Abstract
Objective:
To create a classification system based on stroke-related impairments.
Data source:
All adults with stroke admitted for at least 72 hours in England, Wales and Northern Ireland from July 2013 to July 2015 extracted from the Sentinel Stroke National Audit Programme
Analysis:
Impairments were defined using the National Institute of Health Stroke Scale scores at admission. Common combinations of impairments were identified based on geometric coding and expert knowledge. Validity of the classification was assessed using standard descriptive statistics to report and compare patients’ characteristics, therapy received and outcomes in each group.
Results:
Data from 94,905 patients were extracted. The items of the National Institute of Health Stroke Scale (on admission) were initially grouped into four body systems: Cognitive, Motor, Sensory and Consciousness. Seven common combinations of these impairments were identified (in order of stroke severity); Patients with Loss of Consciousness (n = 6034, 6.4%); those with Motor + Cognitive + Sensory impairments (n = 28,226, 29.7%); Motor + Cognitive impairments (n = 16,967, 17.9%); Motor + Sensory impairments (n = 9882, 10.4%); Motor Only impairments (n = 20,471, 21.6%); Any Non-Motor impairments (n = 7498, 7.9%); and No Impairments (n = 5827, 6.1%). There was a gradation of age, premorbid disability, mortality and disability on discharge. People with the most and least severe categories were least likely to receive therapy, and received least therapy (−20 minutes/day of stay) compared to −35 minutes/day of stay for the moderately severe categories.
Conclusions:
A classification system of seven Stroke Impairment Categories has been presented.
Keywords: Stroke, physiotherapy, occupational therapy, speech and language therapy, psychology, length of stay, disability, rehabilitation
Introduction
Stroke is the most common cause of adult disability in the developed world with two-thirds of survivors left with some degree of disability.1 Although patients’ social situation, life experience and personality play a part in their recovery, the nature of the resulting impairments play a key role. These impairments can lead to wide-ranging activity limitations for which individual patients require personalised rehabilitation.
In order to provide and evaluate effective care, stroke clinicians and researchers need accurate, clinically meaningful ways to define and classify patients’ problems. The most widely adopted stroke classification comes from the Oxford Community Stroke Project, which defined stroke types according to the location and size of the infarct or haemorrhage and is used to predict stroke severity and prognosis.2,3 Although valuable to guide medical care, pathology-based classifications have less to offer when it comes to rehabilitation, which focuses on the effects (i.e. impairments, activity limitations and participation restrictions) of the stroke rather than its cause or location. Thus, an impairment-based system may be more helpful in studying recovery and outcome.
There have been few studies proposing and validating impairment-based classifications. In 1991, Sanchez-Blanco et al classified stroke survivors as having ‘motor only’; ‘motor-sensory’ and ‘motor-sensory-hemianopic’ symptoms and found these patients formed distinct subgroups with respect to recovery of independence in activities of daily living and mobility.4 However, they did not include cognition or communication in their classification. The Southampton Stroke Audit5 was larger but used a (incomplete) mix of impairments and activity limitations to define mild; moderate; severe and very severe strokes. The aim of the current study was to build on these previous studies4,5 by using a large national dataset to develop a simple, pragmatic, clinically meaningful and feasible, impairment-based stroke classification, and to assess its validity by describing the emergent groups in terms of patient and stroke characteristics, the therapy provided and outcomes
Method
We used data from the Sentinel Stroke National Audit Programme (SSNAP). Full details can be found elsewhere6 but in summary, SSNAP is a national audit register of stroke care in England, Wales and Northern Ireland and involves over 95% of all stroke admissions. In this study, we extracted the available patient demographics, stroke characteristics, outcomes and details of the inpatient and community-based therapy for people admitted with stroke between July 2013 and July 2015 who were still inpatients at least 3 days after admission. We also excluded patients with an incomplete entries for the National Institute of Health Stroke Scale7,8 (the measure of stroke severity) on admission as they would contained insufficient information about the impairments the patient suffered.
Firstly, we used the score on the National Institute of Health Stroke Scale7,8 on admission to identify the impairments that each stroke patient suffered. The National Institute of Health Stroke Scale is a simple, valid and reliable measure of stroke severity based on the number and severity of stroke-related impairments.7,8 It contains 15 items (11 questions, but Q5 and Q6 have two parts each) to record: consciousness (responsiveness, orientation, ability to follow commands); cognition (language and neglect); vision (eye movement and visual-field loss); motor control (weakness of the limbs, ataxia, dysarthria); and tactile sensory loss. A trained observer rates the patient’s ability to answer questions and perform activities. They score whether the impairment is present and its severity on 3 or 5 point scales. These are summed to give a total score between zero (normal) to 42 (maximum severity), however we did not use the summed scores for the classification. We merely noted whether the scores for the items were normal (i.e. no impairment present) or not (i.e. the impairment was present), rather than the severity of the impairments.
Secondly, working in a multi-disciplinary group of clinical academics with expertise in stroke care (physiotherapy; stroke medicine; neurology; occupational therapy; speech and language therapy; psychology and nursing) we grouped the impairments into four body systems: consciousness, cognitive (including communication), motor, and sensory (including vision) impairments based on the relevant National Institute of Health Stroke Scale items (detailed in Table 1) and referred to here as the system impairments.
Table 1.
Details of the National Institute of Health Stroke Scale items and the definition of the four body system impairments; consciousness, cognition, motor and sensory impairments.
| National Institute of Health Stroke Scale item label | Score = 0 | Score = 1 | Score = 2 | Score = 3 | Score = 4 | |
|---|---|---|---|---|---|---|
| Body system impairment = consciousness (score > 1) | ||||||
| 1a | LOC responsiveness | Alert, responsive | Not alert but verbally arousable or aroused by minor stimulation to obey, answer, or respond | Only responsive to repeated/strong/painful stimuli | Totally unresponsive, except reflexes/Areflexic | |
| Body system impairment = cognition (any > 0) | ||||||
| 1b | LOC orientation | Correctly answers questions | Correctly answers one question | Nether answer correct | ||
| 1c | LOC commands | Correctly performs tasks | Correctly performs one task | Neither task performed | ||
| 9 | Language/communication | Normal, no speech deficit | Detectable fluency loss, though still able to extract information | Speech fragmented and unable to extract content | Unable to speak/understand speech | |
| 11 | Extinction and inattention | Normal, correctly answers questions | Inattention on one side/modality (visual, tactile, auditory, spatial) | Does not recognise stimuli in multiple modality on same side | ||
| Body system impairment = motor (any > 0) | ||||||
| 4 | Facial palsy | Normal, symmetrical movement | Function less normal, flattened nasolabial fold or minor smile asymmetry | Partial Paralysis, paralysis in lower face | Total paralysis upper and lower portion of one side | |
| 5a,5b | Motor arm | Normal | Patient can hold position against gravity but drifts immediately | Can hold arm up but drops within 10 seconds | Unable to lift but can move arm in some form | No movement |
| 6a,6b | Motor leg | Normal | Can hold position against gravity but drifts within 5 seconds | Can hold leg up but drops within 10 seconds | Unable to lift but can move leg in some form | No movement |
| 7 | Limb ataxia | Normal coordination; smooth, accurate movement | ataxia present In limb | Severe Ataxia (both limbs) | ||
| 10 | Speech/dysarthria | Normal, clear, smooth speech | some slurring speech | cannot be understood | ||
| Body system impairment = sensory (any > 0) | ||||||
| 2 | Best Gaze/ Horizontal Eye Movement | Normal, able to follow pen both sides | Partial gaze palsy, abnormal in one/both eyes | Total gaze paresis, gaze fixed to once side | ||
| 3 | Visual field test | No vision loss | Partial hemianopia or complete quadrantanopia | No visual stimulus in 1/2 of visual field | Bilateral blindness from any cause | |
| 8 | Sensory (pinprick) | Feels pinprick equally both sides | Patient feels pinprick, duller on one side | Total sensory loss on one side | Not aware of being touched in all unilateral extremities | |
Thirdly, to investigate how the system impairments clustered within each patient, geometric coding9 was used. The four system impairments were given a value based on a geometric progression 1,2,4 and 8 and unique combinations of system impairments are identified by adding the corresponding combinations e.g. 1+2 = 3,1+2+4 = 7,etc. This resulted in a list of the frequency of every possible combination of the four system impairments. These combinations were reviewed and refined by the expert group into clinically meaningful groups.
Finally, the emergent categories were named and validated using summary statistics to describe the demographics, stroke characteristics, therapy provided and outcomes in each category. This was to test the hypothesis that different categories involve patients with distinctly different characteristics and thus therapies and outcomes.
Given the potential for reporting bias and as therapy is seldom provided every day, the amount of therapy was defined as the ‘average therapy per day of sta’, (within an inpatient or community team), rather than the average duration of a treatment session (i.e. the average amount of therapy per day when treated).
Due to the large sample size, complex data structure and presence of confounding we have refrained from performing simple comparison tests (e.g. t-test) with factors as the large sample size is likely to produce a statistically significant result regardless of the size of effect, and the result may be subject to bias when confounding and complex data structures are not accounted for.
Results
Data were extracted for 94,905 stroke patients who met the inclusion criteria (Table 2). The cohort has been detailed previously10 but in summary, there were slightly more women than men with a mean age in the mid-seventies. Over three-quarters were independent before their strokes, −10% had a haemorrhage, 40% had a moderately severe stroke (admission National Institute of Health Stroke Scale 5-14) and 81% (n = 76,585) were fully alert (National Institute of Health Stroke Scale Level of Consciousness score = 0) on admission.
Table 2.
Demographics and stroke characteristics in each stroke impairment classification on admission.
| Stroke impairment classification (N, % unless stated) | Loss-Con | Mo-Co-Se | Mo-Co | Mo-Se | Mot-O | No-Mo | None | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Mean (sd) | 79.6 (13) | 77.4 (13) | 78.2 (12) | 71.3 (14) | 74.5 (13) | 75.2 (13) | 74.1 (14) | 76.0 (13.2) |
| Gender | Female | 3698 (7.5) | 15457 (31.4) | 9041 (18.4) | 4775 (9.7) | 9627 (19.6) | 3740 (7.6) | 2861 (5.8) | 49199 |
| Ethnicity | White | 5330 (6.3) | 25231 (29.8) | 15203 (17.9) | 8730 (10.3) | 18303 (21.6) | 6792 (8.0) | 5227 (6.2) | 84816 |
| Stroke severity (NIHSS) median (IQR) | 19 (7,25) | 14 (9,19) | 6 (4,10) | 5 (3,7) | 3 (2,4) | 2 (1,4) | 0 (0,0) | 6 (10) | |
| Intracerebral haemorrhage yes | 1234 (12.2) | 3135 (30.9) | 1557 (15.3) | 922 (9.1) | 1443 (14.2) | 1017 (10.0) | 850 (8.4) | 10158 | |
| Premorbid disability (mRs ⩽ 2) | 3768 (5.0) | 21422 (28.5) | 12411 (16.5) | 8585 (11.4) | 17479 (23.3) | 6528 (8.7) | 4908 (6.5) | 75101 | |
| No. of co-morbidities | 0 | 1304 (5.8) | 6325 (27.9) | 3772 (16.6) | 2629 (11.6) | 5175 (22.8) | 1795 (7.9) | 1673 (7.4) | 22673 |
| 1 to 2 | 3783 (6.3) | 17902 (29.9) | 10813 (18.1) | 6102 (10.2) | 12968 (21.7) | 4764 (8.0) | 3500 (5.8) | 59832 | |
| 3 to 5 | 947 (7.6) | 3999 (32.3) | 2382 (19.2) | 1151 (9.3) | 2328 (18.8) | 939 (7.6) | 654 (5.3) | 12400 | |
| Total | 6034 (6.4) | 28226 (29.7) | 16967 (17.9) | 9882 (10.4) | 20471 (21.6) | 7498 (7.9) | 5827 (6.1) | 94905 | |
N: number; NIHSS: National Institute of Health Stroke Scale; IQR: interquartile range; mRS: modified ranking scale; Loss-Con: Loss of consciousness stroke; Mo-Co-Se: motor-cognitive-sensory stroke; Mo-Co: motor-cognitive stroke; Mo-Se: motor–sensory stroke; Mot-O: motor only stroke; No-Mo: non-motor stroke; None: no impairments stroke.
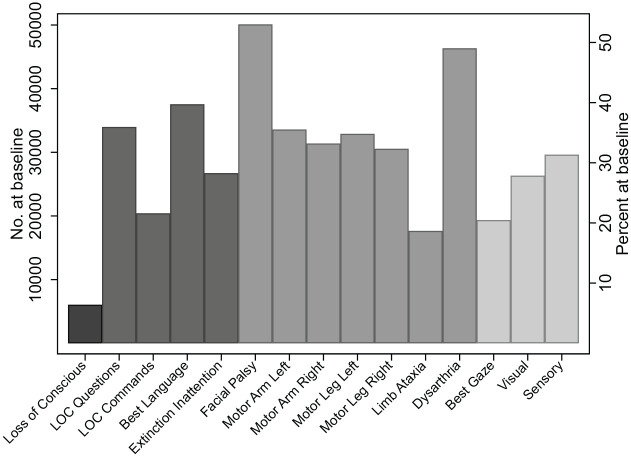
Row percentages are given to reflect the impact of patient characteristics on case-mix. The frequency of each individual impairment is shown in Figure 1. The most common system impairment was motor, >80% of patients had some form of motor impairment (facial palsy (50%), dysarthria (47%), limb weakness (30%) and ataxia (17%)) with similar frequency of upper and lower limb weakness and between right and left limbs. Language was impaired in −50% of patients, while approximately a quarter of patients presented with other cognitive impairments resulting in −58% with cognitive impairments. Sensory impairments (including vision) affected 43% and the least common impairment were loss of consciousness (<5%).
Figure 1.
The number and percentage of stroke patients with each individual National Institute of Health Stroke Scale defined impairments broken down by system impairments. The greyscale represents the different system impairments (left to right; Loss of Consciousness, Cognitive Impairments, Motor Impairments and Sensory Impairments) measured at admission in patients who were hospitalised after 3 days.
Aggregation of the four system impairments (Consciousness, Cognitive, Motor and Sensory Impairments) involved 15 out of the 21 possible combinations of which five involved <1%, and seven involved <2% of the sample. Based on Sanchez-Blanco et al.,4 the Stroke Impairment Categories were initially defined as ‘loss of consciousness’; ‘motor impairments only’; ‘motor impairments plus any other’; ‘any non-motor impairments’ and ‘no impairments’. This resulted in a very large group with ‘motor plus any other impairments’ (n = 55,075, 58%) which included a broad group of impairments. This was split into three categories; ‘motor plus cognitive plus sensory impairments’, ‘motor plus cognitive impairments’ and ‘motor plus sensory impairments’. Thus seven clinically meaningful impairment categories were created:
Loss of consciousness +/- other system impairments, referred to as a ‘loss of consciousness’ stroke (n = 6034, 6.4%)
Motor + cognitive + sensory impairments referred to as a ‘motor-cognitive-sensory’ stroke (n = 28,226, 29.7%)
Motor + cognitive impairments referred to as a ‘motor-cognitive’ stroke (n = 16,967, 17.9%)
Motor + sensory impairments referred to as a ‘motor-sensory’ stroke (n = 9882, 10.4%)
Motor impairments only referred to as a ‘motor only’ stroke (n = 20,471, 21.6%)
Any system impairments without motor impairment or loss of consciousness referred to as a ‘non-motor’ stroke (n = 7498, 7.9%)
No system impairments referred to as a ‘no impairment’ stroke (n = 5827, 6.1%)
The frequency of the individual system impairments in aggregated categories (loss of consciousness; non-motor impairments strokes) are detailed in Table 3. The most commonly observed Stroke Impairment Category was a Motor-Cognitive-Sensory stroke (29.7%), and the least common was a Loss of Consciousness stroke (6.4%).
Table 3.
Combinations of system impairments and the stroke impairment categories showing the frequency of each impairment that contributes to the category (system impairments presented in descending frequency order).
| Stroke impairment categories | Combinations of system impairments | Frequency |
|---|---|---|
| Any loss of consciousness stroke (plus any other impairment) n = 6034 | Loss-Con + motor+ cognitive+ sensory (including vision) | 3380 |
| Loss-Con alone | 1304 | |
| Loss-Con + motor+ cognitive | 983 | |
| Loss-Con + cognitive | 154 | |
| Loss-Con + motor | 110 | |
| Loss-Con + cognitive+ sensory (including vision) | 60 | |
| Loss-Con + motor+ sensory (including vision) | 35 | |
| Loss-Con + sensory (including vision) | 5 | |
| Loss-Con + cognitive+ sensory (including vision) | 3 | |
| Non-Motor Impairments stroke n = 7498 | Cognitive alone | 3606 |
| Sensory (including vision) alone | 2418 | |
| Cognitive + sensory (including vision) | 1474 |
N: number; Loss-Con = loss of consciousness stroke.
Details of the patients’ demographics and stroke characteristics in each Stroke Impairment Category are in Table 2. There was very little difference in ethnic origins or socio-economic status in patients in the different categories. Stroke severity decreased from a Loss-Consciousness stroke (which includes other impairments) to those with a ‘No Impairments’ stroke. Patients who were older, suffered an intracerebral haemorrhage, were dependent before their stroke and had pre-morbid co-morbidities also presented with more impairments post stroke.
People in categories with the greatest and fewest number of impairments (Loss of Consciousness and No Impairment strokes respectively) were least likely to be screened for therapy and to be considered to require each therapy by the treating therapists (Table 4). The numbers considered to require psychology was very low regardless of whether the patient had cognitive impairments or not. Most patients were screened for, and considered to require Physiotherapy and Occupational Therapy. The proportion of patients considered to require Physiotherapy tended to reduce slightly with fewer impairments but for Occupational Therapy it increased. There were marked differences between categories in the need for speech and language therapy. For people with Loss of Consciousness, Motor-Cognitive-Sensory or Motor-Cognitive strokes, this was 60%-70%, compared with 35%-48% for the other categories with fewer impairments which did not include people with communication problems. Unsurprisingly, those with greater number of impairments (except Loss of Consciousness strokes) more frequently required input from multiple therapies.
Table 4.
Therapy assessment with 72 hours of admission in each stroke impairment classification.
| Stroke impairment classification | Loss-Con | Mo-Co-Se | Mo-Co | Mo-Se | Mot-O | No-Mo | None | Total (n %) |
|---|---|---|---|---|---|---|---|---|
| Assessment within 72 hrs (n, column %) | ||||||||
| Swallow assessed by a nurse or SLT | 4415 (73.2) | 23206 (82.2) | 13140 (77.4) | 5885 (59.5) | 12404 (60.6) | 4544 (60.6) | 3889 (66.8) | 67483 (71.1) |
| Communication assessment by SLT | 2186 (36.2) | 15107 (53.5) | 9156 (54.0) | 3036 (30.7) | 6421 (31.4) | 2733 (36.5) | 1438 (24.7) | 40077 (42.2) |
| Physiotherapy assessment | 4418 (73.2) | 25604 (90.7) | 15144 (89.3) | 8957 (90.6) | 18357 (89.7) | 6223 (83.0) | 4501 (77.2) | 83204 (87.7) |
| OT assessment | 3319 (55.0) | 22314 (79.1) | 13451 (79.3) | 8033 (81.3) | 16478 (80.5) | 5808 (77.5) | 3958 (67.9) | 73361 (77.3) |
| The proportion of patients considered to require each therapy on admission (n, column %) | ||||||||
| Physiotherapy | 4937 (81.8) | 26654 (94.4) | 15825 (93.3) | 9318 (94.3) | 19212 (93.9) | 6579 (87.7) | 5036 (86.4) | 87561 (92.3) |
| Occupational therapy | 4098 (67.9) | 25115 (89.0) | 15217 (89.7) | 9017 (91.3) | 18598 (90.9) | 6689 (89.2) | 4841 (83.1) | 83575 (88.1) |
| SLT | 3676 (60.9) | 20352 (72.1) | 12041 (71.0) | 3902 (39.5) | 8427 (41.2) | 3603 (48.1) | 2067 (35.5) | 54068 (57.0) |
| Psychology | 179 (3.0) | 1550 (5.5) | 754 (4.4) | 516 (5.2) | 920 (4.5) | 341 (4.6) | 206 (3.5) | 4466 (4.7) |
| Number of therapies required (n, column %) | ||||||||
| 0 | 926 (15.4) | 1045 (3.7) | 549 (3.2) | 337 (3.4) | 704 (3.4) | 391 (5.2) | 518 (8.9) | 4470 (4.7) |
| 1 | 615 (10.2) | 1071 (3.8) | 707 (4.2) | 515 (5.2) | 1133 (5.5) | 522 (7.0) | 520 (8.9) | 5083 (5.4) |
| 2 | 1360 (22.5) | 7032 (24.9) | 4621 (27.2) | 5130 (51.9) | 10375 (50.7) | 3251 (43.4) | 2856 (49.0) | 34625 (36.5) |
| 3 | 2977 (49.3) | 17776 (63.0) | 10472 (61.7) | 3622 (36.7) | 7762 (37.9) | 3148 (42.0) | 1814 (31.1) | 47571 (50.1) |
| 4 | 156 (2.6) | 1302 (4.6) | 618 (3.6) | 278 (2.8) | 497 (2.4) | 186 (2.5) | 119 (2.0) | 3156 (3.3) |
| Total | 6034 | 28226 | 16967 | 9882 | 20471 | 7498 | 5827 | 94905 |
OT: occupational therapy; SLT: speech and language therapy; N: number; Loss-Con: loss of consciousness stroke; Mo-Co-Se: motor-cognitive-sensory stroke; Mo-Co: motor-cognitive stroke; Mo-Se: motor–sensory stroke; Mot-O: motor only stroke; No-Mo: non-motor stroke; None: no impairments stroke.
The amount of therapy patients received also varied in the different categories (Table 5). Overall, patients with a Loss of Consciousness stroke and No Impairments received the least inpatient and community-based therapy (around 20 minutes per day of stay), while patients in the other, moderately severe categories received ~30–35 minutes/day of stay as an inpatient and 19–23 minutes/day of stay when community-based. Furthermore, relatively few patients with Loss of Consciousness or No Impairment strokes were referred for community-based therapy. For individual therapies, the pattern of provision varied. Physiotherapy followed the overall pattern described above but for inpatient Occupational Therapy the amount of each therapy increased as the number of impairments decreased (i.e. less complex patients received more therapy). During community-based Occupational Therapy and Psychology, all categories received a similar average amount of therapy/day of stay regardless of whether they had cognitive impairments. Patients with Motor-Cognitive and Non-Motor strokes received the most speech and language therapy during both inpatient and community-based therapy, likely reflecting the inclusion of people with aphasia in these categories.
Table 5.
Therapy provision for each stroke impairment classification.
| Stroke impairments classifications (median (IQR)) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Loss-Con | Mo-Co-Se | Mo-Cog | Mo-Se | Mot-O | No-Mo | None | Total | |
| Number of days on which patients received inpatient stroke therapy | ||||||||
| Physiotherapy | 6 (2,14) | 6 (3,15) | 5 (2,10) | 4 (2,10) | 4 (2,9) | 3 (1,6) | 3 (1,8) | 5 (2,11) |
| Occupational therapy | 4 (2,10) | 5 (2,11) | 4 (2,8) | 3 (2,7) | 3 (2,7) | 3 (2,6) | 3 (1,6) | 4 (2,8) |
| Speech and language therapy | 4 (2,9) | 4 (2,8) | 3 (1,6) | 2 (1,5) | 2 (1,4) | 2 (1,4) | 3 (1,6) | 3 (1,7) |
| Psychology | 1 (1,3) | 1 (1,3) | 1 (1,2) | 1 (1,2) | 1 (1,2) | 1 (1,2) | 1 (0,2) | 1 (1,2) |
| Number of days on which patients received community-based stroke therapy | ||||||||
| Physiotherapy | 7 (3,16) | 8 (3,17) | 6 (2,13) | 7 (3,14) | 7 (3,14) | 4 (1,9) | 5.5 (3,11) | 7 (3,14) |
| Occupational therapy | 6 (3,13) | 6 (3,12) | 5 (2,10) | 5 (2.5,10) | 5 (2,9) | 4 (2,9) | 4 (2,8) | 5 (2,10) |
| Speech and language therapy | 4 (2,11) | 5 (2,12) | 5 (2,11) | 2 (1,5) | 2 (1,6) | 5 (2,12) | 3 (1,6) | 4 (1,9) |
| Psychology | 2 (1,3) | 2 (1,3) | 2 (1,3) | 2 (1,4) | 2 (1,3) | 2 (1,4) | 2 (1,3) | 2 (1,3) |
| Amount (minutes) of therapy/inpatient day (mean, (sd)) | ||||||||
| Physiotherapy | 13.0 (10) | 15.7 (10) | 14.1 (10) | 17.0 (10) | 6.5 (11) | 11.7 (9) | 12.7 (9) | 15.1 (10) |
| Occupational therapy | 9.3 (8) | 13.9 (10) | 14.3 (11) | 16.8 (11) | 16.4 (11) | 16.2 (11) | 13.6 (10) | 14.8 (11) |
| Speech and language therapy | 6.6 (6) | 8.7 (7) | 9.8 (8) | 7.6 (7) | 8.1 (7) | 10.4 (9) | 7.2 (7) | 8.7 (8) |
| Psychology | 1.4 (2) | 2.4 (3) | 2.8 (3) | 4.0 (5) | 4.4 (5) | 4.0 (5) | 2.9 (4) | 3.2 (4) |
| Amount (minutes) of therapy/day in community-based ‘stay’ (mean (sd)) | ||||||||
| Physiotherapy | 11.7 (12) | 12.2 (12) | 10.7 (12) | 12.7 (12) | 12.7 (13) | 9.6 (11) | 11.3 (12) | 12.0 (12) |
| Occupational therapy | 10.1 (12) | 11.1 (14) | 10.2 (12) | 10.4 (12) | 10.6 (14) | 11.3 (13) | 10.9 (17) | 10.7 (13) |
| Speech and language therapy | 5.6 (6) | 8.2 (12) | 8.4 (10) | 4.4 (6) | 5.6 (8) | 10.6 (11) | 6.7 (11) | 7.6 (10) |
| Psychology | 4.0 (8) | 1.9 (3) | 2.2 (4) | 2.3 (3) | 2.2 (4) | 2.7 (3) | 2.2 (2) | 2.2 (4) |
Loss-Con: loss of consciousness stroke; Mo-Co-Se: motor-cognitive-sensory stroke; Mo-Co: motor-cognitive stroke; Mo-Se: motor–sensory stroke; Mot-O: motor only stroke; No-Mo: non-motor stroke; None: no impairments stroke; IQR: inter-quartile range.
Outcomes for patients in the different categories differed and are reported in Table 6. The median length of inpatient stay was greater for those with a greater number of impairments (37 days for Loss of Consciousness strokes and 25 days for Motor-Cognitive-Sensory strokes). Similarly, inpatient mortality was −5% for Non-Motor and Motor-Only strokes but 49% for Loss of Consciousness strokes. Overall 14% of all patients were discharged to residential care. Unsurprisingly this proportion was highest for those with a greater number of impairments; a similar proportion of surviving patients with a Loss of Consciousness stroke were discharged home or to a care home. This proportion shifted in favour of patients being discharged home as fewer impairments were present. This pattern was echoed by the proportion of patients who were dependent on discharge: Although 90% of people with a Loss of Consciousness stroke and 72% of people with a Motor-Cognitive-Sensory stroke were dependent on discharge, −40% of people with the fewer impairments (Motor-Only, Non-Motor and No Impairment strokes) were similarly disabled.
Table 6.
Outcomes for each stroke impairment classification.
| Factors | Stroke impairment classification | |||||||
|---|---|---|---|---|---|---|---|---|
| Loss-Con | Mo-Co-Se | Mo-Cog | Mo-Se | Mot-O | No-Mo | None | Total | |
| Length of stay (days) for patients surviving until hospital discharge. Median (IQR) | 37.1 (16,66) | 25.1 (9,54) | 14.1 (6,34) | 9.8 (5,24) | 9.6 (5,22) | 8.2 (4.7,19) | 8.5 (5,21) | 13.6 (6,34) |
| Number who died >3 days after admission (% of all patients) | 2933 (48.6) | 5946 (21.1) | 2064 (12.2) | 531 (5.4) | 1038 (5.1) | 377 (5.0) | 615 (10.6) | 13504 (14.2) |
| Discharged to a care home (n, %) | 1212 (20.1) | 5548 (19.7) | 3056 (18.0) | 726 (7.4) | 1617 (7.9) | 668 (8.9) | 634 (10.9) | 13461 (14.2) |
| Discharged home (n, %) | 1201 (19.9) | 12096 (42.9) | 9252 (54.5) | 7095 (71.8) | 14736 (72.0) | 5484 (73.1) | 3586 (66.2) | 53720 (56.6) |
| Independent at discharge from hospital (mRS ⩽ 2) (n, %) | 661 (11.0) | 7901 (28.0) | 6723 (39.6) | 5726 (58.0) | 12040 (58.8) | 4740 (63.2) | 3456 (59.3) | 41247 (43.5) |
| Total | 6034 | 28226 | 16967 | 9882 | 20471 | 7498 | 5827 | 94905 |
Loss-Con: loss of consciousness stroke; Mo-Co-Se: motor-cognitive-sensory stroke; Mo-Co: motor-cognitive stroke; Mo-Se: motor–sensory stroke; Mot-O: motor only stroke; No-Mo: non-motor stroke; None: no impairments stroke; mRS: modified rankin scale; IQR: inter-quartile range.
NB Column percentages are given to reflect the impact of case-mix on outcome.
Discussion
In this paper we have proposed a new classification based on patients’ stroke-related impairments, identifying seven categories. We have established face, content, construct and ecological validity through the guidance of an expert consultation group and basing the classification on a measurement tool routinely used in all stroke services in the United Kingdom (The National Institute of Health Stroke Scale).7,8 We further tested validity by hypothesising that the Stroke Impairment Categories would identified distinct groups of stroke survivors in terms of demographics and stroke characteristics, therapy and outcomes. The data supports this hypothesis with a gradation of age, co-morbidities, premorbid disability, stroke severity and outcomes from patients in the more severe categories (with greater numbers of impairments) to those with fewer, or no impairments. Further evidence of the validity of the Stroke Impairment Categories is reported in other publications from this project which demonstrates that the Categories are important independent factors associated with the amount and type of therapy patients receive and outcome (in terms of disability on discharge; length of stay; mortality and institutionalisation).10,11
Differences in the need for, and dose of therapy are in line with clinical expectations for patients in the different categories. Nearly all patients were considered to require physiotherapy and the amount of physiotherapy provided showed a bell-shaped curve, with patients in moderately impaired categories receiving most therapy, and the people in the most and least impaired categories receiving less therapy, presumably because they either could not tolerate it or did not need it. The numbers requiring, and the amount of occupational therapy provided increased as category severity decreased, as patients were able to tolerate more therapy and had greater potential to return to activity. The proportion of patients requiring speech and language therapy was higher in the categories which included cognitive (which include communication) impairments, however the amount of therapy received was similar in all categories. This may reflect speech and language therapists’ input to people with motor impairments (dysphagia and dysarthria), as well as those with aphasia. Psychology input showed a different pattern. One might expect psychologists to provide most input to the categories involving cognitive impairments, however, this was not the case. Less than −5% of patients were considered to need psychology input in any of the categories, and when psychology was provided, it typically consisted of a single assessment session without on-going treatment. This clearly sub-optimal given that −30% of stroke survivors suffer cognitive problems post-stroke12 and patient feedback has highlighted the impact of unmet needs for help with cognitive difficulties on ‘life after stroke’.13 Elsewhere we have reported how staffing levels for psychologists were extremely low and few stroke services had access to any psychology services.14 This is an obvious explanation for the low level of recognised need and psychology input. Another possibility is that other members of the multidisciplinary team, such as occupational therapists are providing this input. However, if this were the case, one might expect patients in ‘cognitive categories’ to more frequently require occupational therapy.
All these findings contribute to the validation of the Stroke Impairment Categories. The reliability of the classification is dependent on the scoring of the National Institute of Health Stroke Scale which is reported to be good.8
There is a growing recognition that stroke rehabilitation research needs to stratify stroke patients by recognising their highly varied difficulties, needs and recovery patterns. As well as ensuring that participants are recruited to treatments which more accurately reflect their needs, using a stratification tool has the additional benefit of reducing the sample sizes needed to achieve power, thus improving research efficiency and reducing costs.15 The Stroke Impairment Categories offer a simple potential way to achieve this: further research is needed to develop this further.
The Stroke Impairment Categories also have potential for clinical application. Stroke teams are often required to estimate length of stay (or estimated date of discharge) early after stroke patients’ admission. This is challenging and even experienced clinicians find it difficult to accurately predict outcomes after stroke.16,17 The Stroke Impairment Categories and data presented here could be used as benchmark to inform more specific goal setting, predictions of length of stay and recovery and to benchmark therapy input for patients in the different categories. Predicting outcome/ recovery in practice is moot however, with many clinicians fearing that implementing such processes which could lead to patients being ‘pigeon holed’, denied access to rehabilitation and stifling hope of recovery. There is some evidence to allay these fears. To date, one study examining the implementation of an accurate, valid algorithm to predict upper limb recovery have been published.18,19 This showed that using the algorithm increased therapists’ confidence to predict recovery and focused the content of upper limb therapy more appropriately with greater use of active treatments for the groups with good or excellent predicted recovery. These changes were associated with a reduction in inpatient length of stay of 1 week which, reassuringly did not come at the expense of clinical outcomes. Thus using an algorithm to predict recovery potential for individual patients can increase rehabilitation efficiency after stroke without compromising clinical outcome. The study did not investigate patients’ or families’ experiences and satisfaction, however it is well-established that stroke survivors and their families want consistent information about their progress and predictions of recovery and that these needs are frequently unmet.20–22 Using predictive algorithms have the potential to meet these needs as well as to define and develop more personalised care for different groups of patients.15,23 Further research is clearly needed to develop and evaluate predictive algorithms for other aspects of stroke recovery and (if effective) to understand how to best implement them into practice.
Limitations
This work to develop the Stroke Impairment Categories was heavily dependent on assessment using the National Institute of Health Stroke Scale on admission,7,8 which was developed to measure overall stroke severity, and has limitations as an assessment of stroke-related impairments. Firstly it does not include important stroke-related impairments such as memory, continence or swallowing. It also measures each impairment crudely (on a short Likert scale) and we only evaluated whether impairments were present or absent at admission. Consequently important impairments that could limit everyday activities and participation may go undetected. Finally, the National Institute of Health Stroke Scale does not give any weighting to the impact of the impairments on activity or patients’ lives. Thus it may over- or under-estimate for some patients. However, the National Institute of Health Stroke Scale has good psychometric properties7,8 and is used in practice throughout the UK as well as many other countries. Thus a classification based on this scale has content, face and ecological validity.
Our use of routinely collected observational data also comes with limitations. Although SSNAP has stringent quality control processes, it is dependent on the accuracy of the original data entered and may therefore be open to observer and reporter bias, although this is unlikely to be systemic with such a large sample size. A tendency to over-estimate the duration of therapy treatment sessions has been noted in previous studies24,25 and so the accuracy of estimates of the amount of therapy should be treated with some caution.
Clinical message.
Seven distinct Stroke Impairment Categories have been identified.
The Stroke Impairment Categories were validated by observation of differences in terms of characteristics, therapy provision and outcome between the categories.
Acknowledgments
The authors acknowledge and thank Prof Martin James, Ms Alex Hoffman and the Intercollegiate Stroke Working Party of the Sentinel Stroke National Audit Programme for their invaluable discussion and insights regarding interpretation of the results.
Footnotes
Data sharing statement: This project involved secondary analysis of anonymised routinely collected clinical data. The data were made available under a data sharing agreement between the University of Manchester, Sentinel Stroke National Audit Programme and the Healthcare Quality Improvement Partnership (HQIP). A condition of that agreement was that all the data would be destroyed at the end of the project. Therefore we are unable to make it available to others. Further information can be obtained from the corresponding author.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Professors Tyson, Vail, Bowen and Dr Bray declare research grant funding from NIHR. Prof Bowen was until 2016 a member of the Intercollegiate Stroke Working Party that produces SSNAP from which the SSNAPIEST data were drawn and her University salary is part-funded by a personal award from Stroke Association. Professor Tyson is currently a member of the Intercollegiate Stroke Working Party
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institute for Health Research (NIHR) under its Health Service and Development Research Programme (Grant Reference 14/198/09). The views expressed are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health and Social Care.
ORCID iD: Sarah Tyson  https://orcid.org/0000-0001-6301-8791
https://orcid.org/0000-0001-6301-8791
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bamford J, Sandercock P, Dennis M, et al. Classification and natural-history of clinically identifiable subtypes of cerebral infarction. Lancet 1991; 337: 1521–1526. [DOI] [PubMed] [Google Scholar]
- 3. Bamford JM. The role of the clinical examination in the subclassification of stroke. Cerebrovasc Dis 2000; 10: 2–4. [DOI] [PubMed] [Google Scholar]
- 4. Sanchez-Blanco I, Ochoa-Sangrador C, Lopez-Munain L, et al. Predictive model of functional independence in stroke patients admitted to a rehabilitation programme. Clin Rehabil 1999; 13: 464–475. [DOI] [PubMed] [Google Scholar]
- 5. Tyson ST, Turner GF. Southampton stroke audit: assessing service quality. Br J Occup Ther 1999; 6: 227–232. [Google Scholar]
- 6. Physicians RCo. Sentinel stroke national audit programme (SSNAP) 2015, https://www.rcplondon.ac.uk/projects/outputs/sentinel-stroke-national-audit-programme-ssnap (accessed 1 Nov 2019).
- 7. NIH. The national institutes of health stroke scale (nihss), https://www.stroke.nih.gov/documents/NIH_Stroke_Scale_508C.pdf (accessed 1 Nov 2019). [DOI] [PubMed]
- 8. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction-a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 9. Tyson SF, Connell LA, Lennon S, et al. What treatment packages do UK physiotherapists use to treat postural control and mobility problems after stroke? Disabil Rehabil 2009; 31: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 10. Gittins M, Vail A, Lugo-Palacios, et al. Factors influencing the amount of therapy received during inpatient stroke care. An analysis of data from the UK sentinel stroke national audit programme. Clin Rehabil. Epub ahead of print June 2020. DOI: 10.1177/0269215520927454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gittins M, Lugo-Palacios D, Vail A, et al. Delivery, dose, outcomes and resource use of stroke therapy: the SSNAPIEST observational study. HS & DR 2020; 8: 17. [PubMed] [Google Scholar]
- 12. Rist PM, Chalmers J, Arima H, et al. Baseline cognitive function, recurrent stroke, and risk of dementia in patients with stroke. Stroke 2013; 44: 1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollock A, St George B, Fenton M, et al. Top 10 research priorities relating to life after stroke–consensus from stroke survivors, caregivers, and health professionals. Int J Stroke 2014; 9: 313–320. [DOI] [PubMed] [Google Scholar]
- 14. Gittins M, Lugo-Palacios DG, Paley L, et al. How do patients pass through stroke services? Identifying stroke care pathways using national audit data Clin Rehabil 2020; 34(5): 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 16. Nijland RH, van Wegen EE, Harmeling-van der Wel BC, et al. Early prediction of functional outcome after stroke investigators. Accuracy of physical therapists’ early predictions of upper-limb function in hospital stroke units: the EPOS study. Phys Ther 2013; 93: 460–469. [DOI] [PubMed] [Google Scholar]
- 17. Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol 2010; 9: 1228–1232. [DOI] [PubMed] [Google Scholar]
- 18. Stinear CM, Byblow WD, Ackerley SJ, et al. PREP2: a biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol 2017; 4(11): 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stinear CM, Byblow WD, Ackerley SJ, et al. Predicting recovery potential for individual stroke patients increases rehabilitation efficiency. Stroke 2017; 48(4): 1011–1019. [DOI] [PubMed] [Google Scholar]
- 20. Wiles R, Pain H, Buckland S, et al. Providing appropriate information to patients and carers. J Adv Nurs 1998; 28(4): 794–801. [DOI] [PubMed] [Google Scholar]
- 21. Tyson SF, Burton LJ, McGovern A, et al. Service users’ views of the assessment process in stroke rehabilitation. Clin Rehabil 2014; 28(8): 824–831. [DOI] [PubMed] [Google Scholar]
- 22. McKevitt C, Fudge N, Redfern J, et al. UK stroke survivor needs survey. London: The Stroke Association, 2010. [Google Scholar]
- 23. Rudd AG, Hoffman A, Paley L, et al. 20 Years of researching stroke through audit. Clin Rehabil 2018; 32: 997–1006. [DOI] [PubMed] [Google Scholar]
- 24. Clarke DJ, Burton LJ, Tyson SF, et al. Why do stroke survivors not receive recommended amounts of active therapy? Findings from the ReAcT study, A mixed-methods case-study evaluation in eight stroke units. Clin Rehabil 2018; 32: 1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaur G, English C, Hillier S. Physiotherapists systematically overestimate the amount of time stroke survivors spend engaged in active therapy rehabilitation: an observational study. J Physiother 2013; 59: 45–51. [DOI] [PubMed] [Google Scholar]