Highlights
-
•
IMRT is associated with favorable toxicity rates for patients with anal cancer.
-
•
Outcomes were favorable for those with T3-4 tumors or lymph node involvement.
-
•
Current smokers are at a higher risk of severe dermatologic toxicity.
-
•
Overall treatment duration greater than 39 days is associated with recurrence.
Abbreviations: AE, adverse events; IMRT, intensity modulated radiotherapy; ASCC, anal canal squamous cell carcinoma; OS, overall survival; CFS, colostomy-free survival; PFS, progression-free survival; LR, local recurrence; LRR, locoregional recurrence; DM, distant metastasis; IQR, interquartile range; CI, confidence interval; GI, gastrointestinal; GU, genitourinary; CRT, chemoradiotherapy; 3DCRT, 3-dimensional conformal radiotherapy; RTOG, Radiation Therapy Oncology Group; DP-IMRT, dose-painted intensity modulated radiotherapy; CTV, clinical target volume; LN, lymph node; PTV, planning target volume; DVH, dose-volume histogram; MMC, mitomycin-C; 5-FU, 5-fluorouracil; CTCAE v 4.0, common terminology criteria for adverse events version 4.0; BED, biologically effective dose; HR, hazard ratio; HIV, human immunodeficiency virus; RT, radiotherapy; G, grade; ACT II, United Kingdom Anal Cancer Trial II
Keywords: Anal cancer, Radiation, IMRT
Abstract
Introduction
To report long-term efficacy and adverse events (AEs) associated with intensity modulated radiotherapy (IMRT) for patients with anal canal squamous cell carcinoma (ASCC).
Materials and methods
This was a retrospective review of patients with ASCC who received curative-intent IMRT and concurrent chemotherapy (98%) between 2003 and 2019. Overall survival (OS), colostomy-free survival (CFS), and progression-free survival (PFS) were estimated using the Kaplan-Meier method. The cumulative incidence of local recurrence (LR), locoregional recurrence (LRR), and distant metastasis (DM) were reported. Acute and late AEs were recorded per National Cancer Institute Common Terminology Criteria for AEs.
Results
127 patients were included. The median patient age was 63 years (interquartile range [IQR] 55–69) and 79% of patients were female. 33% of patients had T3-4 disease and 68% had clinically involved pelvic or inguinal lymph nodes (LNs).
The median patient follow-up was 47 months (IQR: 28–89 months). The estimated 4-year OS, CFS, and PFS were 81% (95% confidence interval [CI]: 73%–89%), 77% (95% CI: 68%–86%), and 78% (95% CI: 70%–86%), respectively. The 4-year cumulative incidences of LR, LRR, and DM were 3% (95% CI: 1%–9%), 9% (95% CI: 5%–17%), and 10% (95% CI: 6%–18%), respectively. Overall treatment duration greater than 39 days was associated with an increased risk of LRR (Hazard Ratio [HR]: 5.2, 95% CI: 1.4–19.5, p = 0.015). The most common grade 3+ acute AEs included hematologic (31%), gastrointestinal (GI) (17%), dermatologic (16%), and pain (15%). Grade 3+ late AEs included: GI (3%), genitourinary (GU) (2%), and pain (1%). Current smokers were more likely to experience grade 3+ acute dermatologic toxicity compared to former or never smokers (34% vs. 7%, p < 0.001).
Conclusions
IMRT was associated with favorable toxicity rates and long-term efficacy. These data support the continued utilization of IMRT as the preferred treatment technique for patients with ASCC.
1. Introduction
Concurrent chemoradiotherapy (CRT) is the standard of care curative treatment approach for patients with locoregionally-confined ASCC and is associated with sustained PFS, CFS, and locoregional control in the majority of patients [1], [2], [3], [4], [5], [6], [7], [8]. However, a substantial proportion of patients experience severe acute and late treatment-related AEs [9].
The use of IMRT may reduce treatment-related AEs compared with 3-dimensional conformal radiotherapy (3DCRT), thereby supporting its use as the preferred treatment technique [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. For instance, Radiation Therapy Oncology Group (RTOG) 0529 used a dose-painted IMRT (DP-IMRT) technique and demonstrated a reduction in grade 3+ acute gastrointestinal (GI) and dermatologic AEs and grade 2+ hematologic AEs when compared to historical controls treated with 3DCRT [8]. However, available data evaluating the use of IMRT for patients with ASCC is limited by the small patient numbers, short follow-up, and the relative lack of long-term efficacy and toxicity outcomes.
The purpose of this study is to report a 16-year single institution experience utilizing IMRT for patients with ASCC with an emphasis upon long-term efficacy, toxicity, and disease and treatment-related variables associated with outcomes.
2. Materials and methods
2.1. Patient population
Following institutional review board approval, patients diagnosed with ASCC between 2003 through 2019 were identified. Patients were included for analysis if they were 18 years of age or older, had biopsy confirmed ASCC, and received curative-intent IMRT. Patients with a prior history of pelvic radiotherapy were excluded.
Pre-treatment evaluation included complete history, physical examination, and laboratory evaluation. Systemic staging imaging included positron-emission tomography-CT (73%) and/or CT of the chest, abdomen, and pelvis (27%). Local staging with pelvic MRI was performed per physician discretion. Lymph node status was determined based upon imaging, with biopsy confirmation infrequently performed. Tumor Human Papillomavirus (HPV) status was determined by p16 immunohistochemistry or by in situ hybridization for high risk HPV RNA, when available.
2.2. Treatment techniques
Patients underwent CT-based simulation. Clinical target volumes (CTV) were contoured per consensus guidelines targeting the gross anal canal tumor, grossly involved LNs, and elective inclusion of the mesorectum, presacral, and bilateral inguinal, internal iliac, and external iliac LNs [30], [31]. The planning target volume (PTV) consisted of a 0.5–1.0 cm expansion from the CTV. Median RT doses to the primary tumor, involved LNs, and elective target volumes were 53.2 Gy (IQR: 50.4 Gy–54.0 Gy), 54.0 Gy (IQR: 50.4 Gy–56.25 Gy), and 42.5 Gy (42.0 Gy–45.0 Gy) in 28 fractions (IQR: 25–30). Daily image guidance typically included daily cone beam CT or daily orthogonal kilovoltage images plus once weekly cone beam CT.
Concurrent chemotherapy was administered to most patients (98%). The most commonly prescribed regimen was mitomycin-C (MMC) 10 mg/m2 administered on days 1 and 29 and continuous infusion of 5-fluorouracil (5-FU) 1000 mg/m2 administered on days 1–4 and days 29–32. Supportive care agents (e.g. growth factor support) was delivered per physician discretion.
2.3. Patient assessments
Patients underwent routine oncologic surveillance including digital rectal exam with or without anoscopy and diagnostic imaging at 3–6 month intervals. Sites of recurrence were characterized through the entirety of follow-up and not restricted to first site of recurrence.
LR was defined as recurrence or progression at the primary tumor site; LRR was defined as recurrence or progression within the primary tumor site or non-metastatic regional lymph nodes; DM sites were defined as non-regional lymph nodes or distant organs. Disease recurrence was diagnosed histologically, or when unavailable, clinically per radiographic findings. AEs were assessed and attributed prospectively by the treating physician per common terminology criteria for adverse events version 4.0 (CTCAE v 4.0). When unavailable, AEs were ascertained through retrospective chart review. Acute AEs were defined as those occurring during RT or within 3 months of treatment completion. Late AEs were defined as those occurring greater than 3 months after completion of RT.
2.4. Statistical analysis
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary NC), with p < 0.05 considered statistically significant. OS, CFS, and PFS were estimated using the Kaplan-Meier method. The cumulative incidence of LR, LRR, DM, and post-treatment colostomy (excluding 2 patients who underwent diverting colostomy prior to treatment initiation) were reported with death as a competing risk. Treatment-related AEs were reported descriptively.
The Cox Proportional Hazards model was performed to identify associations between disease and treatment characteristics and oncologic endpoints. Variables examined included American Joint Committee on Cancer Staging 8th edition clinical stage grouping, T-stage, LN status, RT dose, biologically effective dose (BED) assuming an α/β ratio of 10 Gy, chemotherapy regimen, overall treatment duration, and smoking status.
3. Results
A total of 127 patients were included for analysis. Baseline patient and treatment characteristics are reported in Table 1.
Table 1.
Baseline patient and treatment characteristics
| Variable | Value | |
|---|---|---|
| Patient Characteristics | No. patients (%) or median (IQR) | |
| N | 127 | |
| Age | 63 (55-69) | |
| Gender | Female | 100 (79%) |
| Male | 27 (21%) | |
| Smoking Status | Never | 48 (38%) |
| Former | 40 (31%) | |
| Current | 39 (31%) | |
| Histologic Grade | 1 | 9 (7%) |
| 2 | 42 (33%) | |
| 3 | 41 (32%) | |
| 4 | 17 (13%) | |
| X | 18 (14%) | |
| Clinical stage | I | 14 (11%) |
| IIA | 18 (14%) | |
| IIB | 4 (3%) | |
| IIIA | 52 (41%) | |
| IIIB | 4 (3%) | |
| IIIC | 33 (26%) | |
| IV* | 2 (2%) | |
| Primary Tumor size | Median | 4.0 cm (2.4–5.2) |
| T-stage | T0* | 2 (2%) |
| T1 | 27 (21%) | |
| T2 | 56 (44%) | |
| T3 | 27 (21%) | |
| T4 | 15 (12%) | |
| LN Status | Negative | 40 (32%) |
| Positive | 87 (68%) | |
| HIV Status | Negative | 126 (99%) |
| Positive | 1 (1%) | |
| Tumor HPV Status | Positive | 15 (12%) |
| Negative | 7 (6%) | |
| Unknown | 105 (83%) | |
| Treatment Characteristics | ||
| RT dose | Primary | 53.2 Gy (50.4–54.0) |
| Involved LN | 54 Gy (50.4–56.25) | |
| Elective LN | 42.5 Gy (42.0–45.0) | |
| RT fractions | 28 (25–30) | |
| Boost timing | Simultaneous | 120 (95%) |
| Sequential | 7 (5%) | |
| Overall Treatment duration (days) | 39 (36–42) | |
| Chemotherapy | Concurrent | 124 (98%) |
| Induction + Concurrent | 1 (1%) | |
| None | 2 (1%) | |
| Concurrent Chemotherapy Regimen | 5-FU/MMC × 2 | 106 (85%) |
| 5-FU/MMC × 1 | 9 (7%) | |
| 5-FU/Cisplatin | 3 (2%) | |
| Capecitabine/MMC × 2 | 2 (2%) | |
| Capecitabine/MMC × 1 | 1 (1%) | |
| 5-FU alone | 1 (1%) | |
| Capecitabine alone | 3 (2%) |
*2 patients with T0 disease had biopsy proven LN+ disease with anal canal biopsy suggestive of carcinoma in-situ. 2 patients with stage IV disease had metastatic disease limited to retroperitoneal LN.
Abbreviations: LN, lymph node; HIV, human immunodeficiency virus; HPV, human papilloma virus; RT, radiotherapy, 5-FU, 5-fluorouracil; MMC, mitomycin-C.
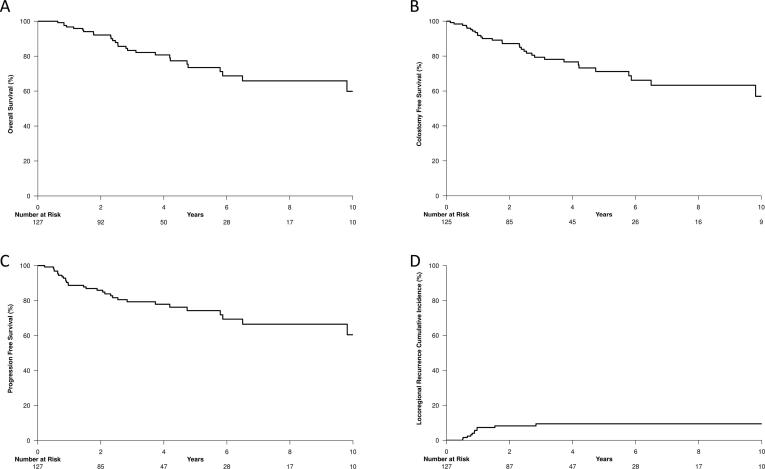
The median patient follow-up was 47 months (IQR: 28–89 months). The estimated 4-year OS, CFS, and PFS were 81% (95% CI: 73%–89%), 77% (95% CI: 68%–86%), and 78% (95% CI: 70%–86%), respectively. Kaplan-Meier estimates of OS, CFS, and PFS and the cumulative incidence of LRR are demonstrated in Fig. 1 along with 4-year estimates for each clinical stage grouping in Supplementary Table A.1. Similarly, 4-year estimates of oncologic end-points stratified by inclusion criteria for the ongoing ECOG-ACRIN Trials 2165 [32] and 2182 [33] and the United Kingdom personalizing anal cancer radiotherapy dose (UK PLATO) ACT3-5 trials [34] are demonstrated in Supplementary Table A.2. Univariate associates with OS, CFS, PFS, and LRR are shown in Table 2. No baseline patient or treatment characteristics were significantly associated with OS, CFS, or PFS.
Fig. 1.
Kaplan-Meier estimates of overall survival (A), colostomy-free survival (B), progression-free survival (C), and the cumulative incidence of locoregional recurrence (D).
Table 2.
Univariate associates with OS, CFS, PFS, LRR
| Variable | OS |
CFS |
PFS |
LRR |
|||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Clinical Stage | I | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| IIA | 1.41 (0.34, 5.78) | 0.634 | 1.58 (0.42, 5.96) | 0.501 | 0.89 (0.23, 3.38) | 0.862 | 2.56 (0.07, 101.02) | 0.616 | |
| IIB | 0.63 (0.07, 5.63) | 0.675 | 0.70 (0.08, 6.28) | 0.749 | 0.53 (0.06, 4.54) | 0.561 | 3.68 (0.04, 331.76) | 0.570 | |
| IIIA | 0.78 (0.25, 2.47) | 0.672 | 0.87 (0.28, 2.72) | 0.814 | 0.68 (0.24, 1.95) | 0.476 | 3.22 (0.12, 89.32) | 0.491 | |
| IIIB | 1.79 (0.40, 8.13) | 0.448 | 4.35 (0.95, 19.94) | 0.058 | 1.60 (0.38, 6.83) | 0.523 | 12.46 (0.32, 491.39) | 0.179 | |
| IIIC | 0.86 (0.24, 3.11) | 0.815 | 0.96 (0.28, 3.33) | 0.954 | 0.76 (0.24, 2.45) | 0.647 | 4.43 (0.16, 126.80) | 0.385 | |
| IV | 2.80 (0.11, 71.8) | 0.534 | 1.80 (0.08, 41.83) | 0.713 | 1.11 (0.05, 24.35) | 0.949 | 7.67 (0.08, 697.85) | 0.376 | |
| T Stage | T0-2 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| T3-4 | 1.07 (0.50, 2.31) | 0.862 | 0.65 (0.32, 1.33) | 0.238 | 1.09 (0.52, 2.26) | 0.824 | 1.81 (0.55, 5.92) | 0.329 | |
| # Positive LN | 0 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 1-2 | 0.48 (0.19, 1.18) | 0.108 | 0.41 (0.17, 1.01) | 0.051 | 0.47 (0.19, 1.14) | 0.093 | 1.23 (0.21, 7.37) | 0.820 | |
| 3+ | 1.11 (0.46, 2.68) | 0.821 | 1.12 (0.50, 2.52) | 0.787 | 1.26 (0.55, 2.85) | 0.584 | 3.80 (0.77, 18.85) | 0.102 | |
| Smoking Status | Never | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| Former | 0.65 (0.28, 1.52) | 0.324 | 0.66 (0.28, 1.57) | 0.351 | 0.67 (0.30, 1.51) | 0.335 | 0.75 (0.21, 2.66) | 0.654 | |
| Current | 0.57 (0.22, 1.48) | 0.245 | 0.96 (0.42, 2.20) | 0.928 | 0.56 (0.23, 1.39) | 0.215 | 0.17 (0.02, 1.43) | 0.103 | |
| Overall Treatment duration (days) | <=39 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| >39 | 1.24 (0.59, 2.61) | 0.566 | 1.54 (0.77, 3.10) | 0.226 | 1.60 (0.80, 3.21) | 0.185 | 5.17 (1.37, 19.51) | 0.015 | |
| BED Primary (Gy10) | <=63.5 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| >63.5 | 1.17 (0.55, 2.50) | 0.680 | 1.27 (0.62, 2.60) | 0.505 | 1.31 (0.64, 2.70) | 0.461 | 1.61 (0.47, 5.50) | 0.448 | |
| BED LN (Gy10) | <=61.0 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| >61.0 | 0.74 (0.35, 1.57) | 0.438 | 0.80 (0.40, 1.61) | 0.525 | 0.83 (0.41, 1.69) | 0.610 | 1.25 (0.38, 4.10) | 0.712 | |
| # MMC Cycles | 1 | 0.81 (0.19, 3.49) | 0.776 | 1.23 (0.37, 4.12) | 0.737 | 1.20 (0.36, 4.04) | 0.770 | 1.24 (0.16, 9.89) | 0.842 |
| 2 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | |||||
Abbreviations: OS, overall survival; CFS, colostomy-free survival; PFS, progression-free survival; LRR, locoregional recurrence; HR, hazard ratio; CI, confidence interval; LN, lymph node; RT, radiotherapy; BED, biologically effective dose assuming an α/β ratio of 10 Gy; MMC, mitomycin-C.
The 4-year cumulative incidences of LR, LRR, and DM were 3% (95% CI: 1%–9%), 9% (95% CI: 5%–17%), and 10% (95% CI: 6%–18%), respectively. The 4-year cumulative incidence of post-treatment colostomy was 8% (95% CI: 4%–15%). Overall treatment duration >39 days (median duration for the group), compared with ≤39 days, was associated with an increased risk of LRR (HR: 5.2, 95% CI: 1.4–19.5, p = 0.015) and an increased risk of colostomy (HR: 4.0, 95% CI: 1.0–16.1, p = 0.049). Overall treatment duration >39 days remained significantly associated with LRR when assessed with bivariate models including overall treatment duration and T-stage (T0-2 vs. T3-4) (HR: 4.9, 95% CI: 1.3–18.7, p = 0.019) or overall treatment duration and LN status (positive vs. negative) (HR: 4.8, 95% CI: 1.3–18.4, p = 0.021). Similarly, on multivariate analysis (demonstrated in Supplementary Table A.3) with covariates of T-stage, LN status, and overall treatment duration, overall treatment duration of >39 days remained associated with an increased risk of LRR (HR: 4.7, 95% CI: 1.2–17.9, p = 0.024), but not OS (HR: 1.4, 95% CI: 0.6–3.0, p = 0.013), CFS (HR: 1.8, 95% CI: 0.9–3.7, p = 0.013), or PFS (HR: 1.7, 95% CI: 0.9–3.6, p = 0.424). The median BED delivered to the GTV primary was 63.5 Gy10 (IQR: 60.0–64.8) with a BED of 61.8 Gy10 (IQR: 60.0–63.7) and a BED of 64.3 Gy10 (IQR: 63.5–68.0) for patients with T0-2 and T3-4 tumors, respectively. The median BED to the GTV (grossly involved) LNs, and CTV (elective regional) LNs were 63.7 Gy10 (IQR: 60.0–68.9), and 49.7 Gy10 (IQR: 48.3–51.8), respectively. When stratified by the median, no associations were identified between BED to the GTV primary or GTV LNs with LRR. Patterns of recurrence are demonstrated in Supplementary Fig. 1. Four patients experienced LR of the GTV primary with a BED range of 61.8 Gy10–68.0 Gy10. Two of these four had isolated LR, one had a synchronous diagnosis of DM, and one had both LR at the primary site and within a previously uninvolved LN treated to a BED of 50.3 Gy10 but no evidence of DM. Seven patients experienced LN recurrence with no evidence for LR. Two recurred at sites of initial LN involvement that received a BED of 78.5 Gy10 and 68.9 Gy10. For the remaining 5 patients who experienced recurrence within previously uninvolved LNs, the elective volume received a median BED of 51.8 Gy10 (range 39.7–51.8 Gy10). Amongst the aforementioned 7 patients who experienced LN recurrence, 2 were isolated LN recurrences where-as 4 had a synchronous diagnosis of DM.
Eleven patients underwent colostomy, of which 2 underwent pre-treatment colostomy due to tumor-related obstruction. One patient developed a bowel obstruction mid-treatment requiring urgent colostomy and another developed a bowel obstruction in the absence of LRR 2 months after treatment and underwent colostomy. Five patients underwent salvage abdominoperineal resection due to LRR or incomplete tumor response at a median of 10 months (range 3–11 months) after RT. Two patients underwent abdominoperineal resection in the absence of LRR-1 patient due to chronic pain and anal sphincter incompetence 7 months after RT and the other was a patient with comorbid inflammatory bowel disease who underwent abdominoperineal resection as part of an inflammatory bowel disease-directed operation approximately 31 months after completion of RT.
Acute grade 2+ and 3+ AEs are shown in Table 3. The most common grade 3+ acute AEs included hematologic (31%), GI (17%), dermatologic (16%), and pain (15%). No patient experienced a grade 3+ acute GU AE. Current smokers were more likely to experience grade 3+ acute dermatologic toxicity compared to former or never smokers (34% vs. 7%, p < 0.001), although there was no correlation between smoking status and grade 3+ acute GI (p = 0.65) or pain (p = 0.65) AEs. Hospitalization occurred in 37 (29%) patients, most commonly due to neutropenic fever (n = 19, 15%).
Table 3.
Acute treatment-related adverse events in comparison to RTOG 0529 and RTOG 9811
| Adverse Event | Current Series | RTOG 0529 | RTOG 9811 |
|---|---|---|---|
| Grade 2+ | |||
| GI/GU | 39% | 77% | 77% |
| Dermatologic | 67% | 75% | 83% |
| GI | 38% | 73% | 73% |
| GU | 5% | 15% | 20% |
| Hematologic | 54% | 73% | 85% |
| Pain | 44% | – | – |
| Grade 3+ | |||
| GI/GU | 17% | 21% | 37% |
| GI | 17% | 21% | 36% |
| GU | 0% | 2% | 3% |
| Dermatologic | 16% | 23% | 49% |
| Hematologic | 32% | 58% | 62% |
| Pain | 15% | – | – |
Abbreviations: GI, gastrointestinal; GU, genitourinary.
Late grade 3+ AEs included: GI (3%), GU (2%), and pain (1%). Five (4%) patients developed small bowel obstruction, all of which were managed non-operatively, and 1 (1%) patient developed a treatment-related fistula in the absence of local disease recurrence.
4. Discussion
We report on a large, 16-year single institution experience utilizing IMRT for patients with ASCC. Treatment was associated with favorable oncologic outcomes with 4-year estimates of OS, CFS, PFS, and LRR of 81%, 77%, 78%, and 9%, respectively. Toxicity rates were comparable to other IMRT series (shown in Table 4), and importantly, demonstrate improvements relative to historical 3DCRT series [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. Prolonged overall treatment duration was associated with an increased risk of LRR and colostomy while continued tobacco smoking during CRT was associated with an increased risk of acute dermatologic toxicity. These data continue to support the use of IMRT as the preferred treatment technique for patients with ASCC.
Table 4.
Select series of intensity-modulated radiotherapy for anal cancer.
| Series, year | N | Technique | Median Follow-up (months) | OS | CFS | Colostomy | LRR | Acute GI (G2+/G3+) | Acute GU (G2+/G3+) | Acute Dermatologic (G2+/G3+) | Acute Hematologic (G2+/G3+) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current | 127 | DP-IMRT (95%) | 47 | 81%4 | 77%4 | 8%4 | 9%4 | 38%/17% | 5%/0% | 67%/16% | 54%/32% |
| Shakir IJROBP 2020 | 385 | IMRT | 24 | 86%3 | 69%3 | – | 20%3 | – | – | – | – |
| Bryant IJROBP 2018 | 367 | IMRT | 72 | 74%5 | – | 10%5 | – | – | – | – | –/47% |
| Call Am J Clin Oncol 2016 | 148 | IMRT | 27 | 87%3 | 92%3 | – | LR: 13%3 | –/11% | – | –/20% | –/41% |
| Jones CM IJROBP 2018 | 147 | IMRT | – | – | 91%1 | – | – | –/14% | – | –/27% | –/18% |
| Mitra Adv Rad Oncol 2017 | 99 | DP-IMRT | 49 | 86%4 | – | 15%4 | 7%4 | 40%/10% | 9%/1% | 76%/13% | 83%/21% |
| Franco Rad Oncol 2018 | 87 | DP-IMRT | 34 | 88%2 | 78%2 | 15%2 | – | – | – | – | – |
| Arcadipane Asia Pac J Clin Oncol 2018 | 87 | DP-IMRT | – | 79%3 | 64%3 | 15%2 | LR: 29%3 | – | – | –/16% | –/26% |
| Mitchell Am J Clin Oncol 2014 | 65 | IMRT | 19 | 96%2 | – | – | 9%2 | –/9% | –/2% | –/17% | 5%/3% |
| Vendrely Rad Oncol 2015 | 64 | Tomotherapy | 23 | 86%2 | 76%1 | – | – | 44%/20% | 13%/2% | 88%/47% | 27%/17% |
| Han IJROBP 2014 | 58 | IMRT | 34 | 90%2 | 84%2 | – | 16%2 | 68%/9% | 19/0% | 97%/46% | 66%/40% |
| Joseph Rad Oncol 2015 | 57 | Tomotherapy | 40 | 91%3 | 77%3 | – | – | 70%/10% | –/4% | 71%/11% | –/46% |
| Franco Cancer Invest 2015 | 54 | DP-IMRT | 33 | 78%4 | 69%4 | – | LR: 15%4 | –/8% | –/2% | –/13% | – |
| Salama JCO 2007 | 53 | IMRT | 15 | 93%1.5 | 84%1.5 | – | LR: 16%1.5 | 72%/15% | 11%/0% | 92%/38% | 79%/59% |
| Foster Plos One 2018 | 52 | IMRT | 33 | 90%3 | – | 9%3 | 6%3 | 71%/7% | 19%/0% | 83%/48% | –/63% |
| RTOG 0529 Kachnic IJROBP 2013 | 52 | DP-IMRT | – | – | – | – | – | 73%/21% | 15%/2% | 75%/23% | 73%/58% |
| RTOG 9811 Ajani JAMA 2008 | 644 | 3DCRT | 30 | 84%3 75%5 | 10%3 | 25%5 | 73%/35% | 22%/3% | 83%/48% | /61% |
1–5Superscript indicates time point in years.
Abbreviations: OS, overall survival; CFS, colostomy-free survival; LRR, locoregional recurrence; LR, local recurrence; GI, gastrointestinal; GU, genitourinary; G, grade; IMRT, intensity modulated radiotherapy; DP-IMRT, dose-painted (simultaneous intregrated boost) intensity modulated radiotherapy.
Interesting aspects of this work include the relatively large patient cohort (n = 127), fairly homogeneous delivery of DP-IMRT (95%), mature median follow-up of 47 months (IQR: 28–89 months), robust collection of acute and late AEs, and the availability of extensive patient, disease, and treatment characteristics which facilitated an analysis of variables associated with oncologic efficacy outcomes.
Our series, in addition to the IMRT series summarized within Table 4, continue to show that IMRT is associated with a favorable toxicity profile compared to historical 3DCRT series [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. We identified rates of grade 3+ acute GI, dermatologic, and hematologic toxicity of 17%, 16%, and 31%, respectively. This is in reference to rates of 7–21%, 10–48%, and 3–63% in other IMRT series, and rates of 36%, 49%, and 62% identified with the use of 3DCRT in RTOG 9811. Severe late toxicities were uncommon, with rates of grade 3+ GI, GU, and chronic pain of 3%, 2%, and 1%, respectively. Patients who continued tobacco smoking during CRT had nearly 5 times the rate of acute dermatologic toxicity (34% vs. 7%, p < 0.001) compared to former or never smokers. Lerman et al. reported on the impact of tobacco smoking upon outcomes after CRT for patients with ASCC [35]. They identified that tobacco smoking had a detrimental impact upon LRR, relapse-free survival, and OS. However, tobacco smoking was not associated with the risk of grade 3 toxicity. Eifel et al. have demonstrated an association between smoking history and an increased risk of acute small bowel toxicity in women receiving pelvic radiotherapy for cervical cancer and Alsadius et al. have reported an association between current smoking and an increased risk of bowel toxicity for men receiving RT for prostate cancer. Therefore, it does seem plausible that smoking may have an adverse impact upon treatment tolerability [36], [37]. Based upon these data, we suggest that utilization of IMRT may offer a reduction in GI, dermatologic, and hematologic toxicity when compared with 3DCRT, while addressing patient modifiable risk factors, such as smoking status, is also an important aspect of care.
In this analysis, prolonged overall treatment duration (>39 days) was associated with an increased risk of LRR. One argument may be that this finding is primarily reflective of a greater risk of LRR for T3-4 tumors as they are more commonly treated with regimens of ≥30 fractions extending >39 days, as was done on RTOG 0529. In our study, the median overall treatment duration for the total cohort was 39 days (IQR: 36–42) and was 38 days and 39 days for patients with T0-2 and T3-4 tumors, respectively. To assess the interactions between T-stage, LN status, and overall treatment duration, analyses were performed as bivariate models incorporating overall treatment duration and either T-stage or LN status, and as a multivariate model. In each instance, only overall treatment duration >39 days remained associated with LRR. These data are consistent with other reports evaluating the impact of overall treatment duration for patients with ASCC and are analogous to data derived from the head and neck cancer population [14], [38], [39], [40]. For example, prolongation of the overall treatment duration greater than 42 days was associated with worse PFS and OS per a post-hoc analysis of the United Kingdom Anal Cancer Trial II (ACT II) and overall treatment duration greater than 53 days has been associated with an increased risk of LRR and colostomy per a pooled analysis of RTOG trials by Ben-Josef et al. [38]. Similarly, a retrospective analysis by Mitra et al. has suggested its detrimental impact upon OS [14]. In contemporary practice, with few patients receiving induction chemotherapy and continued efforts to omit mid-treatment breaks, it is rare for treatment to extend beyond 53 days in the absence of severe toxicity necessitating delays. Therefore, our findings of improved outcomes for patients treated within 39 days supports a continued emphasis on a condensed overall treatment schedule. Assuming treatment commences on a Monday, no intervening holidays, and treatment limited to once per day, this would coincide with limiting treatment to 29 total fractions or less as is currently being utilized in the high-risk cohort of the UK PLATO trial (53.2–61.6 Gy in 28 fractions) [34].
A subset analysis of the RTOG 9811 trial previously reported the impact of tumor stage and LN status upon oncologic outcomes [41]. Patients with T3-4N0 or T3-4N+ disease were at the highest risk for disease relapse or death, with relatively poor disease-free survival of 43% and 27% for patients with T3N+ (IIIC) and T4N+ (IIIC) disease, respectively. These data provide a precedent for treatment intensification strategies in these cohorts [32], [34], [42], [43], [44]. In our series, we identified excellent PFS even in high-risk subgroups with 4-year PFS of 76%, 75%, and 83% for patients with stage IIIA (T1-2N+), IIIB (T4N0), and IIIC (T3-4N+) disease, respectively, and no association between clinical stage grouping and oncologic outcomes. Similarly, we identified 4-year PFS of 78% and 74% in the subsets of patients who meet inclusion criteria for the ongoing high-risk ECOG-ACRIN Trial 2165 and UK PLATO ACT 5 trial, respectively. Apparent improvements in outcomes for patients with stage III disease in our series versus RTOG 9811 may be related to improved diagnostic imaging including PET-CT scan, which may lead to improved targeting and stage migration, lack of induction chemotherapy, shorter overall treatment duration, and improved tolerance of IMRT. As the ECOG-ACRIN Trial 2165 power analysis was based upon event rates from the RTOG 9811 trial with a projected 2-year disease-free survival of 45% in the control group, it is plausible that the study may be underpowered to detect a statistically significant improvement with the addition of maintenance nivolumab in a contemporary cohort of patients receiving high quality IMRT.
Unexpectedly, we did not identify an association between clinical stage and oncologic outcomes, with the most plausible explanation being the limited patient numbers within stage subgroups, low overall event rates, excellent outcomes even amongst high-risk subgroups, and subsequently the inadequate statistical power to identify differences amongst subgroups. However, it is an interesting finding to consider because our practice routinely uses a tailored dosing regimen based upon tumor extent with a particular focus on maintaining a condensed overall treatment duration such that patients with T3-4 tumors received a median dose of RT to the primary tumor and involved LNs of 54 Gy (IQR: 53–57 Gy) and 55 Gy (IQR: 50–58 Gy) in a median of 30 fractions (IQR: 25–30) for a BED of 64 Gy10 over a median duration of 39 days. Additionally, 40% of patients with T3-4 tumor in our series received treatment in 28 fractions or less with daily doses ranging between 1.85 and 2.25 Gy in an attempt to further condense the overall treatment duration. This treatment scheme is most similar to that reported by Mitchell et al. which used DP-IMRT with tailored dosing based upon T-stage (median 54 Gy, range 50–58.8 Gy) and a daily fraction dose of 2–2.1 Gy [17]. Similarly to our series, they demonstrated excellent outcomes with 2-year local control of 91% for the entire cohort and local control of 71% and 80% for patients with T3 tumors and T4 tumors, respectively. Hence, we speculate that the total dose, total number of fractions, and overall treatment duration are at least partially contributing to the favorable outcomes in our series. We must also acknowledge that our cohort was predominately human immunodeficiency virus (HIV)-negative (99%), and thus host immunological factors likely also contributed [45].
These data appear promising and provide continued support of the use of IMRT, although, there are some limitations. This was a retrospective series and is subject to the associated limitations. There was potential underestimation of acute and late treatment-related AEs related to the retrospective nature of data collection for some patients. The addition of patient-reported outcomes would also have strengthened this report, but unfortunately, were unavailable for most patients. Correlation of outcomes with tumor HPV or p16 status may have provided a further understanding of the prognostic significance of this biomarker [46], but unfortunately was unavailable for the majority of patients. Assessments of tumor and treatment characteristics associated with outcomes were limited by low event rates and potentially underpowered statistical comparisons.
5. Conclusion
These data demonstrate that IMRT is associated with favorable toxicity rates and excellent long-term efficacy compared with historical 3DCRT series, and thus serve to support the continued utilization of IMRT as the preferred treatment technique for patients with ASCC. Based upon these data, we speculate that oncologic efficacy may be maintained, even in high-risk subsets, by utilizing simultaneously integrated boost strategies with tailored dosing and a condensed overall treatment duration.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Jethwa report honoraria from RadOncQuestions.com, LLC.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.02.002.
Contributor Information
Krishan R. Jethwa, Email: krishan.jethwa@yale.edu.
Courtney N. Day, Email: day.courtney1@mayo.edu.
William S. Harmsen, Email: harmsen.william@mayo.edu.
Michael G. Haddock, Email: haddock.michael@mayo.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Patterns of recurrence.
Supplementary Table A.1: 4-Year estimates of overall survival, colostomy-free survival, progression-free survival, and locoregional recurrence. Supplementary Table A.2: 4-Year estimates of overall survival, colostomy-free survival, progression-free survival, and locoregional recurrence stratified per inclusion on ECOG-ACRIN Trials 2165/2182 and the UK PLATO Trial. Supplementary Table A.3: Multivariate analysis of OS, CFS, PFS, LRR incorporating T-stage, lymph node status, and overall treatment duration.
References
- 1.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348(9034):1049–54. [PubMed]
- 2.Ajani J.A. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299(16):1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 3.Bartelink H. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15(5):2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 4.Flam M. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14(9):2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson L.L. Long-term update of US GI intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30(35):4344–4351. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James R.D. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 x 2 factorial trial. Lancet Oncol. 2013;14(6):516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 7.Nigro N.D., Vaitkevicius V.K., Considine B., Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17(3):354–356. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 8.Kachnic L.A. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86(1):27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neibart S.S., Manne S.L., Jabbour S.K. Quality of life after radiotherapy for rectal and anal cancer. Curr Colorectal Cancer Rep. 2020;16(1):1–10. doi: 10.1007/s11888-019-00448-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakir R. Patterns and predictors of relapse following radical chemoradiation therapy delivered using intensity modulated radiation therapy with a simultaneous integrated boost in anal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2020;106(2):329–339. doi: 10.1016/j.ijrobp.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant A.K. Intensity modulated radiation therapy versus conventional radiation for anal cancer in the veterans affairs system. Int J Radiat Oncol Biol Phys. 2018;102(1):109–115. doi: 10.1016/j.ijrobp.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Call J.A. Intensity-modulated radiation therapy for anal cancer: results from a multi-institutional retrospective cohort study. Am J Clin Oncol. 2016;39(1):8–12. doi: 10.1097/COC.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones C.M. Toxicity, tolerability, and compliance of concurrent capecitabine or 5-fluorouracil in radical management of anal cancer with single-dose mitomycin-C and intensity modulated radiation therapy: evaluation of a national cohort. Int J Radiat Oncol Biol Phys. 2018;101(5):1202–1211. doi: 10.1016/j.ijrobp.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 14.Mitra D. Long-term outcomes and toxicities of a large cohort of anal cancer patients treated with dose-painted IMRT per RTOG 0529. Adv Radiat Oncol. 2017;2(2):110–117. doi: 10.1016/j.adro.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco P. Comparing simultaneous integrated boost vs sequential boost in anal cancer patients: results of a retrospective observational study. Radiat Oncol. 2018;13(1):172. doi: 10.1186/s13014-018-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arcadipane F. Image-guided IMRT with simultaneous integrated boost as per RTOG 0529 for the treatment of anal cancer. Asia Pac J Clin Oncol. 2018;14(3):217–223. doi: 10.1111/ajco.12768. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell M.P. Intensity-modulated radiation therapy with concurrent chemotherapy for anal cancer: outcomes and toxicity. Am J Clin Oncol. 2014;37(5):461–466. doi: 10.1097/COC.0b013e31827e52a3. [DOI] [PubMed] [Google Scholar]
- 18.Vendrely V. French multicentre clinical evaluation of helical TomoTherapy for anal cancer in a cohort of 64 consecutive patients. Radiat Oncol. 2015;10:170. doi: 10.1186/s13014-015-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han K. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int J Radiat Oncol Biol Phys. 2014;90(3):587–594. doi: 10.1016/j.ijrobp.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 20.Joseph K. Prospective phase II study of tomotherapy based chemoradiation treatment for locally advanced anal cancer. Radiother Oncol. 2015;117(2):234–239. doi: 10.1016/j.radonc.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Franco P. Intensity-modulated radiation therapy with simultaneous integrated boost combined with concurrent chemotherapy for the treatment of anal cancer patients: 4-year results of a consecutive case series. Cancer Invest. 2015;33(6):259–266. doi: 10.3109/07357907.2015.1028586. [DOI] [PubMed] [Google Scholar]
- 22.Salama J.K. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007;25(29):4581–4586. doi: 10.1200/JCO.2007.12.0170. [DOI] [PubMed] [Google Scholar]
- 23.Foster C.C. Treatment outcomes and HPV characteristics for an institutional cohort of patients with anal cancer receiving concurrent chemotherapy and intensity-modulated radiation therapy. PLoS One. 2018;13(3):e0194234. doi: 10.1371/journal.pone.0194234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepek J.M. Intensity-modulated radiation therapy for anal malignancies: a preliminary toxicity and disease outcomes analysis. Int J Radiat Oncol Biol Phys. 2010;78(5):1413–1419. doi: 10.1016/j.ijrobp.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Kachnic L.A. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys. 2012;82(1):153–158. doi: 10.1016/j.ijrobp.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Yates A. Implementing intensity-modulated radiotherapy with simultaneous integrated boost for anal cancer: 3 year outcomes at two Sydney institutions. Clin Oncol (R Coll Radiol) 2015;27(12):700–707. doi: 10.1016/j.clon.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Vieillot S. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol. 2012;7:45. doi: 10.1186/1748-717X-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Call J.A. Concurrent chemotherapy and intensity modulated radiation therapy in the treatment of anal cancer: a retrospective review from a large academic center. Pract Radiat Oncol. 2013;3(1):26–31. doi: 10.1016/j.prro.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Janssen S. Clinical experience of SIB-IMRT in anal cancer and selective literature review. Radiat Oncol. 2014;9:199. doi: 10.1186/1748-717X-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myerson R.J. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74(3):824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng M. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys. 2012;83(5):1455–1462. doi: 10.1016/j.ijrobp.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 32.ECOG-ACRIN trial 2165: nivolumab after combined modality therapy in treating patients with high risk stage II-IIIB anal cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT03233711.
- 33.ECOG-ACRIN trial 2182: lower-dose chemoradiation in treating patients with early-stage anal cancer, the DECREASE study.
- 34.PLATO – personalising anal cancer radiotherapy dose. Available from: http://www.isrctn.com/ISRCTN88455282.
- 35.Lerman J. Impact of tobacco smoking on the patient's outcome after (chemo)radiotherapy for anal cancer. Eur J Cancer. 2020;141:143–151. doi: 10.1016/j.ejca.2020.09.039. [DOI] [PubMed] [Google Scholar]
- 36.Alsadius D. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol. 2011;101(3):495–501. doi: 10.1016/j.radonc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Eifel P.J. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20(17):3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Josef E. Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of Radiation Therapy Oncology Group trials 87–04 and 98–11. J Clin Oncol. 2010;28(34):5061–5066. doi: 10.1200/JCO.2010.29.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glynne-Jones R. Impact of compliance to chemoradiation on long-term outcomes in squamous cell carcinoma of the anus. Results of a post-hoc analysis from the randomized phase III ACT II trial. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal D.I. Final report of a prospective randomized trial to evaluate the dose-response relationship for postoperative radiation therapy and pathologic risk groups in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2017;98(5):1002–1011. doi: 10.1016/j.ijrobp.2017.02.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunderson L.L. Anal carcinoma: impact of TN category of disease on survival, disease relapse, and colostomy failure in US Gastrointestinal Intergroup RTOG 98–11 phase 3 trial. Int J Radiat Oncol Biol Phys. 2013;87(4):638–645. doi: 10.1016/j.ijrobp.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakravarthy A.B. Long-term follow-up of a Phase II trial of high-dose radiation with concurrent 5-fluorouracil and cisplatin in patients with anal cancer (ECOG E4292) Int J Radiat Oncol Biol Phys. 2011;81(4):e607–e613. doi: 10.1016/j.ijrobp.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konski A. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92–08. Int J Radiat Oncol Biol Phys. 2008;72(1):114–118. doi: 10.1016/j.ijrobp.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peiffert D. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30(16):1941–1948. doi: 10.1200/JCO.2011.35.4837. [DOI] [PubMed] [Google Scholar]
- 45.Oehler-Jänne C. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26(15):2550–2557. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert D.C. p16INK4A, p53, EGFR expression and KRAS mutation status in squamous cell cancers of the anus: correlation with outcomes following chemo-radiotherapy. Radiother Oncol. 2013;109(1):146–151. doi: 10.1016/j.radonc.2013.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patterns of recurrence.
Supplementary Table A.1: 4-Year estimates of overall survival, colostomy-free survival, progression-free survival, and locoregional recurrence. Supplementary Table A.2: 4-Year estimates of overall survival, colostomy-free survival, progression-free survival, and locoregional recurrence stratified per inclusion on ECOG-ACRIN Trials 2165/2182 and the UK PLATO Trial. Supplementary Table A.3: Multivariate analysis of OS, CFS, PFS, LRR incorporating T-stage, lymph node status, and overall treatment duration.