Porcine astroviruses are mainly associated with gastroenteritis and neurological diseases in pigs, and five genotypes have been identified (PAstV1-5). However, the clinical manifestations of genotypes other than PAstV1 have not yet been determined because of the failure of in vitro virus isolation. Here, we report a surprising isolation of a PAstV5 strain from a clinical classical swine fever virus (CSFV)-infected tissue sample, which can stably passage in PK-15 cells, and coinfection with CSFV significantly enhanced the replication of PAstV5, possibly through suppression of beta interferon production. Thus, the first isolated PAstV5 strain will be useful for investigating the biological and pathogenic properties of this virus, and the findings obtained in this study provide new insights into defining the interaction mechanism between CSFV and PAstV5.
KEYWORDS: Porcine astrovirus 5, isolation, characterization, classical swine fever virus
ABSTRACT
Many new astroviruses have been identified in humans and other animals in recent years, but only a few have been successfully isolated for extensive biological study. Here, we report an unusual isolation of a porcine astrovirus 5 (PAstV5) strain from a clinical classical swine fever virus (CSFV)-infected tissue sample. Incubation of porcine PK-15 cells with an extract of the CSFV-positive tissue resulted in unexpected cytopathic effects (CPEs), and high-throughput viromic sequencing identified PAstV5 and porcine circovirus type 2 (PCV2) as well as CSFV in the culture. After clearance of CSFV and PCV2, a pure PAstV5 strain, named PAstV5-AH29-2014, was obtained. Analysis revealed virus of typical astroviral morphology with a genome of 6,448 nucleotides, sharing 84.3 to 88.9% nucleotide identity with previously published PAstV5 strains. A mechanistic study showed that CSFV coinfection was likely an important factor for successful isolation by significantly enhancing PAstV5 replication in PK-15 cells via suppression of a type I interferon response. Altogether, PAstV5-AH29-2014, as the first isolated PAstV5 strain, will provide critical material for the investigation of the biological and pathogenic properties of this virus as well as for future development of relevant biological and diagnostic reagents.
IMPORTANCE Porcine astroviruses are mainly associated with gastroenteritis and neurological diseases in pigs, and five genotypes have been identified (PAstV1-5). However, the clinical manifestations of genotypes other than PAstV1 have not yet been determined because of the failure of in vitro virus isolation. Here, we report a surprising isolation of a PAstV5 strain from a clinical classical swine fever virus (CSFV)-infected tissue sample, which can stably passage in PK-15 cells, and coinfection with CSFV significantly enhanced the replication of PAstV5, possibly through suppression of beta interferon production. Thus, the first isolated PAstV5 strain will be useful for investigating the biological and pathogenic properties of this virus, and the findings obtained in this study provide new insights into defining the interaction mechanism between CSFV and PAstV5.
INTRODUCTION
Astroviruses constitute a large group of viruses isolated from a wide variety of mammals and birds, constituting the Mamastrovirus and Avastrovirus genera within the family Astroviridae and currently containing 33 Mamastrovirus and 7 Avastrovirus species (1). The astrovirus genome is a positive-sense single-stranded RNA of 6.17 to 7.78 kb, consisting of 5′ untranslated regions (5′UTR), three overlapping open reading frames (ORF1a, ORF1b, and ORF2), and a 3′UTR (2–5). ORF1a- and ORF1b-encoded nonstructural proteins are further processed to a serine protease and an RNA-dependent RNA polymerase (RdRp), respectively. ORF2 encodes structural capsid protein (6, 7), with its N-terminal half being conserved and C-terminal half highly variable (8).
Astroviruses are mainly associated with gastroenteritis in children and young animals (9–15). Besides enteric diseases, astrovirus infections associated with fatal hepatitis in ducks (3), interstitial nephritis in young chickens, and neurological diseases in pigs have also been reported (16–18). Porcine astrovirus (PAstV) was first identified from the feces of piglets with diarrhea in the 1980s (19) and have been found distributed worldwide, with detections in both asymptomatic pigs and pigs suffering from enteric diseases (14, 20–22).
Currently, five PAstV genotypes (PAstV1-5) have been identified (23, 24), but their pathogenicity is not well understood because of the difficulty in in vitro isolation for experimental infections. To date, PAstV1 is the only PAstV that has been isolated in cell culture (9, 25–27). Experimental infections with isolated strains of this virus have confirmed its pathogenicity, presenting as mild diarrhea in piglets with damage to the villi of the small intestinal mucosa and eventual growth retardation (9, 25). Increasing numbers of reports, however, show frequent detection of PAstVs in both healthy and ill pigs with significantly positive rates. Xiao et al. detected virus in 218 clinical samples of both apparently healthy and diarrheic pigs collected in 2014 from 32 pig farms in Hunan province, China. Results showed that positive rates of PAstV1, -2, -4, and -5 in diarrheic and healthy pig samples (diarrheic/healthy) were 12.2%/15.8%, 8.2%/11.7%, 18.4%/14.2%, and 31.6%/20.0%, respectively, while PAstV3 was not detected (28). Cai et al. detected PAstV infection in 129 samples collected from diarrheic and asymptomatic piglets and healthy wild boars in which 22 PAstV sequences were obtained, 12 of which were PAstV2 and the remainder were PAstV5 with 8 from diarrheic piglets (29). Qin et al. investigated the infection rate of PAstV in 532 fecal samples collected between 2013 and 2015 from clinically healthy pigs at different developmental stages in Guangxi province, with 56.4% testing positive for PAstVs. Among the obtained 72 viral sequences, 44.4%, 31.9%, and 19.4% were PAstV2, PAstV1, and PAstV5, respectively (30). Xiao et al. investigated the prevalence of PAstV infections in 509 fecal samples from 25 swine farms in 19 U.S. states, and PAstV1-5 were identified in both diarrheic and asymptomatic pigs from suckling to adult, with PAstV4 as the dominant type (31). In Japan, an epidemiological investigation showed detection of PAstV2-5 in pigs aged 9 to 100 days with or without diarrhea in 38 commercial farms (32).
So far, multiple PAstVs have been detected in both healthy and ill pigs in many countries, but their causative roles in disease development have not been determined, since none, other than PAstV1, has been successfully isolated for experimental study. Here, we report a surprising isolation of a PAstV5 strain from a CSFV-infected clinical specimen. This PAstV5 strain was then adapted to grow well in porcine PK-15 cells.
RESULTS
Viral metagenomic analysis of PK-15 cells inoculated with CSFV tissue homogenate.
The initial intention was to isolate field CSFV from the clinical specimen. While the virus normally does not cause cytopathic effect (CPE) in cell culture, CPE surprisingly started to appear at passage 2 (P2) and, following two additional passages, became more evident, including cell aggregation and shrinking, followed by detachment. To investigate whether unknown cytopathogenic viruses were isolated together with CSFV, the culture at passage 5 was subjected to viral metagenomic analysis using high-throughput sequencing (HTS), which identified the presence of PAstV5 and PCV2 in addition to CSFV in the culture. The obtained viral reads for PAstV5, PCV2, and CSFV were 476,432, 693, and 6,405, respectively, with PAstV5 reads being dominant. After assembly of the reads, two genomic fragments (positions 1 to 5468 and 5982 to 6378) of PAstV5 were obtained, with the remaining gaps being filled by specific reverse transcription-PCR (RT-PCR) to generate the complete genome (primer sequences are available upon request). The isolate was named PAstV5-AH29-2014, according to its collection time and location.
Genome sequence and phylogenetic analysis of PAstV5.
The whole genome of strain PAstV5-AH29-2014 is 6,448 nucleotides (nt), consisting of a 5′UTR (88 nt, positions 1 to 88), ORF1a (2,586 nt, 89 to 2674), ORF1b (1,452 nt, 2695 to 4146), ORF2 (2,340 nt, 3992 to 6331), and 3′UTR (117 nt, 6332 to 6448). In addition, a conserved frameshift signal composed of the slippery sequence (AAAAAAC) and a stem-loop structure was identified at the 3′ end of ORF1a (44 nt, 2629 to 2672). PAstV5-AH29-2014 shared 84.3 to 88.9% whole-genome identity with published PAstV5 strains in GenBank, showing the highest identity with Japanese strain PAstV5/JPN/Ishi-IM1-1-2015. Sequence comparison of different genomic regions between PAstV5-AH29-2014 and other reference strains showed that ORF1b is the most conserved region and the 5′UTR is the most variable, followed by ORF2 (Table 1). Multiple-sequence alignment of deduced amino acids of putative proteins indicated that PAstV5-AH29-2014, together with PAstV5/JPN/Ishi-IM1-1-2015, carries three deletions (P/T at position 487, S/Y/Q at 488, and Y/F/K at 625) and one insertion (L/I at 776) in the capsid proteins (460 to 782). PAstV5/JPN/Ishi-IM1-1-2015 has one more deletion (D at position 120). In addition, an 11-amino-acid (aa) deletion, SHPSPVESALQ at aa 614 to 624 in the ORF1a-coded serine protease, occurs in the PAstV5-AH29-2014 and Japan PAstV5 strains compared to two U.S. strains (33/USA and AstV5-US-IA122).
TABLE 1.
Percent identity of nucleotide and amino acid sequences between PAstV5-AH29-2014 and PAstV5 reference strains
| Reference strain | Country | GenBank accession no. | Identity (%) |
Subgenotype | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete genome | 5′UTR | ORF1a |
ORF1b |
ORF2 |
3′UTR | ||||||||
| nt | aa | nt | aa | nt | aa | ||||||||
| PAstV5/JPN/Ishi-Im1-2/2015 | Japan | LC201620 | 84.8 | 79.1 | 88.8 | 96.8 | 90.9 | 98.8 | 75.9 | 78.6 | 100 | PAstV5a | 32 |
| PAstV5/JPN/MoI2-1-3/2015 | Japan | LC201617 | 85.2 | 76.1 | 89.2 | 97.2 | 92.1 | 99.0 | 76.3 | 79.3 | 86.0 | 32 | |
| PAstV5/JPN/HgTa2-2/2015 | Japan | LC201616 | 84.8 | 75.6 | 89.0 | 97.4 | 90.4 | 98.8 | 76.7 | 79.7 | 78.9 | 32 | |
| PAstV5/JPN/MoI2-3-1/2015 | Japan | LC201618 | 85.1 | 76.1 | 89.2 | 97.2 | 92.1 | 98.6 | 76.3 | 79.3 | 79.2 | 32 | |
| PAstV5-33/USA | USA | JF713711 | 84.5 | 78.5 | 87.1 | 97.8 | 91.3 | 98.6 | 78.6 | 80.1 | 81.2 | 21 | |
| PAstV5-US-IA122 | USA | JX556693 | 84.3 | 85.2 | 87.0 | 98.1 | 90.8 | 98.1 | 76.9 | 79.0 | 80.5 | 31 | |
| PAstV5-LL-2 | China | KP747574 | 72.8 | 41.2 | 72.0 | 80.1 | 76.9 | 89.7 | 71.3 | 74.4 | 83.2 | 56 | |
| PAstV5/JPN/Ishi-Im1-1/2015 | Japan | LC201619 | 88.9 | 78.4 | 90.4 | 97.0 | 91.5 | 98.6 | 84.8 | 92.9 | 97.7 | PAstV5b | 32 |
| CC12 | Canada | JN088537 | NAa | NA | NA | NA | NA | NA | 74.0 | 78.6 | 76.9 | PAstV5c | 24 |
NA, not available.
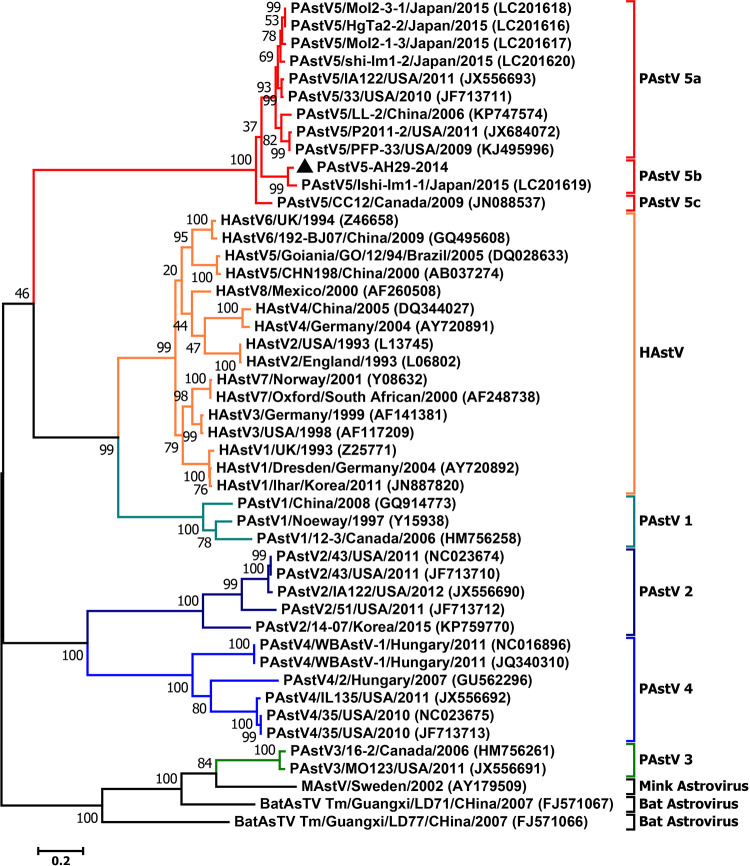
Complete capsid protein-based phylogenetic analysis of all available astrovirus sequences showed that PAstV5 strains can be classified into three subgenotypes: PAstV5a, PAstV5b, and PAstV5c (Fig. 1). PAstV5a comprises isolates from Japan, China, and the United States. PAstV5-AH29-2014 and PAstV5/JPN/Ishi-IM1-1-2015 are grouped together, forming PAstV5b. The PAstV5 CC12 strain, collected from Canada in 2009, is the only member in subgenotype PAstV5c. At the nucleotide level, the intrasubgenotype identity of the complete ORF2 gene was >88.7% and 85.6% for PAstV5a and PAstV5b, respectively, while the intersubgenotype identity was 76.0 to 78.6%. Hence, an 80% complete ORF2 gene identity provides an appropriate demarcation criterion for the PAstV5 subgenotype. At the complete capsid protein level, the amino acid genetic distance (P distance) of PAstV5 between subgenotypes is 0.195 to 0.257, while the P distance of the intrasubgenotype is 0.018 to 0.122 and 0.071 for PAstV5a and PAstV5b, respectively. Hence, the P distance of >0.190 of the complete capsid protein is likely a suitable demarcation criterion for the PAstV5 subgenotype.
FIG 1.
Phylogenetic analysis of PAstVs compared with representative PAstVs from other mammalian hosts. The tree was constructed based on complete capsid protein sequences by MEGA 7.0 with the best fit model (GTR+G+I) and 1,000 bootstrap replicates. All PAstV5 sequences available in GenBank were used, and PAstV5-AH29-2014, obtained in this study, is indicated by a black triangle.
Isolation and identification of PAstV5.
To obtain the pure PAstV5-AH29-2014 isolate from CSFV coinfection, the viral cultures at passage 4 were treated by anti-CSFV serum before propagation to the next passage. Neither RT-PCR nor immunofluorescence assay (IFA) detected CSFV in passages 5 and 6, while PAstV5 was detected, indicating complete clearance of CSFV from PAstV5 culture. To test if PAstV5 can be cultivated stably in PK-15 cells without CSFV, the CSFV-cleared PAstV5 culture (passage 5) was continuously passaged another 16 times to obtain purified PAstV5 stock (i.e., passage 21). In comparison, a culture with CSFV/PAstV5 coinfection was passaged in parallel. As shown in Fig. 2A, PAstV5-AH29-2014 could be stably propagated in PK-15 cells in the absence of CSFV, but PAstV5 genome copies (used as titers rather than 50% tissue culture infectious doses [TCID50]) in the unpurified viral cultures containing CSFV were approximately 10-fold higher. PCV2 was also cleared using d-glucosamine-depleted medium during continuous passage of PAstV5 and had disappeared from passage 12 onward, as confirmed by PCR detection. The clearance of CSFV and PCV2 in the PAstV5-AH29-2014 stock was further confirmed by IFA (Fig. 2B). Further HTS of PAstV5-AH29-2014 stock culture did not identify viral reads of CSFV, PCV2, and others (data not shown). These results showed that the isolated PAstV5-AH29-2014 has been successfully purified, and its growth resulted in stable CPEs, as seen in passage 4 samples coinfected with CSFV and PCV2 (Fig. 2C). Further analysis of replication dynamics showed that PAstV5-AH29-2014 at passage 21 grew efficiently in PK-15 cells and reached a peak titer (106.97 TCID50/ml or 1010.57 genome copies/ml) at 72 or 96 h postinfection (hpi) (Fig. 2D).
FIG 2.
Growth of PAstV5-AH29-2014 in PK-15 cells. (A) PAtV5-AH29-2014 can stably propagate in PK-15 cells, with its titers before purification being higher than those after removal of CSFV by viral neutralizing antibody treatment. (B) Purity detection of PAstV5-AH29-2014 stock by IFA. The upper row was detected by anti-PCV2 MAb 3C1, while the lower row was detected by anti-CSFV MAb WH303. The result showed that both PCV2 and CSFV were detected at passage 4 (P4), but only CSFV, and not PCV2, was detected at passage 21 (P21) of the coinfected culture, AH29-2014. In contrast, in purified PAtV5-AH29-2014 at P21, neither virus was detected. The CSFV positive control is strain JL23-2015, stored in our laboratory, while the PCV2 positive control was provided by Xinglong Yu, from Hunan Agricultural University. (C) CPEs started to show at 48 h and became severe at 96 h postinoculation, and no visible difference was observed in the purified (upper; passage 21) and the unpurified (middle; passage 4) PAstV5 cultures at different time points. (D) Growth dynamic of PAstV5-AH29-2014 at passage 21 was assessed by qRT-PCR (black curve) and TCID50 assay by IFA (red curve). The IFA of PAstV5-AH29-2014 using antiserum against PAstV5 capsid protein is shown on the right. Bars represent the averages and standard deviations from three independent measurements.
Electron microscopy showed that PAstV5-AH29-2014 virions are nonenveloped and spherical, with a diameter of 30 nm. Star-like structures surrounding the icosahedral capsid were also observed (Fig. 3A). Ultrathin sections showed that the virions aggregated as crystalline lattices in the cytoplasm of infected cells (Fig. 3B), which is consistent with reports of human AstVs (33, 34).
FIG 3.
Electron microscopy of PAstV5-AH29-2014 particles following purification by sucrose gradient (A) and in ultrathin cell sections (B).
Enhancement of PAstV5 replication by coculture with CSFV.
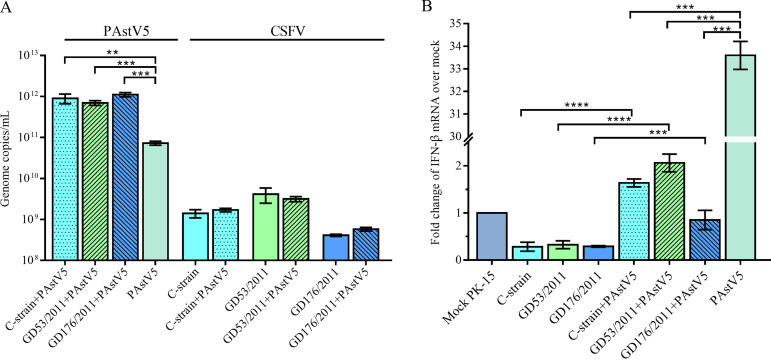
As shown in Fig. 2A, the titer of PAstV5 after CSFV clearance significantly declined compared to that with CSFV coinfection. To test the effect of CSFV coinfection on PAstV5 propagation, PK-15 cells were inoculated simultaneously with both viruses. Compared to that inoculated with PAstV5-AH29-2014 alone, the genome copy titers of PAstV5-AH29-2014 in coinfected cultures with 3 CSFV strains (vaccine C-strain and two field isolates) were increased by 9.6- to 15.3-fold, but PAstV5 coinfection had no significant effect on propagation of 3 CSFV strains (Fig. 4A). This result showed that CSFV can enhance PAstV5 propagation in PK-15 cells.
FIG 4.
Effect of CSFV coinfection on PAstV5 propagation (A) and IFN-β expression (B). (A) Coinfection of both viruses significantly enhanced PAstV5 titer (left) and had no effect on CSFV titers (right). (B) The production of IFN-β is enhanced by PAstV5-AH29-2014 but inhibited by CSFVs. Bars represent the averages and standard deviations from three independent measurements (**, P < 0.01; ***, P < 0.001; both by two-tailed Student's t test).
A recent study demonstrated that HAstV1 infection can induce beta interferon (IFN-β) production, and the induced IFN-β contrarily inhibited HAstV1 replication at certain levels, which was confirmed by the addition of anti-IFN-β antibody upon HAstV1 infection in Caco-2 cells (35). It is well known that CSFV infection can effectively suppress the type I interferon response (36, 37). Therefore, IFN-β production in PAstV5-infected cells was tested, with results showing that IFN-β levels increased by 33-fold during PAstV5-AH29-2014 infection but were significantly decreased during infections with 3 CSFV strains (Fig. 4B). Of note is that IFN-β levels in three PAstV5/CSFV coinfection groups were higher than that following CSFV infection alone but were lower than that following PAstV5 infection alone (Fig. 4B). It is interesting that the addition of swine IFN-β into the cultures significantly inhibited PAstV5 replication in a dose-dependent manner (Fig. 5A), and the inhibition effect could be alleviated upon the addition of anti-swine IFN-β antibody (Fig. 5B). Since PAstV5 replication is sensitive to IFN-β and its production can be inhibited by CSFV infection, it appears that enhancement of PAstV5 replication in PK-15 cells by CSFV coinfection is mediated by the suppression of the IFN-β response.
FIG 5.
Inhibition of PAstV5 replication by swine IFN-β added into the culture is dose dependent (A) and can be alleviated by anti-swine IFN-β antibody (B). Rabbit IgG was used as a negative antibody control. Bars represent the averages and standard deviations from three independent measurements (**, P < 0.01; ***, P < 0.001; both by two-tailed Student's t test).
DISCUSSION
The existence of multiple-virus coinfections has been well established, although isolation of individual viruses in cell cultures from such clinical specimens may present a challenge. In the present study, HTS-based viromic analysis was applied to identify coinfecting viruses in PK-15 cell culture incubated with a CSFV-positive sample, resulting in the identification and the first isolation of PAstV5. As shown by our data, the HTS-based viromic approach effectively identified the coinfecting viruses by viromic scanning and could determine the purity of the isolated virus. In the efforts to purify PAstV5 by clearing unwanted CSFV and PCV2, one round of neutralization at passage 4 with anti-CSFV hyperimmune serum was highly effective, with CSFV no longer being detected in the following passages. To clear PCV2, d-glucosamine, a propagation enhancer for PCV2 (38), was not included in the culture medium; moreover, the PK-15 cell line is not highly susceptible to PCV2. Therefore, after 10 passages in this medium, PCV2 was also completely removed. With this strategy, the pure PAstV5-AH29-2014 strain was successfully obtained.
The gastrointestinal tract, in which trypsin is produced, is a major target of the various AstVs (8, 33, 39). Trypsin digestion of the capsid proteins of mature astrovirus virions into fragments is critical for viral entry to initiate infection (40–42); therefore, the inclusion of trypsin in cell cultures is important for AstV isolation. Using this method, AstVs have been successfully isolated from humans (33, 43–45), calves (46), dogs (12), and pigs (PAstV1) (9, 25). However, PAstVs have remained difficult to isolate (14), and only PAstV1 had been isolated in culture until now. The isolation and stable propagation of PAstV5-AH29-2014 was achieved without using trypsin, the effect of which in PAstV5 propagation remains unknown. The attempt to test the effect of trypsin in the propagation of our PAstV5 isolate failed, since our PK-15 cells grew worse and detached from flask wall when cultured in serum-free medium, a critical condition to test the effect of trypsin. Our further study focused on the IFN signaling pathway, since there are publications showing that replication of HAstVs was inhibited by IFN-β produced during infection (35) and that CSFV significantly inhibits the production of type I IFN via interaction of its Npro protein with host interferon regulatory factors 3 and 7 (36, 37). Our results, summarized in Fig. 4, strongly suggest that CSFV infection enhances PAstV5 replication through inhibition of IFN-β expression, while PAstV5 replication itself had no impact on CSFV propagation.
Astrovirus infections have also been associated with neurological diseases in pigs (17, 18), interstitial nephritis in young chickens (23), and hepatitis in ducks (3). Unfortunately, the lack of clinical information and specimens of tissues other than kidney limits our ability to further investigate the pathogenicity of PAstV5-AH29-2014. However, the isolation of this strain has provided a critical material for further studies into the role of PAstV5 in diseases of pigs.
MATERIALS AND METHODS
Clinical sample.
A kidney sample was collected from a CSF-suspected herd in Bozhou city, Anhui province, China, in December 2014, and the sample-derived pig displayed typical CSF clinical signs and pathogenic lesions, including high fever, anorexia, depression, conjunctivitis, diarrhea, pulmonary edema and hemorrhage, swelling and hemorrhage of lymph nodes, and point hemorrhages in the kidney. The collected sample was confirmed CSFV positive by RT-PCR followed by sequencing as described previously (47).
Viral isolation.
Porcine kidney cells (PK-15) maintained in our laboratory were cultured in minimal essential medium (MEM) supplemented with 8% fetal bovine serum (FBS), 100 U/ml of penicillin G, and 100 μg/ml of sodium streptomycin sulfate at 37°C, 5% CO2. To isolate CSFV from the above-described CSFV-positive sample, a 10% tissue homogenate was prepared and clarified by centrifugation at 12,000 × g for 10 min at 4°C. One milliliter of the resulting supernatant was incubated with a monolayer of PK-15 cells in a T25 flask at 37°C and 5% CO2 for 72 h, followed by passaging using a previously established protocol (47). Unexpectedly, since CSFV does not cause cytopathogenicity in culture, CPEs were observed at P2, and P4 was harvested to identify and isolate the potential cytopathogenic virus.
Viral metagenomic and sequence analysis of isolated virus.
The harvested culture was subjected to high-throughput sequencing (HTS) to identify the cytopathogenic virus(es). Briefly, total RNA was extracted with TRIzol reagent (Invitrogen, CA, USA), in which the rRNA was removed using the Epicentre Ribo-zero rRNA removal kit (Epicentre, USA). The clarified total RNA precipitated by ethanol was used to construct an LncRNA library, followed by sequencing with Hiseq-PE150 by Novogene Solution (Tianjin, China) (48, 49). Using MEGAHIT v1.1.3, sequence reads were assembled de novo into contigs or whole-genome sequences.
Nucleotide and amino acid sequence identities of the assembled contigs and viral genome were analyzed using DNAStar, and multiple-sequence alignment was performed with CLC Sequence Viewer 8.0 (Qiagen, Germany). Phylogenetic analysis based on the complete protein sequences of the cytopathogenic virus was conducted using MEGA 7.0 (1, 50). The maximum-likelihood phylogenetic tree was constructed with the aligned amino acid sequences, and 1,000 bootstrap replicates were applied.
Production of mouse antiserum against PAstV5 capsid protein.
To produce antiserum against capsid protein of PAstV5, truncated capsid protein was first expressed with a baculovirus expression system as previously described (51). Briefly, the gene fragment (nt 1201 to 2337) encoding truncated capsid protein (401 to 779 aa) was amplified with primer pair PAstV5-cap-F (5′-CGGGATCCGCCACCATGGCCCCAGTTGGTGCAGGTATC-3′) and PAstV5-cap-R (5′-CCGGAATTCTTAATGGTGATGGTGATGATGGTCGGACTCAACGAGATTTTC-3′). A 6×His tag was added at the C-terminal end of the truncated capsid protein. After cloning of the fragment into pFastBac1, the recombinant vector was transformed into DH10Bac competent cells to get the bacmid DNA. The latter was then transfected into Sf9 cells to produce the recombinant baculovirus stock. Truncated capsid protein expressed in Sf9 cells infected by passage 3 baculovirus was purified with nickel-nitrilotriacetic acid agarose beads, which was mixed with adjuvant ISA206 (Seppic, France), and then used to immunize BALB/c female mice (40 μg per dose), with two boosts at 2-week intervals. At 2 weeks after the second boost, antiserum against PAstV5 capsid protein was prepared from the immunized mice, and its potency was tested by indirect fluorescent antibody assay (IFA) with Alexa Fluor 488 donkey anti-mouse IgG (H+L) (Life Technologies, USA) as the secondary antibody. The mouse study was reviewed and approved by the Experimental Animal Use and Care Committee, Academy of Military Medical Sciences.
Isolation and characterization of PAstV5 from CSFV coinfection culture.
CSFV was removed from the culture by incubation with anti-CSFV hyperimmune serum with high 50% neutralization doses (ND50) (ND50 = 15,360) (52). Briefly, the coinfected virus culture at P4 was diluted 1:10 and then mixed with an equal volume of anti-CSFV serum to neutralize CSFV. After incubation at 37°C for 1 h, the neutralized viral culture was added to PK-15 cells for incubation at 37°C for 96 h to isolate PAstV5. The resulting PAstV5 cultures were continually passaged in PK-15 cells until viral propagation was stable. To test the purity of isolated PAstV5, the clearance of CSFV from the cultures was confirmed by RT-PCR and IFA with anti-E2 monoclonal antibody (MAb) WH303 as previously described (47). PCV2 contamination was detected by PCR used in our previous study (53), followed by IFA using PCV2 capsid-specific MAb 3C1 as the primary antibody (gifted by Xiaobing Mo at Jilin University) and Alexa Fluor 488 donkey anti-mouse IgG (H+L) (Life Technologies, USA) as the secondary antibody. The final stock of isolated PAstV5 (passage 21) was subjected to HTS to confirm its purity as described above.
The growth and replication dynamics of PAstV5 in PK-15 cells were analyzed by TCID50 assay and quantitative SYBR green RT-PCR (qRT-PCR) as described below. The TCID50 of PAstV5 was determined by IFA as previously described (47) using mouse antiserum against PAstV5 capsid protein prepared as described above.
Detection of viruses.
Growth of PAstV5 and CSFV in the cell cultures was monitored by quantitative SYBR green RT-PCR. Briefly, total RNA of viral culture was extracted with a TIANamp virus RNA kit (Tiangen, Beijing, China) according to the manufacturer’s instructions and then reverse transcribed to cDNA using random primers and M-MLV (TaKaRa, Dalian, China), which served as templates for quantitative PCR (qPCR) with primer pairs for PAstV5 (PAstV5-F, 5′-CAATCGGCGTAACAGGAGACC-3′; PastV5-R, 5′-CTGGCGAGGTGCTGTCTTAGTG-3′) and CSFV (CSFV-F, 5′-GCTCCCTGGGTGGTCTAAGTC-3′; CSFV-R, 5′-CGCYAGGGTTAAGGTGCGTCT-3′). To construct recombinant plasmids for establishing a standard curve, a 491-bp fragment of PAstV5 ORF2 was amplified by TaKaRa LA Taq polymerase with a primer pair (PAstV5-ORF2-F, 5′-ACCACTGCGTCGCTATGTG-3′; PAstV5-ORF2-R, 5′-TTGGTCCGGCACCAGCAG-3′). The 121-bp fragment of the CSFV 5′UTR was also amplified with the above-described CSFV primer pair. The 2 amplicons were separately cloned into pMD-18T according to the manufacturer’s directions to construct plasmids pMD-PAstV5-ORF2 and pMD-CSFV-5UTR for establishing standard curves in qPCR for both viruses. The qRT-PCR (20 μl) consisted of 10 μl 2×SYBR Premix Ex Taq II (TaKaRa, Dalian, China), 0.8 μl forward primer, 0.8 μl reverse primer, 2 μl cDNA, and 6.4 μl H2O, and then we performed experiments on an Mx3000p real-time PCR system (Bio-Rad) with the following steps: 95°C, 3 min; 40 cycles of 95°C, 10 s; and 60°C, 30 s.
Electron microscopy.
PAstV5 cultures prepared as described above were clarified by differential centrifugation at 3,000 × g for 10 min, followed by 12,000 × g for 30 min at 4°C. After passage through a 0.22-μm filter, virus particles in the supernatant were pelleted by centrifugation at 140,000 × g for 3 h and then resuspended in 500 μl phosphate-buffered saline (PBS) and overlaid on a discontinuous (15 to 40%) sucrose density gradient. Following ultracentrifugation at 140,000 × g for 2 h, 2-ml fractions were collected, and sucrose was removed by additional ultracentrifugation. The pelleted virions were dispersed in 100 μl PBS and observed by transmission electron microscopy after negative staining with 2% phosphotungstic acid (pH 7.0). Ultrathin sections of PAstV5-infected cells fixed in 2.5% glutaraldehyde were also processed for electron microscopy by using a published method (54).
Effect of CSFV coinfection and IFN-β on PAstV5 propagation in PK-15 cells.
PK-15 cells were coinfected with PAstV5 (200 genome copies/cell) and different CSFV strains (1 genome copy/cell), modified live attenuated vaccine C-strain, and subgenotype 2.1b (GD53/2011) and 2.1c (GD176/2011) isolates (55). At 96 hpi, the viral cultures were harvested and subjected to qRT-PCR as described above to determine the genomic copies of each virus. To determine if the IFN pathway is involved in the promotion of PAstV5 replication by CSFV, porcine IFN-β mRNA was analyzed with normalization to the porcine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene by the SYBR green comparative quantitation mode, performed as previously described (36). To test if PAstV5 replication is regulated by IFN-β, a multiplicity of infection (MOI) of 10 genome copies per cell of the isolated PAstV5 stock (passage 21) and serial dilutions of swine IFN-β (Kingfisher, USA) in MEM containing 8% fetal bovine serum were mixed and added to overnight-cultured PK-15 cells monolayers in 12-well plates. In parallel, serially diluted rabbit anti-swine IFN-β polyclonal antibody (Kingfisher, USA) was added to PK-15 cell wells already inoculated with at an MOI of 10 genome copies per cell of PAstV5 stock and 1 ng/ml IFN-β. After infection for 48 h, the cultures were collected, and the viral genome copies were quantified by qRT-PCR to determine the effect of swine IFN-β on the viral replication. The statistics and histograms of the data were obtained by two-tailed Student t tests in GraphPad Prism 7.0. Standard deviations (SDs) are presented by error bars, and statistical significance is defined as a P value of <0.05.
Data availability.
The full-genome sequence of porcine astrovirus 5 (PAstV5) strain PAstV5-AH29-2014 is available in GenBank under accession number MT642595.
ACKNOWLEDGMENTS
This work was supported by the following grants: National Key Research and Development Program of China 2017YFD0500104 to C.T. and National Natural Science Foundation of China 31572528 and 32072843 to W.G.
We have no conflict of interest to declare.
REFERENCES
- 1.Guix S, Bosch A, Pintó RM. 2013. Astrovirus taxonomy, p 97–118. In Schultz-Cherry S (ed), Astrovirus research: essential ideas, everyday impacts, future directions. Springer, New York, NY. doi: 10.1007/978-1-4614-4735-1_6. [DOI] [Google Scholar]
- 2.Finkbeiner SR, Kirkwood CD, Wang D. 2008. Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. Virol J 5:117–123. doi: 10.1186/1743-422X-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Y, Pan M, Wang X, Xu Y, Xie X, Knowles NJ, Yang H, Zhang D. 2009. Complete sequence of a duck astrovirus associated with fatal hepatitis in ducklings. J Gen Virol 90:1104–1108. doi: 10.1099/vir.0.008599-0. [DOI] [PubMed] [Google Scholar]
- 4.Monroe SS, Jiang B, Stine SE, Koopmans M, Glass RI. 1993. Subgenomic RNA sequence of human astrovirus supports classification of astroviridae as a new family of RNA viruses. J Virol 67:3611–3614. doi: 10.1128/JVI.67.6.3611-3614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willcocks MM, Carter MJ. 1993. Identification and sequence determination of the capsid protein gene of human astrovirus serotype 1. FEMS Microbiol Lett 114:1–7. doi: 10.1016/0378-1097(93)90133-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortez V, Meliopoulos VA, Karlsson EA, Hargest V, Johnson C, Schultz-Cherry S. 2017. Astrovirus biology and pathogenesis. Annu Rev Virol 4:327–348. doi: 10.1146/annurev-virology-101416-041742. [DOI] [PubMed] [Google Scholar]
- 7.Jiang B, Monroe SS, Koonin EV, Stine SE, Glass RI. 1993. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc Natl Acad Sci U S A 90:10539–10543. doi: 10.1073/pnas.90.22.10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias CF, DuBois RM. 2017. The astrovirus capsid: a review. Viruses 9:15–27. doi: 10.3390/v9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu M, Shirai J, Narita M, Yamane T. 1990. Cytopathic astrovirus isolated from porcine acute gastroenteritis in an established cell line derived from porcine embryonic kidney. J Clin Microbiol 28:201–206. doi: 10.1128/JCM.28.2.201-206.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoor A, Li L, Victoria J, Oderinde B, Mason C, Pandey P, Zaidi SZ, Delwart E. 2009. Multiple novel astrovirus species in human stool. J Gen Virol 90:2965–2972. doi: 10.1099/vir.0.014449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu DK, Poon LL, Guan Y, Peiris JS. 2008. Novel astroviruses in insectivorous bats. J Virol 82:9107–9114. doi: 10.1128/JVI.00857-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toffan A, Jonassen CM, De Battisti C, Schiavon E, Kofstad T, Capua I, Cattoli G. 2009. Genetic characterization of a new astrovirus detected in dogs suffering from diarrhoea. Vet Microbiol 139:147–152. doi: 10.1016/j.vetmic.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Liu L, Li R, Qin Y, Fang Q, Balasubramaniam VR, Wang G, Wei Z, Ouyang K, Huang W, Chen Y. 2017. Detection and genetic characterization of canine astroviruses in pet dogs in Guangxi, China. Virol J 14:156–161. doi: 10.1186/s12985-017-0823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuter G, Knowles NJ. 2019. Astroviruses, p 457–460. In Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J (ed), Diseases of swine, 11th ed. Wiley Blackwell, Hoboken, NJ. [Google Scholar]
- 15.Vilcek S, Salamunova S, Jackova A. 2019. Genetic identification of astroviruses in wild boars. J Vet Sci 20:91–94. doi: 10.4142/jvs.2019.20.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imada T, Yamaguchi S, Kawamura H. 1979. Pathogenicity for baby chicks of the G-4260 strain of the picornavirus “avian nephritis virus.” Avian Dis 23:582–588. doi: 10.2307/1589733. [DOI] [PubMed] [Google Scholar]
- 17.Arruda B, Arruda P, Hensch M, Chen Q, Zheng Y, Yang C, Gatto IRH, Ferreyra FM, Gauger P, Schwartz K, Bradner L, Harmon K, Hause B, Li G. 2017. Porcine astrovirus type 3 in central nervous system of swine with polioencephalomyelitis. Emerg Infect Dis 23:2097–2100. doi: 10.3201/eid2312.170703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boros A, Albert M, Pankovics P, Biro H, Pesavento PA, Phan TG, Delwart E, Reuter G. 2017. Outbreaks of neuroinvasive astrovirus associated with encephalomyelitis, weakness, and paralysis among weaned pigs, Hungary. Emerg Infect Dis 23:1982–1993. doi: 10.3201/eid2312.170804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridger JC. 1980. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus-like particles in the faeces of piglets with diarrhoea. Vet Rec 107:532–533. [PubMed] [Google Scholar]
- 20.Luo Z, Roi S, Dastor M, Gallice E, Laurin MA, L'Homme Y. 2011. Multiple novel and prevalent astroviruses in pigs. Vet Microbiol 149:316–323. doi: 10.1016/j.vetmic.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan T, Li L, Simmonds P, Wang C, Moeser A, Delwart E. 2011. The fecal virome of pigs on a high-density farm. J Virol 85:11697–11708. doi: 10.1128/JVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kattoor JJ, Malik YS, Saurabh S, Sircar S, Vinodhkumar OR, Bora DP, Dhama K, Ghosh S, Banyai K, Touil N, Abdel-Moneim AS, Vlasova AN, Kobayashi N, Singh RK. 2019. First report and genetic characterization of porcine astroviruses of lineage 4 and 2 in diarrhoeic pigs in India. Transbound Emerg Dis 66:47–53. doi: 10.1111/tbed.13058. [DOI] [PubMed] [Google Scholar]
- 23.Owens F, Di S, Li V. 2012. Virus taxonomy: ninth report of the international committee on taxonomy of viruses. Academic Press, New York, NY. [Google Scholar]
- 24.Laurin MA, Dastor M, L'Homme Y. 2011. Detection and genetic characterization of a novel pig astrovirus: relationship to other astroviruses. Arch Virol 156:2095–2099. doi: 10.1007/s00705-011-1088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Q, Wang C, Liu H, Wu Q, Liang S, Cen M, Dong Q, Wei Y, Chen Y, Ouyang K, Wei Z, Huang W. 2019. Pathogenic characteristics of a porcine astrovirus strain isolated in China. Viruses 11:1156–1172. doi: 10.3390/v11121156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Indik S, Valicek L, Smid B, Dvorakova H, Rodak L. 2006. Isolation and partial characterization of a novel porcine astrovirus. Vet Microbiol 117:276–283. doi: 10.1016/j.vetmic.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Qin Y, Fang Q, Liu H, Ji C, Chen Y, Ouyang K, Wei Z, Huang W. 2018. Construction of a reverse genetic system for porcine astrovirus. Arch Virol 163:1511–1518. doi: 10.1007/s00705-018-3771-4. [DOI] [PubMed] [Google Scholar]
- 28.Xiao CT, Luo Z, Lv SL, Opriessnig T, Li RC, Yu XL. 2017. Identification and characterization of multiple porcine astrovirus genotypes in Hunan province, China. Arch Virol 162:943–952. doi: 10.1007/s00705-016-3185-0. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, Yin W, Zhou Y, Li B, Ai L, Pan M, Guo W. 2016. Molecular detection of porcine astrovirus in Sichuan province, China. Virol J 13:6–10. doi: 10.1186/s12985-015-0462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin Y, Fang Q, Li X, Li F, Liu H, Wei Z, Ouyang K, Chen Y, Huang W. 2019. Molecular epidemiology and viremia of porcine astrovirus in pigs from Guangxi province of China. BMC Vet Res 15:471–479. doi: 10.1186/s12917-019-2217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao CT, Gimenez-Lirola LG, Gerber PF, Jiang YH, Halbur PG, Opriessnig T. 2013. Identification and characterization of novel porcine astroviruses (PAstVs) with high prevalence and frequent co-infection of individual pigs with multiple PAstV types. J Gen Virol 94:570–582. doi: 10.1099/vir.0.048744-0. [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Kuroda M, Masuda T, Akagami M, Haga K, Tsuchiaka S, Kishimoto M, Naoi Y, Sano K, Omatsu T, Katayama Y, Oba M, Aoki H, Ichimaru T, Mukono I, Ouchi Y, Yamasato H, Shirai J, Katayama K, Mizutani T, Nagai M. 2017. Whole genome analysis of porcine astroviruses detected in Japanese pigs reveals genetic diversity and possible intra-genotypic recombination. Infect Genet Evol 50:38–48. doi: 10.1016/j.meegid.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Janowski AB, Bauer IK, Holtz LR, Wang D. 2017. Propagation of astrovirus va1, a neurotropic human astrovirus, in cell culture. J Virol 91:e00740-17. doi: 10.1128/JVI.00740-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Méndez E, Aguirre-Crespo G, Zavala G, Arias CF. 2007. Association of the astrovirus structural protein VP90 with membranes plays a role in virus morphogenesis. J Virol 81:10649–10658. doi: 10.1128/JVI.00785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marvin SA, Huerta CT, Sharp B, Freiden P, Cline TD, Schultz-Cherry S. 2016. Type I interferon response limits astrovirus replication and protects against increased barrier permeability in vitro and in vivo. J Virol 90:1988–1996. doi: 10.1128/JVI.02367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggli N, Bird BH, Liu L, Bauhofer O, Tratschin JD, Hofmann MA. 2005. N(pro) of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-alpha/beta induction. Virology 340:265–276. doi: 10.1016/j.virol.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Fiebach AR, Guzylack-Piriou L, Python S, Summerfield A, Ruggli N. 2011. Classical swine fever virus N(pro) limits type I interferon induction in plasmacytoid dendritic cells by interacting with interferon regulatory factor 7. J Virol 85:8002–8011. doi: 10.1128/JVI.00330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz TF, Araujo JP, Jr. 2014. Cultivation of PCV2 in swine testicle cells using the shell vial technique and monitoring of viral replication by qPCR and RT-qPCR. J Virol Methods 196:82–85. doi: 10.1016/j.jviromet.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 39.Koshikawa N, Hasegawa S, Nagashima Y, Mitsuhashi K, Tsubota Y, Miyata S, Miyagi Y, Yasumitsu H, Miyazaki K. 1998. Expression of trypsin by epithelial cells of various tissues, leukocytes, and neurons in human and mouse. Am J Pathol 153:937–944. doi: 10.1016/S0002-9440(10)65635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willcocks MM, Carter MJ, Laidler FR, Madeley CR. 1990. Growth and characterisation of human faecal astrovirus in a continuous cell line. Arch Virol 113:73–81. doi: 10.1007/BF01318354. [DOI] [PubMed] [Google Scholar]
- 41.Méndez E, Fernández-Luna T, López S, Méndez-Toss M, Arias CF. 2002. Proteolytic processing of a serotype 8 human astrovirus ORF2 polyprotein. J Virol 76:7996–8002. doi: 10.1128/jvi.76.16.7996-8002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogdanoff WA, Campos J, Perez EI, Yin L, Alexander DL, DuBois RM. 2017. Structure of a human astrovirus capsid-antibody complex and mechanistic insights into virus neutralization. J Virol 91:e01859-16. doi: 10.1128/JVI.01859-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee TW, Kurtz JB. 1981. Serial propagation of astrovirus in tissue culture with the aid of trypsin. J Gen Virol 57:421–424. doi: 10.1099/0022-1317-57-2-421. [DOI] [PubMed] [Google Scholar]
- 44.Monroe SS, Stine SE, Gorelkin L, Herrmann JE, Blacklow NR, Glass RI. 1991. Temporal synthesis of proteins and RNAs during human astrovirus infection of cultured cells. J Virol 65:641–648. doi: 10.1128/JVI.65.2.641-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brinker JP, Blacklow NR, Herrmann JE. 2000. Human astrovirus isolation and propagation in multiple cell lines. Arch Virol 145:1847–1856. doi: 10.1007/s007050070060. [DOI] [PubMed] [Google Scholar]
- 46.Woode GN, Pohlenz JF, Gourley NE, Fagerland JA. 1984. Astrovirus and Breda virus infections of dome cell epithelium of bovine ileum. J Clin Microbiol 19:623–630. doi: 10.1128/JCM.19.5.623-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong W, Lu Z, Zhang L, Xie X, Jiang D, Jia J, Guo H, Shi J, Tu C. 2016. In vitro adaptation and genome analysis of a sub-subgenotype 2.1c isolate of classical swine fever virus. Virus Genes 52:651–659. doi: 10.1007/s11262-016-1350-x. [DOI] [PubMed] [Google Scholar]
- 48.Ni C, Jiang W, Wang Z, Wang Z, Zhang J, Zheng X, Liu Z, Ou H, Jiang T, Liang W, Wu F, Li Q, Hou Y, Yang Q, Guo B, Liu S, Li S, Li S, Yang E, Zhu XH, Huang X, Wen Z, Zhao C. 3 February 2020. LncRNA-AC006129.1 reactivates a SOCS3-mediated anti-inflammatory response through DNA methylation-mediated CIC downregulation in schizophrenia. Mol Psychiatry doi: 10.1038/s41380-020-0662-3. [DOI] [PubMed] [Google Scholar]
- 49.Xing C, Jiang J, Lu Z, Mi S, He B, Tu C, Liu X, Gong W. 10 April 2020. Isolation and characterization of Getah virus from pigs in Guangdong province of China. Transbound Emerg Dis doi: 10.1111/tbed.13567. [DOI] [PubMed] [Google Scholar]
- 50.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madera R, Gong W, Wang L, Burakova Y, Lleellish K, Galliher-Beckley A, Nietfeld J, Henningson J, Jia K, Li P, Bai J, Schlup J, McVey S, Tu C, Shi J. 2016. Pigs immunized with a novel E2 subunit vaccine are protected from subgenotype heterologous classical swine fever virus challenge. BMC Vet Res 12:197–206. doi: 10.1186/s12917-016-0823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong W, Li J, Wang Z, Sun J, Mi S, Xu J, Cao J, Hou Y, Wang D, Huo X, Sun Y, Wang P, Yuan K, Gao Y, Zhou X, He S, Tu C. 2019. Commercial E2 subunit vaccine provides full protection to pigs against lethal challenge with 4 strains of classical swine fever virus genotype 2. Vet Microbiol 237:108403. doi: 10.1016/j.vetmic.2019.108403. [DOI] [PubMed] [Google Scholar]
- 53.Bao F, Mi S, Luo Q, Guo H, Tu C, Zhu G, Gong W. 2018. Retrospective study of porcine circovirus type 2 infection reveals a novel genotype PCV2f. Transbound Emerg Dis 65:432–440. doi: 10.1111/tbed.12721. [DOI] [PubMed] [Google Scholar]
- 54.Zhao C, Chen C, Li Y, Dong S, Tan K, Tian Y, Zhang L, Huang J, Zhang L. 2019. Genomic characterization of a novel recombinant porcine astrovirus isolated in northeastern China. Arch Virol 164:1469–1473. doi: 10.1007/s00705-019-04162-8. [DOI] [PubMed] [Google Scholar]
- 55.Gong W, Wu J, Lu Z, Zhang L, Qin S, Chen F, Peng Z, Wang Q, Ma L, Bai A, Guo H, Shi J, Tu C. 2016. Genetic diversity of subgenotype 2.1 isolates of classical swine fever virus. Infect Genet Evol 41:218–226. doi: 10.1016/j.meegid.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Li JS, Li MZ, Zheng LS, Liu N, Li DD, Duan ZJ. 2015. Identification and genetic characterization of two porcine astroviruses from domestic piglets in China. Arch Virol 160:3079–3084. doi: 10.1007/s00705-015-2569-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full-genome sequence of porcine astrovirus 5 (PAstV5) strain PAstV5-AH29-2014 is available in GenBank under accession number MT642595.