Abstract
Apex predator reintroductions have proliferated across southern Africa, yet their ecological effects and proposed umbrella benefits of associated management lack empirical evaluations. Despite a rich theory on top-down ecosystem regulation via mesopredator suppression, a knowledge gap exists relating to the influence of lions (Panthera leo) over Africa's diverse mesocarnivore (less than 20 kg) communities. We investigate how geographical variation in mesocarnivore community richness and occupancy across South African reserves is associated with the presence of lions. An interesting duality emerged: lion reserves held more mesocarnivore-rich communities, yet mesocarnivore occupancy rates and evenness-weighted diversity were lower in the presence of lions. Human population density in the reserve surroundings had a similarly ubiquitous negative effect on mesocarnivore occupancy. The positive association between species richness and lion presence corroborated the umbrella species concept but translated into small differences in community size. Distributional contractions of mesocarnivore species within lion reserves, and potentially corresponding numerical reductions, suggest within-community mesopredator suppression by lions, probably as a result of lethal encounters and responses to a landscape of fear. Our findings offer empirical support for the theoretical understanding of processes underpinning carnivore community assembly and are of conservation relevance under current large-predator orientated management and conservation paradigms.
Keywords: lion, Panthera leo, mesopredator release, camera-trap, occupancy, hierarchical Bayesian models
1. Background
Conservation management interventions in southern Africa's network of intensively managed and mostly fenced reserves are disproportionally motivated by maintaining populations of highly charismatic species that have high economic value, including large carnivores [1,2]. These behaviours can generally be attributed to the rise of ecotourism and the game viewing preferences of tourists. While large-predator-centred management of southern Africa's reserves has generated positive conservation outcomes for large carnivores [3], the effects on broader biodiversity patterns are less well understood, particularly for less charismatic and overlooked, yet functionally important, taxa. Evaluating the alignment between commercially motivated carnivore management and biodiversity is, therefore, important for reconciling financial and conservation interests when the maintenance of ecosystem functioning is a conservation priority.
The African lion (Panthera leo) is a large carnivore that has received intensive conservation management in southern Africa. As apex predators, lions may play an important regulatory ecological role [4,5], are considered an indicator of ecosystem health [6] and are susceptible to many of the threats common across African wildlife (e.g. habitat loss, prey depletion and human-wildlife conflict) [5,7]. There is a growing consensus around the role of lions as flagship species [8], their economic and conservation value [2], and the need for increased investment in their conservation [6]. However, despite doubts of the usefulness of lion as umbrella species [9,10], there is a surprising lack of empirical studies evaluating the role of lions as conservation proxies. In South Africa, most lion populations are too small to be independently viable, intensively managed, constrained to small areas, and are reliant on assisted dispersal [11,12]. Lion populations in South Africa are, therefore, not reliable indicators of contiguous, less intensively managed ecosystems [10,13]. Nonetheless, the large capital investment in activities related to lion reintroductions and population maintenance (e.g. prey availability, population control, infrastructure development, anti-poaching efforts [12,14]) are likely to confer umbrella benefits to sympatric species [7,15].
There is, however, little understanding of the direct ecological effects of apex predator reintroductions on sympatric species in South Africa's numerous small, fenced reserves. Past research focus has been on lion-induced top-down effects, specifically the regulation of ungulate species [16], and the relatively few investigations of lions' influence over sympatric carnivores are mostly restricted to other large carnivore species [17–19]. Thus, an important knowledge gap exists relating to the influence lions have on the size, structure and composition of Africa's diverse carnivore assemblages [20], specifically communities of small- and medium-sized species (less than 20 kg), here collectively called mesocarnivores [21]. Yet, mesocarnivore's functional role and susceptibility to suppression or facilitation by larger carnivore species [20,22–24] makes them central to evaluating the ecological outcomes of large-predator-centred conservation efforts and management paradigms [12].
Mesocarnivores are a numerous and diverse, yet understudied [25], group of mammals, and an important component of ecosystem function, structure and dynamics [26,27]. A rich body of theory predicts profound ecosystem-level cascading effects resulting from apex predator mediated top-down processes, such as mesopredator suppression and release [5,23]. While research on intraguild carnivore interactions has traditionally focused on highly competing species, recent evidence has exposed the potential for overlooked suppression pathways by large carnivores over a broader range of sympatric mesopredators [24,28]. Lion-induced suppression of mesocarnivore communities may thus be an important unheeded aspect of ‘lionscapes’ along with proposed umbrella benefits. Such reasoning motivates practical and theoretical interest in relating geographical variation in mesocarnivore diversity to the presence of lions; but this requires linking the scales at which variation in diversity is observed to the ecological levels at which the processes hypothesized to affect diversity operate [29]. Killing, harassment and other kinds of competitive interference by lions on subordinate mesocarnivores [20,30] can theoretically induce local numerical (e.g. population declines) and behavioural (e.g. altering the exploitation of space) responses [23] (but see [17]). Both processes manifest at the population level by the abundance and distribution of a species at the landscape (or reserve) scale. Multiple species responses to apex predators, lion-focused management or associated changes within community dynamics [31], shape higher-level community patterns and can drive spatial variation in composition and diversity of mesocarnivore assemblages. In concert, these patterns are difficult to evaluate as it requires spatially replicated sampling of mesocarnivore communities over large geographical extents which include variation in apex predator populations and environmental contexts [22,32,33]. Fenced reserves operating under a variety of management objectives, including decisions to reintroduce lion populations, provide a unique setting to test multiple hypotheses related to drivers of mesopredator community structure under a natural experimental framework.
Here, we use an extensive camera-trapping dataset collected across South African reserves (figure 1), to investigate correlates of geographical variation in mesocarnivore communities' structure at two distinct community organization levels: we quantify among reserves variation in (i) species richness and (ii) species-specific occupancy rates (i.e. the proportion of occupied/used area within a reserve), a proxy for local relative abundances [34]. Primarily, we are interested in the extent to which geographical variation in mesocarnivore richness and occupancy is linked to the presence or absence of lions; specifically, whether species richness and occupancy are positively associated with lions as would be expected if lion management conferred benefits to sympatric species (a variant of the umbrella-concept) [7,23], or are negatively associated with lion presence as predicted with mesopredator suppression [23,24]. In addition to the association with lion presence, we simultaneously account for inherent anthropogenic and ecological variation among reserves (reserve size, surrounding human population density, structural habitat diversity and baseline top-predator pressure in the absence of lions (leopard (Panthera pardus) density)). Finally, we compared mesocarnivore community evenness-weighted diversity across reserves [35], specifically contrasting communities with and without lion presence. Importantly, including lion presence as a multi-level predictor of geographical variation in mesocarnivore richness and reserve-specific occupancy patterns provides a novel and integrated approach to explore two concepts widely used to advocate the conservation surrogacy of lions—umbrella and keystone species—and how these may act in tandem [9].
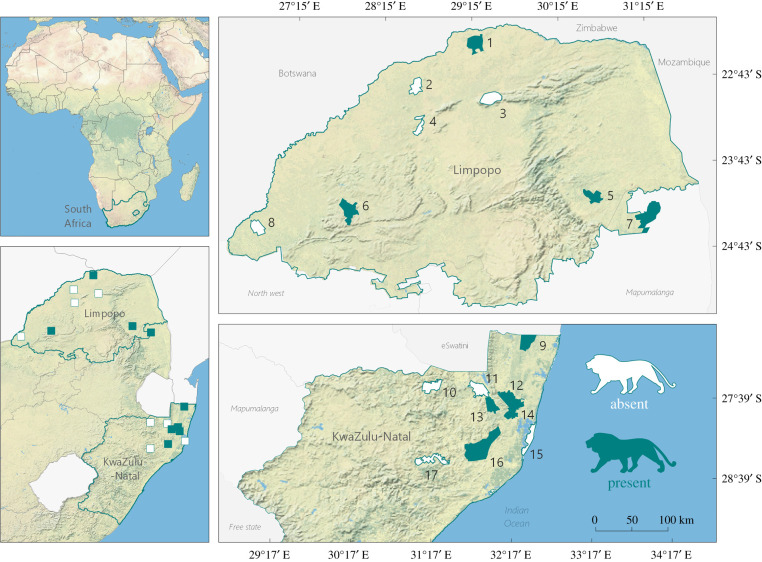
Figure 1.
Reserves target of the 33 camera-trapping surveys implemented across Limpopo and KwaZulu-Natal provinces, South Africa. 1, Venetia-Limpopo GR; 2, Zingela NR; 3, Lajuma RC; 4, Wonderkop NR; 5, Makalali GR; 6, Welgevonden GR; 7, Timbavati PGNR; 8, Atherstone GR; 9, Tembe EP; 10, Ithala GR; 11, Somkhanda GR; 12, uMkhuze GR; 13, Manyoni PGR; 14, Munyawana PGR; 15, iWP E. Shores; 16, Hluhluwe-iMfolozi P; and 17, Ophate GR. See the electronic supplementary material, appendix table S1 for survey details. (Online version in colour.)
2. Material and methods
(a). Study area
We targeted R = 17 reserves across two South African provinces, Limpopo and KwaZulu-Natal (figure 1; electronic supplementary material, appendix table S1). These were a combination of provincial parks (n = 9) and privately managed (n = 8) conservation areas, all providing varying levels of protection to wildlife (International Union for Conservation of Nature categories II–VI) and ranging in size from 150 to 907 km2. Approximately half of these held lion populations during the surveys (n = 9). Reserves were predominantly mixed savannah habitat, but included semi-arid savannah, thicket, forest, montane grassland and coastal belt vegetation. Climate typically varies along a north–south gradient from arid in the north to warm temperate climates at the more southern sites [36].
(b). Carnivore surveys
We used ancillary camera-trap data on small- and medium-sized carnivores collected while documenting leopard densities in target reserves between 2013 and 2016 [37]. From the original dataset, we considered only surveys conducted between April and September, correspondent to the dry season in the region, to avoid confounding aspects associated with seasonality and to increase comparability across surveys. The number of surveys in each reserve varied from 1 to 3. The final dataset comprised S = 33 reserve-by-year surveys. On average in each survey (mean ± s.d.), 40 ± 5 camera-trap stations were deployed, spaced 1935 ± 275 m apart, for 46 ± 4 days; totalling 1318 stations and 61 019 effective trap days (see the electronic supplementary material, appendix table S1 for survey details). Camera locations were selected to target intersections between features commonly used by leopards (i.e. roads, drainage lines and game trails). At each location, two Pantheracam V4, V5 or V6 xenon flash cameras with infrared motion sensors were set at opposite sides of the target feature, at a height of 30–40 cm above the ground and angled parallel to the slope. Cameras were programmed to record a single photograph per trigger. See the electronic supplementary material, appendix SI for additional description of camera-trapping protocols.
Although designed explicitly for the estimation of leopard density, the survey design was adequate for our inference objective. This is achieved by the surveys’ wide spatial coverage, thus allowing for reserve-scale comparisons, with average per-survey number of sites and duration within the recommended guidelines to obtain precise estimates of species richness, occupancy and detection rates with camera-trap arrays [38], and using a trail-based camera placement suggested to increase the detection probability of a wide range of carnivore species [39].
(c). Multi-region community occupancy model
We used a multi-region community model to jointly define geographical variation in community- and species-level attributes, while formally accounting for imperfect detection, heterogeneity in detectability and heterogeneity in occurrence probabilities [40]. The model expands the species-by-site (here, camera-trap stations) data structure typical of multispecies occupancy models [41] to data collected across distinct regions (here, reserve-by-year surveys). This allows for formal testing of hypotheses about drivers of variation in species richness and occupancy across multiple regions and the derivation of biodiversity metrics with full error propagation [40]. Note that the ecological definition of site occupancy and the nature of the occupancy-abundance relationship are species-specific, and our interest was in relative differences in species-specific occupancy rates among regions [34].
We summarized mesocarnivore daily encounter frequency data from S = 33 regions, with reserve-specific observed species richness ranging from 8 to 16 (median 12). Because we were interested in geographical variation at the reserve-scale, we included covariates as a linear combination of effects with a logit-link transformation on two state variables: reserve-specific species richness (Ωr) and species-by-survey occupancy rates (ψis). We modelled occupancy probability using species-by-survey random intercepts with species-specific hyperparameters. Hyperparameters specify the mean community response and variation among species to a covariate. With only 17 reserves and 33 surveys, besides the effect of lion presence, we limited the model's fixed effects on richness and occupancy parameters to three additional broad proxy variables, allowing us to evaluate a global model of community response [42]. Model formulation details are reported in the electronic supplementary material, appendix SII and the JAGS model code is given in the electronic supplementary material, appendix SIII.
(d). Model covariates
We modelled species richness as a linear function of reserve-by-year covariates: (i) lion presence (LION); (ii) reserve size (AREA), based on species-area relationship predictions [43] and potential of larger reserves to buffer edge effects [44]; (iii) surrounding human population density (HUM) [45], as a proxy for human-wildlife conflicts [46–50]; and (iv) structural habitat diversity (HDIV), under expectations that habitat heterogeneity promotes species richness [43] (eqn. (1) in the electronic supplementary material, appendix SII). We simultaneously modelled occupancy probability in relation to lion presence and as a linear function of reserve-scale measures of anthropogenic pressure (human density, as above), assuming human-induced disturbance hampers population densities and/or constrains species distributions, and of local leopard densities (LEOP), to account for the main role of leopards on intraguild dynamics [20], particularly as the apex predator in the absence of lions, and known influence over mesocarnivore species [51] (eqns (2)–(4) in the electronic supplementary material, appendix SII).
We used lion camera-trapping images from our surveys to code lion presence as a binary variable (LION). This information was checked against knowledge on lion population of each reserve to account for the unlikely event that our surveys fail to obtain a single lion record despite the presence of the species. We extracted reserve size (AREA) from area summaries of official property limits in GIS software (Quantum GIS 2.18) and calculated average human population density (HUM) within a 10 km buffer area surrounding each reserve from the 2015 WorldPop estimate for the number of people per 100 m grid square (https://africaopendata.org/dataset/south-africa-population-density-2015). As a proxy for habitat diversity (HDIV), we calculated the Simpson's Landscape Diversity Index for each reserve based on 2013–2014 national remote-sensed land cover data for South Africa (https://egis.environment.gov.za/national_land_cover_data_sa). We used survey-specific leopard density estimates (LEOP) from spatial capture–recapture models applied to leopard data from the same camera-trap surveys [37,52]. We checked for multicollinearity by evaluating pairwise correlations between covariates and ensuring that no highly correlated pairs (r > 0.6) were included in the analysis. All covariates were normalized between 0 and 1.
(e). Diversity metrics
Since species-richness estimates do not account for evenness among species, we used survey-specific occupancy-based Hill number estimators [35] to calculate the effective number of species and further elucidate the potential influence of lion presence on mesocarnivore diversity. Hill numbers are a mathematical family of diversity indices that differ among themselves only by an exponent q. Hill numbers for q > 0 summarize two commonly used biodiversity metrics: (i) Shannon diversity (q = 1, the Shannon entropy exponentiated) and (ii) Simpson diversity (q = 2, the inverse of the complement of the Gini–Simpson index). Both indices translate the degree of dissimilarity across species in each community but differ in relative importance given to rare species. Species richness is a Hill number of order q = 0. Hill numbers were computed as derived quantities within the Bayesian hierarchical model, allowing for error propagation and derivation for these metrics. We compared Hill numbers by deriving the difference in the average index for reserves with and without lions; doing that in a Bayesian framework allowed us to derive the posterior distribution, and therefore summary statistics, of the difference in the effective number of species in relation to lion presence. Because Hill numbers of order q = 1 and q = 2 were highly correlated (r = 0.97), we present results only for the former in the main text.
3. Results
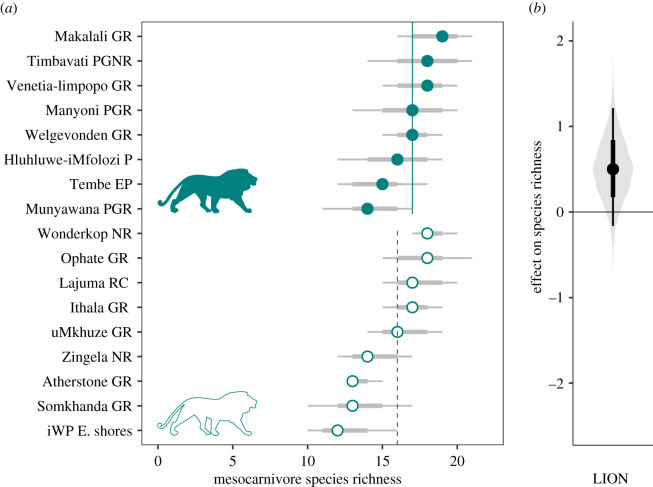
Camera-trapping effort resulted in a total of 13 667 records of 22 mesocarnivore species (electronic supplementary material, appendix table S2). There was considerable variation in estimated median species richness among reserves (figure 2; electronic supplementary material, appendix table S3), ranging from 12 to 19 (95% Bayesian credible interval (BCI): 10–21). Species richness showed a positive relationship with lion presence at the reserve scale (βΩ,LION = 0.51, BCI: −0.16–1.21, 0.93 probability of a greater than zero effect; figure 2b). Mesocarnivore richness was also positively associated with human population density surrounding the reserves (βΩ,HUM = 0.88, BCI: −0.33–2.16, 0.92 probability of a greater than zero effect). Reserve size (βΩ,AREA = −0.20, BCI: −1.94–1.70) and habitat diversity (βΩ,HDIV = 0.21, BCI: −0.62–1.06) did not have clear effects as predictors of relative change in community richness, i.e. low probability that the estimated effect is different than zero (electronic supplementary material, appendix table S4).
Figure 2.
Across reserve variation in mesocarnivore species richness. Points are posterior medians, and error bars represent 66 and 95% Bayesian credible intervals. Vertical lines represent species-richness means across reserves. (a) Reserve-specific species-richness estimates; filled and open symbols represent estimates in the presence and absence of lions, respectively. (b) Coefficient of lion presence (LION) covariate effect on mesocarnivore species richness. (Online version in colour.)
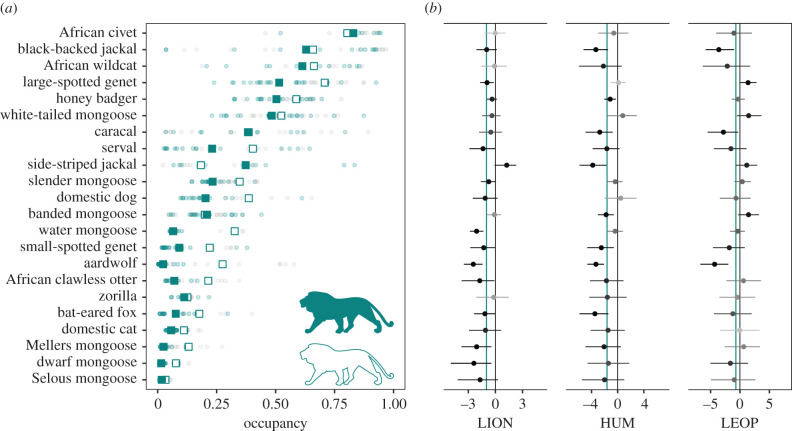
Species-by-survey occupancy estimates (ψ) showed a high degree of inter- and intra-specific heterogeneity (figure 3a). Across surveys, the community mean occupancy probability was moderately low (0.29 ± 0.29; mean ± s.d.), with species-specific means ranging from 0.02 ±0.10 for the Selous's mongoose (Paracynictis selousi) to 0.82 ± 0.15 for the African civet (Civettictis civetta). Lion presence negatively influenced mesocarnivore species occupancy, with a mean community-level response of μβψ,LION = −0.98 (BCI: −1.61 to −0.39; figure 3b). Unlike richness, average mesocarnivore occupancy was higher when lions were absent compared with when they are present (0.35 ± 0.29 and 0.25 ± 0.28, respectively; figure 3). Species-specific responses to lion presence were bimodal, with 16 out of 22 species exhibiting a clear negative response (i.e. probability of a negative effect greater than 0.9), while for other species, the probability of a positive or negative signal for this effect was close to chance. Only the occupancy of side-striped jackals (Canis adustus) was positively associated with the presence of lions. Similarly ubiquitous among species, human population density had a negative effect on mesocarnivore occupancy (μβψ,HUM = −1.66; BCI: −2.65 to −0.74; the probability of a negative effect greater than 0.9 for 13 species; figure 3b). Leopard density had a less clear community-level effect (μβψ,LEOP = −0.69; BCI: −1.83–0.39; figure 3b), with a mix of negative and positive responses (four species each). Full summaries of posterior distributions for community- and species-level covariate coefficients, and species-by-survey occupancy estimates are provided in the electronic supplementary material, appendix tables S5–S9.
Figure 3.
Patterns and drivers of mesocarnivore species occupancy. (a) Species- and survey-specific occupancy estimates. Filled blue symbols represent survey-specific estimates in the presence of lions. Squares represent species-specific mean occupancy across surveys. Error bars were omitted for visual clarity. (b) Effect size of the relationship between species occupancy and lion presence (LION), human population density in reserve's surroundings (HUM) and leopard density (LEOP). Points are posterior distribution means, and error bars represent 95% Bayesian credible intervals, coloured by the probability of an effect, calculated as the proportion of the posterior with the same sign as the mean. Vertical blue lines mark community mean effects. (Online version in colour.)
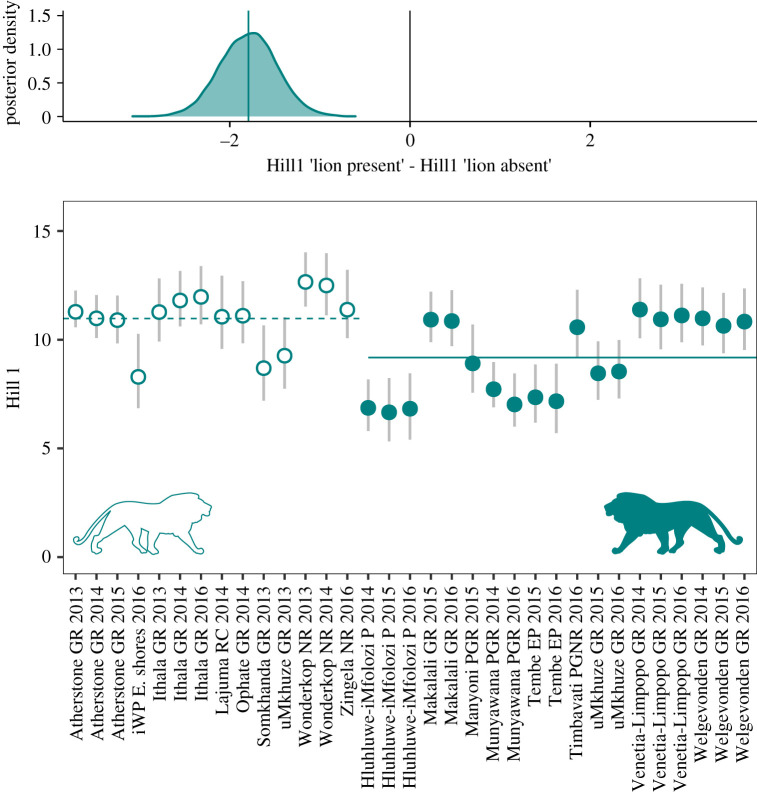
On average, mesocarnivore communities had a higher effective number of species in the absence of lions (figure 4; electronic supplementary material, appendix table S10) as expressed by reserve-by-year-specific occupancy-based Shannon diversity Hill number estimates.
Figure 4.
Reserve-by-year-specific mesocarnivore diversity expressed as occupancy-based Shannon diversity Hill number (q = 1, the Shannon entropy exponentiated). Horizontal bars represent Hill number's means across surveys. Points are posterior distribution means, and error bars represent 95% Bayesian credible intervals. Filled and open symbols represent estimates in the presence and absence of lions, respectively. (Online version in colour.)
4. Discussion
We jointly describe geographical variation in mesocarnivore community size and patterns of species-specific occupancy across a network of reserves in South Africa. We provide empirical support for the potential role of the African lion and lion-focused management as structuring agents of these communities, a matter of theoretical importance for understanding mechanisms underpinning carnivore community assembly, and of conservation relevance in the context of large-predator orientated management paradigms [2,6,49].
(a). Multi-level mesocarnivore community structure
Our findings suggest that lion presence may have a profound influence on the structure of mesocarnivore communities. Namely, an interesting duality emerged: lion presence was associated with slightly more mesocarnivore-rich communities, yet mesocarnivore occupancy rates, a proxy for local abundances [34], were lower in the presence of lions.
Associations of apex predators with high biodiversity have been attributed to common suitable biotic and abiotic conditions, mediated by large predator's sensitivity to disturbance or dependence on productive and heterogeneous ecosystems [9]. Even if decisions to introduce lions in South Africa were largely motivated by the economics of ecotourism rather than biodiversity conservation priorities [14], the sizeable budgets and management capacity necessary to successfully introduce and sustain lion populations [12,14] may favour the species richness of sympatric mesocarnivore communities by mitigating common threats [53,54]. Complementarily, resource facilitation and apex predator-induced cascades are two additional pathways by which lion presence can causatively favour more species-rich mesocarnivore communities. Lions are the single predator of very large ungulates (e.g. giraffe (Giraffa camelopardalis) and buffalo (Syncerus caffer)) which subsequently provide carrion for scavengers [55]. By constraining other large, albeit subordinate, carnivores [18,19], lions can also create enemy-free spaces for sympatric mesocarnivores, thereby promoting species persistence. However, such effects may not be unequivocally present [17]. Increased mesocarnivore richness associated with lion presence corroborates claims for broader biodiversity benefits of maintaining lions [7]. However, in absolute terms, lion presence translated into, on average, just one additional species in the mesocanivore community. While such small a difference may be intrinsically valuable and ecologically relevant for relatively species-poor taxa, depending on species identity and functional redundancy [56], this pattern suggests that the direct and indirect effects of lion presence are more likely to manifest at the population level rather than modulate extreme extinction events.
In contrast to the positive association of lions with mesocarnivore richness, but in accordance with theoretical expectations [23,27,57], we provide rare empirical support for repeated community-wide mesopredator suppression by lions. Lions are predicted to suppress sympatric large carnivores through combinations of direct, i.e. lethal, encounters and indirect, i.e. fear and loathing, responses [18,19] (but see [17]). Here, we propose the same mechanisms may apply across almost the entire South African mesocarnivore assemblage. While lions are among the most frequent intraguild killer species [30], the body-mass ratio between lions and the species that were negatively influenced by lions was outside the range where this behaviour is deemed to be most prevalent and ecologically beneficial (greater than 2 and less than 5.4; [30]). However, these predictions are largely untested empirically, potentially undervaluing alternative competition pathways (e.g. kleptoparasitism) and the role of predatory and incidental killing by hyperpredatory felids [28]. Recent evidence suggests carrion provisioning by large carnivores may potentiate largely asymmetric lethal interactions owing to the scavenging behaviour of mesocarnivore species (i.e. the ‘fatal attraction hypothesis'), therefore enhancing rather than ameliorating suppression at wider spatial scales [24]. Thus, instead of benefiting from cascading effects of antagonistic interactions between lions and sympatric large carnivores [58], African mesocarnivores may experience superadditive suppressive effects [24]. Further research is needed to elucidate net suppressive effects of the complete large carnivore guild over mesocarnivores and how these propagate across guild levels as potentially modulated by lion presence [51]. The risk of killing or harassment by lions may also induce behavioural changes to avoid direct encounters. The ‘landscape of fear’ associated with apex predators [59] can be particularly strong for mesocarnivores as they are poorly adapted to escape [23], especially in fenced environments with artificially high apex predator densities [12]. The resulting numerical reductions and corresponding distributional contractions within reserves are probably mechanisms by which community-wide occupancy reductions in the presence of lions may emerge and outweigh the presumable benefits associated with the increased management capacity of lion reserves.
The apparent dichotomy between the positive and negative effects of lions on species richness and occupancy, respectively, suggest that net suppressive effects by lions may not impact mesocarnivore species to the point of local extinction. The lack of a common negative response in species richness in the presence of lions can also result from synergisms and feedbacks between regulatory processes owing to the smaller carnivore's strong predisposition to intraguild competition [20,31]. Cascading effects of restricted species-specific occupancy may reduce lateral competition among mesocarnivores, thus facilitating coexistence and promoting mesocarnivore persistence by controlling dominant species with the potential to outcompete others [60]. Conversely, competitive processes within the mesocarnivore communities, unaccounted for here but warranting further investigation, can also modulate the observed species-specific associations with lion presence. The net facilitative versus the suppressive effect of an apex predator should also depend on changes in such competitive interactions [24], which may ultimately favour some mesocarnivore species, for example the observed positive response of the side-striped jackal to the presence of lions.
The effective number of mesocarnivore species (i.e. evenness-weighted diversity) was also lower in reserves where lions were present. While descriptive, evenness is regarded as an important component of biodiversity [61], and our result raises further questions about the surrogacy potential of lions in fenced and intensely management reserves. At the same time, we provide support to an additional community-wide intraguild regulation dimension to the usually invoked keystone role of lions. While these results reflect emergent patterns in accordance with standing theoretical expectations, we cannot, however, infer causation. We argue that, despite lacking specific mechanisms, our observational approach provides an informed starting point [62] for detailed investigations of the specific processes that play across complex multi-trophic interaction networks. Future lion reintroductions and translocations create scope for stronger evidence under before-and-after comparisons or, ideally, cross-over designs. Additionally, we did not incorporate information about lion demography and distribution. The adaptive management of lion populations [63] makes it difficult to link fluid population states to emergent ecological effects. Hence, we opted for a conservative presence/absence approach that refers to differences in respect to baseline apex predator states and management intentions. The heterogeneity we observe in mesocarnivore diversity among reserves where lions were present concurs, nonetheless, with growing consensus around context dependency of large-predator effects [64]. The extent to which ecological responses of mesocarnivore communities depend on the structure of the local lion population (e.g. density, sex ratios, age classes) and subjacent management idiosyncrasies [11,12] warrants further research.
There was little support for the effects of habitat diversity or reserve size on mesocarnivore species richness. This is perhaps expected as most of the species we encountered are widely distributed in the region, and as versatile and habitat generalist species [20], possess broader bioclimatic niches. Moreover, reserves are not discrete units within a completely hostile landscape matrix and fences are permeable to most carnivore species [65]. Even land now protected in each reserve was, in many cases, highly disturbed prior to the growth of South Africa's wildlife industry, potentially inducing unaccounted historical ‘extinction filters’ [66]. Indeed, contrary to our initial hypothesis, we found a positive relationship between mesocarnivore richness and human population density in the reserve's surroundings. This result is probably the outcome of human-dominated areas serving as source populations of individuals traversing into the reserve boundaries, particularly the two domestic carnivores included in our study. Nevertheless, anthropogenic pressure did have a pervasive negative influence over species-specific occupancy rates. It is likely that proximity to reserve boundaries or the extent to which external anthropogenic stressors bleed into reserves, i.e. edge effects [53], may result in negative species-specific responses that manifest as variation among species within reserve occupancy rates. Although we did not account for interaction among predictors, the umbrella benefits of more effective protection in lion reserves may counteract potential suppressive effects, particularly for most conflict-prone mesocarnivores. For instance, the caracal did not exhibit a strong negative response to lion presence but was highly impacted by human pressure. Leopards in South Africa are also known to be affected by edge effects [53]; hence, increased protection in lion reserves may similarly mask leopard's influence over sympatric mesocarnivores [51]. Alternatively, leopard effects may not scale proportional to density [67], with observed mesocarnivore occupancies already referring to baseline states under the ubiquitous presence of leopards, as opposed to local lion reintroductions.
(b). Theoretical and applied implications
We provide empirical evidence for geographical variation in mesocarnivore communities’ structure that is associated with the presence of lions. This is of particular conservation relevance considering the disproportional attention given to lions as flagship and umbrella species [6,7], and the potential for lions to modulate the important ecological role of mesocarnivores [27]. Our results highlight the difficulty in disentangling the benefits of the umbrella species concept based on species richness [9] from the ecological effects of highly interactive apex predators. Increasing species richness remains a goal of many conservation practitioners. However, species richness is one, arguably simplistic, biodiversity measure [68] that fails to capture underlying nuances that our multi-level approach has highlighted. More subtle changes in the distribution and abundance of key functional groups, such as mesocarnivore occupancy and evenness, are often unheeded aspects of biodiversity change with far-reaching implications for ecosystem functioning. This presents a fundamental challenge for applied conservation management, where management objectives need to balance ecological responses at multiple levels of spatial and biological organization.
African mesocarnivores are important predators of small vertebrates (e.g. rodents, lagomorphs and birds [20], including pest species [69]). Many are also facultative scavengers significant to waste removal [70]. Moreover, they consume and disperse seeds and prey on a vast array of herbivores and detritivores, thus are also important to vegetation communities [71]. Without adequate conservation benchmarks or baselines [72], it is impossible to ascertain whether apparent mesocarnivore occupancy declines owing to lion presence in small reserves impairs the delivery of such ecosystem functions or if mesopredator release in the absence of lions increases pressure over vulnerable lower trophic levels, with detrimental cascading effects [23]. In this context, the comparison of our results with similar studies carried out in large and unfenced protected areas, home to remaining free-ranging lion populations, could produce valuable insights. Our results make a case for top-down control via mesopredator suppression in small South African reserves with vast applications in the conservation of biodiversity and habitat restoration [9,73]; but the degree of mesocarnivore effects and apex predator dominance are likely to be highly context-dependent and dynamic [64], marked by rapid and variable growth rates of reintroduced lion populations [63]. Although there is an intrinsic value of reintroducing lions as a restored ecosystem-component itself [6] and as an integrative part of metapopulation conservation efforts [74], we call for a more holistic view of African carnivore assemblages and ecosystem-wide implications of management and conservation interventions [75].
Supplementary Material
Acknowledgements
We thank staff at various reserves for their support during data collection. We are grateful for the extensive financial and logistical assistance provided by WildlifeACT, as well as to Lisa Thomas, Gareth Whittington-Jones and Almero Bosch. We also thank Ezemvelo KwaZulu-Natal Wildlife, IsiMangaliso Wetland Park, and the Limpopo Department of Economic Development, Environment and Tourism for their support of this research. We thank Fredrik Dalerum for comments on an early draft of the manuscript.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rxwdbrv77 [76].
Authors' contributions
G.C.S., L.H.S., C.S. and S.T. conceived the ideas and designed the study; R.P. and G.M. collected the data; C.S., S.T. and G.C.S. analysed and interpreted the data with contributions of A.F.C. and L.H.S.; G.C.S. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Peace Parks Foundation; G.C.S. was funded by a doctoral grant from Fundacão para a Ciência e a Tecnologia (FCT: PD/BD/114037/2015); L.H.S. was supported by the National Research Foundation, South Africa (UID: 107099 and 115040) and by the African Institute for Conservation Ecology.
References
- 1.Caro T, Riggio J. 2013. The Big 5 and conservation. Anim. Conserv. 16, 261-262. ( 10.1111/acv.12058) [DOI] [Google Scholar]
- 2.Di Minin E, Fraser I, Slotow R, Macmillan DC. 2013. Understanding heterogeneous preference of tourists for big game species: implications for conservation and management. Anim. Conserv. 16, 249-258. ( 10.1111/j.1469-1795.2012.00595.x) [DOI] [Google Scholar]
- 3.Mossaz A, Buckley RC, Castley JG. 2015. Ecotourism contributions to conservation of African big cats. J. Nat. Conserv. 28, 112-118. ( 10.1016/j.jnc.2015.09.009) [DOI] [Google Scholar]
- 4.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301-306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 5.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 1241484. ( 10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 6.Lindsey PA, et al. 2018. More than $1 billion needed annually to secure Africa's protected areas with lions. Proc. Natl Acad. Sci. USA 115, E10788-E10796. ( 10.1073/pnas.1805048115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsey PA, et al. 2017. The performance of African protected areas for lions and their prey. Biol. Conserv. 209, 137-149. ( 10.1016/j.biocon.2017.01.011) [DOI] [Google Scholar]
- 8.Macdonald EA, Burnham D, Hinks AE, Dickman AJ, Malhi Y, Macdonald DW. 2015. Conservation inequality and the charismatic cat: Felis felicis. Glob. Ecol. Conserv. 3, 851-866. ( 10.1016/j.gecco.2015.04.006) [DOI] [Google Scholar]
- 9.Sergio F, Caro T, Brown D, Clucas B, Hunter J, Ketchum J, McHugh K, Hiraldo F. 2008. Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annu. Rev. Ecol. Evol. Syst. 39, 1-19. ( 10.1146/annurev.ecolsys.39.110707.173545) [DOI] [Google Scholar]
- 10.Dalerum F, Somers MJ, Kunkel KE, Cameron EZ. 2008. The potential for large carnivores to act as biodiversity surrogates in southern Africa. Biodivers. Conserv. 17, 2939-2949. ( 10.1007/s10531-008-9406-4) [DOI] [Google Scholar]
- 11.Bauer H, Chapron G, Nowell K, Henschel P, Funston P, Hunter LTB, Macdonald DW, Packer C. 2015. Lion (Panthera leo) populations are declining rapidly across Africa, except in intensively managed areas. Proc. Natl Acad. Sci. USA 112, 14 894-14 899. ( 10.1073/pnas.1500664112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller SM, et al. 2013. Management of reintroduced lions in small, fenced reserves in South Africa: an assessment and guidelines. S. Afr. J. Wildl. Res. 43, 138-154. ( 10.3957/056.043.0202) [DOI] [Google Scholar]
- 13.Becker M, et al. 2012. The size of savannah Africa: a lion's (Panthera leo) view. Biodivers. Conserv. 22, 17-35. ( 10.1007/s10531-012-0381-4) [DOI] [Google Scholar]
- 14.Slotow R, Hunter LTB. 2009. Reintroduction decisions taken at the incorrect social scale devalue their conservation contribution: the African lion in South Africa. In Reintroduction of top-order predators (eds Alves RRN, Rosa IL), pp. 43-71. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 15.Packer C, et al. 2013. Conserving large carnivores: dollars and fence. Ecol. Lett. 16, 635-641. ( 10.1111/ele.12091) [DOI] [PubMed] [Google Scholar]
- 16.Chizzola M, Belton L, Ganswindt A, Greco I, Hall G, Swanepoel L, Dalerum F. 2018. Landscape level effects of lion presence (Panthera leo) on two contrasting prey species. Front. Ecol. Evol. 6:191. ( 10.3389/fevo.2018.00191) [DOI] [Google Scholar]
- 17.Balme GA, Pitman RT, Robinson HS, Miller JRB, Funston PJ, Hunter LTB. 2017. Leopard distribution and abundance is unaffected by interference competition with lions. Behav. Ecol. 28, 1348-1358. ( 10.1093/beheco/arx098) [DOI] [Google Scholar]
- 18.Vanak AT, Fortin D, Thaker M, Ogden M, Owen C, Greatwood S, Slotow R. 2013. Moving to stay in place: behavioral mechanisms for coexistence of African large carnivores. Ecology 94, 2619-2631. ( 10.1890/13-0217.1) [DOI] [PubMed] [Google Scholar]
- 19.Broekhuis F, Cozzi G, Valeix M, McNutt JW, Macdonald DW. 2013. Risk avoidance in sympatric large carnivores: reactive or predictive? J. Anim. Ecol. 82, 1098-1105. ( 10.1111/1365-2656.12077) [DOI] [PubMed] [Google Scholar]
- 20.Caro T, Stoner C. 2003. The potential for interspecific competition among African carnivores. Biol. Conserv. 110, 67-75. ( 10.1016/S0006-3207(02)00177-5) [DOI] [Google Scholar]
- 21.Carbone C, Teacher A, Rowcliffe JM. 2007. The costs of carnivory. PLoS Biol. 5, 0363-0368. ( 10.1371/journal.pbio.0050022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newsome TM, et al. 2017. Top predators constrain mesopredator distributions. Nat. Commun. 8, 15469. ( 10.1038/ncomms15469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982-998. ( 10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 24.Prugh LR, Sivy KJ. 2020. Enemies with benefits: integrating positive and negative interactions among terrestrial carnivores. Ecol. Lett. 23, 902-918. ( 10.1111/ele.13489) [DOI] [PubMed] [Google Scholar]
- 25.Brooke ZM, Bielby J, Nambiar K, Carbone C. 2014. Correlates of research effort in carnivores: body size, range size and diet matter. PLoS ONE 9, 1-10. ( 10.1371/journal.pone.0093195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roemer GW, Gompper ME, Van Valkenburgh B. 2009. The ecological role of the mammalian mesocarnivore. Bioscience 59, 165-173. ( 10.1525/bio.2009.59.2.9) [DOI] [Google Scholar]
- 27.Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS. 2009. The rise of the mesopredator. Bioscience 59, 779-791. ( 10.1525/bio.2009.59.9.9) [DOI] [Google Scholar]
- 28.de Oliveira TG, Pereira JA. 2014. Intraguild predation and interspecific killing as structuring forces of carnivoran communities in South America. J. Mamm. Evol. 21, 427-436. ( 10.1007/s10914-013-9251-4) [DOI] [Google Scholar]
- 29.Supp SR, Ernest SKM. 2014. Species-level and community-level responses to disturbance: a cross-community analysis. Ecology 95, 1717-1723. ( 10.1890/13-2250.1) [DOI] [PubMed] [Google Scholar]
- 30.Donadio E, Buskirk SW. 2006. Diet, morphology, and interspecific killing in carnivora. Am. Nat. 167, 524-536. ( 10.1086/501033) [DOI] [PubMed] [Google Scholar]
- 31.Mills DR, Do Linh San E, Robinson H, Isoke S, Slotow R, Hunter L. 2019. Competition and specialization in an African forest carnivore community. Ecol. Evol. 9, 10 092-10 108. ( 10.1002/ece3.5391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elmhagen B, Rushton SP. 2007. Trophic control of mesopredators in terrestrial ecosystems: top-down or bottom-up? Ecol. Lett. 10, 197-206. ( 10.1111/j.1461-0248.2006.01010.x) [DOI] [PubMed] [Google Scholar]
- 33.Newsome TM, Ripple WJ. 2015. A continental scale trophic cascade from wolves through coyotes to foxes. J. Anim. Ecol. 84, 49-59. ( 10.1111/1365-2656.12258) [DOI] [PubMed] [Google Scholar]
- 34.Steenweg R, Hebblewhite M, Whittington J, Lukacs P, McKelvey K. 2018. Sampling scales define occupancy and underlying occupancy-abundance relationships in animals. Ecology 99, 172-183. ( 10.1002/ecy.2054) [DOI] [PubMed] [Google Scholar]
- 35.Broms KM, Hooten MB, Fitzpatrick RM. 2015. Accounting for imperfect detection in Hill numbers for biodiversity studies. Methods Ecol. Evol. 6, 99-108. ( 10.1111/2041-210X.12296) [DOI] [Google Scholar]
- 36.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. 2006. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 15, 259-263. ( 10.1127/0941-2948/2006/0130) [DOI] [Google Scholar]
- 37.Miller JRB, Pitman RT, Mann GKH, Fuller AK, Balme GA. 2018. Lions and leopards coexist without spatial, temporal or demographic effects of interspecific competition. J. Anim. Ecol. 87, 1709-1726. ( 10.1111/1365-2656.12883) [DOI] [PubMed] [Google Scholar]
- 38.Kays R, et al. 2020. An empirical evaluation of camera trap study design: how many, how long and when? Methods Ecol. Evol. 11, 700-713. ( 10.1111/2041-210X.13370) [DOI] [Google Scholar]
- 39.Cusack JJ, Dickman AJ, Rowcliffe JM, Carbone C, Macdonald DW, Coulson T. 2015. Random versus game trail-based camera trap placement strategy for monitoring terrestrial mammal communities. PLoS ONE 10, e0126373. ( 10.1371/journal.pone.0126373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland C, Brambilla M, Pedrini P, Tenan S. 2016. A multiregion community model for inference about geographic variation in species richness. Methods Ecol. Evol. 7, 783-791. ( 10.1111/2041-210X.12536) [DOI] [Google Scholar]
- 41.Dorazio RM, Royle JA. 2005. Estimating size and composition of biological communities by modeling the occurrence of species. J. Am. Stat. Assoc. 100, 389-398. ( 10.1198/016214505000000015) [DOI] [Google Scholar]
- 42.Zipkin EF, Royle AJ, Dawson DK, Bates S. 2010. Multi-species occurrence models to evaluate the effects of conservation and management actions. Biol. Conserv. 143, 479-484. ( 10.1016/j.biocon.2009.11.016) [DOI] [Google Scholar]
- 43.Rosenzweig ML. 1995. Species diversity in space and time. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 44.Harcourt AH, Parks SA, Woodroffe R. 2001. Human density as an influence on species/area relationships: double jeopardy for small African reserves? Biodivers. Conserv. 10, 1011-1026. ( 10.1023/A:1016680327755) [DOI] [Google Scholar]
- 45.Cardillo M, Purvis A, Sechrest W, Gittleman JL, Bielby J, Mace GM. 2004. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2, e197. ( 10.1371/journal.pbio.0020197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doughty HL, Karpanty SM, Wilbur HM. 2015. Local hunting of carnivores in forested Africa: a meta-analysis. Oryx 49, 88-95. ( 10.1017/S0030605314000179) [DOI] [Google Scholar]
- 47.Alves RRN, Pinto LCL, Barboza RRD, Souto WMS, Oliveira REMCC, Vieira WLS. 2013. A global overview of carnivores used in traditional medicines. In Animals in traditional folk medicine (eds Hayward MW, Somers MJ), pp. 171-206. Berlin, Germany: Springer. [Google Scholar]
- 48.Vanak AT, Gompper ME. 2009. Dogs Canis familiaris as carnivores: their role and function in intraguild competition. Mamm. Rev. 39, 265-283. ( 10.1111/j.1365-2907.2009.00148.x) [DOI] [Google Scholar]
- 49.Winterbach HEK, Winterbach CW, Somers MJ, Hayward MW. 2013. Key factors and related principles in the conservation of large African carnivores. Mamm. Rev. 43, 89-110. ( 10.1111/j.1365-2907.2011.00209.x) [DOI] [Google Scholar]
- 50.Loveridge AJ, Sousa LL, Seymour-Smith J, Hunt J, Coals P, O'Donnell H, Lindsey PA, Mandisodza-Chikerema R, Macdonald DW. 2020. Evaluating the spatial intensity and demographic impacts of wire-snare bush-meat poaching on large carnivores. Biol. Conserv. 244, 108504. ( 10.1016/j.biocon.2020.108504) [DOI] [Google Scholar]
- 51.Ramesh T, Kalle R, Downs CT. 2017. Staying safe from top predators: patterns of co-occurrence and inter-predator interactions. Behav. Ecol. Sociobiol. 71, 41. ( 10.1007/s00265-017-2271-y) [DOI] [Google Scholar]
- 52.Mann G, Pitman R, Whittington-jones G, Thomas L, Broadfield J, Taylor J, Rogan M, Balme G.. 2020. South African leopard monitoring project. New York, NY: Panthera.
- 53.Burton AC, Sam MK, Kpelle DG, Balangtaa C, Buedi EB, Brashares JS. 2011. Evaluating persistence and its predictors in a West African carnivore community. Biol. Conserv. 144, 2344-2353. ( 10.1016/j.biocon.2011.06.014) [DOI] [Google Scholar]
- 54.Oberosler V, Tenan S, Zipkin EF, Rovero F. 2019. Poor management in protected areas is associated with lowered tropical mammal diversity. Anim. Conserv. 23, 171-181. ( 10.1111/acv.12525) [DOI] [Google Scholar]
- 55.Moleón M, Sánchez-Zapata JA, Selva N, Donázar JA, Owen-Smith N. 2014. Inter-specific interactions linking predation and scavenging in terrestrial vertebrate assemblages. Biol. Rev. 89, 1042-1054. ( 10.1111/brv.12097) [DOI] [PubMed] [Google Scholar]
- 56.Farias AA, Jaksic FM. 2011. Low functional richness and redundancy of a predator assemblage in native forest fragments of Chiloe Island, Chile. J. Anim. Ecol. 80, 809-817. ( 10.1111/j.1365-2656.2011.01824.x) [DOI] [PubMed] [Google Scholar]
- 57.Jiménez J, et al. 2019. Restoring apex predators can reduce mesopredator abundances. Biol. Conserv. 238, 108234. ( 10.1016/j.biocon.2019.108234) [DOI] [Google Scholar]
- 58.Levi T, Wilmers C. 2012. Wolves–coyotes–foxes: a cascade among carnivores. Ecology 93, 921-929. ( 10.1890/11-0165.1) [DOI] [PubMed] [Google Scholar]
- 59.Laundré JW, Hernandez L, Ripple WJ. 2010. The landscape of fear: ecological implications of being afraid. Open Ecol. J. 3, 1-7. ( 10.2174/1874213001003030001) [DOI] [Google Scholar]
- 60.St-Pierre C, Ouellet J-P, Crête M. 2006. Do competitive intraguild interactions affect space and habitat use by small carnivores in a forested landscape? Ecography (Cop.). 29, 487-496. ( 10.1111/j.0906-7590.2006.04395.x) [DOI] [Google Scholar]
- 61.Jost L. 2006. Entropy and diversity. Oikos 113, 363-375. ( 10.1111/j.2006.0030-1299.14714.x) [DOI] [Google Scholar]
- 62.Sagarin R, Pauchard A. 2010. Observational approaches in ecology open new ground in a changing world. Front. Ecol. Environ. 8, 379-386. ( 10.1890/090001) [DOI] [Google Scholar]
- 63.Miller SM, Funston PJ. 2014. Rapid growth rates of lion (Panthera leo) populations in small, fenced reserves in South Africa: a management dilemma. S. Afr. J. Wildl. Res. 44, 43-55. ( 10.3957/056.044.0107) [DOI] [Google Scholar]
- 64.Haswell PM, Kusak J, Hayward MW. 2015. Large carnivore impacts are context-dependent. Food Webs. 12, 3-13. ( 10.1016/j.fooweb.2016.02.005) [DOI] [Google Scholar]
- 65.Curveira-Santos G, Sutherland C, Santos-Reis M, Swanepoel LH. 2020. Responses of carnivore assemblages to decentralized conservation approaches in a South African landscape. J. Appl. Ecol. 58, 92-103. ( 10.1111/1365-2664.13726) [DOI] [Google Scholar]
- 66.Boshoff A, Landman M, Kerley G. 2016. Filling the gaps on the maps: historical distribution patterns of some larger mammals in part of southern Africa. Trans. R. Soc. South Africa 71, 23-87. ( 10.1080/0035919X.2015.1084066) [DOI] [Google Scholar]
- 67.Abrams PA. 1993. Why predation rate should not be proportional to predator density. Ecology 74, 726-733. ( 10.2307/1940800) [DOI] [Google Scholar]
- 68.Cernansky R. 2017. Biodiversity moves beyond counting species. Nature 546, 22-24. ( 10.1038/546022a) [DOI] [PubMed] [Google Scholar]
- 69.Williams ST, Maree N, Taylor P, Belmain SR, Keith M, Swanepoel LH. 2017. Predation by small mammalian carnivores in rural agro-ecosystems: an undervalued ecosystem service? Ecosyst. Serv. 30, 362-371. ( 10.1016/j.ecoser.2017.12.006) [DOI] [Google Scholar]
- 70.O'Bryan CJ, Braczkowski AR, Beyer HL, Carter NH, Watson JEM, McDonald-Madden E. 2018. The contribution of predators and scavengers to human well-being. Nat. Ecol. Evol. 2, 229-236. ( 10.1038/s41559-017-0421-2) [DOI] [PubMed] [Google Scholar]
- 71.Rosalino LM, Santos-Reis M. 2009. Fruit consumption by carnivores in Mediterranean Europe. Mamm. Rev. 39, 67-78. ( 10.1111/j.1365-2907.2008.00134.x) [DOI] [Google Scholar]
- 72.Hayward MW. 2009. Conservation management for the past, present and future. Biodivers. Conserv. 18, 765-775. ( 10.1007/s10531-008-9436-y) [DOI] [Google Scholar]
- 73.Ritchie EG, Elmhagen B, Glen AS, Letnic M, Ludwig G, McDonald RA. 2012. Ecosystem restoration with teeth: what role for predators? Trends Ecol. Evol. 27, 265-271. ( 10.1016/j.tree.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 74.Dolrenry S, Stenglein J, Hazzah L, Lutz RS, Frank L. 2014. A metapopulation approach to African lion (Panthera leo) conservation. PLoS ONE 9, 1-9. ( 10.1371/journal.pone.0088081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montgomery RA, Moll RJ, Say-Sallaz E, Valeix M, Prugh LR. 2019. A tendency to simplify complex systems. Biol. Conserv. 233, 1-11. ( 10.1016/j.biocon.2019.02.001) [DOI] [Google Scholar]
- 76.Curveira-Santos G, Sutherland C, Tenan S, Fernández-Chacón A, Mann GKH, Pitman RT, Swanepoel LH. 2021. Data from: Mesocarnivore community structuring in the presence of Africa’s apex predator. Dryad Digital Repository. ( 10.5061/dryad.rxwdbrv77) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Curveira-Santos G, Sutherland C, Tenan S, Fernández-Chacón A, Mann GKH, Pitman RT, Swanepoel LH. 2021. Data from: Mesocarnivore community structuring in the presence of Africa’s apex predator. Dryad Digital Repository. ( 10.5061/dryad.rxwdbrv77) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rxwdbrv77 [76].