 89) are shown in Table 1.
Fig. 1.
Flow diagram of participant recruitment
Table 1.
Clinical parameters and medication for all the study participants (n = 89), Ht-elevated group (n = 71), and Ht non-elevated group (n = 18) at baseline
| All (n = 89) | Ht-elevated group (n = 71) | Ht non-elevated group (n = 18) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 56.6 ± 11.1 | 56.3 ± 10.8 | 57.6 ± 12.4 | 0.656 | ||||||
| Sex (male (n)/female (n)) | 42/47 | 34/37 | 8/10 | 1.000 | ||||||
| Body weight (kg) | 75.2 ± 12.7 | 75.4 ± 11.7 | 74.4 ± 16.5 | 0.766 | ||||||
| Body mass index (kg/m2) | 28.6 ± 4.2 | 28.7 ± 3.9 | 28.2 ± 5.4 | 0.696 | ||||||
| Waist circumference (cm) | 97.6 ± 10.7 | 97.4 ± 10.2 | 98.2 ± 12.8 | 0.784 | ||||||
| Disease duration (years) | 11.9 ± 8.3 | 11.6 ± 8.4 | 13.2 ± 8.2 | 0.486 | ||||||
| HbA1c (%) | 7.9 ± 0.8 | 7.8 ± 0.7 | 8.1 ± 0.9 | 0.185 | ||||||
| Glycoalbumin (%) | 19.4 ± 3.8 | 19.1 ± 3.7 | 20.4 ± 4.3 | 0.222 | ||||||
| Fasting plasma glucose (mg/dL) | 157.2 ± 36.9 | 154.8 ± 33.5 | 166.2 ± 47.9 | 0.247 | ||||||
| Systolic blood pressure (mmHg) | 132.1 ± 14.4 | 133.2 ± 14.8 | 127.4 ± 11.9 | 0.149 | ||||||
| Diastolic blood pressure (mmHg) | 77.0 ± 11.4 | 77.8 ± 11.8 | 73.8 ± 9.0 | 0.201 | ||||||
| eGFR (mL/min /1.73 m2) | 82.7 ± 22.5 | 84.1 ± 23.1 | 77.2 ± 19.4 | 0.250 | ||||||
| NT-proBNP (pg/mL) | 95.3 ± 253.8 | 78.1 ± 239.5 | 160.4 ± 300.6 | 0.223 | ||||||
| Hematocrit (%) | 42.8 ± 4.2 | 42.2 ± 4.2 | 45.1 ± 3.4 | 0.009* | ||||||
| RBC count (104/µL) | 474 ± 48 | 468 ± 47 | 497 ± 50 | 0.024* | ||||||
| Hemoglobin (g/dL) | 14.3 ± 1.5 | 14.2 ± 1.5 | 14.9 ± 1.4 | 0.090 | ||||||
| Erythropoietin (mIU/mL) | 16.9 ± 13.1 | 17.0 ± 14.5 | 16.2 ± 5.6 | 0.813 | ||||||
| Ret% | 1.65 ± 0.66 | 1.69 ± 0.72 | 1.52 ± 0.35 | 0.356 | ||||||
| Reticulocyte count (104/µL) | 7.81 ± 3.07 | 7.86 ± 2.98 | 7.58 ± 1.85 | 0.739 | ||||||
| Antidiabetic medications (n, (%)) | ||||||||||
| Sulfonylurea | 30 (33.7) | 24 (33.8) | 6 (33.3) | 1.000 | ||||||
| Glinide | 1 (1.1) | 0 (0) | 1 (5.6) | 0.205 | ||||||
| Metformin | 73 (82.0) | 57 (80.3) | 16 (88.9) | 0.726 | ||||||
| Alpha-glucosidase inhibitor | 23 (25.8) | 20 (28.2) | 3 (16.7) | 0.380 | ||||||
| Thiazolidinedione | 3 (3.4) | 3 (4.2) | 0 (0) | 1.000 | ||||||
| DPP-4 inhibitor | 57 (64.0) | 49 (69.0) | 8 (44.4) | 0.055 | ||||||
| GLP-1 receptor agonist | 19 (21.3) | 13 (18.3) | 6 (33.3) | 0.204 | ||||||
| Bolus insulin | 5 (5.6) | 4 (5.6) | 1 (5.6) | 1.000 | ||||||
| Basal insulin | 22 (24.7) | 17 (23.9) | 5 (27.8) | 0.766 | ||||||
| Antihypertensive medications (n, (%)) | ||||||||||
| ARB or ACE inhibitor | 42 (47.2) | 33 (46.5) | 9 (50.0) | 1.000 | ||||||
| CCB | 28 (31.5) | 21 (29.6) | 7 (38.9) | 0.572 | ||||||
| Alpha and/or beta blocker | 14 (15.7) | 8 (11.3) | 6 (33.3) | 0.034 | ||||||
| Diuretics | 10 (11.2) | 7 (9.9) | 3 (16.7) | 0.421 | ||||||
Data are means ± SD with the exception of sex distribution and medication
HbA1c glycated hemoglobin, eGFR estimated glomerular filtration rate, RBC red blood cell, Ret% percentage of reticulocyte, DPP-4 dipeptidyl peptidase, GLP-1 glucagon like peptide-1, ARB angiotensin II receptor blocker, ACE angiotensin-converting-enzyme, CCB calcium channel blocker
*P < 0.05 for comparison between the two groups (unpaired Student’s t test, Mann–Whitney U test, or χ2 test)
The time-dependent change of RBC-related parameters, eGFR, and NT-proBNP
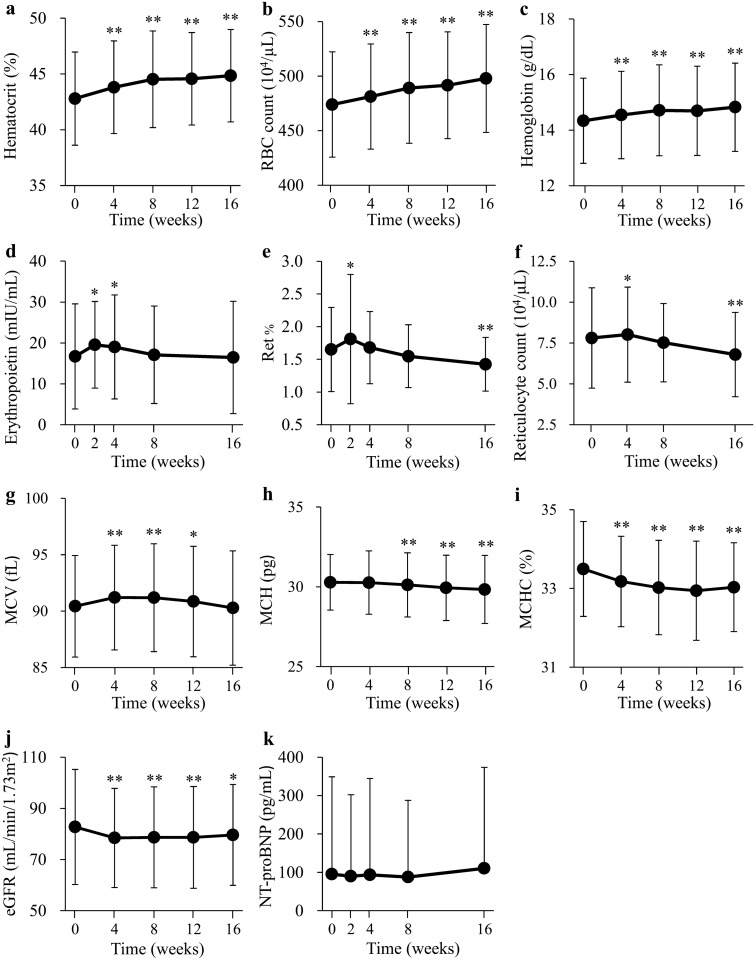
The time-dependent changes of parameters in all participants are shown in Fig. 2a–k. The Ht, RBC count, and blood Hb levels were significantly elevated at 4 weeks after the onset of ipragliflozin treatment and remained higher than baseline throughout the intervention (Fig. 2a–c). The serum concentration of erythropoietin was significantly elevated at 2 weeks, remained increased at 4 weeks, and had returned to the baseline level at 8 weeks (Fig. 2d). The Ret% and reticulocyte count were significantly increased at 2 and 4 weeks and had returned to baseline levels at 4 and 8 weeks, respectively, with both parameters having declined significantly below baseline values at 16 weeks (Fig. 2e,f). MCV was increased at 4 weeks, remained greater than the baseline level at 8 to 12 weeks, and returned to the baseline level at 16 weeks (Fig. 2g). MCH and MCHC were decreased at 8 weeks and 4 weeks, respectively, and remained lower than the baseline levels throughout the intervention period (Fig. 2h,i). The eGFR was significantly decreased at 4 weeks and remained at a level lower than baseline throughout the intervention (Fig. 2j). The serum concentration of NT-proBNP was unaltered throughout the study (Fig. 2k).
Fig. 2.
Changes in the hematocrit (a), RBC count (b), blood hemoglobin level (c), serum erythropoietin concentration (d), Ret% (e), reticulocyte count (f), MCV (g), MCH (h), MCHC (i), eGFR (j), and NT-proBNP (k) during treatment with ipragliflozin. Data are means ± SD (n = 89). *P < 0.01, **P < 0.001 versus baseline by repeated-measures one-way ANOVA and post hoc Bonferroni test
Baseline characteristics in Ht-elevated and non-elevated groups
To further investigate Ht elevation after ipragliflozin initiation, we divided participants into Ht-elevated group (n = 71) and Ht non-elevated group (n = 18) according to the changes of Ht during 16 weeks. The baseline RBC count and Ht of the Ht-elevated group were significantly lower than those of the Ht non-elevated group, but other parameters did not differ between the two groups (Table 1).
We assessed the contributing factors for predicting Ht elevation during 16 weeks treatment. Binomial logistic regression analysis showed that baseline Ht level was significantly related to Ht elevation during ipragliflozin treatment after adjustment of sex, the maximum level of serum erythropoietin level, the change of eGFR, and the change of body weight [odds ratio 0.716 (95% CI 0.581–0.882), p = 0.002] (Table 2).
Table 2.
Binomial logistic regression analysis for hematocrit elevation
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| Baseline hematocrit | 0.716 | (0.581–0.882) | 0.002* |
| Sex | 0.235 | (0.052–1.069) | 0.061 |
| Max of erythropoietin level during 16 weeks | 0.975 | (0.930–1.022) | 0.295 |
| Change in eGFR during 16 weeks | 0.997 | (0.940–1.058) | 0.925 |
| Change in body weight during 16 weeks | 1.089 | (0.896–1.323) | 0.392 |
eGFR estimated glomerular filtration rate, CI confidence interval
*P < 0.05
Optimal cutoff level of baseline Ht to distinguish Ht-elevated or non-elevated group
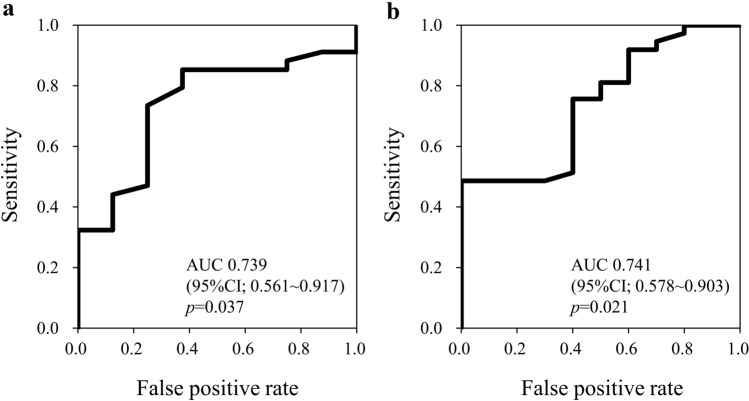
We next tried to determine the optimal cutoff level of baseline Ht to predict the elevation of Ht after ipragliflozin treatment for 16 weeks with the use of ROC analysis (Fig. 3). For males (n = 42), the sensitivity and specificity were revealed to be 73.5% and 75.0%, respectively, when the cutoff value of Ht was set to 46.9%. For females (n = 47), the sensitivity and specificity were revealed to be 75.7% and 60.0%, respectively, when the cutoff value of Ht was set to 41.7%.
Fig. 3.
ROC curves and area under the curve (AUC) for baseline Ht level to distinguish Ht-elevated or non-elevated group; (a) male cases (n = 42). When the cutoff value of Ht was set to 46.9%, the sensitivity and specificity were 73.5% and 75.0%, respectively; (b) female cases (n = 47). When the cutoff value of Ht was set to 41.7%, the sensitivity and specificity were 75.7% and 60.0%, respectively
Comparison of time-dependent changes of parameters between Ht-elevated and non-elevated groups
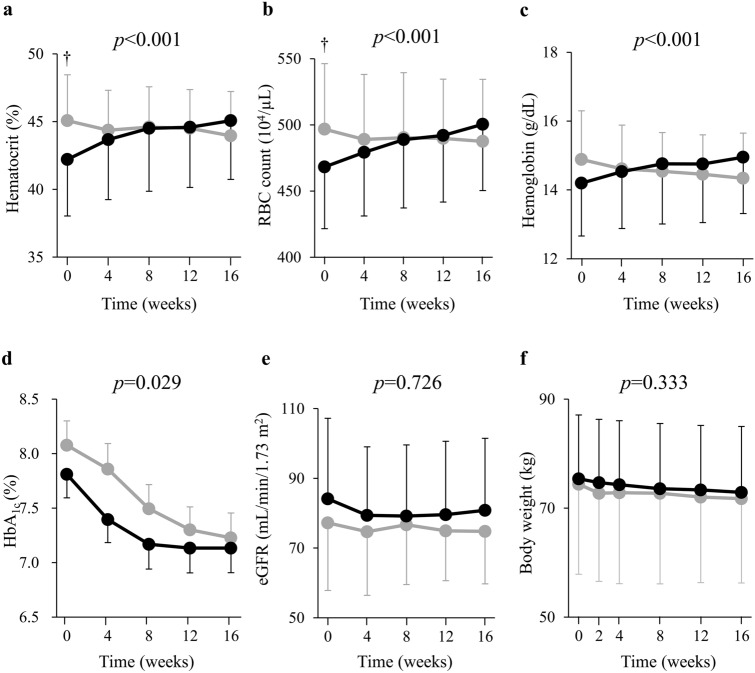
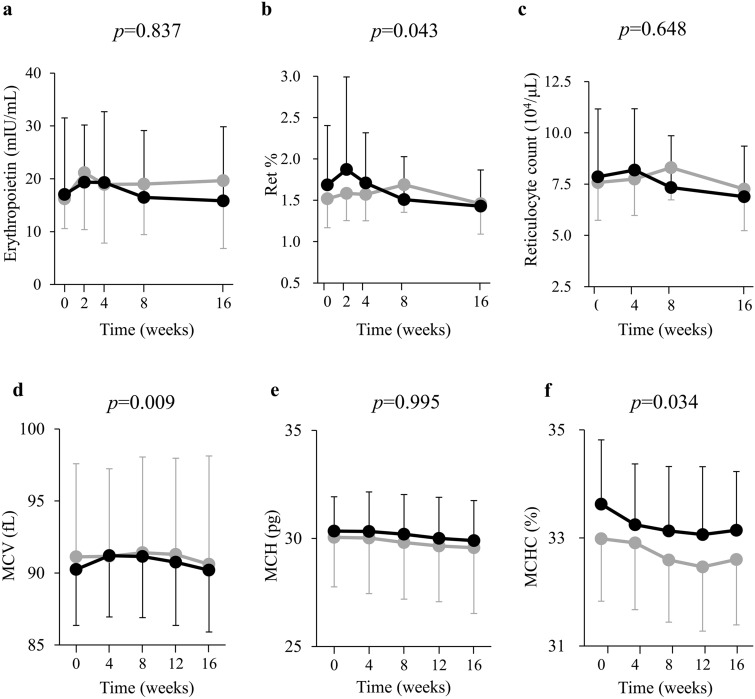
The time-dependent changes in the Ht, RBC count, and Hb level for the two groups are shown (Fig. 4a–c). In the random intercept model analysis, the changes in Ht, RBC, and hemoglobin were significantly increased in Ht-elevated group compared with Ht non-elevated group (all of p < 0.001) during the intervention period. This analysis showed that HbA1c significantly decreased in Ht non-elevated group compared with Ht-elevated group (p = 0.029) (Fig. 4d), whereas eGFR and body weight did not differ between the two groups (eGFR p = 0.726, body weight p = 0.333, respectively) (Fig. 4e,f). Serum erythropoietin concentration was elevated at 2 and 4 weeks in Ht-elevated and non-elevated groups, respectively (Fig. 5a). Indeed, in the random intercept model analysis, serum erythropoietin did not show a significant group–time interaction (p = 0.837), and both groups had similar elevations. Ret% was elevated only in Ht-elevated group at 4 weeks. The change in Ret% was increased in Ht-elevated group compared with Ht non-elevated group (p = 0.043) during the intervention period (Fig. 5b). Reticulocyte count did not change significantly in either group (p = 0.648) (Fig. 5c). While MCH did not change in either group (p = 0.995) (Fig. 5e), MCV and MCHC were increased and decreased, respectively, in Ht-elevated group compared with Ht non-elevated group (MCV p = 0.009, MCHC p = 0.034) (Fig. 5d,f).
Fig. 4.
Changes in the hematocrit (a), RBC count (b), blood hemoglobin level (c), HbA1c (d), eGFR (e), and body weight (f) during treatment with ipragliflozin for study participants in the Ht-elevated (n = 71, black circles) and non-elevated (n = 18, gray circles) groups. Data are means ± SD.†P < 0.05 versus the corresponding value for the Ht-elevated group (unpaired Student’s t test). P values are indicated for the group × time interaction terms of the random intercept model
Fig. 5.
Changes in serum erythropoietin concentration (a), Ret% (b), and reticulocyte count (c), MCV (d), MCH (e), and MCHC (f) during treatment with ipragliflozin in both Ht-elevated (n = 71, black columns) and non-elevated (n = 18, gray columns) groups. Data are means ± SD. P values are indicated for the group × time interaction terms of the random intercept model
Discussion
Novel finding in our study is that ~ 80.0% of the study subjects showed and ~ 20.0% did not show an increase in the hematocrit after the administration of ipragliflozin. We have found that serum erythropoietin concentration was increased both in Ht-elevated and non-elevated groups, whereas erythropoiesis appeared to be stimulated only in the former. These findings suggest that the difference in erythropoiesis between the two groups is attributable to the responsiveness to, but not to the production of, erythropoietin. It remains to be elucidated why the responsiveness to erythropoietin differs.
We have shown here that MCH and MCHC were decreased in response to ipragliflozin treatment. These findings are consistent with a recent study showing that these two indices were decreased after 6 months treatment with empagliflozin [11]. In that study, serum concentration of ferritin, which reflects iron storage in body, was also decreased [11], suggesting that MCH and MCHC were decreased as a result of relative iron deficiency induced by augmented erythropoiesis. In contrast, we have shown that MCV was transiently increased during the treatment with the SGLT2 inhibitor. Given that the volume of reticulocytes is greater than that of erythrocytes [15], the transient increase in MCV is likely attributable to the generation of reticulocytes. These results all suggest the enhancement of erythropoiesis after administration of SGLT2 inhibitors.
It was controversial whether SGLT2 inhibitors-induced increase in Ht would be caused by hydration or by erythropoiesis [3, 16]. Evidence suggests that the decrease in body weight at an early phase of the treatment with SGLT2 inhibitors is mainly attributable to the reduction of water (hydration) in the body [5, 16]. We have found that the extents of the decrease in the eGFR and body weight were similar between Ht-elevated and Ht non-elevated groups. In contrast, Ret%, MCV, and MCHC were altered only in Ht-elevated group, suggesting that the difference in the change in the hematocrit between the two groups is attributable not to hydration status but to erythropoiesis. Furthermore, positive linear relationship in area under the curve between erythropoietin concentration and the Ret% level during the first 4 weeks was observed in Ht-elevated group (r = 0.330, p = 0.005), but not in Ht non-elevated group (r = 0.053, p = 0.836). This suggests the Ht elevation is attributable to erythropoietin-induced erythropoiesis in Ht-elevated group.
We also found that baseline Ht level was the predictive factor for determining Ht elevation or not. In addition, ROC analysis revealed the optimal cutoff level of baseline Ht which predicts the elevation of Ht after ipragliflozin treatment. Given that the increase in Ht level was the most prominently contributing factor to the favorable effects of the drug in decreasing cardiovascular mortality [9], our results may present a useful marker that determines the preferable subjects for the administration of SGLT2 inhibitors.
The mechanism how the serum concentration of erythropoietin is increased during the treatment of SGLT2 inhibitors is unclear. Erythropoietin is produced by fibroblast-like cells located in the interstitium near proximal straight tubule of the kidney [17]. An SGLT1, another sodium-glucose cotransporter localized in the proximal straight tubule, is overloaded because of the compensation in urine glucose reabsorption after inhibition of SGLT2 [18]. Given that hypoxia is an important stimulus to erythropoietin production [19], it is possible that the enhanced glucose reabsorption mediated by the SGLT1 may increase oxygen consumption and induce the subsequent hypoxia, which stimulates erythropoietin-producing cells localized near proximal straight tubules.
In our study, HbA1c decreased to the larger extent in Ht non-elevated group than in Ht-elevated group. SGLT2 inhibitor not only induces glycosuria and lowers blood glucose level, but also alters serum insulin and glucagon levels [20]. These changes induce the shift in the metabolic fuel source from glucose toward fatty acid [21, 22]. Such changes in metabolic fuel source might affect erythropoiesis, although further investigations will be necessary.
Strengths of the present study include the comprehensive analyses of time-dependent changes in parameters related erythrocytes as well as of the analysis of the difference between subjects who showed or did not show the elevation of the hematocrit. Its open-label, one-arm design is a limitation of the study.
In conclusion, our results suggest that the increase in the serum erythropoietin concentration, which occurs shortly after the treatment onset likely contribute, at least in part, to the elevation of the hematocrit during ipragliflozin treatment and that the difference between subjects who showed or did not show the increase in hematocrit is attributable to the responsibility to, but not to the production of, erythropoietin. Further studies are required to understand the pharmacological properties of this relatively new class of drugs on erythrocyte parameters and erythropoiesis.
Acknowledgements
We thank all the patients, SOAR-KOBE study investigators, and staff at participating sites for their contributions to this study. We also thank Prof. Takashi Omori for the helpful suggestion in statistics. The company was not involved in study design, patient selection, data collection/analysis, interpretation of results, or preparation of the manuscript.
Appendix
Principal investigators at the participating centers are as follows: Kazuhiko Sakaguchi (Kobe University Hospital, Obara Hospital), Takeshi Ohara (Hyogo Brain and Heart Center), Yasuo Kuroki (Kobe Century Memorial Hospital), Kenta Hara (Kita-harima Medical Center), Tomokazu Matsuda (Kaisei Hospital), Minoru Kishi (Nishiwaki Municipal Hospital), Akihiko Takeda (Shinko Hospital), and Kazuki Yokota (Yokota Medical Clinic).
Funding
This investigator-initiated trial was funded by Astellas Pharma Inc. (Tokyo, Japan).
Compliance with ethical standards
Conflict of interest
KS, YO, YH, YT, and WO have received unlimited grant support as well as lecture fees from MSD and Astellas Pharma Inc. TO, KH, MK, TM, and AT have received lecture fees from MSD and Astellas Pharma Inc. YK has received lecture fees from MSD. The remaining authors (TY, HM, NO-S, AS, HK, and KY) have nothing to disclose.
Human rights statement
The study was conducted in accordance with the Declaration of Helsinki and its amendments, and it was approved by the institutional review boards of the participating facilities. Written informed consent was obtained from all participants. Date of approval: Apr 13, 2015. Approval number: 1713.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi T, Dohi K, Omori T, Moriwaki K, Sato Y, Nakamori S, Fujimoto N, Fujii E, Yamada N, Ito M. Diuretic effects of sodium-glucose cotransporter 2 inhibitor in patients with type 2 diabetes mellitus and heart failure. Int J Cardiol. 2015;201:1–3. doi: 10.1016/j.ijcard.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 3.LambersHeerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kambara T, Shibata R, Osanai H, Nakashima Y, Asano H, Sakai K, Murohara T, Ajioka M. Use of sodium-glucose cotransporter 2 inhibitors in older patients with type 2 diabetes mellitus. Geriatr Gerontol Int. 2018;18:108–114. doi: 10.1111/ggi.13149. [DOI] [PubMed] [Google Scholar]
- 5.Hirose S, Nakajima S, Iwahashi Y, Seo A, Takahashi T, Tamori Y. Impact of the 8-week administration of tofogliflozin for glycemic control and body composition in Japanese patients with type 2 diabetes mellitus. Intern Med. 2016;55:3239–3245. doi: 10.2169/internalmedicine.55.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–391. doi: 10.1111/jdi.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawata T, Iizuka T, Iemitsu K, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, Kanamori A, Takeda H, Ito S, Kikuchi T, Amemiya H, Kaneshiro M, Mokubo A, Takuma T, Machimura H, Tanaka K, Asakura T, Kubota A, Aoyanagi S, Hoshino K, Ishikawa M, Matsuzawa Y, Obana M, Sasai N, Kaneshige H, Minagawa F, Saito T, Shinoda K, Miyakawa M, Tanaka Y, Terauchi Y, Matsuba I. Ipragliflozin improves glycemic control and decreases body fat in patients with type 2 diabetes mellitus. J Clin Med Res. 2017;9:586–595. doi: 10.14740/jocmr3038w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynmic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 9.Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? insights from a mediation analysis of the EMPA-REG Outcome trial. Diabetes Care. 2018;41:356–363. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Barsotti E, Clerico A, Muscelli E. Renal handling of ketones in response to sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40:771–776. doi: 10.2337/dc16-2724. [DOI] [PubMed] [Google Scholar]
- 11.Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Jüni P, Zuo F, Mistry N, Thorpe KE, Goldenberg RM, Yan AT, Connelly KA, Verma S. Effect of empagliflozin on erythropoietin levels, iron stores and red blood cell morphology in patients with type 2 diabetes and coronary artery disease. Circulation. 2020;141:704–707. doi: 10.1161/CIRCULATIONAHA.119.044235. [DOI] [PubMed] [Google Scholar]
- 12.Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, Chaudhuri A, Dandona P. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020;105:057. doi: 10.1210/clinem/dgz288. [DOI] [PubMed] [Google Scholar]
- 13.Osonoi T, Gouda M, Kubo M, Arakawa K, Hashimoto T, Abe M. Effect of canagliflozin on urinary albumin excretion in Japanese patients with type 2 diabetes mellitus and microalbuminuria: a pilot study. Diabetes Technol Ther. 2018;20:681–688. doi: 10.1089/dia.2018.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura H, Sakaguchi K, Okada Y, Yamada T, Otowa-Suematsu N, So A, Komada H, Hirota Y, Ohara T, Kuroki Y, Hara K, Matsuda T, Kishi M, Takeda A, Yokota K, Tamori Y, Ogawa W. Effects of ipragliflozin on glycemic control, appetite and its related hormones: a prospective, multicenter, open-label study (SOAR-KOBE Study) J Diabetes Investig. 2019;10:1254–1261. doi: 10.1111/jdi.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ovchynnikova E, Aglialoro F, von Lindern M, van den Akker E. The shape shifting story of reticulocyte maturation. Front Physiol. 2018;9:829. doi: 10.3389/fphys.2018.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Mörschel L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087–1095. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- 17.Souma T, Suzuki N, Yamamoto M. Renal erythropoietin-producing cells in health and disease. Front Physiol. 2015;6:167. doi: 10.3389/fphys.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell DR, DaCosta CM, Gay J, Ding ZM, Smith M, Greer J, Doree D, Jeter-Jones S, Mseeh F, Rodriguez LA, Harris A, Buhring L, Platt KA, Vogel P, Brommage R, Shadoan MK, Sands AT, Zambrowicz B. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab. 2013;304:E117–E130. doi: 10.1152/ajpendo.00439.2012. [DOI] [PubMed] [Google Scholar]
- 19.Shih HM, Wu CJ, Lin SL. Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc. 2018;117:955–963. doi: 10.1016/j.jfma.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2013;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]