Abstract
Toll-like receptors (TLRs) are a family of pattern recognition receptors (PRRs) in the first line defense system of our bodies; they are widely expressed on leukocytes and kidney epithelial cells. Infections due to pathogens or danger signals from injured tissues often activate several TLRs and these receptors mediate their signal transduction through the activation of transcription factors that regulate the expression of cytokine interleukin-1β (IL-1β), type I interferons (IFNs), and nuclear factor kappa light chain enhancer of activated B cells (NF-κB) dependent cytokines and chemokines. Acute kidney injury (AKI) involves early Toll-like receptors driven immunopathology, while resolution of inflammation is needed for rapid regeneration of injured tubular cells. Despite their well known function in the progression of inflammation; interestingly, activation of TLRs also has been implicated in renal epithelial repair through the induction of certain interleukins and improvement in autophagy mechanism. Studies have found that although the blockade of TLRs during the early injury phase of renal tissues prevented tubular necrosis, suppression of interleukins production and impaired kidney regeneration due to their blockade has been observed during the healing phase of tissue. Taken together, these results suggest that the two danger response programs of renal cells i.e. renal inflammation and regeneration may link at the level of TLRs. This review aims to emphasize on the role of TLRs signaling in different acute kidney injury phases. Understanding of these pathways may turn out to be effective as therapeutic option for kidney diseases.
Keywords: Toll-like receptors, Epithelial cells, Danger associated molecular patterns, Acute kidney injury, Inflammation, Renal repair, Cytokines
Toll-like receptors; epithelial cells; danger associated molecular patterns; acute kidney injury; inflammation; renal repair; cytokines.
1. Introduction
Toll-like receptors (TLRs) are integral part of the innate immune system.They are a classical type of pattern-recognition receptors (PRRs) which can identify two classes of ligands. First, special conserved molecules present on the surface of foreign pathogens, pathogen-associated molecular patterns (PAMPs) and second are the host endogenous damage-associated molecular pattern molecules (DAMPs), which are released by injured body cells [1]. A variety of different TLRs have been identified in many invertebrate and vertebrate species since the first discovery of this receptor in Drosophila melanogaster [2] and up till now 11 TLRs in humans and 13 mouse TLRs have been reported.The structure of transmembrane proteins TLRs is composed of three structural domains: outer leucine-rich repeats (LRRs) motif which recognizes their ligands, a transmembrane domain and a cytoplasmic Toll/IL-1 receptor (TIR) domain which recruit signal transduction adaptor molecules and initiates signaling [3]. Five adaptor proteins have been reported: i) myeloid differentiation factor 88 (MyD88); ii) MyD88 adaptor-like (MAL, also known as TIRAP); iii) TIR domaincontaining adaptor protein-inducing IFN-β (TRIF, also known as TICAM1); iv) TRIF-related adaptor molecule (TRAM, also known as TICAM2); and v) sterile α and armadillo motif-containing protein [4]. TLRs recognize self and non-self antigens of body and by regulating the production of cytokines, cell proliferation, survival and death they bridge innate and adaptive immune system [5,6].
Currently, the signaling pathways of TLR are broadly categorized into two distinct classes on the basis of adaptor usage, namely, the MyD88 dependent pathways which is the most widely used pathway by almost all the TLRs and the TIR domain-containing adaptor-inducing IFNβ (TRIF) dependent pathways which is considered to be specific for only few TLRs, such as TLR3 and TLR4 in mammals [7]. These pathways ultimately lead to activation of different transcription factors mainly thorough nuclear factor kappa light chain enhancer of activated B cells (NF-κB). The NF-κB is an evolutionarily conserved and ubiquitously expressed, inducible transcription factor that is expressed in all mammalian cells types and involved in the coordination of cell cycle progression, differentiation, migration, and survival. It is a heterogeneous collection of homo and heterodimers, the subunits of dimers are comprised of five members of Rel family of proteins including, p50, p52, p65, c-Rel, and RelB. These members of the Rel family can dimerize to make up to 15 different NF-κB dimmers combinations, in which p50/p65 is the most physiologically active heterodimer. Inhibitor of κB (IκB) molecules renders inactive dimers in the cell's cytoplasm. Therefore, phosphorylation dependent ubiquitination (carried out by the IκB kinase, also known IKK complex) and proteasomal degradation of IκB proteins result in activation of NF-κBdimmers [8].
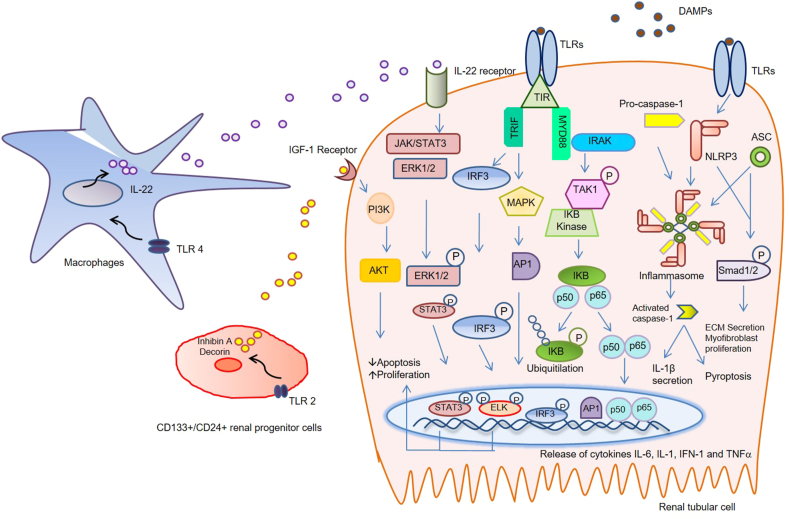
The TRIF-dependent pathway is involved in the activation of transcription factors, including NF-κB, activator protein-1 (AP-1), mitogen-activated protein kinase (MAPKs) and interferon (IFN) regulatory factor 3 (IRF3) family members, to induce the production of type 1 IFNs and pro-inflammatory cytokines [9,10]. After activation, adaptor molecule MyD88 forms a complex with members of interleukin receptor–associated kinase (IRAK) family. This complex then activate mitogen-activated protein kinase kinase kinase 7 (MAP3K7), also known as TAK1. The TAK1 binds to IκB kinase, which is a central regulator of NF-κB activation and allowing it to translocate into the nucleus. TAK1 activation also results in activation of MAPK family members such as ERK1/2, p38 and c-Jun kinase (JNK), which mediates activation of AP-1 family transcription factors [10]. Activation of NF-κB and AP-1 result in expression of proinflammatory cytokines such as IL-6, IL-1β, and TNF-α. Lipopolysaccharide (LPS) mediated MyD88-dependent inflammatory signaling, can also leads to reactive oxygen species (ROS) generation and a reduction in antioxidant enzymes via NADPH oxidase 1 (NOX1) adjustment [11]. Independent of MyD88, activation of NF-κB can also be mediated through PI3K by TLR1, TLR2, and TLR6 as these receptors contain a phosphatidylinositol 3-kinase (PI3K) binding motif. Activation of Fas-associated protein with death domain and, finally, caspase 8 or 10 activation can also lead to cell apoptosis by both MyD88 and TRIF pathways [12]. Ultimately, the induction or suppression of genes that fine tune the inflammatory response essentially drive by the TLRs signaling pathways [13]. (Figure 1).
Figure 1.
Overview of the TLRs multiple signaling pathways. DAMPs activate TLRs then a cytoplasmic receptor domain TIR recruits signal transduction adaptor molecules to initiate signaling. Signaling pathways are broadly categorized into two distinct classes on the basis of adaptor usage, MyD88-dependent pathways and TRIF-dependent pathways. Adaptor molecule MyD88 forms a complex with IRAK family. This complex then activates TAK1. TAK1 binds to the IκB kinase, which is a central regulator of NF-κB activation and allowing it to translocate into the nucleus. Transcription factors, including NF-κB, AP-1, MAPKs and IRF3 family members, can be activated by the TRIF-dependent pathway to induce the production of pro-inflammatory cytokinessuch as IL-6, INF-1, IL-1β, and TNF-α.Pathways of fibrosisproceed with activation of SMAD2 phosphorylation which is triggered by TLRs activated NLRP3 inflammasome formation and ASC expression. The inflammasomes also trigger caspase-1-dependent pyroptosis and IL-1β secretion. Activated TLR2 on CD133+/CD24+ renal progenitor cells can accelerate tubular repair via release of soluble factors inhibin A and decorinand inhibiting apoptosis of renal tubular epithelial cells via the IGF type I receptor/Akt-signaling pathway. TLR4 agonists induce the release of IL-22 secretion by macrophages, which in turn activates its receptor (exclusively present on tubular epithelia) to accelerate re-epithelialization in vivo through activation of the Jak/STAT3 and ERK1/2 pathways. TLRs, Toll like receptors; DAMPs, damage-associated molecular patterns; TIR, Toll/IL-1β receptor; MyD88, myeloid differentiation factor 88; IRAK, interleukin receptor–associated kinase; IκB, inhibitor of kappa B; NF-κB, nuclear factor kappa B; AP-1, activating protein-1; MAPKs, mitogen-activated protein kinase; IRF3, interferon regulatory factor 3; TRIF, TIR domain containing adaptor protein-inducing IFN-β; IL, interleukin; INF, interferon; TNF-α, tumor necrosis factor alpha; NLRP3, NLR family pyrin domain containing 3; ASC, apoptosis-associated speck-like protein; IGF, Insulin-like growth factor; Jak/STAT3, janus kinase-signal transducer and activator of transcription 3; ERK, extracellular-signal-regulated kinase.
TLRs have been shown to be involved in the development and progression of acute kidney injury [14,15]. Further to this their role in kidney regeneration has also been evolved reflecting there multifaceted roles in cell biology [16,17]. In this review, we present an overview and possible progression and regeneration signaling patterns of TLRs in acute kidney injury, hoping that this will provide notions for further investigations in this field.
2. Signaling of TLRs in acute kidney injury
Acute kidney injury (AKI) is a complex disorder manifested by a rapid loss of renal function that results in retention of metabolic waste products [18]. Injury results in rapid loss of cytoskeletal integrity and membrane polarity and cells die by apoptosis and necrosis. Renal proximal tubular epithelial cells are particular targets of drug-induced AKI [19,20]. Injury causes dysfunction of the tubular epithelial cells, which may promote the release of cytokines and recruitment of inflammatory cells [21]. Both tubular epithelial cells, and activated leukocytes are involved in releasing a variety of inflammatory mediators including, TNF-α, IL-1β, IL-6, IL-8, TGF-β, MCP-1, RANTES, IL-18 and MIP-2 [19,22,23]. A transcription factor interferon regulatory factor-1 (IRF-1) is reported to be involved in pro-inflammatory signal stimulated by reactive oxygen species during acute kidney injury [19,24].
In a setting of AKI, Toll-like receptors play important role in disease progression. They translate danger recognition into the secretion of pro-inflammatory cytokines and chemokines and expressed by both immune and non immune cells and this expression is rapidly altered in response to foreign antigens and environmental stressors [25]. TLR1/2/4/5/6/10 are present on the cell surface while TLR3/7/8/9 in intracellular endosomes and mostly recognize nucleic acids of pathogens [26]. Binding of DAMPs to TLR1/2/4/5 is a central element of danger signaling because these TLRs activate NF-kB–dependent cytokines pathway. NF-kB induces the expression of pro-IL-1β (which activates apoptotic cell death) as well as expression of TNF-α and IL-6 mainly in tubular cells [27]. For sterile inflammatory mechanism, histones, heat shock proteins, uricacid and high-mobility group box 1 nuclear protein (HMGB1), fragments of extracellular matrix as well as necrotic cellular debris functions as DAMPs of danger signaling [28].
In the kidney, the tubular epithelial cells and mesangial cells express TLR1/2/3/4 and TLR6. TLR4 expression is predominant at proximal and collecting tubules [29]. Many cell types in the kidney including tubules, medulla, glomeruli, and renal vasculature has been shown TLR2 protein expression [30]. TLR2 antisense oligonucleotides, and chimeric mice deficient in leukocyte or renal TLR2 were used to explore the role of TLR2 in kidney injury using TLR2−/− and TLR2+/+ mice. Injury was established by ischemia reperfusion (I/R) and marked reduction of local cytokines, chemokines, and leukocytes with decreased renal injury was observed in TLR2−/− mice compared with controls which confirms the pro-inflammatory role of this receptor in injury [31]. Marked increase in the synthesis of TLR2 and TLR4 mRNA in ischemic tubular epithelium was shown to be completely dependent on the action of IFN-γ and TNF-α [32,33]. Lipopolysaccharide (LPS) induced septic AKI mice showed that TLR2 overexpression results in inflammatory cytokines IL-6 and TNF-α expression along with increase in MyD88/NF-κB and p65 signaling pathway [34]. Synergistic effect of TLR2 and nucleotide-binding oligomerization domain-like receptors (NLRs) NOD1/2 showed chemokine CXCL1 production in neutrophils, macrophages and dendritic cells of mice renal ischemic kidney model. However, TLR2−/−and NOD1/2−/− mice showed inhibitory effect on neutrophil infiltration only and despite the apparent lack of injury in the TLR2−/− and NOD1/2−/− kidneys (reflected by normal serum creatinine values), macrophage or dendritic cell infiltrates remains unaffected. It suggests that either TLR2 or NOD1/2 blockade could decrease neutrophil inflammation following an ischemic insult to the kidney [35]. In contrast, a protective role of TLR2 via autophagy induction was demonstrated on cisplatin induced AKI. The cisplatin treatment resulted in decreased autophagy related genes, microtubule-associated protein light chain 3 (Lc3) and autophagy related gene 5 (Atg5) expression levels in wild type (WT) mice and same observation (decreased autophagy related genes) was found on TLR2 knockout mice not treated with cisplatin while, TLR4 knockout mice not treated with cisplatin presented elevated index in comparison with TLR2 knockout mice group which depicts the role of TLR2 in the expression of molecules responsible for autophagosome formation. It was observed that only the absence of TLR2 but not TLR4 in mice exacerbated the renal dysfunction, tissue injury and mortality rate and less heme oxygenase-1(HO-1) mediated renal protection was evident in TLR2 knockout mice which further demonstrate the putative cross-talk interactions between HO-1 and TLR2 system [36].
Release of fully reduced HMGB1 and HMGB1 from damaged cells with a disulfide bond results in cytokine induction via TLR4 binding [37]. HMBG-1 proteins can induce inflammatory signaling in human renal proximal tubules and endothelial cells from wild type mice, while dull induction of cytokines and chemokines in TLR4 and TLR2 null mice with reduced leukocytes infiltration was observed in response to ischemia and cisplatin kidney injury models [19]. In hypertension mediated renal injury macrophages mediated inflammation is confirmed by increased levels of MCP-1 chemokines mRNA levels in angiotensin II (Ang-II) treated mice with normal TLR4. Increased expression of NADPH oxidase 4 (Nox4) confirms oxidative stress in mice with normal TLR4 while there was no change in mice with TLR4 deficiency [38]. Exclusive role of TLR4 in renal ischemia/reperfusion injury is proposed when no significant differences were found in renal function and inflammation in TLR4−/−, MyD88−/− and TRIF-mutant mice when compared to their wild types, demonstrate that renal tubular epithelial cells can coordinate an immune response to ischemic injury in a TLR4 dependent manner [39]. However, in another study MyD88-dependent pathway also appeared crucial for the full development of kidney IRI as both TLR4−/− and MyD88−/− mice appeared protected against kidney damage, pro-inflammatory cytokines production and inflammatory cells accumulation [40]. Early renal injury at the sub cellular level by hypoxia due to obstructive sleep apnea may result in increased expression of HMGB1. Hypoxia was shown to stimulate HMGB1, receptor for advanced glycosylation end products (RAGE) and TNF-α pathway in kidney tissue and increased expression of soluble TLR4, TNF-α and IL-6 in the peripheral blood of hypoxic rates. This suggested that the HMGB1-RAGE/TLR-TNF-α pathway may contribute to the molecular mechanisms of early renal injury induced by hypoxia [41]. Freely filtered toxins and LPS can also induce TLR4-mediated renal inflammation. Proximal tubular epithelial cells recognize and internalized LPS via membrane TLR4 receptors, this recognition additionally requires LPS-binding protein (LBP) and interaction with the CD14 membrane bound molecules. This LPS-LBP-CD14-TLR4 complex then initiates an inflammatory downstream signaling cascade via the MyD88 pathway [25].
Pathogenesis of early acute kidney injury after ischemia and reperfusion is shown by early participation of nucleic acid binding TLR-3. After 24 h of reperfusion TLR-3 mRNA was significantly upregulated in renal tubular cells versus sham treatment in wild type mice which result in strong proinflammatory response, significant elevation in intercellular adhesion molecule 1(ICAM-1) and C-X-C chemokine receptor type 4 (CXCR4) gene expression. In contrast, these levels remained unchanged in TLR-3−/− mice [42].
Sang et al suggest complex nature of TLR9 (a cytosolic receptor) signaling in renal ischemia reperfusion (IR) injury as they observed TLR9 mediated renal tubular epithelial cell injury whereas TLR9 signaling in other cell types showed cyto-protective effects. Notable differences in renal inflammatory response between renal proximal tubular TLR9 deficient mice and global TLR9 knockout mice were observed. In renal proximal tubular TLR9 deficient mice MIP-2 and MCP-1 expression was significantly reduced, lesser induction of IL-6, ICAM-1 and reduced renal tubular necrosis, inflammation and apoptosis was observed when compared to wild type mice. However, no significant difference in TNF-α, MIP-2 and MCP-1 but interestingly reduced kidney IL-6 mRNA expression was showed by global TLR9 knockout mice. In the same way, ODN-1668, a TLR9 agonist intensify renal IR injury via NF-κB and caspase activation in wild type mice but not in renal proximal tubular TLR9 deficient mice. It has been suggested that TLR9 in other cell types may promote tissue protection by promoting accumulation of beneficial regulatory T-cells [43].
Recently, the role of NADPH oxidase (Nox5) mediated ROS generation is identified in a lipopolysaccharide model of acute kidney injury. Mice with podocyte-specific Nox5 (Nox5pod+) expression showed more expression of Toll-like receptors and pro-inflammatory cytokines with ROS production. The Interaction between interleukin-1 receptor-associated kinases (IRAK1) and Nox5 is established and it is demonstrated that Nox5-derived ROS may be regulated by IRAK1/4 protein inhibitors [44].
Inappropriate inflammatory reactions involve in proliferation and formation of myofibroblasts which results in activation of fibrosis pathways. TGF-ß receptor signaling activate with SMAD2 phosphorylation which is triggered by TLRs activated NOD-like receptor, pyrin domain containing-3 (NLRP3) inflammasome formation and apoptotic speck protein (ASC) expression [16,45]. The inflammasomes also trigger caspase-1 dependent pyroptosis, a programmed form of inflammatory cell death characterized by apoptosis-like chromatin condensation but rupture of the plasma membrane [46]. The expression of NLRP3 and ASC is induced by TLRs, such inductions are needed for SMAD2 phosphorylation as a critical step in TGF-β receptor signaling.
3. TLRs in kidney regeneration
Kidney repair process also involves complex interplay of events. Recovery of proximal tubular cells begins with reassembly of the actin cytoskeleton, and repolarization of the surface membranes. The renal response of repair is replacement of injured tubular epithelium with functional tubular cells but it happens in a restricted manner as kidney has a limited capacity to undergo endogenous tissue remodeling. During renal development mesenchymal to epithelial transition (MET) follows and this process is controlled by different growth factors e.g. hepatocyte growth factor (HGF) and bone morphogenetic protein-7 (BMP-7). Therefore, a transition of the metanephric mesenchymal cells into polarized epithelial cells may be required in kidney regeneration [47]. A significant elevation in tubular cell proliferation that peaks on 3rd day of ischemia reperfusion injury indicates beginning of repair process. However, resolution of sterile inflammation and influence of pro-regeneratory factors are the prerequisite of surviving tubular epithelial cells (TECs) functional recovery. Besides, surviving TECs, other intra-tubular progenitor cells, bone marrow derived stem cells and immune cells provided these factors in a paracrine manner [48].
The activation of TLRs also has been implicated in epithelial repair by regulating clearance of cellular debris and initiating tissue-repair programs. Inhibin A and decorin belong to the TGF-β signaling pathway and there is evidence for an antifibrotic activity of soluble decorin directly interacting with another member of TGF-β superfamily, connective tissue growth factor (CTGF), and inhibiting apoptosis of renal tubular epithelial cells via the insulin-like growth factor 1 (IGF-1) receptor/Akt-signaling pathway [16]. It has been observed that certain DAMPs activate TLR2 on CD133+/CD24+renal progenitor cells and accelerate tubular repair via release of soluble factors inhibin A and decorin. Blocking of TLRs2 completely abrogate this regenerative effect. This regenerative capacity of CD133+/CD24+ renal progenitor cells was further confirmed using an in vitrotranswells co-culture system in which cisplatin induced damaged proximal tubular epithelial cells showed significant proliferation co-cultured with renal progenitor cells and besides proliferation other mechanism i.e. suppression of apoptosis was also observed with no expression of cleaved caspase-3 after 48 h. This data show an essential role of TLR2 in the damage repair process [49].
Study of Kulkarni et al support the idea that to support their regeneration through a specific TLR4–IL-22 pathway, dying TECs activate interstitial dendritic and macrophage cells. They found IL-22 secretion to be selectively induced by TLR4 agonists released from necrotic tubular cells, which in turn activates its receptor (exclusively present on tubular epithelia) to accelerate re-epithelization in vivo through activation of the Jak/STAT3 and ERK1/2 pathways. Neutralization of IL-22 in the healing phase of AKI (2–5 days after injury) by injecting anti–IL-22 antibody significantly impaired tubular recovery. This study demonstrates the link of two danger response programs, renal inflammation and regeneration at the level of TLR4 [17].
Temporal participation of interleukins in regeneration of acute kidney injury may appear an interesting venue to further explore TLRs involvement in AKI. It has been noted that IL-6, locally expressed in renal tubular cells plays a protective role in the development of cisplatin-induced acute renal failure through up regulation of anti-oxidative stress factors and IL-6 knockout (IL-6−/−) mice appeared more sensitive to injury induced by cisplatin than in wild-type mice, possibly via an increase in pro-apoptotic regulatory signals. Activity of superoxide dismutase, an anti-oxidative enzyme, was significantly decreased in the kidney obtained from IL-6−/− mice after cisplatin administration [50]. However, during the development of injury, significant increased in renal IL-6 expression and signal transducer and activator of transcription 3 (STAT3) activation in renal tubular epithelial cells suggests active IL-6 signaling. Besides, renal IL-6 receptors (IL-6R), IL-6 can also stimulate target cells together with a soluble form of the IL-6R (sIL-6R) in a process termed trans-signaling. During AKI, serum sIL-6R levels increased three-fold, suggesting a possible role for IL-6 trans-signaling in injury. This signaling reduced lipid peroxidation after injury, suggesting that IL-6 simultaneously promotes an injurious inflammatory response and, through a mechanism of trans-signaling, protects the kidney from oxidative stress [51]. Interestingly it has been shown that activation of Toll-like receptor 2 (TLR2) on primary human peripheral blood mononuclear cells (PBMCs) and on the monocytic cell line THP-1 induces expression and secretion of IL-6 and the generation of sIL-6R [52]. However same role in the context of AKI is not yet demonstrated.
IL-10 is a potent anti-inflammatory cytokine that inhibits inflammatory pathways. A greater increase in serum creatinine with increased expression of TNF-α in IL-10−/− mice than in wild-type mice was observed in renal IR injury [53]. Study of Ashim et al demonstrated the effect of IL-10 in the activation of TLR4 downstream signals which resulted in cardiomyocytes survival and there data on cardiomyocytes suggest that IL-10 induced anti-apoptotic signaling involves upregulation of TLR4 through MyD88 activation [54]. It has been hypothesized that a subtype of T lymphocytes, regulatory T-cells (Treg) predominantly produce IL10 which play important role in suppression of inflammatory response and here comes the role of activation of IKKα-dependent NF-κB non canonical pathway which unlike IKKβ pathway, induce the resolution of inflammation and tissue regeneration during the recovery phase of AKI through the recruitment of Treg cells. This data support the concept that IKKα mediates renal repair in recovery phase of kidney IR injury [55].
It has been noted that phenotypic switch of pro-inflammatory macrophages (M1) to anti-inflammatory/pro-regeneratory macrophage cells (M2) is essential for recovery on AKI and IL-4 stimulated macrophages with an M2 phenotype, but not IFN-γ stimulated pro-inflammatory macrophages, promoted renal tubular cell proliferation at 3–5 days after injury. IL-10 and myeloid cell-derived mediators improve epithelial healing which are produced by M2 macrophages [56]. This concept is further supported by Chiara et al and in a recent study it has been reported that biglycan proteoglycan promotes macrophages autophagy mechanism by CD44 and TLR4 signaling axis in ischemia/reperfusion injury and evokes anti-inflammatory response. This study demonstrates the importance of interaction between a DAMP ligand and specific TLR co-receptors in curtailing kidney inflammation. Interestingly, on the other side they observed biglycan dependent regulation of inflammation via TLR2 with CD14 co-receptor resulted in renal M1 macrophage polarization in renal IRI. This study shed light on co-receptor dependent TLR4 mediated tubular recovery [57].
4. Rationale of use of TLR inhibitors in kidney injury
As inflammation during AKI leads to the extension phase of injury, it is suggested that interventions that could curtail the inflammatory response may represent a potential area of treatment with notable impact. Till recent years, various therapeutic compounds (including small molecule inhibitors, oligonucleotides, antibodies, lipid-A analogs, microRNAs, and nano-inhibitors) have been developed to control extended inflammation as they inhibit TLRs signaling; however with the perspective of AKI injury their therapeutic role require more research.
TLRs inhibition can be achieved by two generalstrategies: either blocking the ligands -receptor binding or interfering the intracellular TLRs signaling pathways. Antimalarial drugs, including hydroxychloroquine sulfate (HCQ), chloroquine (CQ), and quinacrine have been using in treating autoimmune diseases like arthritis but besides this their additional mechanisms of action on endosomal TLRs signaling (TLR7/8/9) was also identified. They can accumulate in the acidic intracellular compartments like endosomes and lysosomes, and modulate the pH in these vesicles which lead to blockade of endosomal TLRs signaling, and decrease in cytokine production.These mechanisms of action suggest their anti-inflammatory activity in AKI as well [58]. TAK-242 (Resatorvid) is an anti-sepsis small molecule inhibitor which binds to the intracellular TIR domain of TLR4 at cysteine 747 position and it inhibits the interaction between TLR4 and the adaptor proteins-TIR domain containing adaptor protein (TIRAP) and TRIF-related adaptor molecule (TRAM), thereby may appear as useful anti-inflammatory compound by targeting LPS-induced TLR4 signaling [59]. The in vivo role of TAK242 in mice cyclosporine A (CsA) nephrotoxicity model reflects reduced activation of proinflammatory signaling, including JNK/c-jun, JAK2/STAT3 and NF-κB. This TLR4 inhibition also results in reduced tubular damage and alleviates the development of kidney fibrosis [60]. Another small molecule, TJ-M2010-2, which inhibits MyD88 homo-dimerization, showed better survival rate, reduced tubular interstitial fibrosis and reduced inflammatory response in mice subjected to kidney IRI [61].
Regarding targeting TLR2 signaling in LPS-challenged AKI, a recent finding indicate that ortho-vanillin (OV), an inhibitor of TLR2, interrupted the interaction between TLR2 and its downstream adaptor MyD88 in vitro, resulting in the reduction of inflammatory cytokines IL-6 and TNF-α expression. In vivo OV treatment in an LPS-challenged mouse model showed significantly reduced expression of inflammatory cytokines TNF-α, IL-6 and IL-1β due to suppressed p65 phosphorylation [62].
OPN-305 is the first fully humanized IgG4 monoclonalTLR2-specific antibody. In vitro as well as ischemia reperfusion injury animal models studies have shown the effect of this compound in inhibiting TLR2 mediated pro-inflammatory cytokine production [63]. Now, a phase II clinical trial (NCT01794663) of this antibody is under progress in renal transplant patients. NI-0101 and 1A6 are anti-TLR4 antibodies showed protective effects in vivo and successfully block cytokine release. However, it has been noted that blockade of TLR4 signaling by 1A6 antibodies could also delay mucosal healing in the recovery stage [64]. It reflects the importance of timings on which TLR4 signaling blockade could provide maximal therapeutic effect.
Altogether, these studies suggest that TLRs are potential pharmacological targets in AKI and blocking the TLRs signaling may appear therapeutically beneficial in AKI related extended inflammation.
5. Conclusion
Exploring the signaling mechanisms of TLRs may provide a useful tool to investigate pathophysiological mechanisms and protection strategies against acute kidney injury. Binding to TLRs is a central element of sterile inflammation or danger signaling and a single stimulus can activate various types of TLRs to induce the expression of pro-IL-1β, IFN-α/β and NF- κB dependent cytokines. However, over activation of TLRs can ultimately lead to disruption of immune homeostasis, and thus implicated in the pathogenesis of extended kidney inflammation. Further to this their role in kidney regeneration by regulating the production of cytokines, cell proliferation and survival, has also been evolved, which reflect their multifaceted roles mediated through different co-receptors activations in a time dependent fashion. Therefore, keeping in mind the dual role of TLRs in both kidney injury and healing processes in a temporal fashion, it would be interesting to explore the therapeutic role of interventions that could control these receptors signaling mechanisms and ultimately leads to attenuate the inflammatory response and improve tissue repair process.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data availability is not applicable as this is a review article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nie L., Cai S.Y., Shao J.Z., Chen J. Toll-like receptors, associated biological roles, and signaling networks in non-mammals. Front. Immunol. 2018;9:1523. doi: 10.3389/fimmu.2018.01523. Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson K.V., Bokla L., Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42(3):791–798. doi: 10.1016/0092-8674(85)90275-2. Oct. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 4.Vallés P.G., Lorenzo A.G., Bocanegra V., Vallés R. Acute kidney injury: what part do Toll-like receptors play? Int J NephrolRenovasc Dis. 2014;7:241–251. doi: 10.2147/IJNRD.S37891. Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidya M.K., Kumar V.G., Sejian V., Bagath M., Krishnan G., Bhatta R. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2018;37(1):20–36. doi: 10.1080/08830185.2017.1380200. Jan 2. [DOI] [PubMed] [Google Scholar]
- 6.Reuven E.M., Fink A., Shai Y. Regulation of innate immune responses by transmembrane interactions: lessons from the TLR family. Biochim. Biophys. Acta. 2014;1838(6):1586–1593. doi: 10.1016/j.bbamem.2014.01.020. Jun. [DOI] [PubMed] [Google Scholar]
- 7.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. Jul. [DOI] [PubMed] [Google Scholar]
- 8.Herrington F.D., Carmody R.J., Goodyear C.S. Modulation of NF-κB signaling as a therapeutic target in autoimmunity. J. Biomol. Screen. 2016;21(3):223–242. doi: 10.1177/1087057115617456. Mar. [DOI] [PubMed] [Google Scholar]
- 9.Hoebe K., Du X., Georgel P., Janssen E., Tabeta K., Kim S.O., Goode J., Lin P., Mann N., Mudd S., Crozat K., Sovath S., Han J., Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–748. doi: 10.1038/nature01889. Aug 14. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A.J., Cho K.J., Kim J.H. MyD88-BLT2-dependent cascade contributes to LPS-induced interleukin-6 production in mouse macrophage. Exp. Mol. Med. 2015;47:e156. doi: 10.1038/emm.2015.8. Apr 3. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. Mar 19. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S., Yu M., Guo Q., Li R., Li G., Tan S., Li X., Wei Y., Wu M. Annexin A2 binds to endosomes and negatively regulates TLR4-triggered inflammatory responses via the TRAM-TRIF pathway. Sci. Rep. 2015;5:15859. doi: 10.1038/srep15859. Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenhammar J., Rundgren M., Forestier J., Kalman S., Eriksson S., Frithiof R. Toll-like receptor 4 inhibitor TAK-242 attenuates acute kidney injury in endotoxemic sheep. Anesthesiology. 2011;114(5):1130–1137. doi: 10.1097/ALN.0b013e31820b8b44. May. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda H., Leelahavanichkul A., Tsunoda S., Dear J.W., Takahashi Y., Ito S., Hu X., Zhou H., Doi K., Childs R., Klinman D.M., Yuen P.S., Star R.A. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008;294(5):F1050–F1058. doi: 10.1152/ajprenal.00461.2007. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anders H.J., Schaefer L. Beyond tissue injury-damage-associated molecular patterns, Toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. 2014;25(7):1387–1400. doi: 10.1681/ASN.2014010117. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni O.P., Hartter I., Mulay S.R., Hagemann J., Darisipudi M.N., Kumar Vr S., Romoli S., Thomasova D., Ryu M., Kobold S., Anders H.J. Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J. Am. Soc. Nephrol. 2014;25(5):978–989. doi: 10.1681/ASN.2013050528. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Kader K., Palevsky P.M. Acute kidney injury in the elderly. Clin. Geriatr. Med. 2009;25(3):331–358. doi: 10.1016/j.cger.2009.04.001. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basile D.P., Anderson M.D., Sutton T.A. Pathophysiology of acute kidney injury. Comp. Physiol. 2012;2(2):1303–1353. doi: 10.1002/cphy.c110041. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonventre J.V., Yang L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonventre J.V. Pathophysiology of AKI: injury and normal and abnormal repair. Contrib. Nephrol. 2010;165:9–17. doi: 10.1159/000313738. [DOI] [PubMed] [Google Scholar]
- 22.Haq M., Norman J., Saba S.R., Ramirez G., Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J. Am. Soc. Nephrol. 1998;9(4):614–619. doi: 10.1681/ASN.V94614. Apr. [DOI] [PubMed] [Google Scholar]
- 23.Akcay A., Nguyen Q., Edelstein C.L. Mediators of inflammation in acute kidney injury. Mediat. Inflamm. 2009:137072. doi: 10.1155/2009/137072. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., John R., Chen J., Richardson J.A., Shelton J.M., Bennett M., Zhou X.J., Nagami G.T., Zhang Y., Wu Q.Q., Lu C.Y. IRF-1 promotes inflammation early after ischemic acute kidney injury. J. Am. Soc. Nephrol. 2009;20(7):1544–1555. doi: 10.1681/ASN.2008080843. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radi Z.A. Immuno pathogenesis of acute kidney injury. Toxicol. Pathol. 2018;46(8):930–943. doi: 10.1177/0192623318799976. Dec. [DOI] [PubMed] [Google Scholar]
- 26.Chen L., DiPietro L.A. Toll-like receptor function in acute wounds. Adv. Wound Care. 2017;6(10):344–355. doi: 10.1089/wound.2017.0734. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anders H.J. Toll-like receptors and danger signaling in kidney injury. J. Am. Soc. Nephrol. 2010;21(8):1270–1274. doi: 10.1681/ASN.2010030233. Aug. [DOI] [PubMed] [Google Scholar]
- 28.Ma K.C., Schenck E.J., Pabon M.A., Choi A.M.K. The role of danger signals in the pathogenesis and perpetuation of critical illness. Am. J. Respir. Crit. Care Med. 2018;197(3):300–309. doi: 10.1164/rccm.201612-2460PP. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batsford S., Duermueller U., Seemayer C., Mueller C., Hopfer H., Mihatsch M. Protein level expression of Toll-like receptors 2, 4 and 9 in renal disease. Nephrol. Dial. Transplant. 2011;26(4):1413–1416. doi: 10.1093/ndt/gfq752. Apr. [DOI] [PubMed] [Google Scholar]
- 30.Shigeoka A.A., Holscher T.D., King A.J., Hall F.W., Kiosses W.B., Tobias P.S., Mackman N., McKay D.B. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J. Immunol. 2007;178(10):6252–6258. doi: 10.4049/jimmunol.178.10.6252. May 15. [DOI] [PubMed] [Google Scholar]
- 31.Leemans J.C., Stokman G., Claessen N., Rouschop K.M., Teske G.J., Kirschning C.J., Akira S., van der Poll T., Weening J.J., Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J. Clin. Invest. 2005;115(10):2894–2903. doi: 10.1172/JCI22832. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arumugam T.V., Okun E., Tang S.C., Thundyil J., Taylor S.M., Woodruff T.M. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32(1):4–16. doi: 10.1097/SHK.0b013e318193e333. Jul. [DOI] [PubMed] [Google Scholar]
- 33.Gill R., Tsung A., Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010;48(9):1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Peng, Zhang Xi, Wang Yongfang, Yuan Hua, Zhang Shaoyu, Liu Long. Toll like receptor 2 induces kidney inflammation via MyD88/NF-κB signaling pathway. Int. J. Clin. Exp. Med. 2018;11(4):3494–3503. [Google Scholar]
- 35.Kasimsetty S.G., Hawkes A., Barekatain K., Soo E., Welch A.K., McKay D.B. TLR2 and NODs1 and 2 cooperate in inflammatory responses associated with renal ischemia reperfusion injury. Transpl. Immunol. 2020;58:101260. doi: 10.1016/j.trim.2019.101260. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrade-Silva M., Cenedeze M.A., Perandini L.A., Felizardo R.J.F., Watanabe I.K.M., Agudelo J.S.H., Castoldi A., Gonçalves G.M., Origassa C.S.T., Semedo P., Hiyane M.I., Foresto-Neto O., Malheiros D.M.A.C., Reis M.A., Fujihara C.K., Zatz R., Pacheco-Silva A., Câmara N.O.S., de Almeida D.C. TLR2 and TLR4 play opposite role in autophagy associated with cisplatin-induced acute kidney injury. Clin. Sci. (Lond.) 2018;132(16):1725–1739. doi: 10.1042/CS20170262. Aug 22. [DOI] [PubMed] [Google Scholar]
- 37.Magna M., Pisetsky D.S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 2014;20(1):138–146. doi: 10.2119/molmed.2013.00164. Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pushpakumar S., Ren L., Kundu S., Gamon A., Tyagi S.C., Sen U. Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci. Rep. 2017;7(1):6349. doi: 10.1038/s41598-017-06484-6. Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulskens W.P., Teske G.J., Butter L.M., Roelofs J.J., van der Poll T., Florquin S., Leemans J.C. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PloS One. 2008;3(10) doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H., Chen G., Wyburn K.R., Yin J., Bertolino P., Eris J.M., Alexander S.I., Sharland A.F., Chadban S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 2007;117(10):2847–2859. doi: 10.1172/JCI31008. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C., Dong H., Chen F., Wang Y., Ma J., Wang G. The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. ExpTher Med. 2019;17(1):17–26. doi: 10.3892/etm.2018.6932. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulus P., Rupprecht K., Baer P., Obermüller N., Penzkofer D., Reissig C., Scheller B., Holfeld J., Zacharowski K., Dimmeler S., Schlammes J., Urbschat A. The early activation of Toll-like receptor (TLR)-3 initiates kidney injury after ischemia and reperfusion. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0094366. Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han S.J., Li H., Kim M., Shlomchik M.J., Lee H.T. Kidney proximal tubular TLR9 exacerbates ischemic acute kidney injury. J. Immunol. 2018;201(3):1073–1085. doi: 10.4049/jimmunol.1800211. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holterman C.E., Boisvert N.C., Thibodeau J.F., Kamto E., Novakovic M., Abd-Elrahman K.S., Ferguson S.S.G., Kennedy C.R.J. Podocyte NADPH oxidase 5 promotes renal inflammation regulated by the toll-like receptor pathway. Antioxidants Redox Signal. 2019 May 20;30(15):1817–1830. doi: 10.1089/ars.2017.7402. [DOI] [PubMed] [Google Scholar]
- 45.Wang W., Wang X., Chun J., Vilaysane A., Clark S., French G., Bracey N.A., Trpkov K., Bonni S., Duff H.J., Beck P.L., Muruve D.A. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J. Immunol. 2013;190(3):1239–1249. doi: 10.4049/jimmunol.1201959. Feb 1. [DOI] [PubMed] [Google Scholar]
- 46.Anders H.J., Lech M. NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int. 2013;84(2):225–228. doi: 10.1038/ki.2013.122. Aug. [DOI] [PubMed] [Google Scholar]
- 47.Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. Faseb. J. 1989;3(10):2141–2150. doi: 10.1096/fasebj.3.10.2666230. Aug. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji K., Kitamura S. Trophic factors from tissue stem cells for renal regeneration. Stem Cell. Int. 2015:537204. doi: 10.1155/2015/537204. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sallustio F., Curci C., Aloisi A., Toma C.C., Marulli E., Serino G., Cox S.N., De Palma G., Stasi A., Divella C., Rinaldi R., Schena F.P. Inhibin-A and decorin secreted by human adult renal stem/progenitor cells through the TLR2 engagement induce renal tubular cell regeneration. Sci. Rep. 2017;7(1):8225. doi: 10.1038/s41598-017-08474-0. Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitazaki S., Honma S., Suto M., Kato N., Hiraiwa K., Yoshida M., Abe S. Interleukin-6 plays a protective role in development of cisplatin-induced acute renal failure through upregulation of anti-oxidative stress factors. Life Sci. 2011;88(25-26):1142–1148. doi: 10.1016/j.lfs.2011.04.016. Jun 20. [DOI] [PubMed] [Google Scholar]
- 51.Nechemia-Arbely Y., Barkan D., Pizov G., Shriki A., Rose-John S., Galun E., Axelrod J.H. IL-6/IL-6R axis plays a critical role in acute kidney injury. J. Am. Soc. Nephrol. 2008;19(6):1106–1115. doi: 10.1681/ASN.2007070744. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flynn C.M., Garbers Y., Lokau J., Wesch D., Schulte D.M., Laudes M., Lieb W., Aparicio-Siegmund S., Garbers C. Activation of toll-like receptor 2 (TLR2) induces interleukin-6 trans-signaling. Sci. Rep. 2019;9(1):7306. doi: 10.1038/s41598-019-43617-5. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan X., Huang W.J., Chen W., Xie H.G., Wei P., Chen X., Cao C.C. IL-10 deficiency increases renal ischemia-reperfusion injury. Nephron Exp. Nephrol. 2014;128(1-2):37–45. doi: 10.1159/000366130. [DOI] [PubMed] [Google Scholar]
- 54.Bagchi A.K., Sharma A., Dhingra S., LehenbauerLudke A.R., Al-Shudiefat A.A., Singal P.K. Interleukin-10 activates Toll-like receptor 4 and requires MyD88 for cardiomyocyte survival. Cytokine. 2013;61(1):304–314. doi: 10.1016/j.cyto.2012.10.013. Jan. [DOI] [PubMed] [Google Scholar]
- 55.Wan X., Hou L.J., Zhang L.Y., Huang W.J., Liu L., Zhang Q., Hu B., Chen W., Chen X., Cao C.C. IKKα is involved in kidney recovery and regeneration of acute ischemia/reperfusion injury in mice through IL10-producing regulatory T cells. Dis Model Mech. 2015;8(7):733–742. doi: 10.1242/dmm.018200. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S., Huen S., Nishio H., Nishio S., Lee H.K., Choi B.S., Ruhrberg C., Cantley L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011;22(2):317–326. doi: 10.1681/ASN.2009060615. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poluzzi C., Nastase M.V., Zeng-Brouwers J., Roedig H., Hsieh L.T., Michaelis J.B., Buhl E.M., Rezende F., Manavski Y., Bleich A., Boor P., Brandes R.P., Pfeilschifter J., Stelzer E.H.K., Münch C., Dikic I., Brandts C., Iozzo R.V., Wygrecka M., Schaefer L. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 2019;95(3):540–562. doi: 10.1016/j.kint.2018.10.037. Mar. [DOI] [PubMed] [Google Scholar]
- 58.Gao W., Xiong Y., Li Q., Yang H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front. Physiol. 2017;8:508. doi: 10.3389/fphys.2017.00508. Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsunaga N., Tsuchimori N., Matsumoto T., Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 2011;79(1):34–41. doi: 10.1124/mol.110.068064. Jan. [DOI] [PubMed] [Google Scholar]
- 60.González-Guerrero C., Cannata-Ortiz P., Guerri C., Egido J., Ortiz A., Ramos A.M. TLR4-mediated inflammation is a key pathogenic event leading to kidney damage and fibrosis in cyclosporine nephrotoxicity. Arch. Toxicol. 2017;91(4):1925–1939. doi: 10.1007/s00204-016-1830-8. Apr. [DOI] [PubMed] [Google Scholar]
- 61.Kezić A., Stajic N., Thaiss F. Innate immune response in kidney ischemia/reperfusion injury: potential target for therapy. J Immunol Res. 2017:6305439. doi: 10.1155/2017/6305439. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng Y., Liu L., Wang Y., Yao J., Jin F., Tao T., Yuan H., Shi L., Lu S. Treatment with Toll-like receptor 2 inhibitor ortho-vanillin alleviates lipopolysaccharide-induced acute kidney injury in mice. ExpTher Med. 2019 Dec;18(6):4829–4837. doi: 10.3892/etm.2019.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farrar C.A., Keogh B., McCormack W., O'Shaughnessy A., Parker A., Reilly M., Sacks S.H. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. Faseb. J. 2012;26(2):799–807. doi: 10.1096/fj.11-195396. Feb. [DOI] [PubMed] [Google Scholar]
- 64.Ungaro R., Fukata M., Hsu D., Hernandez Y., Breglio K., Chen A., Xu R., Sotolongo J., Espana C., Zaias J., Elson G., Mayer L., Kosco-Vilbois M., Abreu M.T. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(6):G1167–G1179. doi: 10.1152/ajpgi.90496.2008. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable as this is a review article.