Abstract
Introduction:
Recently, initiation and enhancement of extraction socket healing has been amplified by platelet concentrates, whereas the positive role of Sticky bone has been focused on maintaining alveolar bone dimensions. This study aimed to determine the effectiveness of Sticky Bone for socket grafting of mandibular third molars (M3Ms) in terms of soft- and hard-tissue healing.
Materials and Methods:
This split-mouth prospective trial constituted prophylactic removal of M3Ms with Sticky bone grafted in the study site as a primary predictor variable. Patients underwent 3 months of mandatory follow-up where pain, swelling, interincisal mouth opening, and gingival healing were measured on the 3rd, 7th, and 14th day using the Numeric Rating Scale, anatomic landmarks, steel metric ruler, and criteria given by Landry et al. respectively. Radiological healing was calculated based on the height of the socket, Kelly's Index, and histogram values immediately after the procedure at 1 week, 1 month, and 3 months, respectively. Statistical comparison was made using Paired t-test. P < 0.05 was considered significant.
Results:
Forty-seven patients (mean 26.83 ± 6.58 years) demonstrated significantly lesser pain, swelling, and better gingival healing at the study site on multiple periods of follow-up. Rapid bone formation with superior density, lesser alveolar resorption, earlier bone blending, and trabecular formation were noticed on the study site with a significant difference at all time intervals.
Discussion:
Sticky bone was chosen as the graft owing to advantages such as simple preparation, convenient handling characteristics, safety, evident postoperative patient comfort, better retention of the clot, enhanced soft-tissue healing, absence of infection, and decreased osseous deformation as compared to the control site. This study validated the role of Sticky bone as an indispensable component of regenerative therapy in the orofacial osseous tissues as it was an ideal biologic graft with fibrin rich structure.
Conclusion:
This study validated the role of Sticky bone as an indispensable component of regenerative therapy in the orofacial osseous tissues as it was an ideal biologic graft with fibrin rich structure.
Keywords: Bone density, mandibular third molar, soft tissue healing, sticky bone
INTRODUCTION
Although removal of the mandibular third molar (M3M) is a routinely attempted procedure, the anatomy of adjacent structures and difficult accessibility impair its smooth surgical management.[1] The removal of adjacent bone for safe delivery of tooth dictates that bony healing of the extraction socket should be studied and discussed thoroughly.
The osteoclastic activity post-extraction is markedly seen on the buccal wall in comparison to lingual and more in the mandible than the maxilla for about 3–6 months.[2] Clinicians often wait for the cessation of physiological resorption before opting for prosthetic rehabilitation, resulting in the deficient alveolar ridge.[3] To prevent dimensional changes of the socket, many studies have employed autogenous bone grafts or substitutes, guided bone regeneration with resorbable or nonresorbable membranes, and various bone promoting molecules such as enamel matrix derivative, recombinant growth, and differentiation factors, and autologous platelet derivatives to augment the regenerative process of the socket.[4] Autografts are considered as GOLD STANDARD owing to its osteogenicity, osteoinductivity, and osteoconductivity. However, due to operational pitfalls and limited quantity, they have been replaced with allografts such as Demineralized Freeze-Dried Bone Allografts or Xenografts such as Bovine bone.[5] Over the past few decades, application of platelet concentrates in socket healing has been explored to harness favorable properties of platelets such as cellular differentiation and angiogenesis.[4]
The specific use of platelets in grafting procedures is credited to Whitman et al. and Marx et al. through their use of platelet-rich plasma (PRP) in mandibular continuity defects.[6,7] However, long preparation time, addition of bovine thrombin, and variable quality of preparation of PRP forced researchers to create platelet-rich fibrin (PRF) and concentrated growth factor (CGF) as second-generation platelet concentrates which exhibited greater promotion of wound healing, eliminated the role of thrombin, had easier preparation and denser matrix of boosted growth factors.[8,9]
Despite the numerous advantages of the above mentioned autologous grafts, space maintenance of defect and subsequent stability of grafts was always questionable. Unlike PRP, PRF and CGF layer failed to stabilize particulate or powder bone. To contain the particulate bone graft within the cavity during the postoperative healing period in attempt to repair bony defects or for three dimensional ridge augmentation, the use of bone tack, collagen membrane, or titanium mesh was almost inevitable. However, these procedures are surgically time-consuming, technique sensitive, and cause an additional financial burden. Therefore, Sticky Bone was introduced in 2010 by Sohn et al. as a solidified bone graft entrapped in fibrin network. It is a growth factor enriched bone graft matrix prepared using autologous fibrin glue (AFG) and an alloplast.[10] Stabilization of bone graft in the defect to accelerate tissue healing and elimination of loss of graft is a characteristic feature of Sticky bone. It also prevents the ingrowth of soft tissue in the graft.[11] The study objective was to demonstrate successfully the use of Sticky Bone as a graft material in osseous healing of socket after the M3M extractions.
MATERIALS AND METHODS
This prospective clinical study was conducted at the Department of Oral and Maxillofacial Surgery at our institute after due authorization from the Institutional Ethics Committee (AMC Institutional Ethics Committee Reg No: ECG/236/Indt/GJ/2015/RR-18). The split-mouth study, conducted from December 2017 till December 2019 with a minimum follow-up period of 3 months, constituted of a sample of 47 patients.
Inclusion criteria
All healthy patients aged 18–45 years with bilaterally symmetrical M3M indicated for extraction (Class 1/2; Position A/B-according to Winter's and Pell and Gregory classification) [Table 1 and Figure 1]
ASA Class 1 patients
Patients without any oral destructive habits such as smoking, chewing tobacco, bruxism, etcetera
Patients who were presurgically asymptomatic.
Table 1.
Data regarding the type of impactions included in the sample
| Angulation | Position | Class | n |
|---|---|---|---|
| Vertical | A | 1 | 6 |
| A | 2 | 4 | |
| B | 1 | 4 | |
| B | 2 | 7 | |
| Distoangular | A | 1 | 6 |
| A | 2 | 7 | |
| B | 1 | 8 | |
| B | 2 | 5 | |
| Total | 47 |
Figure 1.
Preoperative orthopantomogram
Exclusion criteria
Patients with a history of allergy to any drugs or biomaterial to be used in the procedure or with a history of intake of drugs which may alter the physiologic healing potential
Patients with a history of radiotherapy/chemotherapy
Patients who were pregnant, lactating, or on oral contraceptives
Patients having acute/chronic infections or any kind of pathology (evaluated and confirmed radiographically)
Cases with complications such as – fracture of tooth crown or root, fracture of the adjacent alveolus, fracture of mandible, dislocation of the adjacent tooth, or excessive intraoperative hemorrhage.
Surgical procedure
Before the study, its purpose was explained to the patients and routine blood investigations were advised. They were informed about possible complications and follow-up visits. Detailed clinical history was recorded in a proforma sheet, and preoperative clinical plus radiographic records were taken (Intraoral Periapical Radiographs [IOPA] with GRID (Bluedent India, Chennai, India) and Orthopantomogram [OPG]). Patients were prescribed standard prophylactic medications (capsule amoxicillin 500 mg TDS, tablet diclofenac sodium 50 mg + tablet paracetamol 500 mg BD and tablet ranitidine 150 mg BD) 1 day before the procedure and were advised to be continued for 4 days postoperatively.
Under strict asepsis, surgical removal of bilateral M3Ms was performed using local anesthesia. The standard surgical technique was followed for each patient and was performed by a single operator. The operated sites were randomly divided, by toss of a coin, into control and study groups. Primary closure was done at the control site using 3-0 black braided silk suture, and study site was grafted with Sticky Bone (prepared as per the protocol laid by Sohn et al., and primary closure was achieved.[10] Immediate postoperative radiographs were taken, and patients were recalled on the 3rd day, 7th day, 14th day, 1 month, and 3 months for follow-up.
Preparation of sticky bone
Around 20 cc of venous blood was taken from the cubital vein and centrifuged at 2400–2700 rotations per minute (rpm) using a specific centrifuge machine (REMI R4C) (REMI Laboratory Instruments, Mumbai, India) running at alternated and controlled speed for 2 min. The upper layer of AFG thus prepared was aspirated and mixed with an alloplastic particulate bone graft (mixture of hydroxyapatite + tricalcium phosphate) to polymerize for 10–15 min to form Sticky Bone [Figure 2].
Figure 2.
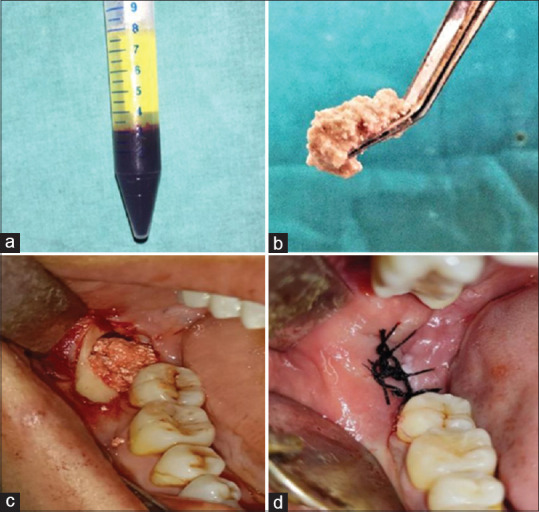
(a) Autologous fibrin glue after centrifugation. (b) Polymerised sticky bone. (c) Sticky bone grafted in socket. (d) Primary closure done
Clinical evaluation
The pain was assessed at Day 3, Day 7 and Day 14 using the Numerical Rating Scale to subjectively record the pain score from 0 to 10.[12]
Assessment of swelling was made through the distance measured between three anatomical points: lateral canthus of eye to gonial angle, tragus to the commissure of the mouth, and tragus to Pogonion[13] [Figure 3]. Evaluation of soft-tissue healing was done at Day 3, Day 7, and at Day 14 using the index given by Landry et al. based on tissue color, bleeding on palpation, epithelialization of incision margins, and suppuration.[14]
Figure 3.
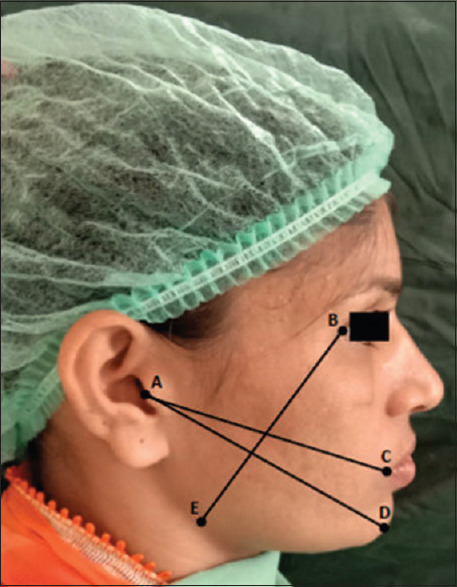
Three imaginary lines joining AC, AD and BE for calculation of postoperative facial swelling
Interincisal mouth opening was recorded by measuring the distance between the incisal edges of maxillary and mandibular central incisors on Day 3, Day 7, and Day 14.[15]
Radiographic evaluation
IOPA with Grid, at the control and the study sites, were taken at immediate postoperative, 7th day, 1 month, and 3 months to observe the bone re-fill in the socket. The scores for healing were recorded as per the modification of Kelly's Index given by Ogundipe et al.[15]
The IOPA radiographs were also evaluated to measure the height of the alveolar socket. A straight line was drawn parallel to the occlusal plane from the cementoenamel junction on the distal surface of the second mandibular molar. Then another straight line was drawn from the base of the socket perpendicular to the previously drawn line to measure the height of the socket. OPG, taken at a similar time period, was analyzed using Mean grayscale measurement of the extraction sockets employing C.S. Imaging Software 7.0.3 (Carestream Health, Inc, 2010, Rochester New York, United States) [Figure 4].
Figure 4.
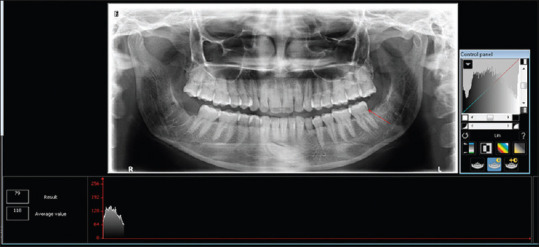
Densitometric Analysis done with Carestream Imaging Software 7.0.3
Bone density measurement was done using the radiographic landmarks delineated over the area of the extraction socket, as described by Kaul et al.[16]
Statistical analysis
The mean value and standard deviation (SD) of each parameter were calculated and checked for statistical significance using the Paired Samples t-test. All the data were compiled and analysis was completed using IBM SPSS Statistics for Windows (version 11, IBM Corp, Armonk, NY, USA).
RESULTS
The study sample constituted of 16 male and 31 female patients with a Male:Female ratio of 1:1.93 showing a clear female predominance [Figure 5]. The participants of the study ranged from 18 to 39 years with a mean (SD) age of 26.83 ± 6.58 years.
Figure 5.
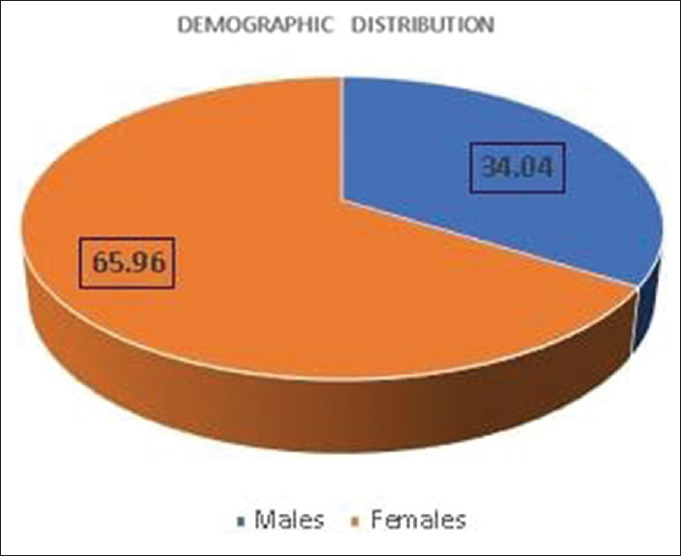
Distribution of gender in the sample
The mean difference between experimental and control group with respect to the class, position, and angulation of the tooth was − 0.83, −0.83 and 9.00, showing no statistical significance. The difficulty index of the sample was constant on both sides, with a mean (SD) of 6.08 ± 1.165.
Assessment of pain and swelling
The mean (SD) score for pain on the 3rd day for the study site was 7.25 ± 0.62 and for the control site was 7.75 ± 0.62. Both the study and control sites showed a gradual reduction in pain, which was negligible at 2 weeks interval with a common mean (SD) value of 0.08 ± 0.289 for both the sites.
The results of Table 2 show a gradual reduction in facial swelling over a period of 2 weeks with a significant reduction on study site at Day 3 and Day 7 with a mean difference of 0.792 (P = 0.005) and 0.642 (P = 0.000), respectively.
Table 2.
Comparison of pain, swelling and gingival healing scores between the two groups at postoperative day 3rd, 7th and 14th
| Pain | Swelling | Gingival healing | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3rd day | 7th day | 14th day | Preoperative | 3rd day | 7th day | 14th day | 3rd day | 7th day | 14th day | |||||||||||
| S | C | S | C | S | C | S | C | S | C | S | C | S | C | S | C | S | C | S | C | |
| Mean | 7.25 | 7.75 | 4.25 | 4.83 | 0.08 | 0.08 | 36.46 | 36.69 | 38.68 | 39.48 | 37.28 | 37.92 | 36.65 | 36.94 | 2.08 | 2.08 | 3.08 | 2.75 | 4.58 | 4.08 |
| Standard deviation | 0.622 | 0.622 | 0.622 | 0.835 | 0.289 | 0.289 | 1.113 | 0.977 | 1.416 | 1.786 | 1.284 | 1.356 | 1.157 | 1.016 | 0.289 | 0.289 | 0.515 | 0.754 | 0.515 | 0.515 |
| Mean difference | 0.500 | 0.583 | 0.000 | −0.233 | 0.792 | 0.642 | −0.292 | −23.333 | 12.833 | 0.000 | ||||||||||
| P* | 0.053 | 0.002 | 1.000 | 0.097 | 0.005 | 0.000 | 0.075 | 1.000 | 0.039 | 0.026 | ||||||||||
| Significance | NS | S | NS | NS | S | S | NS | NS | S | S | ||||||||||
*Paired t-test. S=Study group; C=Control group; NS=Not significant; S=Significant
Assessment of gingival healing
Both study and control sites showed similar healing scores of the mean (SD) 2.08 ± 0.289 on Day 3, which improved comparatively on study site on Day 7 and Day 14 with a mean difference of 12.833 (P = 0.039) and 0.000 (P = 0.026), respectively [Table 2].
Assessment of postoperative mouth opening
Patients had a statistically significant increase in their interincisal mouth opening at each follow-up intervals with the lowest mean (SD) values of 15.58 ± 3.059 mm seen at Day 3 and the mean (SD) values of 37.42 ± 1.88 mm seen at Day 14, which were nearly similar to the preoperative measurements [Table 3].
Table 3.
Data representing the increase in interincisal mouth opening at each follow-up interval
| Interincisal mouth opening | ||||
|---|---|---|---|---|
| Preoperative | 3rd day | 7th day | 14th day | |
| Mean | 38.92 | 15.58 | 24.58 | 37.42 |
| Standard deviation | 2.392 | 3.059 | 3.232 | 1.881 |
| Mean difference | −23.33 | −14.33 | −1.50 | |
| P* | 0.000 | 0.000 | 0.026 | |
| Significance | S | S | S | |
*Paired t-test. S=Significant
Assessment of height of the socket
The decrease in the height of the socket measured at 1 week showed a mean (SD) value of 10.08 ± 0.996 mm, which then reduced to 9.42 ± 0.900 mm and 8.58 ± 7.17 mm at 1 month and 3 months, respectively, at the study site. The reduction in the height of the socket was markedly more at the control site (P = 0.000) at all-time intervals as its height of socket reduced to mean (SD) 7.17 ± 1.193 mm, whereas at the study site mean (SD) reduced to 8.58 ± 0.996 mm at the end of 3 months [Table 4 and Figure 6].
Table 4.
Comparison of height of the socket - preoperatively, at immediate postoperatively, at 1 week, 1 and 3 months and radiographic healing scores between the two groups at immediate postoperative period, 1 week, 1 and 3 months respectively
| Height of the socket | Kelley’s index | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Immediate | 1 week | 1 month | 3 months | Immediate | 1 week | 1 month | 3 months | ||||||||||
| S | C | S | C | S | C | S | C | S | C | S | C | S | C | S | C | S | C | |
| Mean | 10.50 | 10.25 | 10.50 | 10.25 | 10.08 | 9.00 | 9.42 | 8.17 | 8.58 | 7.17 | −0.75 | −3.25 | 1.08 | −1.17 | 2.92 | 0.75 | 4.75 | 2.42 |
| Standard deviation | 1.168 | 1.215 | 1.168 | 1.215 | 0.996 | 1.348 | 0.900 | 1.267 | 0.996 | 1.193 | 1.357 | 1.422 | 1.240 | 1.586 | 0.669 | 0.965 | 0.622 | 0.515 |
| Mean difference | −0.333 | −0.500 | −0.250 | −0.250 | −1.083 | −1.250 | −1.417 | −2.500 | −2.250 | |||||||||
| P* | 0.339 | 0.339 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||||||
| Significance | NS | NS | S | S | S | S | S | S | S | |||||||||
*Paired t-test. S=Study group; C=Control group; NS=Not significant; S=Significant
Figure 6.
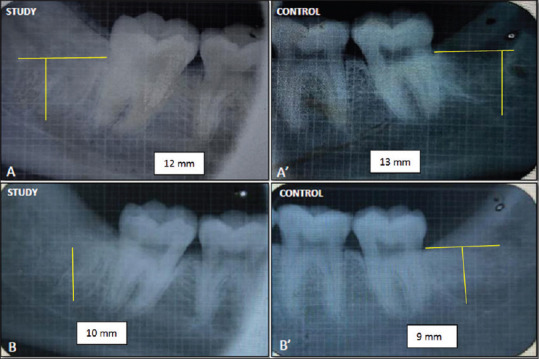
(A) Immediate (Grid) imaging of the study site. (A') (Grid) imaging of the study site after 3 months. (B) Immediate (Grid) imaging of the control site (B') (Grid) imaging of the study site after 3 months
Assessment of radiographic healing (Kelly's Index)
The mean (SD) radiographic healing scores at 1 week, 1 month and 3 months for the study site were 1.08 ± 1.290, 2.92 ± 0.669, and 4.75 ± 0.622, respectively, which showed a statistical significance (P = 0.000) over the mean (SD) scores of − 1.17 ± 1.586, 0.75 ± 0.965 and 2.42 ± 0.515 of the control group at the same time of follow-up [Table 4].
Assessment of mean bone density
The bone histogram analysis at the sites of extraction showed mean (SD) preoperative density values of 170.17 ± 31.866 and 160.92 ± 28.523 at the site of study and control. The increased density at study site in the immediate postoperative period was 202.08 ± 38.068, which was significantly higher (P = 0.000) than the decrease in mean (SD) bone density at the control site. The mean bone density difference of −9.250, −45.083 and −52.667 between study and control sites observed at the end of 1 week, 1 month, and 3 months was statistically significant (P = 0.000) although a decrease in bone density was observed at both the sites in comparison to preoperative values [Table 5 and Figure 7].
Table 5.
Comparison of bone density levels - preoperatively, at immediate postoperatively at 1 week, 1 and 3 month
| Immediate | 1 week | 1 month | 3 months | |||||
|---|---|---|---|---|---|---|---|---|
| S | C | S | C | S | C | S | C | |
| Mean | 202.08 | 157.00 | 188.25 | 135.58 | 166.92 | 108.58 | 143.67 | 77.67 |
| Standard deviation | 38.068 | 28.626 | 39.162 | 21.940 | 37.999 | 24.138 | 33.765 | 22.552 |
| Mean difference | −2.333 | −9.250 | −45.083 | −52.667 | ||||
| P* | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Significance | S | S | S | S | ||||
*Paired t-test. S=Study group; C=Control group; NS=Not significant; S=Significant
Figure 7.

Variation in bone density levels with time
DISCUSSION
The concept of early and superior new bone formation has rapidly gained momentum as newer treatment modalities pertaining to oral and maxillofacial reconstruction are contingent upon faster bony regeneration and lesser alveolar resorption.[17] Restoration of bony defect subsequent to surgical trauma represents a challenge in comprehensively treating patients who demand early, socially pleasing, and esthetic restorative options.[18] Healing of the socket is an intricate process wherein the clot fills the alveolus immediately after extraction, followed by the recruitment of constructive inflammatory cells and growth factors.[19] The accumulation of granulation tissue within the socket is followed by epithelium migration and the activity of osteoblasts and osteoclasts demonstrates alterations in the dimensions of alveolus, which leads to the deposition of bone within the entire socket with its radiopacity comparable to the adjacent bone at the end of 15 weeks.[19,20,21]
The rationale of socket grafting intends to hasten this physiologic healing by providing a solid scaffold to strengthen the coagulum during early phases of healing using a plethora of biomaterials. The recent surge in the use of platelet concentrates for superior epithelial and osseous regeneration has provided substantial evidence which display reduced inflammation, untoward complications, and stimulated ossification.[4,22,23,24,25]
However, conditions such as technique sensitivity, prolonged preparation time of PRP, the addition of chemical additives in PRGF, and limited graft stability achieved with PRF and CGF demanded the advent of a newer autologous graft material which could be amenable for use in all conditions.[8,10] Sticky bone, the latest among the autologous concentrates, was introduced by Sohn et al. in 2015 as a biologically solidified bony matrix trapped within a fibrin meshwork, prepared by alternated and controlled centrifugation of venous blood at a variable speed of 2400–2700 rpm. The blood collection excluded prior addition of anticoagulant and centrifugation time of only 2–12 min was required to obtain AFG.[8,10] After centrifugation, AFG was aspirated and mixed with particulate bone powder, and after a polymerization period of 10–15 min, yellow-colored Sticky bone was formed. This method of preparation was simple, cost-effective, and could be readily incorporated in surgical practice.
Although the use of sticky bone has been successfully demonstrated in multiple case reports, the authors have largely concentrated on the dimensional stability of the alveolus with respect to implant placement in the anterior regions.[10,26,27,28] Interestingly, the mandibular posterior region is also vulnerable for vertical bone resorption with subsequent soft tissue recession as the cleft between the mandibular second molar, and a mesioangular/horizontally inclined M3M attracts colonization of potential pathogens leading to postextraction periodontal defects, although conflicting evidence have emerged which support vertical impactions as having the highest potential (18.8%) to cause distal bone loss.[29,30] The grafting of sticky bone in mesioangular or horizontally impacted molars was absent in this study due to the unavailability of bilaterally symmetrical cases, which may have served as a better indicator for its use as a graft material. Furthermore, it is binding to acknowledge that the soft tissue defect may appear over a long period of 6–36 months and is also dependent upon other factors such as iatrogenic trauma during extraction, increased age, status of eruption and preexisting periodontal defects, all of which have been taken into consideration during the design of this study.[30,31,32] In addition, as M3M removal is one of the most frequently attempted surgical procedure across the world, it was chosen as a template for grafting, the results of which can be meticulously replicated in areas which demand enhanced bone regeneration, comparable to other similar trials conducted earlier.[13,15,17,33]
This study hypothesized sticky bone as a biologic model socket graft material due to ease of its preparation, better handling properties, mechanical retention in socket evident through a number of clinical and radiographic parameters. The confounding factors such as gender, oral hygiene, age and smoking influencing pain, edema, trismus, and subsequent healing were eliminated as it was a split-mouth trial. The gender variation in the study sample was in line with the inclusion criteria of previous studies, which exhibited a clear female preponderance, and hence it justifies our sample selection. In addition, all surgical procedures were carried out by a single surgeon to remove any possibility of operator variability.[17,34]
The NRS score, originally given by Downe in 1978, offered great accuracy and was used to record the pain score, which was highest initially, decreasing subsequently with lesser scores for the study group at all times, showing statistical significance only at one week. Similarly, percentage increase in facial swelling, which was measured using fixed anatomic landmarks in accordance with the findings of multiple studies, reflected significantly better results for the study site up to one week with comparable results present at the end of 14 days.[13,18,35] These findings reinforce the efficacy of sticky bone in mitigating the adverse postoperative findings of pain and swelling as both sites undergo similar inflammatory process and eliminate any concern due to the presence of alloplastic bone graft in the polymerized mixture.[13,15,18,19,35,36] Moreover, as socket healing commences, hemostasis and coagulation result in the formation of a clot,[37] similar for both sites, but with better retention in the study group due the fibrin network of the AFG imparting an obvious mechanical advantage. Thus, the positive results for the study site promulgate its use to lessen patient's discomfort and derive better patient co-operation and compliance.
Maximum reduction in the mouth opening in the immediate postoperative period was followed by notable improvement with no comparison of the degree of trismus between the two extraction sites as it was a split-mouth study, unlike previous studies.[15,18] Preoperative mouth opening achieved in 41.66% of patients by Day 14 and at the end of 1 month, for the rest of the sample, followed normal operative healing pattern after third molar impactions and was not adversely hindered by the addition of the graft.
Even though sticky bone did not affect the immediate gingival healing index scores, calculated using criteria given by Landry et al. to judge the degree of gingival inflammation and repair, it resulted in better healing for the study site ultimately by the end of 2 weeks, comparable to other similar studies done using PRF as a graft material.[1,13,17,19] Biologically, the inflammatory phase witnesses the action of neutrophils and macrophages in the form of phagocytosis and the release of growth factors that are concentrated, larger and denser in the fibrin matrix of Sticky Bone due to lesser time of centrifugation used to prepare AFG.[10] This might expedite the cellular processes of chemotaxis and angiogenesis, leading to rapid tissue repair, as evident by the tissue color and margin on the study site within 14 days of extraction.[37] Progressively, as the socket gets impregnated with intense fibroblast migration and extracellular collagen during the proliferative stage, it allows enhanced adhesion and anchorage.[37] This process, evident by the absence of exposed granulation tissue, was precipitated on the study site as AFG may lead to sustained release of plasma-derived growth factors, insulin-like growth factors, and transforming growth factor-beta.
Owing to the nature of the surgical procedure, patients of both the groups experienced minor discomfort in the form of postoperative edema, reduced mouth opening, and a case of buccal sulcus ecchymosis, which were efficiently managed conservatively using warm saline gargles and mouth opening exercise along with medications. None of the patients in either group experienced episodes of any major complications such as alveolar osteitis or postoperative infections owing to strict adherence to standard aseptic protocols and comprehensive prophylactic medications.
The use of grids for determination of the height of the socket proved to be an efficient method of saving undue cost and time, and it showed a significant decrease in the rate of resorption of the socket heights of M3M on the study site as radiographic healing progressed periodically till 3 months. The positional stability of sticky bone prevented fibrous in-growth, which reduced alveolar resorption and helped in preserving the postoperative socket height. Hence, the present study affirms the positive role of sticky bone in maintaining the dimensions of the M3M socket and adds to the existing reports promoting the favorable properties of the graft in the anterior region.[10,30,38]
The quality of osseous regeneration was studied by evaluating IOPA for the presence or absence of lamina dura, increase or decrease in density, and coarseness or fineness of the trabeculations as stated by Kelly in 1980.[39] The results of the present study simulated the findings of earlier studies done using PRF, as it showed marked absence of the lamina dura, denser refilled bone, and coarser trabecular patterns on the study site at follow up intervals of 1 month and 3 months.[15,18] Furthermore, panoramic radiographs were used to judge the density of the refilled bone using densitometric analysis, as they were conveniently available, caused reduced radiation exposure and allowed simultaneous observation of both extraction sites. It revealed significantly higher bone density at the study site due to the presence of graft in the immediate postoperative period. The mean bone density subsequently decreased with lesser grayscale values over the time interval of 3 months for both the groups with a significantly lesser reduction seen over the study socket. Growth factors present within AFG might stimulate the deposition of precursors of bone-forming cells and hinder the osteoclast activity. Consequently, deposition of sticky bone acts as a nidus for the accelerated conversion of osteoid into mineralized tissue having superior bone density and elimination of lamina dura within 4–8 weeks as compared to inferior bone density seen on the control site for the same time interval.
These observations made by clinical and image histogram analysis were encouraging as parallel inferences have been made with respect to older generations of platelet concentrates,[17,18,19,38,40] but further histomorphometric studies are warranted to establish the precise roles of chemical mediators in sticky bone for providing radiographically detectable favorable bone healing.
Although the use of autologous concentrates such as PRP, PRF, and CGF has been widely documented in interventions such as alveolar ridge augmentation, improving implant stability and marginal bone loss and alveolar grafting in cases of cleft lip and palate, recent evidence have presented confounding results doubting the potential of PRF and PRGF in osseous healing of the extraction sockets.[41,42] Conversely, over and above the clinical benefits of sticky bone usage, it is a fairly ideal autologous graft which can be rapidly prepared to fill up the entire socket cavity according to the shape of the socket due to its excellent mouldability without disintegration into adjacent soft tissues, as its inherent mass is made up of particulate bone which had fibrin interconnections. This obviated the incorporation of titanium mesh and bone blocks, and a CGF membrane can also be prepared using the same methodology to cover the grafted site instead of an alloplastic option. All these properties have been previously affirmed to distinguish sticky bone as the first choice for any surgeon who wishes to opt for an autologous graft with minimum morbidity, relatively decreased burden on the cost and time of the surgery.[8,30,43,44]
Even though the observations derived from this study were consistent in the support of the use of Sticky bone in bony defects, recent advances such as laser scanning for swelling and quantitative computed tomography, bone scintigraphy, and dual-energy X-ray absorptiometry (DEXA) for measuring bone density would be a more conclusive option for assessing the osseous healing potential of Sticky bone.[17,42] Owing to the practical difficulties in pursuing these options in multiple follow-ups for a developing country like India, we believe that we were able to carry out the present study in the most comprehensive, convenient, and fairly accurate manner.
CONCLUSION
The findings of the present study confirmed the hypothesis that utilization of AFG enriched bone graft matrix (Sticky Bone) leads to promising results for gingival healing and reossification of alveolar defects. The preparation of sticky bone was convenient, and it was suitable for grafting as it has superior handling characteristics. The strong fibrin interconnection allowed it to be retained within the socket while maintaining the socket dimensions.[10] Further exhaustive trials with sticky bone engaging newer investigative techniques such as cone beam computed tomography, stereo lithography, DEXA scan or scintigraphy are warranted in all kinds of osseous defects which demand superior and rapid ossification to conclusively establish it as an indispensable part of any surgical intervention which necessitates fixed prosthetic replacements, especially in younger individuals.[40,45]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh V, Alex K, Pradhan R, Mohammad S, Singh N. Techniques in the removal of impacted mandibular third molar: A comparative study. Eur J Gen Dent. 2013;2:25. [Google Scholar]
- 2.Moraschini V, Barboza ES. Effect of autologous platelet concentrates for alveolar socket preservation: A systematic review. Int J Oral Maxillofac Surg. 2015;44:632–41. doi: 10.1016/j.ijom.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Irinakis T, Tabesh M. Preserving the socket dimensions with bone grafting in single sites: An esthetic surgical approach when planning delayed implant placement. J Oral Implantol. 2007;33:156–63. doi: 10.1563/0.824.1. [DOI] [PubMed] [Google Scholar]
- 4.Del Fabbro M, Bortolin M, Taschieri S. Is autologous platelet concentrate beneficial for post-extraction socket healing. A systematic review? Int J Oral Maxillofac Surg. 2011;40:891–900. doi: 10.1016/j.ijom.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Misch CM. Autogenous bone: Is it still the gold standard? Implant Dent. 2010;19:361. doi: 10.1097/ID.0b013e3181f8115b. [DOI] [PubMed] [Google Scholar]
- 6.Whitman DH, Berry RL, Green DM. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–9. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 7.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 8.Upadhayaya V, Arora A, Goyal A. Bioactive platelet aggregates: Prp, Prgf, Prf, Cgf and sticky bone. J Dent Med Sci. 2017;16:5–11. [Google Scholar]
- 9.Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, Choung HW, et al. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2011;17:349–59. doi: 10.1089/ten.TEA.2010.0327. [DOI] [PubMed] [Google Scholar]
- 10.Sohn DS, Huang B, Kim J, Park WE, Park CC. Utilization of autologous concentrated growth factors (CGF) enriched bone graft matrix (Sticky bone) and CGF-enriched fibrin membrane in implant dentistry. J Implant Adv Clin Dent. 2015;7:11–29. [Google Scholar]
- 11.HalimAyoub A, Ramadan O. Comparative study socket preservation using PRF and MPM platelet concentrates. I J Pre Clin Dent Res. 2015;2:1–4. [Google Scholar]
- 12.Sirintawat N, Sawang K, Chaiyasamut T, Wongsirichat N. Pain measurement in oral and maxillofacial surgery. J Dent Anesth Pain Med. 2017;17:253–63. doi: 10.17245/jdapm.2017.17.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta SR, Passi D, Singh P, Sharma S, Singh M, Srivastava D. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. Natl J Maxillofac Surg. 2016;7:45–51. doi: 10.4103/0975-5950.196124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landry RG, Turnbull RS, Howley T. Effectiveness of benzydamyne HCl in the treatment of periodontal post surgical patients. Res Clin Forums. 1988;10:105–18. [Google Scholar]
- 15.Ogundipe OK, Ugboko VI, Owotade FJ. Can autologous platelet-rich plasma gel enhance healing after surgical extraction of mandibular third molars? J Oral Maxillofac Surg. 2011;69:2305–10. doi: 10.1016/j.joms.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Kaul RP, Godhi SS, Singh A. Autologous platelet rich plasma after third molar surgery: A comparative study. J Maxillofac Oral Surg. 2012;11:200–5. doi: 10.1007/s12663-011-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varghese MP, Manuel S, LK Kumar Surej. Potential for osseous regeneration of platelet-rich fibrin A comparative study in mandibular third molar impaction sockets. J Oral Maxillofac Surg. 2017;75:1322–9. doi: 10.1016/j.joms.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Jeyaraj PE, Chakranarayan A. Soft tissue healing and bony regeneration of impacted Mandibular third molar extraction sockets, following postoperative incorporation of platelet-rich fibrin. Ann Maxillofac Surg. 2018;8:10–8. doi: 10.4103/ams.ams_185_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Post extraction alveolar ridge preservation: Biological basis and treatments. Int J Dent. 2012;151030:1–13. doi: 10.1155/2012/151030. doi:10.1155/2012/151030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amler MH. The time sequence of tissue regeneration in human extraction wounds. Oral Surg Oral Med Oral Pathol. 1969;27:309–18. doi: 10.1016/0030-4220(69)90357-0. [DOI] [PubMed] [Google Scholar]
- 21.Mangos JF. The healing of extraction wounds. N Z Dent J. 1941;37:4–23. [Google Scholar]
- 22.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 23.Carlson NE, Roach RB., Jr Platelet-rich plasma: Clinical applications in dentistry. J Am Dent Assoc. 2002;133:1383–6. doi: 10.14219/jada.archive.2002.0054. [DOI] [PubMed] [Google Scholar]
- 24.Gruber R, Varga F, Fischer MB, Watzek G. Platelets stimulate proliferation of bone cells: Involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002;13:529–35. doi: 10.1034/j.1600-0501.2002.130513.x. [DOI] [PubMed] [Google Scholar]
- 25.Weibrich G, Gnoth SH, Otto M, Reichert TE, Wagner W. Growth stimulation of human osteoblast-like cells by thrombocyte concentrates in vitro. Mund Kiefer Gesichtschir. 2002;6:168–74. doi: 10.1007/s10006-002-0367-6. [DOI] [PubMed] [Google Scholar]
- 26.Atia WM, Khalil AA, Melek LN. Sticky bone in dehiscence defect around dental implant. Alex Dent J. 2018;43:35–40. [Google Scholar]
- 27.Ayoub AH, Agbor RO. Tissue engineering, platelets concentrates and its role in dental implant treatment. EC Dent Sci. 2016;5:969–80. [Google Scholar]
- 28.Soni R, Priya A, Yadav H, Mishra N, Kumar L. Bone augmentation with sticky bone and platelet-rich fibrin by ridge-split technique and nasal floor engagement for immediate loading of dental implant after extracting impacted canine. Natl J Maxillofac Surg. 2019;10:98–101. doi: 10.4103/njms.NJMS_37_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravikumar KK, Jamal BT, Ageel R, Binaffif AA, Ageel B, Karkashan L, et al. Prevalence of impacted teeth and their associated pathologies on panoramic films in the Saudi population. Int J Soc Rehab. 2019;3:40–6. [Google Scholar]
- 30.Kan KW, Liu JK, Lo EC, Corbet EF, Leung WK. Residual periodontal defects distal to the mandibular second molar 6-36 months after impacted third molar extraction. J Clin Periodontol. 2002;29:1004–11. doi: 10.1034/j.1600-051x.2002.291105.x. [DOI] [PubMed] [Google Scholar]
- 31.Stumbras A, Kuliesius P, Januzis G, Juodzbalys G. Alveolar Ridge Preservation after Tooth Extraction Using Different Bone Graft Materials and Autologous Platelet Concentrates: A Systematic Review. J Oral Maxillofac Res. 2019;10:e2. doi: 10.5037/jomr.2019.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabrizi R, Khorshidi H, Shahidi S, Gholami M, Kalbasi S, Khayati A. Use of lincomycin-impregnated demineralised freeze-dried bone allograft in the periodontal defect after third molar surgery. J Oral Maxillofac Surg. 2014;72:850–7. doi: 10.1016/j.joms.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Nathani DB, Sequeira J, Rao BH. Comparison of platelet rich plasma and synthetic graft material for bone regeneration after third molar extraction. Ann Maxillofac Surg. 2015;5:213–8. doi: 10.4103/2231-0746.175762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eshghpour M, Dastmalchi P, Nekooei AH, Nejat A. Effect of platelet-rich fibrin on frequency of alveolar osteitis following mandibular third molar surgery: A double-blinded randomized clinical trial. J Oral Maxillofac Surg. 2014;72:1463–7. doi: 10.1016/j.joms.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Quadri A, Quadri S, Khan TA. Potential for osseous regeneration of platelet rich fibrin: A comparitive study in mandibular third molar socket. IJSS. 2016;2:75. [Google Scholar]
- 36.Kedarnath S, Abhilash R. Role of platelet rich plasma in healing after impacted Mandibular 3rd molar surgery. J Orofac Res. 2011;1:1–5. [Google Scholar]
- 37.de Sousa Gomes P, Daugela P, Poskevicius L, Mariano L, Fernandes MH. Molecular and cellular aspects of socket healing in the absence and presence of graft materials and autologous platelet concentrates: A focused review. J Oral Maxillofac Res. 2019;10:e2. doi: 10.5037/jomr.2019.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly WH, Mirahmadi MK, Simon JH, Gorman JT. Radiographic changes of the jawbones in end stage renal disease. Oral Surg Oral Med Oral Pathol. 1980;50:372–81. doi: 10.1016/0030-4220(80)90423-5. [DOI] [PubMed] [Google Scholar]
- 39.Dutta SR, Singh P, Passi D, Patter P. Mandibular third molar extraction wound healing with and without platelet rich plasma: A comparative prospective study. J Maxillofac Oral Surg. 2015;14:808–15. doi: 10.1007/s12663-014-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baslarli O, Tumer C, Ugur O, Vatankulu B. Evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. Med Oral Patol Oral Cir Bucal. 2015;20:e111–6. doi: 10.4317/medoral.19999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Annunziata M, Guida L, Nastri L, Piccirillo A, Sommese L, Napoli C. The role of autologous platelet concentrates in alveolar socket preservation: A systematic review. Transfus Med Hemother. 2018;45:195–203. doi: 10.1159/000488061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Hamed FS, Tawfik MA, Abdelfadil E, Al-Saleh MA. Efficacy of platelet-rich fibrin after mandibular third molar extraction: A systematic review and meta-analysis. J Oral Maxillofac Surg. 2017;75:1124–35. doi: 10.1016/j.joms.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Samir E, Hicham S, Keltoum E, Ismaili Z. Management of post -extractional alveolar socket with mineralized plasmatic matrix before implant placement: A case report. Asian Pac J Health Sci. 2017;4:220–7. [Google Scholar]
- 44.Vijay Sunil Reddy P, Singh R, Raj S, Chourasia S, Majumdar S, Bharti A. Sticky bone: Boon to regeneration a review. Int J Med Applied Sci. 2019;8:1–5. [Google Scholar]
- 45.Srinivas B, Das P, Rana MM, Qureshi AQ, Vaidya KC, Ahmed Raziuddin SJ. Wound healing and bone regeneration in postextraction sockets with and without platelet-rich fibrin. Ann Maxillofac Surg. 2018;8:28–34. doi: 10.4103/ams.ams_153_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


