Abstract
Background
The purpose of this study was to research the molecular transmission and genetic evolutionary characteristics among CRF07_BC-infected patients in a developed area in Eastern China.
Methods
Plasma samples from newly diagnosed HIV-1-positive patients from 2015–2018 and basic demographic and epidemiological information were obtained. Pol sequences from CRF07_BC-infected patients were selected for phylogenetic, molecular transmission network, and Bayesian evolutionary analyses.
Results
Pol sequences were successfully obtained from 258 samples of CRF07_BC. Phylogenetic analysis revealed 2 distinct lineages: lineage 1 (66.3%, 171/258), primarily from men who have sex with men (MSM) and some heterosexual individuals, and lineage 2 (33.7%, 87/258), primarily from heterosexual individuals. Under an optimal genetic distance of 0.01 substitutions/site, 163 individuals (63.2%, 163/258) formed 23 groups comprising 6 clusters and 17 dyads in the networks. A distinctly large and rapidly growing cluster (C1) containing 105 individuals was identified, in which MSM with ≥4 links had quite a high transmission risk (low educational background, active sexual behavior, low sexual protection awareness, etc.). According to Bayesian analyses, most C1 clades formed from 2005 to 2009, most of which were closely geographically related to CRF07_BC epidemic strains from Anhui province.
Conclusions
Here, we elucidated the local transmission characteristics and epidemic pattern of HIV-1 CRF07_BC, revealing that MSM (especially with ≥4 links) may be a significant driver in the formation of active and rapid growth networks in regional CRF07_BC epidemics. Thus, unique region– and risk group–specific transmission network analysis based on a molecular approach can provide critical and insightful information for more effective intervention strategies to limit future HIV-1 transmission.
Keywords: CRF07_BC, HIV-1, molecular epidemiology, transmission network
Although HIV-1 is preventable through effective public health measures, the global HIV-1 epidemic continues to rise and has developed into numerous heterogeneous subepidemics worldwide due to the genetic diversity and high variability of HIV-1 and the impacts of human migration and globalization patterns [1–4]. Similar to what happened worldwide, the HIV-1 epidemic in China has evolved through several stages. Over the past nearly 30 years, the 4 National HIV Molecular Epidemiological Surveys revealed that CRF07_BC and CRF01_AE have become the most prevalent HIV subtypes and are widely epidemic in most parts of the country, creating a considerable challenge for intervention [5–7].
CRF07_BC appeared among intravenous drug users (IDUs) in Yunnan province in the late 1980s due to the coexistence of Thai subtypes B (B’) and C, which was the first circulating recombinant form (CRF) discovered in China [8]. Subsequently, CRF07_BC rapidly spread throughout China (eg, spread to Xinjiang, Guangxi, and Sichuan provinces during 1994–1996; spread to Jiangsu and Liaoning provinces during 1997–1998), and the epidemic of CRF07_BC has since shifted from primarily injecting drug users (IDUs) to include a diverse range of overlapping risk groups with unprotected sexual contact, particularly among men who have sex with men (MSM) [5, 9–12]. Previous research has shown that there are between 3 100 000 and 6 300 000 MSM in China [13], and the prevalence of HIV in MSM increased from 0.9% to 8.0% between 2003 and 2015 [14, 15]. In recent years, the MSM population has become the group with the most significant increased HIV-1 risk; it accounts for >50% of new HIV diagnoses in some developed areas and large cities [16–18]. With the prevalence of the MSM population, the second spreading wave of CRF07_BC was driven, forcing CRF07_BC to become the major recombinant form in the epidemic subtype of the MSM population in China, especially in economically developed areas [19–22]. Consequently, sufficient understanding about the epidemic patterns and transmission characteristics of CRF07_BC in the MSM population and other risk groups in these areas is critical to controlling HIV transmission and improving the efficiency of targeted prevention efforts.
With the continuous development of molecular epidemiology, HIV-1 molecular transmission networks play an increasingly important role in analyzing and studying the epidemic pattern and transmission characteristics of HIV-1 in different risk groups [23–26]. This method could be reliable for accurately identifying potential transmission network characteristics (including recent infection clusters and faster-growing clusters), which could provide empirical evidence to better evaluate the at-risk population, reveal the dynamics of the HIV epidemic, and identify infections in relation to time, geographic area, and risk group [27–29].
Therefore, in this paper, our study combined phylogenetic inference, transmission network analysis, and molecular epidemiology to construct a relatively deeply sampled transmission network of CRF07_BC to describe temporal and spatial transmission networks and explore the factors affecting linkages among genetic transmission networks. These findings are expected to provide a new perspective for local HIV-1 intervention and prevention through in-depth analysis of the CRF07_BC transmission networks and effectively reduce the HIV-1 infection rate, which has great public health significance.
METHODS
Study Population and Sample Collection
In this study, we selected a medium-sized and developed city (Jiaxing) in Eastern China as the research area in which to conduct a prospective molecular epidemiological study. Jiaxing city is located in the central area of the Yangtze River Delta in China (population of 4.726 million, among which the migrant population is ~2.47 million), bordered by many developed cities such as Hangzhou, Shanghai, and Suzhou. In recent years, the number of newly diagnosed HIV-1 patients in Jiaxing city had continuously increased, and the growth ratio has ranked first in Zhejiang province for several successive years. Furthermore, sexual transmission accounts for >90% of newly diagnosed patients, and more than half of all non–Jiaxing residents are diagnosed at Jiaxing Municipal Center for Disease Control and Prevention (Jiaxing CDC) every year.
Blood samples were obtained from 806 newly confirmed HIV-1-positive patients by the Jiaxing CDC through the first follow-up between January 2015 and December 2018, representing 87.5% (806/921) of the newly diagnosed patients in Jiaxing city in this study. All patients were newly confirmed HIV-1-positive and treatment-naive cases during sampling and had no previous exposure to highly active antiretroviral therapy. Demographic and epidemiological data (eg, age, gender, ethnicity, education, current residence, registered residence, transmission route, and other HIV infection-related information) were collected by the staff of the Jiaxing CDC and Zhejiang Provincial CDC through face-to-face interviews as covariates for transmission network analysis. Cases with incomplete information or without the target gene fragment were not included in this study.
Amplification of HIV-1 pol for Phylogenetic Analyses
Viral RNA was extracted from 140 μL of plasma using a QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNA samples were directly subjected to reverse transcription polymerase chain reaction (PCR) and nested PCR to generate the partial HIV-1 pol coding region (HXB2: 2253–3306), which included the entire protease (PR) and the first 300 codons of the reverse transcriptase (RT) gene. Amplification and sequencing were performed as previously described [30]. Target PCR products were sent to Hangzhou TsingKe Bio-Tech Co. for purification and sequencing using 5 overlapping primers.
Phylogenetic and Drug Resistance Mutation Analyses
The trimming and assembly of sequences were performed using Sequencher, version 5.0 (Gene Codes, Ann Arbor, MI, USA) [31]. Ambiguous bases were considered when the secondary peak was >30% of the main peak. Sequences with ≥5% ambiguous bases were excluded in the study, as previously described [32]. Sequences were subjected to multiple alignment with ClustalW and trimmed to identical lengths (>1000 bp, HXB2: 2253 to 3265 nt) using Bio-Edit, version 7.0 [33]. Reference sequences were obtained from the Los Alamos National Laboratory (LANL) HIV sequence database (https://www.hiv.lanl.gov), which covers the major HIV-1 subtypes and circulating recombinant forms (CRFs). Subtypes were identified using 2 methods: the online automated HIV-1 subtyping tool COntext-based Modeling for Expeditious Typing (COMET) [34] and phylogenetic analyses. Phylogenetic analyses for pol sequences were performed based on the approximate maximum likelihood method with FastTree, version 2.3 [35], under the GTR + G + I nucleotide substitution model to further confirm subtyping. Local support values were computed with the Shimodaira-Hassegawa (SH) test [36]. If phylogenetic tree nodes had SH-like support values ≥0.90, the viral sequences were identified as the subtypes of reference sequences. Finally, 258 available CRF07_BC sequences (>1000 bp) were selected for further analysis. Transmitted drug resistance (TDR) was evaluated using the Calibrated Population Resistance (CPR) tool from the Stanford HIV Database.
Identification and Analysis of the HIV-1 CRF07_BC Transmission Network
All sequences were aligned, and pairwise genetic distances were computed using the Tamura-Nei 93 (TN93) method [37]. To construct an effective transmission network for identifying potential transmission clusters using the visualization software Cytoscape, version 3.6.2 [38], an optimal genetic distance threshold was selected from 0.001 substitutions/site to 0.02 substitutions/site (the optimal threshold was established by the maximum number of clusters below this threshold). In such a network, a node represents a sequence or an individual, and links (edges) that connect different individuals represent their potential transmission relationships; with more links, the node may have higher transmission risk (the node with ≥4 links indicates a suspected high-risk case) [39]. Dyads (connected with a unique participant) and clusters were defined as groups of linked sequences in the network containing 2 nodes and >2 nodes, respectively. Singletons were defined as individuals with genetic distances greater than the threshold.
Bayesian Markov Chain Monte Carlo Evolutionary Analysis
To better understand the evolutionary history of HIV-1 CRF07_BC in Jiaxing city, a total of 2969 available CRF07_BC reference sequences were downloaded from the Los Alamos National Laboratory (LANL) HIV sequence database (https://www.hiv.lanl.gov), in which 85 reference sequences were selected based on the highest similarity (>98.0%) with CRF07_BC sequences of cluster C1, after excluding repeated sequences. Eventually, 190 CRF07_BC sequences were included for Bayesian analysis.
Bayesian analyses were performed using BEAST, version 1.7.2. Bayesian skyline plot (BSP) analysis was conducted to explore the changes in the evolutionary rate and the effective population size of CRF07_BC in cluster C1 over time. The most recent common ancestor (tMRCAs) and rates of evolution (in units of nucleotide substitutions/site/year) were calculated. The best-fitting model of evolution according to the Akaike information criterion (AIC) was identified using jModelTest, version 2.0.1. The operating parameters of the BEAUti program were modified, and the 2 independent chains, both adjusted to 50 million steps, were run under the strict clock model and the relaxed clock model, assuming a constant substitution rate as estimated from the data set. Time scales of the trees were calibrated with the sampling dates available. In general, only the effective sample size (ESS) values >200 were accepted after a 20% burn-in using Tracer, version 1.6, to minimize the effects of standard errors. The maximum clade credibility (MCC) tree constructed with an optimal clock model was displayed and edited using FigTree, version 1.4.0 [40].
Statistical Analyses
EpiData 3.0 software was used to establish the database, and Statistical Product and Service Solutions (SPSS) 20.0 software was used to organize and analyze the data. A chi-square test was used for comparisons between groups. Statistical significance was set at P < .05.
Patient Consent Statement
This study was reviewed and approved by the Zhejiang Provincial Center of Disease Control and Prevention (Zhejiang CDC) Institutional Ethics Committee (Approval ID: X140617334), and written informed consent was obtained from the study participants. All experiments were performed following the approved guidelines and regulations, and the experimental protocols were approved by the institutional review boards of the Zhejiang CDC.
RESULTS
Demographic Characteristics of CRF07_BC-Infected Patients
In this prospective study, sequences were successfully obtained from a total of 716 (89%, 716/806) newly diagnosed HIV-positive patients, among which CRF07_BC was a dominant circulating strain (36%, 258/716) in Jiaxing from 2015 to 2018 (unpublished data). Of the 258 CRF07_BC-infected patients, most were male (79.1%, 204/258), the median age (range) was 38 (17–79) years, the vast majority (58.9%, 152/258) were between 20 and 40 years of age, 93% were of Han ethnicity (186/258), and the proportion of married individuals was 43.8% (113/258). Only 95 (36.8%) of these cases were born in Jiaxing, while a majority (63.2%, 163/258) were domestic migrants, who were mainly from eastern provinces and Southwest China (eg, Anhui, Jiangsu, Sichuan, Chongqing, and Yunnan). Heterosexual transmission was the main route of infection (53.1%, 137/258), and homosexual transmission was reported in 45.7% of cases. The majority of the individuals had a middle school education or below (70.9%, 183/258). Occupations were widely distributed, with farmers/migrant workers accounting for the largest proportion (112 cases, 42.6%). A demographic comparison of 2 groups (individuals in the networks [≥1 link] and individuals not in the networks [singletons; 0 links]) is shown in Table 1.
Table 1.
Sociodemographic Characteristics of CRF07_BC-Infected Patients From 2015 to 2018
| Total | 0 Links | ≥1 Link | ||||
|---|---|---|---|---|---|---|
| No. | Column, % | No. | Row, % | No. | Row, % | |
| Total | 258 | 95 | 163 | |||
| Collection time | ||||||
| 2015–2016 | 71 | 27.5 | 17 | 17.9 | 54 | 33.1 |
| 2017 | 77 | 29.9 | 29 | 30.5 | 48 | 29.4 |
| 2018 | 110 | 42.6 | 49 | 51.6 | 61 | 37.4 |
| Sex | ||||||
| Male | 204 | 79.1 | 72 | 75.8 | 132 | 81.0 |
| Female | 54 | 20.9 | 23 | 24.2 | 31 | 19.0 |
| Age | ||||||
| <20 y | 7 | 2.7 | 2 | 2.1 | 5 | 3.1 |
| 20–40 y | 152 | 58.9 | 65 | 68.4 | 87 | 53.4 |
| >40 y | 99 | 38.4 | 28 | 29.5 | 71 | 43.6 |
| Marital status | ||||||
| Single | 95 | 36.8 | 37 | 38.9 | 58 | 35.6 |
| Married | 113 | 43.8 | 42 | 44.2 | 71 | 43.6 |
| Divorced/widowed | 50 | 19.4 | 16 | 16.8 | 34 | 20.9 |
| Transmission category | ||||||
| MSM | 118 | 45.7 | 48 | 50.5 | 70 | 42.9 |
| Heterosexual male | 83 | 32.2 | 23 | 24.2 | 60 | 36.8 |
| Heterosexual female | 54 | 20.9 | 23 | 24.2 | 31 | 19.0 |
| Other/unknown | 3 | 1.2 | 1 | 1.1 | 2 | 1.2 |
| Country of birth | ||||||
| Jiaxing city | 95 | 36.8 | 24 | 25.3 | 71 | 43.6 |
| Other cities in Zhejiang province | 4 | 1.6 | 3 | 3.2 | 1 | 0.6 |
| Other provinces | 159 | 61.6 | 68 | 71.6 | 91 | 55.8 |
| Current address | ||||||
| Jiaxing city | 193 | 74.8 | 69 | 72.6 | 124 | 76.1 |
| Other cities in Zhejiang province | 1 | 0.4 | 1 | 1.1 | 0 | 0.0 |
| Other provinces | 64 | 24.8 | 25 | 26.3 | 39 | 23.9 |
| Education | ||||||
| Primary school and below | 64 | 24.8 | 26 | 27.4 | 38 | 23.3 |
| Junior middle school | 119 | 46.1 | 39 | 41.1 | 80 | 49.1 |
| High school and above | 75 | 29.1 | 30 | 31.6 | 45 | 27.6 |
Phylogenetic and Drug Resistance Mutation Characteristics of CRF07_BC-Infected Patients
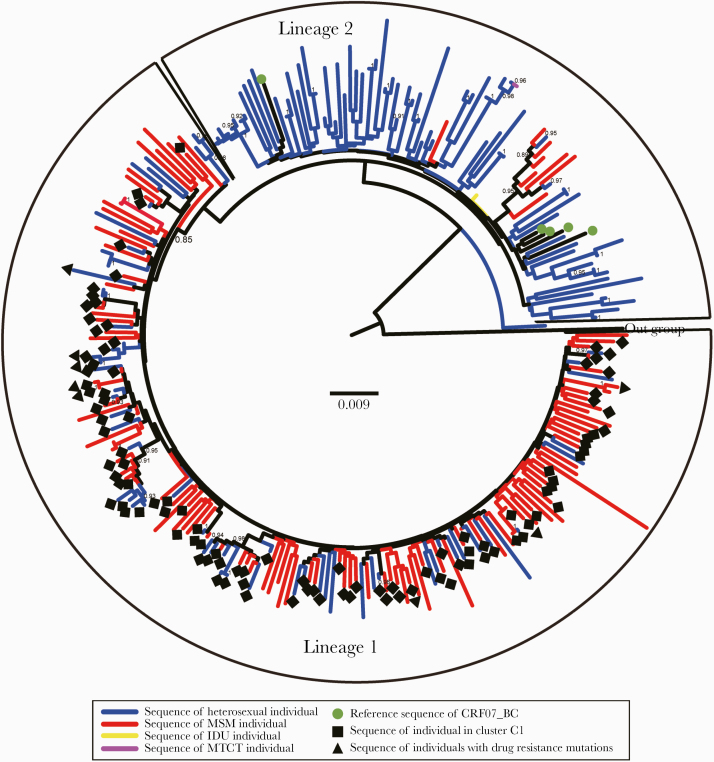
A phylogenetic tree was constructed based on the sequences from CRF07_BC-infected patients, and the results showed that the epidemic of CRF07_BC in Jiaxing city mainly consisted of 2 distinct lineages (Figure 1). The median average pairwise genetic distance of sequences within lineage 1 (SD) was 0.026 (0.002), and that of sequences outside lineage 2 (SD) was 0.04 (0.003). The distributions of heterosexual individuals and MSM were significantly different in the phylogenetic tree. Lineage 1 included 171 cases (66.3%, 171/258), with a higher bootstrap value, which included 110 cases of MSM (93.2%, 110/118) and 61 cases of heterosexual individuals (44.5%, 61/137), suggesting that MSM with CRF07_BC infection may form a dense and complex transmission network and interact with other risk groups in the area. Among the 171 cases with pol sequences from lineage 1, 8 were found to harbor a single drug resistance mutation (DRM). Except for 1 protease inhibitor (PI)–related mutation (G73S) and 1 NRTI-related mutation (D67N), nonnucleoside reverse transcriptase inhibitor (NNRTI)–related mutations (K103N and K103N) were detected 3 times. Lineage 2 included 87 cases (33.7%, 87/258), with a bootstrap support value lower than that predefined, but was composed of sequences primarily isolated from heterosexual individuals (39 cases of heterosexual female, 38 cases of heterosexual male, 8 cases of MSM, and 2 case of IDU/mother-to-child transmission [MTCT]), and no drug-resistant mutation sites were detected.
Figure 1.
Phylogenetic analysis of CRF07_BC-infected patients from 2015 to 2018. The phylogenetic tree based on pol region of CRF07_BC (>1000 bp, HXB2: 2253 to 3265 nt) was constructed using the approximate maximum likelihood method with FastTree, version 2.3. The node SH-like support value (≥0.90) was indicated and considered credible. HIV-1 subtype K was chosen as an out-group in the rooted tree. Abbreviations: IDU, intravenous drug user; MSM, men who have sex with men; MTCT, mother-to-child transmission.
Identification and Characterization of Molecular Transmission Networks Among CRF07_BC-Infected Patients
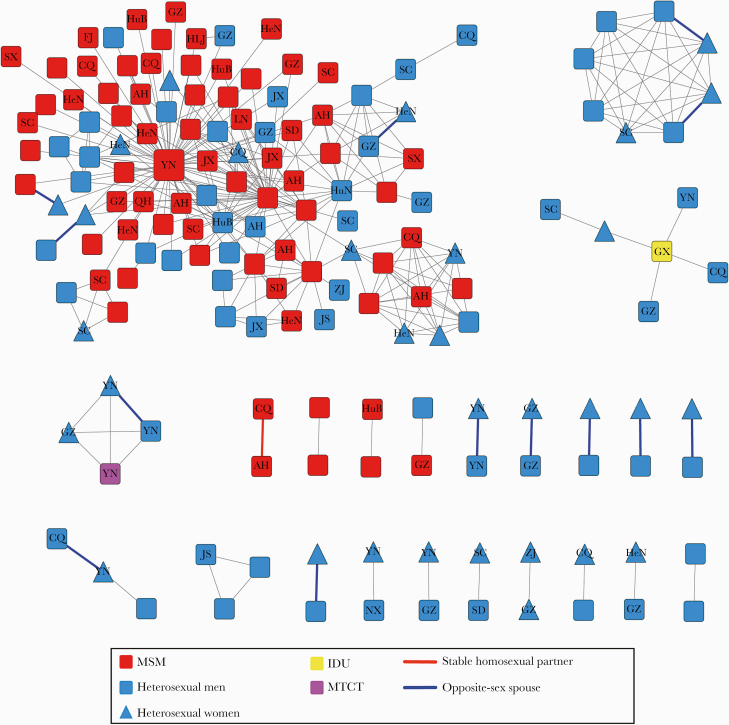
To clearly understand the relationship between cluster characteristics and demographic characteristics among CRF07_BC-infected patients, we plotted the molecular transmission networks based on pol sequences of CRF07_BC generated from each individual from 2015 to 2018 using the optimal genetic distance of 0.01 substitutions/site. Using this threshold, the maximum number of clusters obtained was below the threshold, and 163 individuals (63.2%, 163/258) were connected to at least 1 other study participant and formed 23 groups comprising 6 clusters (cluster sizes ranging from 3 to 105) and 17 dyads. Of the individuals with sequences included in the networks, 81.0% were male, 72.4% had a low level of education (junior high and below), 53.4% were aged 20–40 years (mean age [SD], 38.5 [4.2] years), 55.8% included sequences isolated only from heterosexual individuals, 42.9% included sequences only from MSM, 1.2% included sequences only from other sources (1 case of MTCT and 1 case of IDU), 55.8% were non–Jiaxing residents from 19 provinces (who were mainly from the Southwestern and Eastern regions in China), and 76.1% were currently local (Table 1). We compared individuals in the networks (≥1 link) with individuals not in the networks (singletons; 0 links), which revealed that individuals who were linked in the networks included larger proportions of MSM (59% vs 41%; P < .01) and heterosexual males (72% vs 28%; P < .01) and had a larger proportion of cases over 40 years old (44% vs 29%; P < .01) than singletons.
Within the transmission networks, a distinctly large and rapidly growing cluster (C1) containing 105 individuals was identified and found to be a mixture of different infectious risk factors. In cluster C1, the vast majority of individuals were male (89.5%, 94/105), 63 were MSM (88.7%, 63/70), 49 were Jiaxing residents, and 56 were non–Jiaxing residents from the Southwestern region of China and other locations throughout the country, most of whom currently live in Jiaxing city. In addition to the individuals in the large cluster, the other individuals in the networks were mainly heterosexual. Among the remaining 5 clusters, 3 clusters (C2, C5, and C6) included sequences isolated only from heterosexual individuals and were identified with respective sizes of 3, 3, and 8. Specifically, 8 individuals (3 had commercial heterosexual behavior) in cluster C2 were diagnosed in the same county (Haiyan County) and included 2 pairs of spouses, with extremely high homology, and the pairwise average genetic distance (SD) was 0.004 (0.002), suggesting that the cluster may be caused by commercial heterosexual behavior and related to undetected transmission by commercial sex workers (CSWs). Cluster C3 comprised 5 cases of heterosexual individuals and 1 case of IDU. Cluster C4 included 3 cases of heterosexual individuals and 1 case of MTCT, in which 3 cases labeled “YN” came from the same family and 1 case labeled “GZ” was identified as a case of CSW transmission. Among the 17 dyads, 13 were formed with heterosexual individuals, 3 were formed with MSM, and 1 was formed with MSM and heterosexual individuals, including 6 pairs of opposite-sex spouses and a pair of stable homosexual partners (Figure 2).
Figure 2.
Molecular transmission network analysis of CRF07_BC-infected patients; HIV-1 transmission cluster diagrams illustrating the structure and demographics of the putative transmission clusters defined in CRF07_BC-infected patients from 2015 to 2018. All edges represent a genetic distance between nodes of <0.01 substitutions/site, and the color of the node indicates the reported transmission risk. Nodes with no labels represent Jiaxing residents, and nodes with labels represent non–Jiaxing residents from different provinces or cities (the labels refer to their place of origin; eg, AH represents Anhui province, YN represents Yunnan province, and CQ represents Chongqing city). Different shapes denote gender, and the color of the bold edges represents the relationship between 2 linked individuals. Abbreviations: IDU, intravenous drug user; MSM, men who have sex with men; MTCT, mother-to-child transmission.
We further analyzed different risk factors in the networks. First, the 163 individuals within the networks exhibited a total of 311 links (edges), in which 67 individuals had 1 link and 96 individuals had ≥2 links. Of the MSM, 179 links included 94 individuals, 63.9% of whom were linked to other MSM. A total of 129 links of heterosexual individuals included 63 individuals, 63.3% of whom were linked to other heterosexual individuals. We further examined links (35.5%, 109/311 links) between MSM and heterosexual individuals associated with 33 cases of MSM and 39 cases heterosexual individuals, among whom MSM were more likely to be linked to heterosexual males (69.7%, 76/109 links) than to heterosexual females (30.3%, 33/109 links). A comparison of links between different infected groups suggested the existence of clusters among individuals with the same or distinct sexual orientations or among some bisexual male (in particular, men who define themselves as heterosexual but who have sex with other males) during the local epidemic of CRF07_BC.
Then, we investigated the influence of links among different areas on transmission to improve our understanding of the roles that Jiaxing residents and non–Jiaxing residents played in the networks by place of origin. The networks contained 92 non–Jiaxing residents from different areas, including 50 from the Southwestern regions of China (Yunnan, Sichuan, Chongqing, and Guizhou), and the remaining residents were from other regions, including 15 provinces. A comparison of links between Jiaxing residents and non–Jiaxing residents showed that MSM among Jiaxing residents were more likely to be associated with MSM among non–Jiaxing residents (52.6%, 61/114 links), while heterosexual individuals among Jiaxing residents tended to be interrelated (42.5%, 34/80 links). Moreover, the individuals with more links in the networks (≥4 links) were mainly Jiaxing residents, who were associated with individuals from multiple regions.
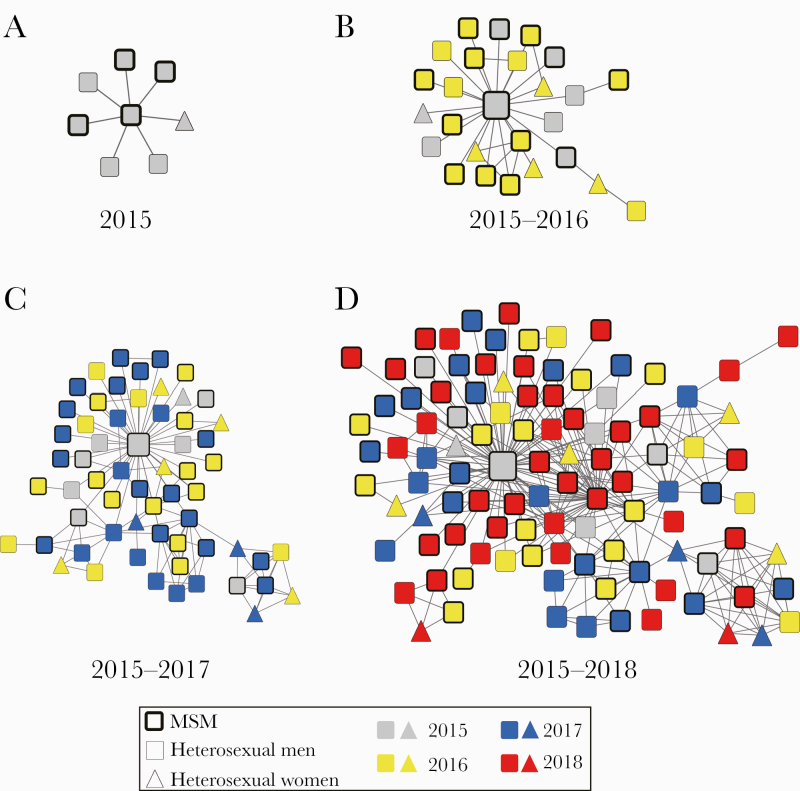
Active Transmission Cluster Dynamics
Furthermore, we analyzed the time dynamics of cluster C1, which included 105 individuals. In 2015, 2016, 2017, and 2018, the number of individuals detected in cluster C1 was 8, 26, 61, and 105, respectively. As shown in Figure 3, we found that the individual with 70 edges was diagnosed in 2015, and this individual was at the core of the cluster in the 4 consecutive years of network monitoring. The individual was registered in Yunnan province and had returned to Yunnan after confirmation. According to HIV BLAST analysis, the CRF07_BC strain isolated from the individual had high homology with the CRF07_BC endemic strains from Yunnan province (97%–98%).
Figure 3.
Dynamic characteristics analysis of active and rapid growth cluster C1 from 2015 to 2018. Molecular transmission network of newly diagnosed cases was drawn year by year: (A) in 2015, (B) during 2015 to 2016, (C) during 2015 to 2017, (D) during 2015 to 2018. The color of the node indicates confirmation year (gray represents newly diagnosed patients in 2015, yellow represents newly diagnosed patients in 2016, blue represents newly diagnosed patients in 2017, and red represents newly diagnosed patients in 2018); different shapes denote gender. Abbreviations: IDU, intravenous drug user; MSM, men who have sex with men; MTCT, mother-to-child transmission.
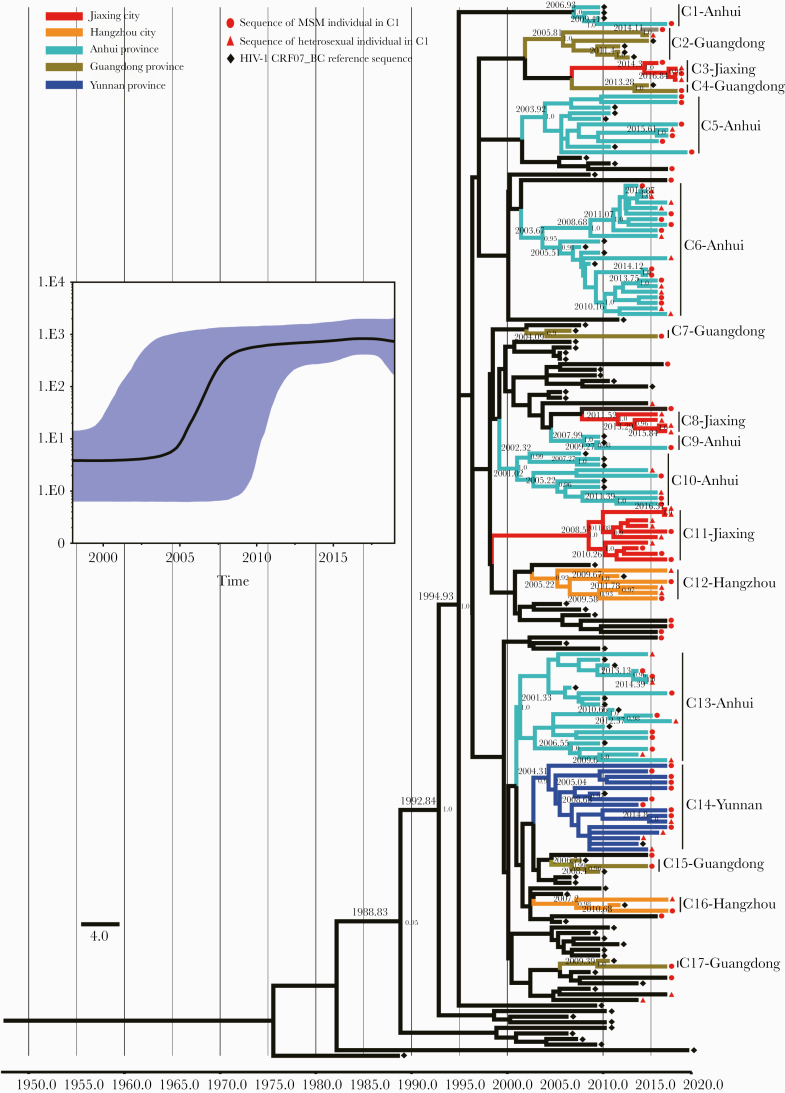
BSP analysis revealed that the CRF07_BC strains in cluster C1 started the initial growth phase in ~2004, followed by exponential expansion during 2005–2009, and reached steady growth thereafter (Figure 4). For the evolutionary tree constructed with a molecular clock, the estimated mean evolutionary rate was 1.07 × 10–3 nucleotide substitutions/site/year. Combining the mean evolutionary rate and the pairwise genetic distances for estimating evolutionary time (which can be regarded as infection time), the results showed that most of the individuals in the large cluster C1 had been infected for between 4.67 and 9.35 years. In addition, with high posterior probability support, we found that the largest cluster C1 gradually formed 17 unique phylogenetic clades during the evolutionary process, which showed a close geographical relationship with the different CRF07_BC epidemic strains from Yunnan, Guangdong, Anhui, and Zhejiang provinces in China. Most of these clades evolved independently from 2003 to 2010; of them, 6 clades had higher homology with different CRF07_BC epidemic strains from Anhui, 5 clades had higher homology with different CRF07_BC epidemic strains from Guangdong, 3 clades had higher homology with Yunnan and Hangzhou CRF07_BC epidemic strains, and the other 3 clades were suspected to be the Jiaxing epidemic strains (Figure 4).
Figure 4.
Maximum clade credibility (MCC) tree of the active and large cluster C1 of CRF07_BC-infected patients based on HIV-1 pol sequences from 2015 to 2018. Bayesian analyses were performed using BEAST, version 1.75 (see the “Methods” for details). Bayesian skyline plot of the cluster C1 was demonstrated (the x-axis represents the time in units of years, and the y-axis represents the effective population size); the median effective population size is shown in black, and the 95% highest probability density (HPD) credible region is shown in blue. In the MCC tree, sequences from190 CRF07_BC sequences (85 reference sequences and 105 sequences of the cluster C1) form 17 unique phylogenetic clades, labeled C1 through C17; the branch lengths reflect evolutionary time, and nodes labeled with evolutionary time are supported by a high posterior probability (≥0.90). The corresponding time scale was marked at the bottom of the MCC tree; different colors of branches indicate that the formed clades contain reference sequences from different provinces/cities in China. Abbreviation: MSM, men who have sex with men.
Risk Factor Analysis of Key Individuals in Cluster C1
For a total of 50 individuals with more links (≥4 links, 4~70 links) in cluster C1, a detailed epidemiological survey (eg, previous high-risk behavior, history of diagnosis, determination of infection time and location, and number of sexual partners) was conducted (Table 2). The results showed that the infection time of individuals with more links was mainly >2 years, and high-risk behaviors mainly occurred in Jiaxing city. In addition, we found that MSM who were >40 years old had more sexual partners (≥10; including anonymous partners) 2 years before diagnosis and reported clustered sexual activity (meeting partners) in the local park or temporary homosexual behavior in baths in Hangzhou, Shanghai, and other surrounding cities. These individuals had been infected for a long time (≥5 years) and had a high viral load (≥10 000 copies/mL) when they were diagnosed.
Table 2.
Risk Factors for Suspected Key Individuals in Cluster C1
| Total | MSM | Heterosexual | Unknown | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Column, % | No. | Row, % | No. | Row, % | No. | Row, % | |
| Gender | ||||||||
| Male | 45 | 90.0 | 31 | 68.9 | 13 | 28.9 | 1 | 2.2 |
| Female | 5 | 10.0 | 0 | 0.0 | 4 | 80.0 | 1 | 20.0 |
| Age | ||||||||
| 17–29 y | 18 | 36.0 | 12 | 66.7 | 5 | 27.8 | 1 | 5.6 |
| 30–39 y | 11 | 22.0 | 8 | 72.7 | 2 | 18.2 | 1 | 9.1 |
| ≥40 y | 21 | 42.0 | 10 | 47.6 | 10 | 47.6 | 1 | 4.8 |
| Infection time | ||||||||
| ≤2 y | 30 | 60.0 | 17 | 56.7 | 12 | 40.0 | 1 | 3.3 |
| >2 y | 20 | 40.0 | 13 | 65.0 | 5 | 25.0 | 2 | 10.0 |
| Infection location | ||||||||
| Jiaxing city | 39 | 78.0 | 24 | 61.5 | 13 | 33.3 | 2 | 5.1 |
| Ambiguous | 11 | 22.0 | 6 | 54.5 | 4 | 36.4 | 1 | 9.1 |
| Fixed venues for sexual contacts | ||||||||
| Yes | 13 | 26.0 | 5 | 38.5 | 6 | 46.2 | 2 | 15.4 |
| No | 37 | 74.0 | 25 | 67.6 | 11 | 29.7 | 1 | 2.7 |
| Sexual contacts outside Jiaxing city | ||||||||
| Yes | 11 | 22.0 | 5 | 45.5 | 4 | 36.4 | 2 | 18.2 |
| No | 39 | 78.0 | 25 | 64.1 | 13 | 33.3 | 1 | 2.6 |
Abbreviation: MSM, men who have sex with men.
DISCUSSION
In recent years, some researchers have used genetic surveillance or regional molecular networks for major HIV subtypes to explore the characteristics of HIV transmission patterns and networks [25, 41–43]. Nevertheless, most of these molecular epidemiological studies were conducted on HIV-1-infected patients in high-income countries or large cities. In this study, due to the characteristics of the special geographical location (Jiaxing city) considered (the central area of the Yangtze River Delta, medium-sized city, more developed economy, convenient transportation, and high population mobility), we were able to more easily conduct in-depth sampling of research subjects, explore the characteristics of regional HIV-1 transmission, and perform dynamic monitoring in different risk groups using prospective studies. Moreover, field epidemiological verification was easily implemented, and the reliability of HIV transmission analysis was improved. Hence, we conducted high-density sampling and a detailed molecular epidemiological survey in Jiaxing city from 2015 to 2018. In this study, we focused on comprehensive phylogenetic, transmission network, and risk factor analyses of 258 cases of newly diagnosed CRF07_BC-infected patients. This study is unique in that it is the first to use the HIV molecular transmission networks method among CRF07_BC-infected patients in developed eastern China by combining HIV molecular transmission networks with epidemiological, demographic, and behavioral data to explore the local transmission characteristics and epidemic pattern of subtype CRF07_BC.
Although the proportion of heterosexual individuals among CRF07_BC-infected patients was larger than that of MSM in our study, through phylogenetic analysis and transmission networks analysis, we found that the MSM population played an important role in the prevalence of subtype CRF07_BC. According to phylogenetic analysis, CRF07_BC formed 2 distinct lineages, with lineage 1 primarily from MSM and some heterosexual individuals and lineage 2 mainly composed of sequences from heterosexual individuals. The mean genetic distance of lineage 1 was significantly lower than that of lineage 2. These results indicated that the lineage 1 sequences were more genetically homogeneous, suggesting highly related and rapidly expanding transmission networks among MSM and heterosexual individuals. Additionally, 8 cases of individuals harboring a single DRM were identified and concentrated in lineage 1. Although the level of DRM (3.1%) in CRF07_BC-infected patients was lower than the baseline (5%), these DRM individuals were associated with the highly homologous and active lineage 1, increasing the risk of drug resistance transmission. Hence, continuous and detailed drug resistance monitoring is still essential.
As one of the fastest developing methods in HIV molecular evolutionary analysis in recent years, HIV-1 transmission network analysis makes up for the lack of credibility of information based only on social behavior and field epidemiology and can effectively identify key transmission clusters and targets of precise intervention and reveal the relevant factors driving rapid spread of the virus in the networks [25, 41, 42, 44–46]. Moreover, a project titled “Ending the HIV Epidemic: A Plan for America” was announced by the US Department of Health and Human Services (HHS) in 2019, which identified the monitoring of HIV molecular clusters as 1 of the 4 pillars of a strategic initiative to reduce new HIV infections [47, 48]. In this study, we thoroughly analyzed the transmission networks of CRF07_BC among infected patients below the optimal genetic distance of 0.01 substitutions/site (Figure 2). By analyzing the different risk factors of individuals in the networks and individuals outside the networks, we found that the majority of MSM were linked to other MSM and were more inclined to link with heterosexual males than with heterosexual females. Interestingly, the percentage of heterosexual males in the networks was significantly higher than that of heterosexual males outside the networks. Many studies have reported clusters consisting of MSM and heterosexual individuals, especially mixed clusters including heterosexual females, suggesting that some heterosexual males may act as a transmission bridge between mixed clusters, as bisexual males promote the rapid spread of clusters locally [49, 50]. Due to pressure from their parents and public opinion, some MSM have chosen to marry heterosexual females, which leads to some MSM reporting themselves as having been heterosexually infected when investigated [51].
Further analysis of the relationships among different areas in the networks showed that the majority of non–Jiaxing residents with CRF07_BC infection were from the Southwest (eg, Yunnan and Guizhou) and surrounding provinces (eg, Jiangsu and Anhui), indicating obvious cross-regional transmission events. Of these non–Jiaxing residents, MSM were more likely to link to other MSM who lived in Jiaxing city, while heterosexual individuals from the same registered residence were more likely to cluster, revealing intricate HIV-1 transmission patterns and the difficulty of intervention among MSM. Due to the far-reaching influence of traditional Chinese culture, MSM tend to hide their homosexual behavior and seek sexual partners in other cities (especially economically developed areas) for fear of being recognized by acquaintances and being greatly stigmatized and discriminated against [52–54]. Due to the rapid economic development in Eastern China and the uneven development of the Eastern and Western regions, areas such as Shanghai city and Hangzhou city, which represent the most economically developed regions and attract large floating populations each year, also contain the largest concentrations of MSM. With the rapid development of social networks and the convenience of transportation, the cities (eg, Jiaxing city and Huzhou city) surrounding these developed cities are also among the main locations of MSM, resulting in the rapid spread of HIV among MSM and the tendency to form dense and complex transmission networks in these regions, further increasing the region’s HIV prevention and control difficulties [28, 41, 55].
Many studies have indicated that the size and dynamic nature of active clusters tend to actively grow over time and that such clusters tend to include more individuals than inactive clusters [25, 56, 57]. In our study, 63.2% of individuals formed 6 clusters and 17 dyads in the networks. In contrast to the distinctly large cluster (C1) with infection risk factors, the other clusters and dyads were especially small and mostly composed of heterosexual individuals, suggesting that heterosexual clusters and dyads are limited to transmission pairs and small clusters, without substantial further spread of the infection. Surprisingly, the distinctly larger cluster (C1) included 89.5% males and 88.7% MSM. The cluster, which was first identified in 2015 and consisted of 8 individuals, rapidly expanded to 105 cases in 2018 and increased >13-fold, demonstrating that clustering is becoming more active and uncontrolled over time (Figure 3). Through BSP and Bayesian analyses, we found that this large cluster was mainly formed via independent evolution of several CRF07_BC clades with genetic and geographical relationships with the different regions that underwent exponential expansion from 2005 to 2009, and then experienced slower growth. Most clades formed during this period, most of which were epidemic clades of CRF07_BC among MSM from Anhui province, indicating that this regional epidemic of CRF07_BC was closely geographically related to CRF07_BC epidemic strains from Anhui province. Previous research has shown that CRF07_BC was first transmitted to the MSM population in about 2004–2006 [20], then rapidly spread among the MSM population during the following years, consistent with our study results.
In addition, we conducted a detailed epidemiological investigation of individuals with more links in the cluster C1 (≥4 links) and multiple risk factors, and we found that MSM over 40 years old had a high risk of transmission. These individuals generally had a low educational background, active sexual behaviors but low awareness of sexual protection, and more sexual partners, which drove the rapid spread of the networks. Moreover, due to a lack of awareness of their infection status, these individuals could not be diagnosed in a timely manner, and uncontrolled and rapidly spreading clusters become more frequent over time. Our results indicate that these individuals with more links may act as superspreaders and have a higher risk of infecting other individuals, suggesting that some superspreaders indeed drive the formation and rapid growth of transmission networks to a significant extent. Further, the high transmission dynamics among these MSM in the cluster revealed by our results emphasize the necessity for active testing and early treatment in this group. However, it needs to be noted that there could also be undiagnosed individuals driving transmission due to the limitations of genetic linkage in the network. Thus, although these suspected key individuals in the active cluster are important targets of interventions, further expanded network monitoring measures are also crucial, such as increasing the frequency of follow-up, partner notification and contact tracing, and regular syphilis testing.
Our study provided some insights and information to explore the local transmission characteristics and epidemic patterns of HIV-1 CRF07_BC. However, our study has several limitations. First, in our study we assume high sampling density (>85%) in Jiaxing city, but Jiaxing city is just one of the cities in Eastern China and may not fully represent the epidemic characteristics of HIV-1 CRF07_BC in these regions. The lack of multiregional and multisectoral joint prevention and control mechanisms is one of the limitations of this study and will be the focus of further research. Second, due to the certain lag between the collection of samples and the surveillance analysis of molecular networks, we mainly used epidemiological investigations to determine the infection time of infected persons (especially large cluster C1 of individuals), not supplementing serological testing in time. Third, due to the stigma and discrimination associated with HIV/AIDS, people with HIV fear that their status will be disclosed and might hide information on HIV-related risky behaviors, leading to biases.
CONCLUSIONS
Our study is the first to use the HIV-1 molecular transmission network method among CRF07_BC-infected patients in developed Eastern China, combining an HIV-1 molecular transmission network with epidemiological, demographic, and behavioral data to explore the local transmission characteristics and epidemic pattern of subtype CRF07_BC. These results, combined with epidemiological data, emphasize the importance of the unique region– and risk group–specific transmission network and provide critical and insightful information for more effective intervention strategies to limit HIV-1 transmission in the future. Understanding these epidemic dynamics in real time is increasingly of public health importance in terms of guiding prevention efforts.
Acknowledgments
We appreciate all the volunteer patients enrolled in this study.
Financial support. This work was supported by the Zhejiang Provincial Medicine Science and Technology Plan (grant number: 2019KY359) and the Public Technology Research Project of Zhejiang province (grant number: LGF20H260002).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stecher M, Hoenigl M, Eis-Hübinger AM, et al. Hotspots of transmission driving the local human immunodeficiency virus epidemic in the Cologne-Bonn Region, Germany. Clin Infect Dis 2019; 68:1539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bbosa N, Kaleebu P, Ssemwanga D. HIV subtype diversity worldwide. Curr Opin HIV AIDS 2019; 14:153–60. [DOI] [PubMed] [Google Scholar]
- 3. Pimentel V, Pingarilho M, Alves D, et al. Molecular epidemiology of HIV-1 infected migrants followed up in portugal: trends between 2001–2017. Viruses 2020; 12:268–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faria NR, Rambaut A, Suchard MA, et al. The early spread and epidemic ignition of HIV-1 in human populations. Science 2014; 346:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xin R, He X, Xing H, et al. Genetic and temporal dynamics of human immunodeficiency virus type 1 CRF07_BC in Xinjiang, China. J Gen Virol 2009; 90:1757–61. [DOI] [PubMed] [Google Scholar]
- 6. Feng Y, He X, Hsi JH, et al. The rapidly expanding CRF01_AE epidemic in China is driven by multiple lineages of HIV-1 viruses introduced in the 1990s. AIDS 2013; 27:1793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meng Z, Xin R, Zhong P, et al. A new migration map of HIV-1 CRF07_BC in China: analysis of sequences from 12 provinces over a decade. PLoS One 2012; 12:e52373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su L, Graf M, Zhang Y, et al. Characterization of a virtually full-length human immunodeficiency virus type 1 genome of a prevalent intersubtype (C/B’) recombinant strain in China. J Virol 2000; 74:11367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen ZW, Liu L, Chen G, et al. Surging HIV-1 CRF07_BC epidemic among recently infected men who have sex with men in Fujian, China. J Med Virol 2018;. 90:1210–21. [DOI] [PubMed] [Google Scholar]
- 10. Ma L, Guo Y, Yuan L, et al. Phenotypic and genotypic characterization of human immunodeficiency virus type 1 CRF07_BC strains circulating in the Xinjiang province of China. Retrovirology 2009; 6:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng Z, Xin R, Zhong P, Zhang C, Abubakar YF, et al. A new migration map of HIV-1 CRF07_BC in China: analysis of sequences from 12 provinces over a decade. PLoS One 2012; 7:e52373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Zang X, Ning C, et al. Molecular epidemiology of HIV-1 in Jilin province, Northeastern China: emergence of a new CRF07_BC transmission cluster and intersubtype recombinants. PLoS One 2014; 9:e110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Wang N, Wang L, et al. The 2007 estimates for people at risk for and living with HIV in China: progress and challenges. JAIDS 2009; 50:414–8. [DOI] [PubMed] [Google Scholar]
- 14. Hu M, Xu C, Wang J. Spatiotemporal analysis of men who have sex with men in mainland China: social app capture-recapture method. JMIR mHealth uHealth 2020; 8:e14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong MJ, Peng B, Liu ZF, et al. The prevalence of HIV among MSM in China: a large-scale systematic analysis. BMC Infect Dis 2019; 19:1000–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang JZ, Chen WJ, Zhang WJ, et al. Molecular epidemiology and transmission of HIV-1 infection in Zhejiang province, 2015. Chin J Epidemiol 2017; 38:1551–6. [DOI] [PubMed] [Google Scholar]
- 17. Lu X, Kang X, Liu Y, et al. HIV-1 molecular epidemiology among newly diagnosed HIV-1 individuals in Hebei, a low HIV prevalence province in China. PLoS One 2017; 12:e0171481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng MN, Ning TL, Zhao X, et al. Molecular epidemiology and transmission of HIV in Tianjin, 2015. Chin J Epidemiol 2016; 37:1142–7. [DOI] [PubMed] [Google Scholar]
- 19. Zhang M, Jia D, Li H, et al. Phylodynamic analysis revealed that epidemic of CRF07_BC strain in men who have sex with men drove its second spreading wave in China. AIDS Res Hum Retroviruses 2017; 33:1065–9. [DOI] [PubMed] [Google Scholar]
- 20. Peng X, Xu Y, Wu N. Differences of cytotoxic T-lymphocyte pressure and divergent evolution of several CRF07_BC clusters circulating in men who have sex with men in China. Infect Genet Evol 2020; 85:104486. [DOI] [PubMed] [Google Scholar]
- 21. Yuan H, Liu Z, Wu X, et al. Evolutionary characteristics and genetic transmission patterns of predominant HIV-1 subtypes among men who have sex with men in China. Int J Infect Dis 2020; 90:125–31. [DOI] [PubMed] [Google Scholar]
- 22. Li Z, Liao L, Feng Y, et al. Trends of HIV subtypes and phylogenetic dynamics among young men who have sex with men in China, 2009-2014. Sci Rep 2015; 5:16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wertheim JO, Leigh Brown AJ, Hepler NL, et al. The global transmission network of HIV-1. J Infect Dis 2014; 209:304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wertheim JO, Kosakovsky Pond SL, Forgione LA, et al. Social and genetic networks of HIV-1 transmission in New York City. PLoS Pathog 2017; 13:e1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wertheim JO, Murrell B, Mehta SR, et al. Growth of HIV-1 molecular transmission clusters in New York City. J Infect Dis 2018; 218:1943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han X, Zhao B, An M, et al. Molecular network-based intervention brings us closer to ending the HIV pandemic. Front Med 2020; 14:136–48. [DOI] [PubMed] [Google Scholar]
- 27. Chan PA, Hogan JW, Huang A, et al. Phylogenetic investigation of a statewide HIV-1 epidemic reveals ongoing and active transmission networks among men who have sex with men. J Acquir Immune Defic Syndr 2015; 70:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X, Zhu K, Xue Y, et al. Multiple introductions and onward transmission of HIV-1 subtype B strains in Shanghai, China. J Infect 2017; 75:160–8. [DOI] [PubMed] [Google Scholar]
- 29. Chang D, Sanders-Buell E, Bose M, et al. ; RV254/SEARCH 010 Study Group . Molecular epidemiology of a primarily MSM acute HIV-1 cohort in Bangkok, Thailand and connections within networks of transmission in Asia. J Int AIDS Soc 2018; 21:e25204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Guo Z, Yang J, et al. Genetic diversity of HIV-1 and transmitted drug resistance among newly diagnosed individuals with HIV infection in Hangzhou, China. J Med Virol 2015; 87:1668–76. [DOI] [PubMed] [Google Scholar]
- 31. Fan Q, Zhang Y, Hu L, et al. A novel recombinant enterovirus type EV-A89 with low epidemic strength in Xinjiang, China. Sci Rep 2015; 5:18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang J, Fan Q, Zhang J, et al. A geographic hotspot and emerging transmission cluster of the HIV-1 epidemic among older adults in a rural area of Eastern China. AIDS Res Hum Retroviruses 2020; 36:712–20. [DOI] [PubMed] [Google Scholar]
- 33. Tippmann H-F. Analysis for free: comparing programs for sequence analysis. Brief Bioinform 2004; 5:82–7. [DOI] [PubMed] [Google Scholar]
- 34. Daniel S, Lawyer G, Ternes A-M, et al. COMET: adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nuclc Acids Res 2014; 42:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Price MN, Dehal PS, Arkin AP, Poon AFY. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 2010; 5:e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu K, Linder CR, Warnow T. RAxML and FastTree: comparing two methods for large-scale maximum likelihood phylogeny estimation. PLoS One 2011; 6:e27731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahn MY, Wertheim JO, Kim WJ, et al. HIV-1 transmission networks across South Korea. AIDS Res Hum Retroviruses 2017; 33:827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang J, Yao J, Jiang J, et al. Migration interacts with the local transmission of HIV in developed trade areas: a molecular transmission network analysis in China. Infect Genet Evol 2020; 84:104376. [DOI] [PubMed] [Google Scholar]
- 39. Oster AM, Wertheim JO, Hernandez AL, et al. Using molecular HIV surveillance data to understand transmission between subpopulations in the United States. J Acquir Immune Defic Syndr 2015; 70:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 2012; 29:1969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, Xue Y, Lin Y, et al. Evolutionary dynamics and complicated genetic transmission network patterns of HIV-1 CRF01_AE among MSM in Shanghai, China. Sci Rep 2016; 6:34729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pérez-Losada M, Castel AD, Lewis B, et al. ; DC Cohort Executive Committee . Characterization of HIV diversity, phylodynamics and drug resistance in Washington, DC. PLoS One 2017; 12:e0185644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Z, Dai L, Jiang Y, et al. Transmission network characteristics based on env and gag sequences from MSM during acute HIV-1 infection in Beijing, China. Arch Virol 2017; 162:3329–38. [DOI] [PubMed] [Google Scholar]
- 44. Dalai SC, Junqueira DM, Wilkinson E, et al. Combining phylogenetic and network approaches to identify HIV-1 transmission links in San Mateo County, California. Front Microbiol 2018; 9:2799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Metzig C, Ratmann O, Bezemer D, Colijn C. Phylogenies from dynamic networks. PLoS Comput Biol 2019; 15:e1006761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang D, Wu J, Zhang Y, et al. Genetic characterization of HIV-1 epidemic in Anhui province, China. Virol J 2020; 17:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oster AM, France AM, Mermin J. Molecular epidemiology and the transformation of HIV prevention. JAMA 2018; 319:1657–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 49. Esbjornsson J, Albert J, Esbjornsson J, et al. HIV-1 transmission between MSM and heterosexuals, and increasing proportions of circulating recombinant forms in the Nordic countries. Virus Evol 2016; 2:vew010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dennis AM, Hué S, Hurt CB, et al. Phylogenetic insights into regional HIV transmission. AIDS 2012; 26:1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang D, Wang J, Yang T. Mapping the spatial-temporal distribution and migration patterns of men who have sex with men in mainland China: a web-based study. Int J Environ Res Public Health 2020; 17:1469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu J, Han X, Shang H, et al. New features of the HIV epidemic among men who have sex with men in China. Emerg Microbes Infect 2013; 2:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen M, Ma Y, Su Y, et al. HIV-1 genetic characteristics and transmitted drug resistance among men who have sex with men in Kunming, China. PLoS One 2014; 9:e87033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shang H, Zhang L. MSM and HIV-1 infection in China. Natl Sci Rev 2015; 2:388–91. [Google Scholar]
- 55. Zhang J, Guo Z, Pan X, et al. Highlighting the crucial role of Hangzhou in HIV-1 transmission among men who have sex with men in Zhejiang, China. Sci Rep 2017; 7:13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jovanović L, Šiljić M, Ćirković V, et al. Exploring evolutionary and transmission dynamics of HIV epidemic in Serbia: bridging socio-demographic with phylogenetic approach. Front Microbiol 2019; 10:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tanaka TSO, Leite TF, Freitas SZ, et al. HIV-1 molecular epidemiology, transmission clusters and transmitted drug resistance mutations in central Brazil. Front Microbiol 2019; 10:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]