Abstract
Background
Acute coronary syndrome (ACS) remains one of the leading causes of mortality for women, increasing with age. There is an unmet need regarding this condition in a fast‐growing and predominantly female population, such as nonagenarians.
Hypothesis
Our aim is to compare sex‐based differences in ACS management and long‐term clinical outcomes between women and men in a cohort of nonagenarians.
Methods
We included consecutive nonagenarian patients with ACS admitted at four academic centers between 2005 and 2018. The study was approved by the Ethics Committee of each center.
Results
A total of 680 nonagenarians were included (59% females). Of them, 373 (55%) patients presented with non‐ST‐segment elevation ACS and 307 (45%) with ST‐segment elevation myocardial infarction (STEMI). Men presented a higher disease burden compared to women. Conversely, women were frailer with higher disability and severe cognitive impairment. In the STEMI group, women were less likely than men to undergo percutaneous coronary intervention (PCI) (60% vs. 45%; p = .01). Overall mortality rates were similar in both groups but PCI survival benefit at 1‐year was greater in women compared to their male counterparts (82% vs. 68%; p = .008), persisting after sensitivity analyses using propensity‐score matching (80% vs. 64%; p = .03).
Conclusion
Sex‐gender disparities have been observed in nonagenarians. Despite receiving less often invasive approaches, women showed better clinical outcomes. Our finding may help increase awareness and reduce the current gender gap in ACS management at any age.
Keywords: acute coronary syndrome, elderly, myocardial infarction, ST‐segment elevation myocardial infarction, women
1. INTRODUCTION
Over the coming years we shall witness a progressive increase in the aging of the population that will lead to several social and medical challenges. In the field of cardiovascular diseases, aging causes a significant clinical heterogeneity in which sex differences play a key role. Acute coronary syndrome (ACS) remains one of the leading causes of morbidity and mortality for women, and increases with age. Nevertheless, older women continue to be underrepresented in clinical trials and their management and outcomes are underexplored. 1 This fact is even more relevant in patients with extreme age, such as nonagenarians, who are usually excluded from the main clinical trials because a prolonged follow‐up may be compromised by limited life expectancy. However, the nonagenarian population will become clinically and numerically relevant in our daily routine practice in the near future and data about their prognosis in ACS context is scarce, especially in women. It is expected that female life expectancy will break the 90‐year barrier by 2030, a level that was deemed unattainable by some at the beginning of the 21st century. 2 Therefore, there is an unmet need regarding ACS in this fast‐growing and predominantly female population. The aim of this study was to compare sex‐based differences in ACS management and long‐term clinical outcomes between women and men in a cohort of nonagenarians.
2. METHODS
This multicentre observational study included all consecutive patients aged ≥90 years who were hospitalized for ACS between 2005 and 2018 across four academic institutions. Only patients with type 1 myocardial infarction were included. The choice of treatment was based on the criteria of the attending medical staff. The flowchart of the study is presented in Figure 1. The follow‐up protocol included a review of medical records at 30 days and 1 year after hospital discharge. The four academic institutions are characterized by being tertiary and high complexity hospitals. Each serves a total catchment area of over half a million population and provides 24/7 ST‐segment elevation myocardial infarction (STEMI) percutaneous coronary intervention (PCI). The study was approved by the Ethics Committee and adhered to the principles outlined in the Declaration of Helsinki. All data were obtained by a retrospective review of cases using standardized report forms (Appendix S1). Standardized definitions of all patient‐related variables, clinical diagnoses, and hospital complications and outcomes were used. All patients admitted with an ACS were classified in STEMI or non‐ST‐segment elevation ACS (NSTE‐ACS), where unstable angina was included, according to current clinical practice guidelines 3 , 4 and fourth definition of myocardial infarction. 5 The following outcomes were evaluated: mortality, recurrent myocardial infarction, stroke, and major bleeding. Cardiovascular mortality was defined as any death due to proximate cardiac cause (MI, low‐output failure, fatal arrhythmia), unwitnessed death, stroke, cardiovascular hemorrhage, death of unknown cause, and all procedure‐related deaths, including those related to concomitant treatment. 6 Recurrent myocardial infarction was defined as any myocardial infarction according to the World Health Organization's extended definition. 7 Stroke was defined as focal neurologic deficit lasting ≥24 hours or focal neurologic deficit lasting <24 hours with imaging findings of acute infarction or hemorrhage. 8 Major bleeding was defined as Bleeding Academic Research Consortium score ≥ 3. 9 The primary endpoint was sex‐based differences in ACS management. The second endpoints were 1‐year all‐cause mortality by sex. Long‐term survival was compared between patients undergoing PCI and those managed with medical treatment alone by sex.
FIGURE 1.
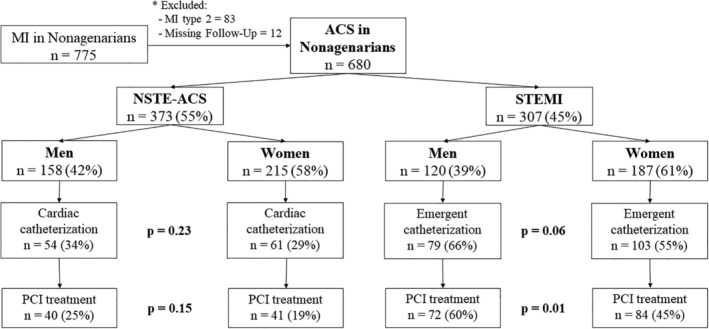
Title: Study Flowchart. Legend: From 2005 to 2018, 680 nonagenarian patients with acute coronary syndrome (ACS) were included. MI, myocardial infarction; STEMI, ST‐elevation myocardial infarction; NSTE‐ACS, non‐ST‐elevation acute coronary syndrome; PCI, percutaneous coronary intervention
Categorical variables are presented as frequencies (percentages), assessing the differences by χ 2 test (or Fisher test when necessary). Continuous variables are presented as a mean ± standard deviation or as a median (interquartile range). The Kolmogorov–Smirnov test was applied to ensure normal distribution. Continuous variables were compared using the analysis of variance test or the Kruskal–Wallis test, as appropriate. Survival curves were constructed for the time‐to‐event variables using the Kaplan–Meier method. Because differences in baseline characteristics could substantially interfere with the outcomes, propensity‐score matching was performed. Propensity score matching yielded 185 patients in the men group and 185 control subjects in the women group as Table S1. The balance between the two groups after propensity‐score matching was assessed by calculating percent standardized mean differences. Percent standardized mean differences after propensity‐score matching adjustment were within 15% across all matched covariates, demonstrating the achievement of successful balance between comparative groups. To identify independent predictors of PCIs by ACS and 1‐year all‐cause death in each group, we used a multivariable logistic and Cox proportional hazard regression, respectively. For all analyses, a two tailed p value <.05 was used as the criterion for statistical significance. Analyses were performed using STATA software (V 14.0, StataCorp LP, College Station, TX).
3. RESULTS
A total of 680 nonagenarian patients with an admission diagnosis of ACS were enrolled between January 2005 and December 2018. The diagnosis of STEMI was stablished in 307 (45%) whereas the number of patients with NSTE‐ACS was 373 (55%). Women were predominant in both groups (58% and 61%, respectively) as Figure 1 shows. The main baseline characteristics and clinical presentation of the two groups are presented in Table 1. Men presented a higher disease burden, including higher prevalence of peripheral artery disease (9% vs. 4%; p = .002), chronic obstructive pulmonary disease (19% vs. 4%; p < .001), active oncology disorder (11% vs. 4%; p = .006) and prior myocardial infarction (26% vs. 18%; p = .01) compared to women. Conversely, women were frailer with higher disability (5% vs. 11%; p < .001) and severe cognitive impairment (5% vs. 10%; p = .01).
TABLE 1.
Baseline clinical characteristics
| Variable | All patients (N = 680) | Men (n = 278) | Women (n = 402) | p value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 92.6 ± 2.4 | 92.4 ± 2.2 | 92.6 ± 2.5 | .35 |
| Medical history | ||||
| Hypertension | 541 (80) | 208 (75) | 333 (82) | .01 |
| Hyperlipidemia | 261 (38) | 107 (39) | 154 (38) | .96 |
| Diabetes mellitus | 203 (30) | 82 (30) | 121 (30) | .87 |
| Chronic kidney disease | 144 (21) | 59 (21) | 85 (21) | .98 |
| Prior stroke | ||||
| Ischemic | 93 (13) | 38 (14) | 55 (14) | .66 |
| Hemorrhagic | 3 (1) | 2 (1) | 1 (1) | |
| Peripheral artery disease | 41 (6) | 26 (9) | 15 (4) | .002 |
| Chronic obstructive pulmonary disease | 71 (10) | 54 (19) | 17 (4) | <.001 |
| Oncology disorders | ||||
| Previous | 48 (7) | 18 (7) | 30 (8) | .006 |
| Active | 46 (7) | 29 (10) | 17 (4) | |
| Previous myocardial infarction | 145 (21) | 72 (26) | 73 (18) | .01 |
| Previous percutaneous coronary intervention | 64 (9) | 35 (13) | 29 (7) | .01 |
| Previous coronary artery bypass grafting | 27 (4) | 18 (7) | 9 (2) | .005 |
| Previous heart failure | 111 (16) | 47 (17) | 64 (16) | .73 |
| Frailty Characteristics | ||||
| Disability (activities of daily living) | ||||
| None | 421 (62) | 198 (74) | 223 (57) | <.001 |
| Semi‐independent | 183 (28) | 56 (21) | 127 (32) | |
| Dependent | 55 (8) | 13 (5) | 42 (11) | |
| Moderate or severe cognitive impairment | 55 (8) | 14 (5) | 41 (10) | .01 |
| Initial presentation | ||||
| Atypical symptoms | 125 (18) | 42 (15) | 83 (21) | .07 |
| Killip class at admission | ||||
| I | 403 (59) | 178 (64) | 225 (56) | .09 |
| II | 184 (27) | 71 (26) | 113 (28) | |
| III | 70 (11) | 20 (7) | 50 (12) | |
| IV | 23 (3) | 9 (3) | 14 (3) | |
| GRACE score | 173.4 ± 24 | 170.8 ± 22 | 175 ± 25 | .03 |
| CRUSADE score | 46.5 ± 12 | 42.4 ± 10 | 49.8 ± 12 | <.001 |
| Serum creatinine at admission (mg/dl) | 1.37 ± 0.67 | 1.50 ± 0.8 | 1.28 ± 0.6 | <.001 |
| Hemoglobin at admission (mg/dl) | 12.2 ± 1.8 | 12.3 ± 1.9 | 12.1 ± 1.7 | .08 |
| Management approach | ||||
| Coronary angiogram | 297 (44) | 133 (48) | 164 (41) | .07 |
| PCI approach | 237 (35) | 112 (41) | 125 (31) | .01 |
Note: Values are n (%) or mean ± SD.
Abbreviations: NSTE ACS, non‐ST‐elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
“There was a non‐significant tendency to perform less emergent catheterization in women compared to men (48% vs. 41%; p = .07). These differences are mainly driven by the STEMI group (66% vs. 55%; p = .06). Regarding this, women were less likely than men to undergo a PCI (40% vs. 31%; p = .01), mainly in the STEMI group with a rate of subsequent PCI lower in women (60% vs. 45%; p = .01). These differences were not found in the NSTE‐ACS group as Figure 1 shows. Angiographic findings and interventional procedures are detailed in Table S2. No differences were found in catheterization access between groups, being the radial access predominant in both. There were no significant differences between the groups in terms of the TIMI flow grade III after the procedure (93% vs. 84%, p = .05). No differences were found in the medical treatment at discharge (Table S3).
A comparison of clinical outcomes between the women and men groups is presented in Table 2 and Figure 2. At 1‐year follow‐up, a total of 263 patients had died (39%). Overall mortality rates in men and women were similar both in‐hospital and at 1‐year follow‐up (16% vs. 18%; p = .4 and 41% vs. 37%; p = .3, respectively). However, patients treated with PCI showed better survival rates than those managed with medical therapy alone. Furthermore, PCI survival benefit at 1‐year was greater in women compared to their male counterparts (82% vs. 68%; p = .008; Figure 2(A)), persisting after sensitivity analyses using propensity‐score matching (80% vs. 64%; p = .03; Figure 2(B)). Female sex remains in the multivariate logistic regression analysis (Table 3) as an independent predictor for not undergoing a PCI in STEMI (OR: 0.62; 95% CI: 0.38–0.97; p = .03) along with age (OR: 0.88; 95% CI: 0.79–0.98; p = .02). The absence of disability was related to undergoing PCI in STEMI (OR: 1.82; 95% CI: 1.03–3.22; p = .04) as well as in NSTE‐ACS (OR: 3.34; 95% CI: 1.83–6.08; p < .001). On the other hand, in multivariate Cox proportional hazard models (Table S4), PCI was independently associated with a lower risk of 1‐year all‐cause death in both groups (HR men: 0.63; 95% CI: 0.41–0.96; p = .03; HR women: 0.37; 95% CI: 0.23–0.60; p < .001).
TABLE 2.
Outcomes
| Variable | All patients (N = 680) | Men (n = 278) | Women (n = 402) | p value |
|---|---|---|---|---|
| In‐hospital | ||||
| Death | 115 (17) | 43 (16) | 72 (18) | .40 |
| Major or clinically relevant bleeding | 10 (2) | 8 (3) | 2 (1) | .01 |
| Acute kidney injury stage 3 | 45 (7) | 18 (7) | 27 (7) | .90 |
| Mechanical complications a | 22 (3) | 9 (3) | 13 (3) | .99 |
| Left ventricular ejection fraction ≤30% | 84 (12) | 35 (13) | 49 (12) | .88 |
| 1‐year follow‐up | ||||
| All‐cause death | 263 (39) | 113 (41) | 150 (37) | .38 |
| Cardiovascular death | 162 (24) | 63 (12) | 97 (24) | .18 |
| Recurrent MI | 73 (11) | 33 (12) | 40 (10) | .41 |
| Stroke | 25 (4) | 9 (3) | 16 (4) | .59 |
| Major or clinically relevant bleeding | 17 (3) | 9 (3) | 8 (2) | .30 |
| Rehospitalization by heart failure | 127 (19) | 49 (18) | 78 (19) | .86 |
Note: Values are n (%) or mean ± SD.
Abbreviations: MI, myocardial infarction; NSTE ACS, non‐ST‐elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
Ventricular septal rupture, free wall rupture and ischemic mitral regurgitation by papillary muscle rupture.
FIGURE 2.
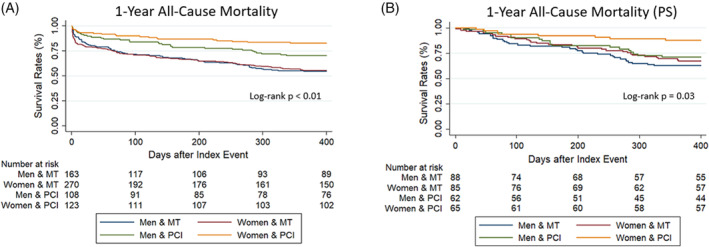
Title: Kaplan–Meier survival estimates for 1‐year All‐Cause Death. Legend by sex and treatment: 1‐year survival rates: All cohort (A) and propensity‐matching score's cohort (B). PCI, primary percutaneous coronary intervention. MT, medical treatment. PS, propensity‐matching score
TABLE 3.
Independent predictors for percutaneous coronary intervention by ACS
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Non‐ST‐elevation acute coronary syndrome | ||||
| Women | 0.70 (0.43–1.14) | 0.15 | ‐ | ‐ |
| Age | 0.90 (0.80–1.01) | 0.08 | ‐ | ‐ |
| Chronic obstructive lung disease | 1.02 (0.46–2.23) | 0.97 | ‐ | ‐ |
| Chronic kidney disease | 3.92 (2.03–7.54) | < 0.001 | ‐ | ‐ |
| Peripheral vascular disease | 1.48 (0.55–3.94) | 0.44 | ‐ | ‐ |
| Active oncology disorder | 0.87 (0.34–2.20) | 0.77 | ‐ | ‐ |
| No disability for activities of daily living | 3.33 (1.86–5.96) | < 0.001 | 3.34 (1.83–6.08) | < 0.001 |
| Moderate or severe cognitive impairment | 0.45 (0.25–0.80) | 0.006 | ‐ | ‐ |
| Atypical symptoms | 0.32 (0.14–0.72) | 0.006 | ‐ | ‐ |
| Killip class >2 at admission | 0.51 (0.22–1.17) | 0.11 | ‐ | ‐ |
| Institutions | ‐ | ‐ | ||
| A | Ref. | Ref. | ||
| B | 0.53 (0.19–1.7) | 0.19 | ||
| C | 1.47 (0.82–2.66) | 0.21 | ||
| D | 1.26 (0.59–2.80) | 0.57 | ||
| ST‐elevation myocardial infarction | ||||
| Women | 0.54 (0.34–0.87) | 0.01 | 0.62 (0.38–0.97) | 0.03 |
| Age | 0.86 (0.77–0.95) | 0.002 | 0.88 (0.79–0.98) | 0.02 |
| Chronic Kidney Disease | 0.79 (0.62–1.00) | 0.05 | ‐ | ‐ |
| Peripheral vascular disease | 0.39 (0.15–1.05) | 0.06 | ‐ | ‐ |
| Chronic Obstructive Lung Disease | 1.12 (0.53–2.38) | 0.77 | ‐ | ‐ |
| Active oncology disorder | 0.64 (0.23–1.72) | 0.37 | ‐ | ‐ |
| No disability for activities of daily living | 2.63 (1.61–4.30) | < 0.001 | 1.82 (1.03–3.22) | 0.04 |
| Moderate or severe cognitive impairment | 0.50 (0.31–0.76) | 0.002 | ‐ | ‐ |
| Atypical symptoms | 0.63 (0.34–1.15) | 0.134 | ‐ | ‐ |
| Killip class >2 at admission | 0.61 (0.31–1.19) | 0.145 | ‐ | ‐ |
| Institutions | ‐ | ‐ | ||
| A | Ref. | Ref. | ||
| B | 1.47 (0.82–2.66) | 0.19 | ||
| C | 1.77 (0.88–2.93) | 0.23 | ||
| D | 2.10 (0.97–4.55) | 0.06 | ||
Note: Odd ratios and their 95% confidence intervals were calculated by multivariate logistic regression analysis.
Abbreviation: ACS, acute coronary syndrome.
4. DISCUSSION
There are two main findings of our study. First, nonagenarian women were less likely to undergo an emergent catheterization compared to men, mainly in a STEMI context. Second, coronary revascularization in women was associated with a lower risk of 1‐year all‐cause mortality compared to men even after sensitivity analysis.
Cardiovascular disease is the leading cause of death in women in developed countries and its incidence is increasing in developing countries, with ischemic heart disease being the main etiology. 10 Despite the proven efficacy of coronary revascularization, many studies have shown that women are less likely to be referred for revascularization in ACS in different contexts: chronic coronary syndrome, NSTE‐ACS, and STEMI. 11 On the other hand, older patients and women are underrepresented in contemporary ACS trials, since the development of observational studies is necessary to measure the real impact of ACS in this subgroup. 12 In our study, female nonagenarians admitted for ACS were treated significantly less often with an invasive approach compared to men, leading to lower revascularization rates, which is significant in the STEMI group. This sex‐gender disparity in ACS has been previously described mainly in young populations, as shown by the study VIRGO (The Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) where young women with STEMI were less likely to receive reperfusion therapy and more likely to have reperfusion delays than similarly aged men. Similar results have been observed in women with NSTE‐ACS 13 and chronic coronary syndrome. 14 We evidenced the same pattern in nonagenarians despite the fact that PCI appeared to be beneficial in women. Sulzgruber et al. 15 showed that elderly women (≥80 years) with ACS obtained a higher benefit of any coronary intervention in cardiovascular mortality rate compared to men. Hao et al. evaluated sex differences in acute management, medical therapies for secondary prevention, and in‐hospital mortality in 82 196 patients admitted for ACS at 192 hospitals in China, using data from the Improving Care for Cardiovascular Disease in China‐Acute Coronary Syndrome project (CCC‐ACS). 16 They found that women were less likely to receive evidence‐based acute treatments for ACS than men, including early dual antiplatelet therapy, heparins during hospitalization, and reperfusion therapy for ST‐segment‐elevation myocardial infarction and fewer strategies for secondary prevention after hospital discharge. Similar results were found by Hvelplund et al. 17 In a cohort of 9561 women and 16 406 men, significantly fewer women underwent coronary angiogram (cumulative incidence 64% for women vs. 78% for men, p < .05) compared with men. Subsequently, revascularization was also less likely in women compared to men (HR = 0.68, 95% CI = 0.66–0.71, p < .0001). This gender gap in optimal medical treatment is also observed in other clinical conditions such as atrial fibrillation. 18 At this point it is very important to underline that ACS guidelines highlight that primary PCI is recommended in STEMI regardless of age and sex. 3 To the best of our knowledge, our study is the first where these sex‐gender differences are evaluated in a fast‐growing and predominantly female population, such as nonagenarians.
A higher burden of frailty characteristics observed in women may influence physicians to adopt less invasive strategies. Subsequently, attending medical staff may have selected a non‐invasive approach due to less anticipated life expectancy. In our study, frailty, measured as the presence of disability and/or cognitive dysfunction, could influence clinical decisions in women. It has been broadly reported that women at any age have a higher frailty index compared to men. Nevertheless, their life expectancy used to be longer than men. This inverse association between health and longevity is known as the morbidity–mortality paradox. 19 This phenomenon is not fully understood, but hypotheses include higher self‐reported deficits, 20 more diseases that affect quality of life rather than mortality, and higher physiological reserve in women compared to men. 21 Psychosocial factors, such as healthcare utilization and self‐reported behaviors have also been implicated in the morbidity‐mortality paradox. 22 On the other hand, another possible explanation for the lower intention of coronary intervention in women could be a higher rate of understanding of postprocedural complications in this group. Several studies have demonstrated a higher rate of in‐hospital complications and higher rates of bleeding in women, but these sex differences disappear after adjusting for different comorbidities 13 and the use of appropriate antithrombotic treatment and vascular access. 23 Awareness of cardiovascular disease (CVD) among women has improved over the past 15 years, but awareness of atypical symptoms remains low. 24 This might be conducive to misdiagnosis, resulting in a delay in receiving appropriate acute treatments for ACS, including a coronary angiogram and subsequent PCI if indicated. This conservative approach in the female group compared to men could be explained by the wrong perception that ischaemic cardiac symptoms, often less specific in women, are related to other pathologies. 25 The use of a systems‐based approach to STEMI care may help reduce sex disparities and improves STEMI care and outcomes in women, regardless of age. 26
5. STUDY LIMITATIONS
The main limitation of this study is its observational design, which implies an inherent selection bias. Moreover, it is difficult to capture and control all potential confounders when using a registry. Despite the use of a propensity‐matching score, we cannot control for all potential confounders when using data from a registry. Therefore, the purpose of the study is exploratory, and our results should be considered as hypothesis‐generating. In addition, the sample size may lack the power to detect other statistically significant differences in outcomes between groups. Also, we lacked data regarding cardiac rehabilitation reference at hospital discharge and during follow‐up. Therefore, we cannot compare cardiac rehabilitation reference rates at hospital discharge between groups.
6. CONCLUSION
In conclusion, sex‐gender disparities have been observed in nonagenarians. Despite receiving less often invasive approaches, women showed better clinical outcomes. This finding may help increase awareness and reduce the current gender gap in ACS management at any age.
CONFLICT OF INTEREST
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
AUTHOR CONTRIBUTION
Pedro L. Cepas‐Guille takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supporting information
Appendix S1. Supporting Information.
TABLE S1. Percent standardized differences of variables among unadjusted and propensity‐score matched, and baseline characteristics of propensity‐score matched cohort.
TABLE S2. Baseline Lesion‐ and Procedure‐Related Profiles.
TABLE S3. A, Treatment at hospital discharge in NST‐ACS. B, Treatment at hospital discharge in STEMI.
TABLE S4. Independent predictors for 1‐year all‐cause death by sex.
Cepas‐Guillen PL, Echarte‐Morales J, Flores‐Umanzor E, et al. Sex‐gender disparities in nonagenarians with acute coronary syndrome. Clin Cardiol. 2021;44:371–378. 10.1002/clc.23545
REFERENCES
- 1. Aggarwal NR, Patel HN, Mehta LS, et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. 2018;11(2):e004437. 10.1161/CIRCOUTCOMES.117.004437. [DOI] [PubMed] [Google Scholar]
- 2. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323‐1335. 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2018;39(2):119‐177. 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 4. Roffi M, Patrono C, Collet J‐P, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2016;37(3):267‐315. 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 5. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231‐2264. 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 6. Garcia‐Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium‐2 consensus document. Circulation. 2018;137(24):2635‐2650. 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 7. Vranckx P, Cutlip DE, Mehran R, et al. Myocardial infarction adjudication in contemporary all‐comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5(7):871‐874. [DOI] [PubMed] [Google Scholar]
- 8. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century. Stroke. 2013;44(7):2064‐2089. 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials. Circulation. 2011;123(23):2736‐2747. 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 10. Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age‐sex specifc mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1151‐1210. 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Onofrio G, Safdar B, Lichtman JH, et al. Sex differences in reperfusion in young patients with ST‐segment‐elevation myocardial infarction: results from the VIRGO study. Circulation. 2015;131(15):1324‐1332. 10.1161/CIRCULATIONAHA.114.012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial/ethnic minority groups in contemporary acute coronary syndrome clinical trials: a systematic review. JAMA Cardiol. 2020;5(6):714‐722. 10.1001/jamacardio.2020.0359. [DOI] [PubMed] [Google Scholar]
- 13. Blomkalns AL, Chen AY, Hochman JS, et al. Gender disparities in the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes: large‐scale observations from the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the American College of Cardiology/American Heart Association guidelines) National Quality Improvement Initiative. J Am Coll Cardiol. 2005;45(6):832‐837. 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 14. Flores‐Umanzor EJ, Cepas‐Guillen PL, Caldentey G, et al. Sex‐based differences in chronic total occlusion management and long‐term clinical outcomes. Int J Cardiol. 2020;319:46‐51. 10.1016/j.ijcard.2020.05.090. [DOI] [PubMed] [Google Scholar]
- 15. Sulzgruber P, Koller L, Pavo N, et al. Gender‐related differences in elderly patients with myocardial infarction in a European Centre. Eur J Clin Invest. 2016;46(1):60‐69. 10.1111/eci.12567. [DOI] [PubMed] [Google Scholar]
- 16. Hao Y, Liu J, Liu J, et al. Sex differences in in‐hospital management and outcomes of patients with acute coronary syndrome: findings from the CCC project. Circulation. 2019;139(15):1776‐1785. 10.1161/CIRCULATIONAHA.118.037655. [DOI] [PubMed] [Google Scholar]
- 17. Hvelplund A, Galatius S, Madsen M, et al. Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur Heart J. 2010;31(6):684‐690. 10.1093/eurheartj/ehp493. [DOI] [PubMed] [Google Scholar]
- 18. Politi C, Ciarambino T, Riva L, et al. Sex‐gender and atrial fibrillation treatment in the AntiThrombotic agents in atrial fibrillation (ATA‐AF) study. Ital J Med. 2016;10(3):207‐212. 10.4081/itjm.2016.649. [DOI] [Google Scholar]
- 19. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta‐analysis. Exp Gerontol. 2017;89:30‐40. 10.1016/j.exger.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 20. Crimmins EM, Kim JK, Solé‐Auró A. Gender differences in health: results from SHARE, ELSA and HRS. Eur J Public Health. 2011;21(1):81‐91. 10.1093/eurpub/ckq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi J, Yang Z, Song X, et al. Sex differences in the limit to deficit accumulation in late middle‐aged and older Chinese people: results from the Beijing longitudinal study of aging. J Gerontol Ser A. 2014;69(6):702‐709. 10.1093/gerona/glt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20:91‐102. 10.1007/BF03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rao SV, Hess CN, Barham B, et al. A registry‐based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE‐PCI for women (study of access site for enhancement of PCI for women) trial. JACC Cardiovasc Interv. 2014;7(8):857‐867. 10.1016/j.jcin.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 24. Mosca L, Hammond G, Mochari‐Greenberger H, Towfighi A, Albert MA, American Heart Association Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on High Bloo . Fifteen‐year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation. 2013;127(11):1254‐1263. 10.1161/CIR.0b013e318287cf2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wada H, Miyauchi K, Daida H. Gender differences in the clinical features and outcomes of patients with coronary artery disease. Expert Rev Cardiovasc Ther. 2019;17:127‐133. 10.1080/14779072.2019.1561277. [DOI] [PubMed] [Google Scholar]
- 26. Huded CP, Johnson M, Kravitz K, et al. 4‐step protocol for disparities in STEMI care and outcomes in women. J Am Coll Cardiol. 2018;71(19):2122‐2132. 10.1016/j.jacc.2018.02.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
TABLE S1. Percent standardized differences of variables among unadjusted and propensity‐score matched, and baseline characteristics of propensity‐score matched cohort.
TABLE S2. Baseline Lesion‐ and Procedure‐Related Profiles.
TABLE S3. A, Treatment at hospital discharge in NST‐ACS. B, Treatment at hospital discharge in STEMI.
TABLE S4. Independent predictors for 1‐year all‐cause death by sex.


