Abstract
Background
Rectal cancer accounts for approximately 30–50% of colorectal cancer. Despite its widespread use and convenience, the American Joint Committee on Cancer (AJCC) staging system for predicting survival is prone to inaccuracy, even including a survival paradox for locally advanced rectal cancer (LARC). An accurate risk stratification of LARC is essential for proper treatment selection and prognostic evaluation. Therefore, we aimed to create prognostic nomograms for LARC capable of assessing overall survival (OS) and cancer-specific survival (CSS) precisely and intuitively.
Methods
The Surveillance, Epidemiology, and End Results (SEER) database was accessed. All of the significant variables in the multivariate analysis were integrated to build the nomograms.
Results
Data for a total of 23,055 patients with LARC were collected from the SEER database in this study. Based on the multivariate Cox regression analysis, both OS and CSS were significantly associated with 13 variables: age, marital status, race, pathological grade, histological type, T stage, N stage, surgery, radiotherapy, chemotherapy, regional nodes examined (RNE), tumor size, and carcinoembryonic antigen (CEA). These were included in the construction of nomograms for OS and CSS. Time-dependent receiver operating characteristic (ROC) curves, decision curve analysis (DCA), concordance index, and calibration curves demonstrated the discriminative superiority of the nomograms.
Conclusions
The nomograms, which effectively solve the issue of the survival paradox in the AJCC staging system regarding LARC, may act as excellent tools for integrating clinical characteristics and to guiding therapeutic choices for LARC patients.
Keywords: Locally advanced rectal cancer (LARC), overall survival (OS), cancer-specific survival (CSS), nomogram
Introduction
Rectal cancer accounts for approximately 30–50% of all colorectal cancer cases (1), placing it third as the most common malignancy worldwide (2). With the advances in treatment technology, the survival rates of patients with locally advanced rectal cancer (LARC) have improved significantly over the past few decades (3).
The combination of surgical resection, chemotherapy, and/or radiation therapy is the conventional treatment for LARC (3). Updated surgical equipment and concepts constitute the major advancements in surgical resection technology. Total mesorectal resection (TME) has become the standard surgical procedure for radical resection of rectal cancer (4,5). In addition, the refinement of colorectal cancer surgery is attributed to the application of laparoscopy and robot-assisted laparoscopy (6,7). Chemotherapy for patients with rectal cancer has evolved substantially over the past decades, together with the concept of neoadjuvant therapy, as well as the increased marketing of irinotecan, oxaliplatin, bevacizumab, and cetuximab. The adoption of TME combined with adjuvant oncological treatment for LARC has reduced local recurrence rates and improved long-term survival (8). In particular, advancements in chemotherapy regimens have been the main contributor to the upswing of colorectal cancer survival in the past decades (3).
Patients who have colon and rectal cancers are generally analyzed in the context of statistical homogeneity, despite having different etiologies, anatomy, and treatments (9). Thus, it is necessary to conduct a specific analysis for LARC that is different from colon cancer owing to the apparent distinctions in treatment, the universal involvement of neoadjuvant chemoradiotherapy (nCRT), and the performance of TME in the surgical technique (10,11).
Despite its widespread use and convenience, the American Joint Committee on Cancer (AJCC) staging system for the prediction of survival with this malignancy has proven inaccurate. The AJCC staging has even produced a survival paradox for LARC, in that those patients with T3–4N− were found to develop worse survival outcomes than those with T1–2N+ (12-14). A precise risk stratification of LARC is imperative for proper treatment selection and prognostic evaluation. As a visible representation of a mathematical model, a nomogram can not only integrate certain features together to estimate specific endpoints, but also provide pragmatic and comprehensive prediction for clinical practice. Meanwhile, national databases, such as the Surveillance, Epidemiology, and End Results (SEER) database, can provide the available clinical factors and ample patient data to build a reliable statistical model for the prediction of survival.
Therefore, we aimed to create SEER-based prognostic nomograms for patients with LARC based that could accurately and conveniently assess overall survival (OS) and cancer-specific survival (CSS). We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4144).
Methods
Data collection
Data in this retrospective analysis were extracted from the SEER Linked database. The SEER Program of the National Cancer Institute is an authoritative source of information on cancer incidence and survival in the United States that is updated annually. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval from the ethical board for this study was not required because of the public nature of all the data. Patients’ informed consent was waived because of the retrospective nature of the study design.
Patient screening
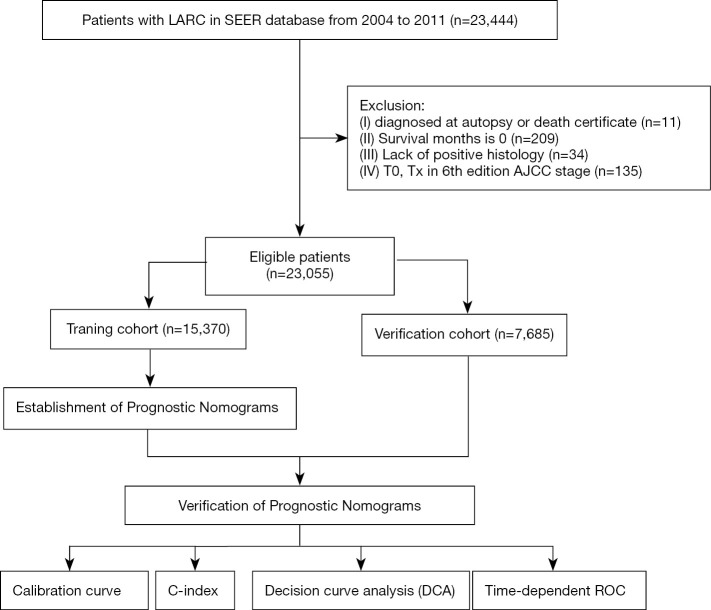
The target population was limited to patients with stage II and III (T34 and/or N+) rectal adenocarcinoma [International Classification of Diseases for Oncology 3rd edition (ICD-O-3): 8,140, 8,144, 8,210, 8,211, 8,213, 8,245, 8,255, 8,260, 8,261, 8,262, 8,263, 8,310, 8,323, 8,480, 8,481, 8,490], resulting in a total of 23,444 patients. The exclusion criteria were as follows: diagnosed at autopsy or death certificate (n=11); survival months 0 (n=209); lack of positive histology (n=34); and T0 and Tx according to the 6th edition AJCC staging (n=135). The final study sample contained 23,055 patients (Figure 1).
Figure 1.
The workflow of the establishment of nomograms to predict OS and CSS of patients with LARC. OS, overall survival; CSS, cancer-specific survival; LARC, locally advanced rectal cancer.
Patients were chosen from the period between 2004 and 2011, since the follow-up time of who after 2011 was less than 5 years. The cutoff for follow-up was December 31, 2016. The endpoints of this study were OS and CSS. The median follow-up was estimated as the median observed survival time. OS was computed from the time of diagnosis to the time of death due to any cause or the time of last follow-up for patients still alive. CSS was computed as the time of diagnosis to the time of death attributed to rectal cancer or survival at last follow-up. The OS and CSS curves were evaluated by the Kaplan-Meier method and compared by the log-rank test. For each patient, the following data were acquired: age at diagnosis, marital status, gender, race, tumor size, grade, histological type, T stage, N stage, regional nodes examined (RNE), carcinoembryonic antigen (CEA), surgery, radiotherapy, and chemotherapy. All patients were randomly separated into 2 groups (training group, n=15,370 and validation group, n=7,685).
Construction and validation of the nomogram
Univariate and multivariate Cox regression analyses were applied to calculate the weight of variables in OS and CSS, as presented with odds ratio (OR), and were used to identify independent risk factors. The variables with significant differences in the univariate analysis were included in the Cox regression model for multivariate analysis. All of the significant variables in the multivariate analysis were integrated to build the nomograms for OS and CSS. The probabilities could be estimated for 2-, 3-, and 5-year OS and CSS after summing the scores related to each variable and casting total scores to the bottom scale. The total points in each case of the 2 survival groups were calculated using the established nomograms to verify the effect. The calibration curves were used to demonstrate the reliability of the nomograms. The distinguishing ability of the nomogram was evaluated by concordance index (C-index) and receiver operating characteristic (ROC) curve analysis. Decision curve analysis (DCA) was carried out to compare the latent profit of the prognostic nomograms.
Statistical analysis
The OR and a 95% confidence interval (CI) were evaluated by univariate and multivariate Cox regression analysis. Variables with significant differences in the univariate analysis were included in the Cox regression model for multivariate analysis. Missing data were marked as NOS (not otherwise specified) for analysis. R software (version 3.6.1, http://www.r-project.org) was used to build the nomograms, plot the calibration curves, Sankey diagrams, ROC curves, and DCA curves, and to calculate the C-index. The survival curves were drawn by GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). The univariate and multivariate Cox regression models were performed with IBM SPSS statistics trial ver. 22.0 (IBM, Armonk, NY, USA). All reported P values <0.05 were considered significant.
Results
Patient characteristics
A total of 389 patients with rectal cancer were not included in the final study [diagnosed at autopsy or death certificate (n=11); survival months 0 (n=209); lack of positive histology (n=34); T0 and Tx according to the 6th edition AJCC staging (n=135)] (Figure 1). Eventually, data for 23,055 eligible patients with LARC were collected from the SEER database in this study. The characteristics of the patients are summarized in Table 1. More than half of the patients were male (59.97%), of whom 68.80% had moderately differentiated adenocarcinoma. The patients with mucinous cell carcinoma (MCC) or signet ring cell carcinoma (SRCC) accounted for 8.55% of the total population. The majority of LARCs were smaller than 5 cm in size (57.41%), and the proportion of patients with increased levels of CEA reached 26.54% in this study. The median OS and CSS were 69 and 72 months, respectively.
Table 1. Characteristics of patients with LARC in the training and validation group.
| Characteristics | Total (n=23,055) | Training group (n=15,370) | Validation group (n=7,685) | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Gender | ||||||||
| Female | 9,229 | 40.03 | 6,138 | 39.93 | 3,091 | 40.22 | ||
| Male | 13,826 | 59.97 | 9,232 | 60.07 | 4,594 | 59.78 | ||
| Age (years) | ||||||||
| ≤60 | 9,650 | 41.86 | 6,403 | 41.66 | 3,247 | 42.25 | ||
| 61–70 | 5,710 | 24.77 | 3,839 | 24.98 | 1,871 | 24.35 | ||
| >70 | 7,695 | 33.38 | 5,128 | 33.36 | 2,567 | 33.40 | ||
| Marital status | ||||||||
| Married | 13,269 | 57.55 | 8,845 | 57.55 | 4,424 | 57.57 | ||
| Unmarried/NOS | 9,786 | 42.45 | 6,525 | 42.45 | 3,261 | 42.43 | ||
| Race | ||||||||
| White | 18,811 | 81.59 | 12,534 | 81.55 | 6,277 | 81.68 | ||
| Black | 1,961 | 8.51 | 1,325 | 8.62 | 636 | 8.28 | ||
| Other/NOS | 2,283 | 9.90 | 1,511 | 9.83 | 772 | 10.05 | ||
| Pathological grade | ||||||||
| I | 1,398 | 6.06 | 947 | 6.16 | 451 | 5.87 | ||
| II | 15,861 | 68.80 | 10,570 | 68.77 | 5,291 | 68.85 | ||
| III | 3,452 | 14.97 | 2,303 | 14.98 | 1,149 | 14.95 | ||
| IV | 281 | 1.22 | 179 | 1.16 | 102 | 1.33 | ||
| Unknown | 2,063 | 8.95 | 1,371 | 8.92 | 692 | 9.00 | ||
| Histologic type | ||||||||
| Adenocarcinomas | 21,083 | 91.45 | 14,073 | 91.56 | 7,010 | 91.22 | ||
| MCC/SRCC | 1,972 | 8.55 | 1,297 | 8.44 | 675 | 8.78 | ||
| T stage | ||||||||
| T1 | 772 | 3.35 | 504 | 3.28 | 268 | 3.49 | ||
| T2 | 1,768 | 7.67 | 1,161 | 7.55 | 607 | 7.90 | ||
| T3 | 18,184 | 78.87 | 12,114 | 78.82 | 6,070 | 78.99 | ||
| T4 | 2,331 | 10.11 | 1,591 | 10.35 | 740 | 9.63 | ||
| N stage | ||||||||
| N0 | 10,506 | 45.57 | 6,965 | 45.32 | 3,541 | 46.08 | ||
| N1 | 8,903 | 38.62 | 5,941 | 38.65 | 2,962 | 38.54 | ||
| N2 | 3,646 | 15.81 | 2,464 | 16.03 | 1,182 | 15.38 | ||
| Surgery | ||||||||
| Yes | 20,693 | 89.75 | 13,788 | 89.71 | 6,905 | 89.85 | ||
| No | 2,362 | 10.25 | 1,582 | 10.29 | 780 | 10.15 | ||
| Radiotherapy | ||||||||
| Neoradiotherapy | 11,002 | 47.72 | 7,338 | 47.74 | 3,664 | 47.68 | ||
| Radiotherapy* | 6,139 | 26.63 | 4,102 | 26.69 | 2,037 | 26.51 | ||
| No/unknown | 5,914 | 25.65 | 3,930 | 25.57 | 1,984 | 25.82 | ||
| Chemotherapy | ||||||||
| Yes | 17,763 | 77.05 | 11,866 | 77.20 | 5,897 | 76.73 | ||
| No/unknown | 5,292 | 22.95 | 3,504 | 22.80 | 1,788 | 23.27 | ||
| RNE | ||||||||
| <3 | 4,290 | 18.61 | 2,848 | 18.53 | 1,442 | 18.76 | ||
| 3–5 | 1,697 | 7.36 | 1,116 | 7.26 | 581 | 7.56 | ||
| 6–8 | 2,375 | 10.30 | 1,579 | 10.27 | 796 | 10.36 | ||
| 9–11 | 2,799 | 12.14 | 1,831 | 11.91 | 968 | 12.60 | ||
| ≥12 | 11,642 | 50.50 | 7,832 | 50.96 | 3,810 | 49.58 | ||
| NOS | 252 | 1.09 | 164 | 1.07 | 88 | 1.15 | ||
| Tumor size (cm) | ||||||||
| ≤5 | 13,237 | 57.41 | 8,752 | 56.94 | 4,485 | 58.36 | ||
| 5–10 | 5,176 | 22.45 | 3,498 | 22.76 | 1,678 | 21.83 | ||
| >10 | 338 | 1.47 | 243 | 1.58 | 95 | 1.24 | ||
| NOS | 4,304 | 18.67 | 2,877 | 18.72 | 1,427 | 18.57 | ||
| CEA | ||||||||
| Negative | 7,813 | 33.89 | 5,191 | 33.77 | 2,622 | 34.12 | ||
| Positive | 6,119 | 26.54 | 4,149 | 26.99 | 1,970 | 25.63 | ||
| NOS | 9,123 | 39.57 | 6,030 | 39.23 | 3,093 | 40.25 | ||
| OS (months) | 69 (33 to 101) | 69 (33 to 100) | 69 (33 to 102) | |||||
| CSS (months) | 72 (37 to 104) | 72 (37 to 103) | 72 (37 to 105) | |||||
*, not neoadjuvant. MCC, mucinous cell carcinoma; SRCC, signet ring cell carcinoma; RNE, regional nodes examined; NOS, not otherwise specified.
In addition, 10.25% of patients with LARC did not undergo surgical resection, 25.65% did not undergo radiotherapy, and 22.95% did not undergo chemotherapy. As an important indicator of surgical quality in the SEER database (3), RNE >12 was only present in 50.50% of patients in this study.
Establishment of prognostic nomograms
Univariate and multivariate Cox regression analyses were applied to calculate the weight of variables in OS and CSS (presented as OR) and were used to identify independent risk factors.
The variables with significant differences in the univariate analysis were included in the Cox regression model for multivariate analysis, where both OS and CSS were significantly associated with 13 variables, namely, age, marital status, race, pathological grade, histological type, T stage, N stage, surgery, radiotherapy, chemotherapy, RNE, tumor size, and CEA (Tables 2,3).
Table 2. Univariable and multivariable Cox regression model analyses of OS for nomogram.
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Gender | 0.373 | ||||||
| Female | Reference | 1 | NA | ||||
| Male | 1.021 | 0.976–1.068 | 0.373 | ||||
| Age (years) | <0.001 | <0.001 | |||||
| ≤60 | Reference | 1 | Reference | 1 | |||
| 61–70 | 1.410 | 1.327–1.499 | <0.001 | 1.366 | 1.284–1.452 | <0.001 | |
| >70 | 3.011 | 2.859–3.171 | <0.001 | 2.565 | 2.427–2.710 | <0.001 | |
| Marital status | <0.001 | <0.001 | |||||
| Married | Reference | 1 | Reference | 1 | |||
| Unmarried/NOS | 1.478 | 1.414–1.544 | <0.001 | 1.203 | 1.150–1.258 | <0.001 | |
| Race | <0.001 | <0.001 | |||||
| White | Reference | 1 | Reference | 1 | |||
| Black | 1.264 | 1.174–1.361 | <0.001 | 1.256 | 1.165–1.354 | <0.001 | |
| Other/NOS | 0.869 | 0.804–0.940 | <0.001 | 0.904 | 0.836–0.977 | 0.011 | |
| Pathological grade | <0.001 | <0.001 | |||||
| I | Reference | 1 | Reference | 1 | |||
| II | 0.998 | 0.909–1.096 | 0.970 | 1.024 | 0.932–1.125 | 0.622 | |
| III | 1.412 | 1.273–1.567 | <0.001 | 1.338 | 1.204–1.486 | <0.001 | |
| IV | 1.709 | 1.398–2.087 | <0.001 | 1.471 | 1.203–1.799 | <0.001 | |
| Unknown | 1.149 | 1.023–1.291 | 0.019 | 1.007 | 0.896–1.132 | 0.907 | |
| Histological type | <0.001 | <0.001 | |||||
| Adenocarcinomas | Reference | 1 | Reference | 1 | |||
| MCC/SRCC | 1.344 | 1.249–1.445 | <0.001 | 1.262 | 1.171–1.359 | <0.001 | |
| T stage | <0.001 | <0.001 | |||||
| T1 | Reference | 1 | Reference | 1 | |||
| T2 | 1.024 | 0.866–1.211 | 0.781 | 1.015 | 0.857–1.201 | 0.864 | |
| T3 | 1.481 | 1.284–1.709 | <0.001 | 1.482 | 1.280–1.717 | <0.001 | |
| T4 | 2.786 | 2.391–3.246 | <0.001 | 2.469 | 2.109–2.890 | <0.001 | |
| N stage | <0.001 | <0.001 | |||||
| N0 | Reference | 1 | Reference | 1 | |||
| N1 | 0.924 | 0.880–0.971 | 0.002 | 1.262 | 1.197–1.330 | <0.001 | |
| N2 | 1.430 | 1.348–1.518 | <0.001 | 2.035 | 1.908–2.172 | <0.001 | |
| Surgery | <0.001 | <0.001 | |||||
| Yes | Reference | 1 | Reference | 1 | |||
| No | 2.938 | 2.764–3.122 | <0.001 | 2.024 | 1.839–2.227 | <0.001 | |
| Radiotherapy | <0.001 | <0.001 | |||||
| Neoradiotherapy | Reference | 1 | Reference | 1 | |||
| Radiotherapy* | 1.577 | 1.495–1.664 | <0.001 | 1.043 | 0.979–1.111 | 0.194 | |
| No/unknown | 2.046 | 1.942–2.157 | <0.001 | 1.220 | 1.132–1.315 | <0.001 | |
| Chemotherapy | <0.001 | <0.001 | |||||
| Yes | Reference | 1 | Reference | 1 | |||
| No/unknown | 2.030 | 1.936–2.129 | <0.001 | 1.448 | 1.351–1.551 | <0.001 | |
| RNE | <0.001 | <0.001 | |||||
| <3 | Reference | 1 | Reference | 1 | |||
| 3–5 | 0.556 | 0.508–0.610 | <0.001 | 0.856 | 0.771–0.951 | 0.004 | |
| 6–8 | 0.541 | 0.498–0.586 | <0.001 | 0.794 | 0.721–0.875 | <0.001 | |
| 9–11 | 0.503 | 0.465–0.544 | <0.001 | 0.730 | 0.663–0.803 | <0.001 | |
| ≥12 | 0.473 | 0.448–0.500 | <0.001 | 0.651 | 0.601–0.705 | <0.001 | |
| NOS | 0.738 | 0.604–0.903 | 0.003 | 0.965 | 0.786–1.183 | 0.730 | |
| Tumor size (cm) | <0.001 | <0.001 | |||||
| ≤5 | Reference | 1 | Reference | 1 | |||
| 5–10 | 1.281 | 1.214–1.351 | <0.001 | 1.113 | 1.053–1.175 | <0.001 | |
| >10 | 1.561 | 1.323–1.840 | <0.001 | 1.360 | 1.152–1.606 | <0.001 | |
| NOS | 1.158 | 1.092–1.227 | <0.001 | 1.051 | 0.988–1.117 | 0.117 | |
| CEA | <0.001 | <0.001 | |||||
| Negative | Reference | 1 | Reference | 1 | |||
| Positive | 1.543 | 1.457–1.633 | <0.001 | 1.354 | 1.278–1.435 | <0.001 | |
| NOS | 1.341 | 1.271–1.414 | <0.001 | 1.215 | 1.151–1.282 | <0.001 | |
*, not neoadjuvant. MCC, mucinous cell carcinoma; SRCC, signet ring cell carcinoma; RNE, regional nodes examined; NOS, not otherwise specified, NA, Unavailable.
Table 3. Univariable and multivariable Cox regression model analyses of CSS for nomogram.
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Gender | 0.486 | ||||||
| Female | Reference | 1 | NA | ||||
| Male | 0.979 | 0.921–1.040 | 0.486 | ||||
| Age (years) | <0.001 | <0.001 | |||||
| ≤60 | Reference | 1 | Reference | 1 | |||
| 61–70 | 1.115 | 1.032–1.204 | 0.006 | 1.119 | 1.035–1.209 | 0.005 | |
| >70 | 2.008 | 1.876–2.150 | <0.001 | 1.840 | 1.712–1.979 | <0.001 | |
| Marital status | <0.001 | <0.001 | |||||
| Married | Reference | 1 | Reference | 1 | |||
| Unmarried/NOS | 1.498 | 1.412–1.590 | <0.001 | 1.247 | 1.173–1.326 | <0.001 | |
| Race | <0.001 | <0.001 | |||||
| White | Reference | 1 | Reference | 1 | |||
| Black | 1.421 | 1.291–1.564 | <0.001 | 1.322 | 1.199–1.457 | <0.001 | |
| Other/NOS | 0.926 | 0.836–1.025 | 0.137 | 0.925 | 0.835–1.024 | 0.134 | |
| Pathological grade | <0.001 | <0.001 | |||||
| I | Reference | 1 | Reference | 1 | |||
| II | 1.075 | 0.941–1.227 | 0.285 | 1.078 | 0.944–1.231 | 0.269 | |
| III | 1.696 | 1.468–1.960 | <0.001 | 1.519 | 1.313–1.758 | <0.001 | |
| IV | 1.997 | 1.520–2.624 | <0.001 | 1.594 | 1.211–2.098 | 0.001 | |
| Unknown | 1.352 | 1.153–1.586 | <0.001 | 1.042 | 0.887–1.225 | 0.615 | |
| Histological type | <0.001 | <0.001 | |||||
| Adenocarcinomas | Reference | 1 | Reference | 1 | |||
| MCC/SRCC | 1.535 | 1.396–1.689 | <0.001 | 1.410 | 1.279–1.555 | <0.001 | |
| T stage | <0.001 | <0.001 | |||||
| T1 | Reference | 1 | Reference | 1 | |||
| T2 | 0.936 | 0.741–1.181 | 0.576 | 0.925 | 0.732–1.169 | 0.514 | |
| T3 | 1.472 | 1.210–1.791 | <0.001 | 1.561 | 1.277–1.907 | <0.001 | |
| T4 | 3.344 | 2.720–4.110 | <0.001 | 2.898 | 2.343–3.586 | <0.001 | |
| N stage | <0.001 | <0.001 | |||||
| N0 | Reference | 1 | Reference | 1 | |||
| N1 | 1.147 | 1.072–1.228 | <0.001 | 1.505 | 1.401–1.616 | <0.001 | |
| N2 | 2.001 | 1.851–2.164 | <0.001 | 2.717 | 2.496–2.957 | <0.001 | |
| Surgery | <0.001 | <0.001 | |||||
| Yes | Reference | 1 | Reference | 1 | |||
| No | 3.295 | 3.044–3.567 | <0.001 | 2.221 | 1.947–2.533 | <0.001 | |
| Radiotherapy | <0.001 | 0.003 | |||||
| Neoradiotherapy | Reference | 1 | Reference | 1 | |||
| Radiotherapy* | 1.534 | 1.431–1.644 | <0.001 | 0.994 | 0.914–1.081 | 0.891 | |
| No/unknown | 1.638 | 1.519–1.765 | <0.001 | 1.178 | 1.062–1.306 | 0.002 | |
| Chemotherapy | <0.001 | <0.001 | |||||
| Yes | Reference | 1 | Reference | 1 | |||
| No/unknown | 1.553 | 1.447–1.667 | <0.001 | 1.293 | 1.172–1.427 | <0.001 | |
| RNE | <0.001 | <0.001 | |||||
| <3 | Reference | 1 | Reference | 1 | |||
| 3–5 | 0.504 | 0.444–0.572 | <0.001 | 0.800 | 0.690–0.928 | 0.003 | |
| 6–8 | 0.485 | 0.434–0.542 | <0.001 | 0.728 | 0.635–0.833 | <0.001 | |
| 9–11 | 0.463 | 0.416–0.515 | <0.001 | 0.662 | 0.579–0.756 | <0.001 | |
| ≥12 | 0.441 | 0.410–0.475 | <0.001 | 0.589 | 0.527–0.657 | <0.001 | |
| NOS | 0.710 | 0.546–0.925 | 0.011 | 0.864 | 0.660–1.131 | 0.288 | |
| Tumor size (cm) | <0.001 | .001 | |||||
| ≤5 | Reference | 1 | Reference | 1 | |||
| 5–10 | 1.341 | 1.248–1.441 | <0.001 | 1.114 | 1.035–1.200 | 0.004 | |
| >10 | 1.925 | 1.569–2.361 | <0.001 | 1.407 | 1.144–1.730 | 0.001 | |
| NOS | 1.282 | 1.187–1.385 | <0.001 | 1.088 | 1.002–1.180 | 0.043 | |
| CEA | <0.001 | <0.001 | |||||
| Negative | Reference | 1 | Reference | 1 | |||
| Positive | 1.702 | 1.577–1.836 | <0.001 | 1.450 | 1.342–1.566 | <0.001 | |
| NOS | 1.336 | 1.242–1.438 | <0.001 | 1.251 | 1.162–1.346 | <0.001 | |
*, not neoadjuvant. MCC, mucinous cell carcinoma; SRCC, signet ring cell carcinoma; RNE, Regional nodes examined; NOS, Not otherwise specified, NA, Unavailable.
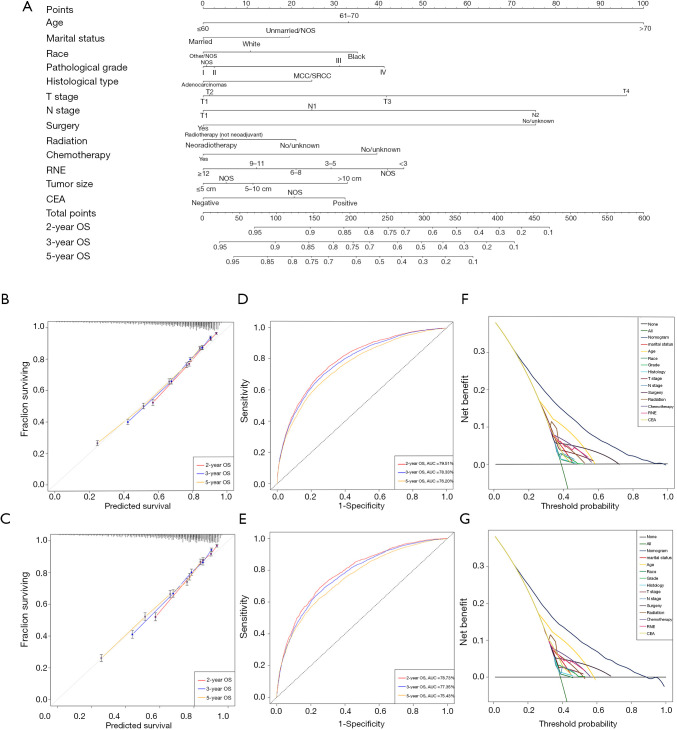
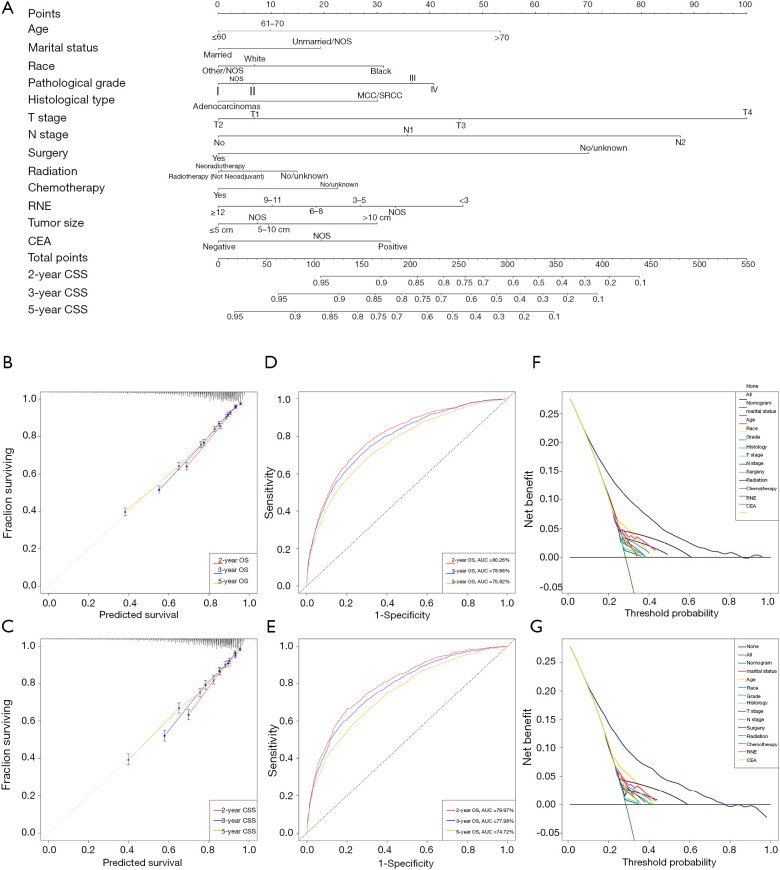
All of the significant variables were integrated to build the nomograms for OS and CSS. The prognostic nomogram for 2-, 3-, and 5-year OS is shown in Figure 2, and the nomogram for 2-, 3-, and 5-year CSS is shown in Figure 3. The probabilities could be estimated for 2-, 3-, and 5-year OS and CSS after summing the scores related to each variable and casting total scores to the bottom scale.
Figure 2.
Development and validation of the nomogram predicting OS. (A) The nomogram predicting OS for patients with locally advanced rectal cancer (LARC). (B) The calibration curves predicting OS in the training group. (C) The calibration curves predicting OS in the validation group. (D) The time-dependent ROC curves of the nomogram predicting OS in the training group. (E) The time-dependent ROC curves of the nomogram predicting OS in the validation group. (F) The decision curve analysis of the nomogram and all prognostic factors for OS in the training cohort. (G) The decision curve analysis of the nomogram and all prognostic factors for OS in the validation group. ROC, receiver operating characteristic; OS, overall survival; LARC, locally advanced rectal cancer.
Figure 3.
Development and validation of the nomogram predicting CSS. (A) The nomogram predicting CSS for patients with LARC. (B) The calibration curves predicting CSS in the training group. (C) The calibration curves predicting CSS in the validation group. (D) The time-dependent receiver operating characteristic (ROC) curves of the nomogram predicting CSS in the training group. (E) The time-dependent ROC curves of the nomogram predicting CSS in the validation group. (F) The decision curve analysis of the nomogram and all prognostic factors for CSS in the training cohort. (G) The decision curve analysis of the nomogram and all prognostic factors for CSS in the validation group. CSS, cancer-specific survival; LARC, locally advanced rectal cancer.
Validation of prognostic nomograms
Various methods have been used to demonstrate the superiority of nomograms, including C-index, time-dependent ROC curves, DCA, and calibration curves. C-indices were used to comprehensively assess the discriminatory power of the predictive models in this study. The nomograms obtained a superior C-index compared with the AJCC staging system [OS: 0.718 (95% CI, 0.712–0.723) vs. 0.597 (95% CI, 0.588–0.605) in the training cohort; 0.712 (95% CI, 0.704–0.720) vs. 0.579 (95% CI, 0.567–0.591) in the validation cohort; CSS: 0.718 (95% CI, 0.710–0.725) vs. 0.646 (95% CI, 0.635–0.656) in the training cohort; 0.711 (95% CI, 0.700–0.722) vs. 0.625 (95% CI, 0.610–0.640) in the validation cohort] (Table 4).
Table 4. The C-indices for predictions of OS and CSS.
| Group | OS | CSS | |||
|---|---|---|---|---|---|
| C-index | 95% CI | C-index | 95% CI | ||
| Training group-nomogram | 0.718 | 0.712–0.723 | 0.718 | 0.710–0.725 | |
| Training group-AJCC stage | 0.597 | 0.588–0.605 | 0.646 | 0.635–0.656 | |
| Validation group-nomogram | 0.712 | 0.704–0.720 | 0.711 | 0.700–0.722 | |
| Validation group-AJCC stage | 0.579 | 0.567–0.591 | 0.625 | 0.610–0.640 | |
OS, overall survival; CSS, cancer-specific survival; C-index, index of concordance; CI, confidence interval.
The sensitivity and specificity of predicting the prognosis of LARC were identified by time-dependent ROC curves. Figure 2B,C illustrates the 2-, 3-, and 5-year values of the area under the curve (AUC) regarding the nomogram for OS (training group: 2-year OS 79.51%; 3-year OS 78.33%; 5-year OS 76.20%; validation group: 2-year OS 78.73%; 3-year OS 77.35%; 5-year OS 75.43%). The AUC values of the nomogram predicting CSS are displayed in Figure 3B,C (training group: 2-year CSS 80.26%; 3-year CSS 78.66%; 5-year CSS 75.82%; validation group: 2-year CSS 79.97%; 3-year CSS 77.98%; 5-year CSS 74.72%).
In addition, the calibration curves demonstrated a high degree of reliability of the nomograms in this study owing to the minor deviations from the reference line (Figure 2D,E for OS; Figure 3D,E for CSS). DCA is able to identify predictive models that help clinicians make better decisions (15). The DCA curves for the novel nomograms and each predictor are presented in Figure 2F,G for OS and Figure 3F,G for CSS. The superior net benefits revealed that the nomograms in this study showed more pinpoint values than individual predictors in clinical application.
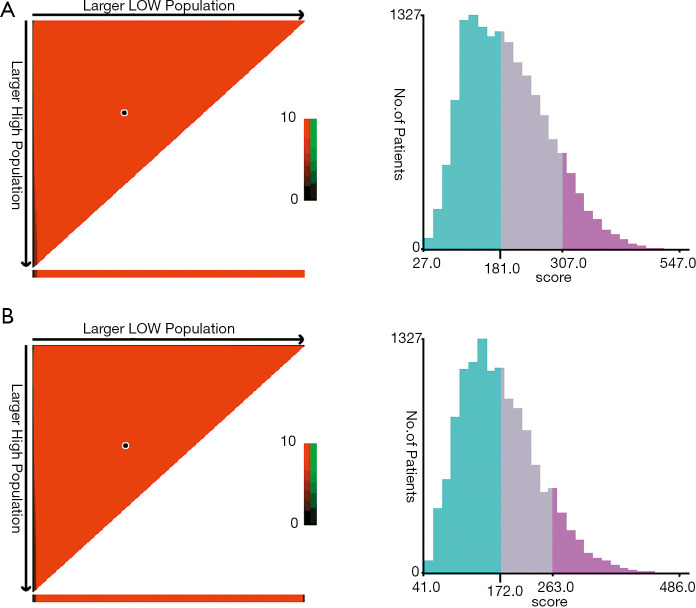
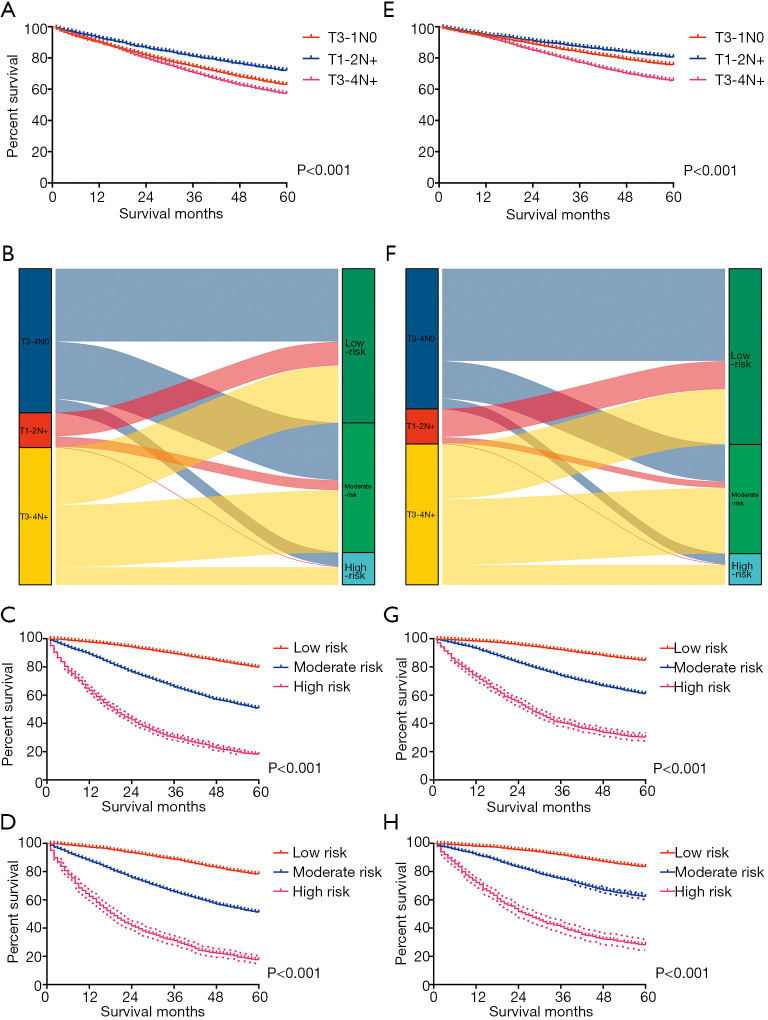
Risk stratification
X-tile software (version 3.6.1; Yale University, New Haven, CT, USA) was used to calculate the cutoff values concerning the total scores of LARC patients by summing the ones related to each variable. The cutoff values were 181 and 307 for OS, and 172 and 263 for CSS (Figure 4). Therefore, LARC patients were classified as high risk (score >307), moderate risk (181< score ≤307), and low risk (score ≤181) for OS. In addition, patients with LARC were classified as high risk (score >263), moderate risk (172< score ≤263), and low risk (score ≤172) for CSS. Although it is widely used to evaluate the prognosis of various tumors, the AJCC staging system produces a survival paradox for LARC, in that rectal cancer patients with T3–4N0 (stage II) showed worse survival compared to patients with T1–2N+ (stage III) (Figure 5; Figure 5A for OS and Figure 5E for CSS). Figure 5B,F show the correspondence between AJCC stage and the risk stratification in this study. The risk stratification effectively avoided the survival paradox in this study. The low-risk group had the highest 5-year CSS rate of 84.71% and a 5-year OS rate of 79.71%, followed by the moderate-risk group (61.06% for CSS and 50.78% for OS), and the high-risk group (30.05% for CSS and 17.86% for OS) in the training cohort (Figure 5C,G). The validation group confirmed the results of the low-risk group having the highest 5-year OS (78.17%) and CSS (83.48%) rate, followed by the moderate-risk group (51.09% for OS and 62.25% for CSS), and the high-risk group (17.58% for OS and 28.26% for CSS) (Figure 5D,H).
Figure 4.
The cutoff values were calculated by using X-tile based on the total scores of patients summing the ones related to each variable. (A) The cutoff values were 181 and 307 for OS. (B) The cutoff values were 172 and 263 for CSS. OS, overall survival; CSS, cancer-specific survival.
Figure 5.
Performance of the nomograms in stratifying on the basis of risk points. (A) The difference in OS among T3–4N0, T1–2N+, and T3–4N+ patients. (B) The correspondence between the AJCC stage and the risk stratification based on the nomogram predicting OS. (C) OS in the subgroups according to the risk stratification in the training cohort. (D) OS in the subgroups according to the risk stratification in the validation cohort. (E) The difference in CSS among T3–4N0, T1–2N+, and T3–4N+ patients. (F) The correspondence between AJCC stage and the risk stratification based on the nomogram predicting CSS. (G) CSS in the subgroups according to the risk stratification in the training cohort. (H) CSS in the subgroups according to the risk stratification in the validation cohort. OS, overall survival; CSS, cancer-specific survival; AJCC, American Joint Committee on Cancer.
Discussion
Numerous studies have reported that the AJCC staging system’s ability to predict survival is insufficiently inaccurate for the medical demands of rectal cancer (16-18), especially for LARC. In order to develop a precise scoring system with clinical value, nomograms that could evaluate OS and CSS in patients with LARC were constructed and examined based on a large population from the SEER database. The nomograms not only incorporated pathological variables but also therapeutic and demographic ones, and can therefore provide comprehensive guidance for clinical practice.
The positive status of regional lymph nodes, without the intervention of T stage, is classified as stage III in the AJCC staging system. However, those patients with T3–4N− developed worse survival outcomes than T1–2N+ (12-14), which was consistent with our study. Increasing research has focused on the survival paradox in the AJCC staging system, suggesting that the T stage has more influence than the N stage on survival in rectal cancer (19), which was further demonstrated by the nomograms of OS and CSS in our study. The poor predictive performance of the AJCC staging system for LARC has spurred clinicians to seek a new method of risk stratification that would effectively avoid the survival paradox.
Currently, (nCRT) is recommended for patients with LARC (20). Consequently, numerous studies have actively explored the positive response of LARC to nCRT (21-23). However, our study did not find that neoadjuvant radiotherapy (nRT) conferred significantly superior survival to other radiotherapy regimens (OS: P=0.194; CSS: P=0.891). It is well-established that nRT can be conducive to sphincter preservation for low rectal cancer. Nevertheless, nRT may be abandoned in patients with mid-high rectal cancer without the issue of sphincter preservation, due to increased surgical complications after nRT. In addition, the intuitive nomograms, which showed noteworthy survival benefits from surgery, chemotherapy, and radiotherapy, support the active treatment of LARC. Furthermore, RNE has been utilized to measure the quality of surgery in other research (3) and is a major factor in the nomograms, which can remind surgeons of the importance of regional lymph node dissection.
The effect of tumor size on survival has long been ignored in cavity organs. However, many studies have suggested that tumor size is related to the response of LARC to chemoradiotherapy (21,22), which may also affect the prognosis of LARC. CEA has been revealed to have a close association with chemosensitivity and survival of rectal cancer patients in various studies (24,25). Similarly, an elevated CEA was confirmed to be an indicator of poor prognosis in this study. Other essential prognostic factors were also incorporated into the study, including age, marital status, pathological grade, and histological type. Cancers can increase the risk of death from geriatric diseases, which is why age contributed a higher weight in the nomogram of OS compared to CSS. Furthermore, it is worth noting that patients with MCC/SRCC had a worse survival. The prognosis of LARC that was well/moderately differentiated was significantly better than that of poorly differentiated/undifferentiated LARC, which was in agreement with previous studies. Interestingly, marital status has been found to correlate with the prognosis of various tumors (26-28), which was also applicable to rectal cancer in our study.
One-third of the patients were randomly selected as the validation group to confirm the superiority of the nomograms in this study. The excellent results, including C-index, time-dependent ROC curves, DCA, and calibration curves, in the validation group ensure the generalizability of the novel nomograms. However, some limitations were nonetheless present in our study. Firstly, as a retrospective study, the nomograms still need to be validated in the future by prospective studies. Secondly, we adopted the sixth edition of AJCC staging, rather than the latest editions, since the cases studied were taken from 2004 to 2011, which reduced, to some extent, the accuracy of the AJCC stage in that it lacked the T4 and N+ subgroups. Moreover, we still need more real-world data to verify the efficacy of the nomograms. These limitations notwithstanding, the study results attest to the excellent sensitivity, specificity, and outstanding clinical value of the nomograms.
Conclusions
Our nomograms, which effectively solved the issue of the survival paradox of the AJCC staging system regarding LARC, may serve as excellent tools for integrating clinical characteristics and guiding therapeutic choices for LARC patients.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database. The interpretation and reporting of these data are the sole responsibility of the authors. The first author, Yuqiang Li, gratefully acknowledges financial support from China Scholarship Council.
Funding: This study received funding from The Nature Scientific Foundation of China (No. 81702956).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval from the ethical board for this study was not required because of the public nature of all the data. Patients’ informed consent was waived because of the retrospective nature of the study design.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4144
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4144). The authors have no conflicts of interest to declare.
References
- 1.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150:17-22. 10.1001/jamasurg.2014.1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Zhao L, Gungor C, et al. The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database. Therap Adv Gastroenterol 2019;12:1756284819862154. 10.1177/1756284819862154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miskovic D, Foster J, Agha A, et al. Standardization of laparoscopic total mesorectal excision for rectal cancer: a structured international expert consensus. Ann Surg 2015;261:716-22. 10.1097/SLA.0000000000000823 [DOI] [PubMed] [Google Scholar]
- 5.Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 2015;16:161-8. 10.1016/S1470-2045(14)71168-4 [DOI] [PubMed] [Google Scholar]
- 6.van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. 10.1016/S1470-2045(13)70016-0 [DOI] [PubMed] [Google Scholar]
- 7.Mushtaq HH, Shah SK, Agarwal AK. The Current Role of Robotics in Colorectal Surgery. Curr Gastroenterol Rep 2019;21:11. 10.1007/s11894-019-0676-7 [DOI] [PubMed] [Google Scholar]
- 8.Brown KGM, Solomon MJ. Progress and future direction in the management of advanced colorectal cancer. Br J Surg 2018;105:615-7. 10.1002/bjs.10759 [DOI] [PubMed] [Google Scholar]
- 9.Doumouras AG, Tsao MW, Saleh F, et al. A population-based comparison of 30-day readmission after surgery for colon and rectal cancer: How are they different? J Surg Oncol 2016;114:354-60. 10.1002/jso.24334 [DOI] [PubMed] [Google Scholar]
- 10.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. 10.1056/NEJMoa010580 [DOI] [PubMed] [Google Scholar]
- 11.Tamas K, Walenkamp AM, de Vries EG, et al. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev 2015;41:671-9. 10.1016/j.ctrv.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 12.Gunderson LL, Jessup JM, Sargent DJ, et al. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol 2010;28:256-63. 10.1200/JCO.2009.23.9194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 2010;28:264-71. 10.1200/JCO.2009.24.0952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MJ, Jeong SY, Choi SJ, et al. Survival paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon cancer. Ann Surg Oncol 2015;22:505-12. 10.1245/s10434-014-3982-1 [DOI] [PubMed] [Google Scholar]
- 15.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. 10.1177/0272989X06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Dai W, Li Y, et al. Nomograms for predicting the prognostic value of serological tumor biomarkers in colorectal cancer patients after radical resection. Sci Rep 2017;7:46345. 10.1038/srep46345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan S, Li T, Zhou P, et al. Development and validation of nomogram combining serum biomarker for predicting survival in patients with resected rectal cancer. Biosci Rep 2019;39:BSR20192636. 10.1042/BSR20192636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son HJ, Park JW, Chang HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol 2013;20:2908-13. 10.1245/s10434-013-2968-8 [DOI] [PubMed] [Google Scholar]
- 19.Li J, Guo BC, Sun LR, et al. TNM staging of colorectal cancer should be reconsidered by T stage weighting. World J Gastroenterol 2014;20:5104-12. 10.3748/wjg.v20.i17.5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1-42. 10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Liu W. Predicting pathological complete response by comparing MRI-based radiomics pre- and postneoadjuvant radiotherapy for locally advanced rectal cancer. Cancer Med 2019;8:7244-52. 10.1002/cam4.2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Li Y, Zhu H, et al. The Relationship between Primary Gross Tumor Volume and Tumor Response of Locally Advanced Rectal Cancer: pGTV as a More Accurate Tumor Size Indicator. J Invest Surg 2021;34:181-90. 10.1080/08941939.2019.1615153 [DOI] [PubMed] [Google Scholar]
- 23.Yi X, Pei Q, Zhang Y, et al. MRI-Based Radiomics Predicts Tumor Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Front Oncol 2019;9:552. 10.3389/fonc.2019.00552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Pei Q, Zhu H, et al. Survival nomograms for stage III colorectal cancer. Medicine (Baltimore) 2018;97:e13239. 10.1097/MD.0000000000013239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CH, Huh JW, Yeom SS, et al. Predictive value of serum and tissue carcinoembryonic antigens for radiologic response and oncologic outcome of rectal cancer. Pathol Res Pract 2020;216:152834. 10.1016/j.prp.2020.152834 [DOI] [PubMed] [Google Scholar]
- 26.Alamanda VK, Song Y, Holt GE. Effect of marital status on treatment and survival of extremity soft tissue sarcoma. Ann Oncol 2014;25:725-9. 10.1093/annonc/mdt583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo P, Zhou JG, Jin SH, et al. Influence of marital status on overall survival in patients with ovarian serous carcinoma: finding from the surveillance epidemiology and end results (SEER) database. J Ovarian Res 2019;12:126. 10.1186/s13048-019-0600-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Yin K, Zheng D, et al. Marital status independently predicts non-small cell lung cancer survival: a propensity-adjusted SEER database analysis. J Cancer Res Clin Oncol 2020;146:67-74. 10.1007/s00432-019-03084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as