Immune responses to RSV in infants can be reduced due to immunological immaturity and immunosuppression by RSV-specific maternal antibodies. In infants and young children, two infections with wild-type RSV typically are needed to achieve the titers of RSV-specific serum antibodies and protection against illness that are observed in adults. Therefore, a boost might substantially improve the performance of live pediatric RSV vaccines presently being developed. Hamsters and African green monkeys received a primary intranasal infection with RSV and were given a boost with RSV or a parainfluenza virus (PIV) vector expressing RSV fusion protein engineered for enhanced immunogenicity. The RSV boost was highly restricted but induced a significant increase in serum RSV-neutralizing antibodies. The PIV vectors replicated efficiently and induced significantly higher antibody responses. The use of an attenuated PIV vector expressing RSV antigen to boost a primary immunization with an attenuated RSV warrants further evaluation.
KEYWORDS: DS-Cav1, fusion protein, mucosal vaccines, parainfluenza virus type 3, pediatric immunization, prefusion, prime boost, respiratory syncytial virus, vaccine
ABSTRACT
Live-attenuated pediatric vaccines for intranasal administration are being developed for human respiratory syncytial virus (RSV), an important worldwide pediatric respiratory pathogen that lacks a licensed vaccine or suitable antiviral drug. We evaluated a prime-boost strategy in which primary immunization with RSV was boosted by secondary immunization with RSV or with a chimeric recombinant bovine/human parainfluenza virus type 3 (rB/HPIV3) vector expressing the RSV fusion F protein. The vector-expressed F protein had been engineered (DS-Cav1 mutations) for increased stability in the highly immunogenic prefusion (pre-F) conformation, with or without replacement of its transmembrane and cytoplasmic tail domains with their counterparts from bovine parainfluenza virus type 3 (BPIV3) F protein to direct incorporation into the vector virion for increased immunogenicity. In hamsters that received a primary infection with RSV, a booster infection with RSV ∼6 weeks later was completely restricted for producing infectious virus but induced a significant increase in the serum RSV-plaque-reduction neutralizing antibody titer (RSV-PRNT). Boosting instead with the rB/HPIV3-RSV-pre-F vectors resulted in efficient replication and induced significantly higher RSV-PRNTs than RSV. In African green monkeys that received a primary infection with RSV, a booster infection with RSV ∼2, ∼6, or ∼15 months later was highly restricted, whereas booster infections with the vectors had robust replication. Compared with RSV, boosts with the vectors induced 7- to 15-fold higher titers of RSV-specific serum antibodies with high neutralizing activity, as well as significantly higher titers of RSV-specific mucosal IgA antibodies. These findings support further development of this heterologous prime-boost strategy.
IMPORTANCE Immune responses to RSV in infants can be reduced due to immunological immaturity and immunosuppression by RSV-specific maternal antibodies. In infants and young children, two infections with wild-type RSV typically are needed to achieve the titers of RSV-specific serum antibodies and protection against illness that are observed in adults. Therefore, a boost might substantially improve the performance of live pediatric RSV vaccines presently being developed. Hamsters and African green monkeys received a primary intranasal infection with RSV and were given a boost with RSV or a parainfluenza virus (PIV) vector expressing RSV fusion protein engineered for enhanced immunogenicity. The RSV boost was highly restricted but induced a significant increase in serum RSV-neutralizing antibodies. The PIV vectors replicated efficiently and induced significantly higher antibody responses. The use of an attenuated PIV vector expressing RSV antigen to boost a primary immunization with an attenuated RSV warrants further evaluation.
INTRODUCTION
Human respiratory syncytial virus (RSV) is the leading viral agent of severe acute respiratory infections in infants and young children worldwide (1). Every year, RSV is responsible for an estimated 118,200 deaths worldwide among children <5 years of age, with 99% of these deaths occurring in developing countries (2, 3). RSV disease and the associated economic burden also are substantial in developed countries (4, 5). While severe RSV disease has been commonly thought to occur predominantly in young infants <6 months of age, it was recently recognized that >50% of hospitalizations and in-hospital deaths of children with RSV occur among infants ≥6 months of age (2). Thus, RSV morbidity and mortality are frequent throughout infancy and young childhood. Infants at high risk for severe RSV disease due to premature birth or underlying illness can receive passive immunoprophylaxis with a monoclonal antibody against RSV called palivizumab, with substantial protective efficacy (6). However, this is not indicated for infants in general and is not cost-effective for use in resource-limited settings. A pediatric RSV vaccine is needed to reduce the morbidity and mortality associated with RSV infection. Despite decades of effort and recent progress, a licensed RSV vaccine is not yet available.
RSV is classified in the family Pneumoviridae of the order Mononegavirales. RSV is an enveloped virus with a nonsegmented negative-sense RNA genome of approximately 15,200 nucleotides. The genome is expressed as 10 separate mRNAs encoding 11 proteins. The 3′ to 5′ gene order (identified by encoded proteins) is as follows: nonstructural protein 1 (NS1), NS2, nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), small hydrophobic protein (SH), attachment protein (G), fusion protein (F), RNA synthesis factors M2-1 and M2-2 (encoded by overlapping open reading frames [ORFs] in the M2 mRNA), and polymerase protein (L); there also are short leader and trailer regions at the 3′ and 5′ genome ends, respectively. The RSV G protein is the major viral attachment protein. RSV F mediates fusion of the viral envelope with the cellular membrane during viral entry and may also have attachment activity (7).
The RSV F and G proteins are the two RSV neutralization antigens and the major protective antigens. F is generally considered to be a more potent neutralization and protective antigen than G, and its amino acid sequence is much more conserved among RSV strains. RSV F is produced in a prefusion (pre-F) conformation that is metastable and can be readily triggered to undergo a major irreversible conformational rearrangement that drives membrane fusion and leaves F in a highly stable postfusion (post-F) conformation (8–10). Pre-F and post-F share some neutralizing epitopes, but most of the neutralizing activity in convalescent human sera recognizes epitopes specific to pre-F (11). RSV F can be substantially stabilized in the pre-F conformation by structure-based engineering, such as by the introduction of a disulfide bond called DS and two hydrophobic cavity-filling amino acid substitutions called Cav1 (12). DS-Cav1-stabilized pre-F is substantially more immunogenic in rodents and nonhuman primates than post-F either as a subunit vaccine or expressed by a parainfluenza virus (PIV) vector (13).
Development of a pediatric RSV vaccine has been challenging. Inactivated RSV and purified RSV subunit vaccines are associated with enhancement of RSV disease in RSV-naive young children and experimental animals, respectively (14–16), and are contraindicated for administration to RSV-naive infants and young children (although they appear to be safe in RSV-experienced recipients). In contrast, two types of vaccines are not associated with priming for enhanced RSV disease, i.e., (i) live-attenuated RSVs (17) and (ii) a live vectored vaccine candidate consisting of chimeric recombinant bovine/human PIV type 3 (rB/HPIV3) expressing the RSV F protein from an added gene (18). These presently are being developed as candidate pediatric RSV vaccines for intranasal (i.n.) administration.
We have developed a number of attenuated derivatives of RSV strain A2 as candidate live-attenuated i.n. RSV vaccines (19–26) (reviewed in reference 27). The attenuating mutations in these viruses variously include deletion of the M2-2, NS2, NS1, or SH gene, as well as introduction of missense or codon-deletion mutations that mostly are in the L polymerase gene and usually confer temperature sensitivity in addition to attenuation (19, 20, 23–26). In addition, principal missense and codon-deletion mutations have been stabilized genetically by the identification of alternate codons involving that codon or nearby codons that render the mutations refractory to deattenuation (23, 24). Some of these attenuated viruses have been shown to be well tolerated and immunogenic in phase I studies in infants and young children (19–22).
We also have been pursuing the PIV vector strategy noted above to express RSV antigen, principally the F protein, from an added gene (13, 28–33). HPIV serotypes 1, 2, and 3 are important pediatric respiratory viruses, and HPIV3 is second only to RSV as a major cause of severe pediatric respiratory infection. The PIVs are enveloped nonsegmented negative-sense RNA viruses that are related to RSV and belong to the family Paramyxoviridae of the order Mononegavirales. We have focused on the chimeric rB/HPIV3 vector noted above. rB/HPIV3 consists of the bovine PIV3 (BPIV3) genome (which confers attenuation in humans and nonhuman primates by host range restriction) in which the BPIV3 F and hemagglutinin-neuraminidase (HN) glycoprotein genes (encoding the two PIV3 neutralization and major protective antigens) were replaced by their counterparts from human PIV3 (HPIV3). Compared with live-attenuated RSV, PIV vector-based RSV vaccines have a number of potential advantages, including providing a bivalent vaccine against the PIV vector and RSV, providing more efficient manufacture and greater physical stability, and providing the ability to express RSV F that has been engineered for increased stability in the nonfunctional and highly immunogenic pre-F conformation (e.g., DS-Cav1). The immunogenicity of RSV F protein expressed from a PIV vector also can be enhanced by replacing its transmembrane (TM) and cytoplasmic tail (CT) domains with those of the BPIV3 F protein (a modification called B3TMCT), which results in efficient incorporation of RSV F into the PIV vector virion (30). Yet another potential advantage is that a PIV-vectored RSV vaccine might be particularly effective in boosting following immunization with an RSV vaccine because, in addition to expressing an F protein engineered for enhanced immunogenicity, the PIV vector might be less sensitive to restriction by preexisting RSV-specific immunity, compared to an attenuated RSV (see Discussion). This would provide a greater antigenic load of RSV F and increased immunogenicity, compared to an attenuated RSV.
In the present study, we evaluated the boosting effect of rB/HPIV3 vectors expressing DS-Cav1-stabilized RSV pre-F protein, with or without the B3TMCT mutation, in comparison with RSV. Boosting was evaluated in hamsters and African green monkeys (AGMs) that previously had a primary RSV infection. In addition, the effect of different time intervals (∼2, ∼6, and ∼15 months) between the prime and boost was evaluated in AGMs. The results showed that, in either experimental animal and for any of the time intervals, a boost by a rB/HPIV3 vector expressing RSV F engineered for enhanced immunogenicity induced significantly higher titers of serum RSV-neutralizing antibodies, compared to a boost by RSV, particularly antibodies that are capable of neutralizing RSV in vitro without added complement and thus are particularly potent.
RESULTS
Comparison of booster immunization with RSV versus rB/HPIV3-RSV-pre-F vectors in hamsters previously infected with RSV.
Two rB/HPIV3 vectors were constructed in previous work (31), i.e., rB/HPIV3/DS-Cav1 (abbreviated DS-Cav1 vector), which expresses RSV F with greatly increased stability in the pre-F conformation due to the DS-Cav1 mutations, and rB/HPIV3/DS-Cav1/B3TMCT (abbreviated DS-Cav1/B3TMCT vector), which expresses RSV F with the same DS-Cav1 mutations plus replacement of its TM and CT domains with those of the BPIV3 F protein to achieve efficient incorporation into the vector virion (see the introduction). These two vectors were compared with wild-type (wt) RSV for the ability to infect and to induce a secondary or booster response of serum RSV-neutralizing antibodies in hamsters that previously had received a single primary i.n. infection with wt RSV (Fig. 1A). Wild-type RSV was used for the infections because attenuated RSV strains are poorly infectious in hamsters due to a strong host range restriction.
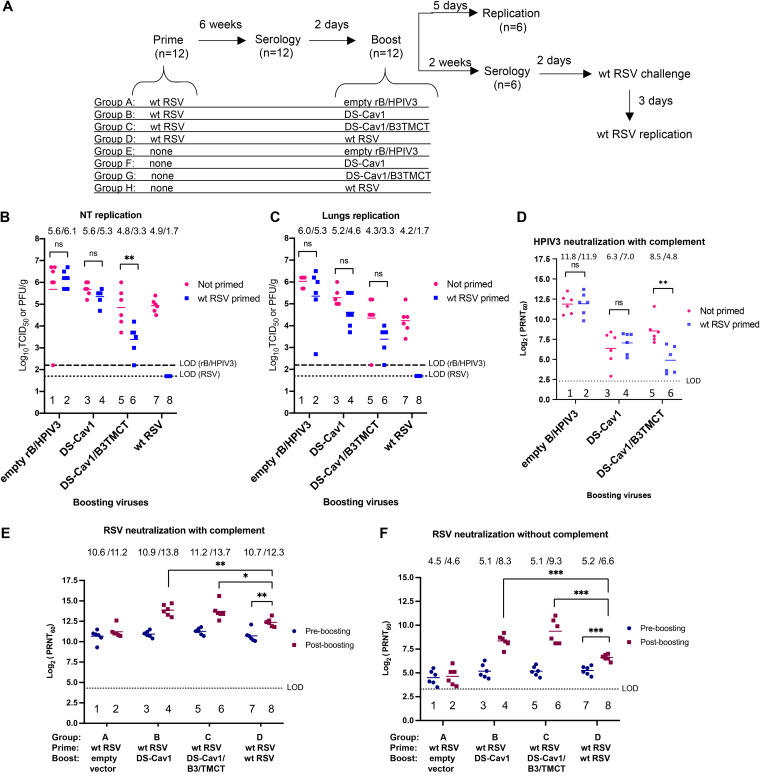
FIG 1.
Evaluation of a prime-boost regimen in hamsters. (A) Study design. Ninety-six female hamsters were confirmed to be RSV and HPIV3 seronegative and were assigned into eight groups of 12 animals each. On day 0, groups A to D were given a primary i.n. infection with 106 PFU of wt RSV in 0.1 ml of L15 medium, and groups E to H were left uninfected. Six weeks later, sera were collected for measurement of pre-boost 60% RSV-PRNTs. Two days later, primed and unprimed groups in pairs were boosted i.n. (105 TCID50 for vectors and 106 PFU for RSV) with one of the four viruses, i.e., (i) the empty rB/HPIV3 vector (groups A and E), (ii) DS-Cav1 vector (groups B and F), (iii) DS-Cav1/B3TMCT vector (groups C and G), or (iv) wt RSV (groups D and H). Five days after boosting, NTs and lungs of 6 hamsters per group were collected for virus titration. From the other 6 hamsters per group, sera were collected 2 weeks after boosting to measure RSV- and HPIV3-PRNTs; 2 days later, the animals were challenged i.n. with 106 PFU wt RSV. Three days following the challenge, NTs and lungs were collected for titration of RSV replication. (B and C) Titers of boosting viruses in NT (B) and lung (C) tissue homogenates 5 days post-boost. Dashed and dotted lines indicate the limit of detection (LOD) for the rB/HPIV3 vectors and wt RSV, respectively. (D) Serum HPIV3-PRNTs 2 weeks post-boost in primed and unprimed hamsters, assayed with complement. (E and F) Pre-boost and post-boost serum RSV-PRNTs in animals from groups A to D, which were primed with wt RSV and boosted with the indicated viruses, assayed with (E) and without (F) added complement. The significance of differences between the indicated comparisons was determined by Student's t test. ns, not significant (P > 0.05); *, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, 0.0001 < P < 0.001.
Forty-eight hamsters were confirmed to be seronegative for RSV and HPIV3 (see Materials and Methods), placed into four groups (groups A to D [n = 12 each]), and primed by i.n. inoculation with 106 PFU wt RSV per animal (Fig. 1A). Forty-eight additional RSV- and HPIV3-seronegative hamsters in four groups (groups E to H [n = 12 each]) were left unprimed for comparison.
Six weeks post-priming, sera were collected to measure RSV- and HPIV3-plaque-reduction neutralizing antibody titers (PRNTs). Two days later, pairs of primed and unprimed groups were boosted i.n. with (i) empty rB/HPIV3 vector (groups A and E), (ii) DS-Cav1 vector (groups B and F), (iii) DS-Cav1/B3TMCT vector (groups C and G), or (iv) wt RSV (groups D and H). The vectors were given at a dose of 105 times the 50% tissue culture infective dose (TCID50) per animal and wt RSV at a dose of 106 PFU. Five days after boosting, six hamsters per group were sacrificed and nasal turbinates (NTs) and lungs were harvested and assayed by immunoplaque assay (RSV) or limiting dilution assay (rB/HPIV3 vectors) to measure infectious virus titers. For the other six hamsters per group, sera were collected 2 weeks post-boost to measure RSV- and HPIV3-PRNTs. Two days later, the animals were challenged i.n. with wt RSV as described below.
Analysis of virus titers in unprimed animals 5 days following the boost showed that the DS-Cav1 and DS-Cav1/B3TMCT vectors replicated to lower titers than the empty vector in the NTs and lungs (Fig. 1B and C, lanes 3 and 5 versus lane 1), indicating attenuation due to the presence of the RSV F insert, as observed previously (28). The DS-Cav1/B3TMCT vector was somewhat more attenuated than the DS-Cav1 vector, in agreement with previous observations (30, 31) and consistent with the idea that incorporation of RSV F into the vector envelope might interfere with vector replication.
Comparison of RSV-primed versus unprimed animals (Fig. 1B and C, even lanes versus odd lanes) showed that the priming immunization completely restricted the replication of the wt RSV boost (Fig. 1B and C, lane 8 versus lane 7) but had no effect on replication of the empty rB/HPIV3 vector (Fig. 1B and C, lane 2 versus lane 1), consistent with the expectation that RSV-specific immunity would restrict the replication of RSV but not rB/HPIV3. Priming with RSV resulted in modest restriction (2-fold in the NTs and 4-fold in the lungs) of the replication the DS-Cav1 vector (Fig. 1B and C, lane 4 versus lane 3) and somewhat greater restriction of the DS-Cav1/B3TMCT vector (32-fold in the NTs and 10-fold in the lungs) (Fig. 1B and C, lane 6 versus lane 5), although the difference between primed and unprimed groups was statistically significant only for the DS-Cav1/B3TMCT vector in the NTs (Fig. 1B, lane 6 versus lane 5). These observations suggested that the expression of RSV F by rB/HPIV3 conferred a low level of sensitivity to restriction by RSV-specific immunity that was increased somewhat when RSV F was packaged into the vector virion (see Discussion).
Analysis of the titers of HPIV3-neutralizing antibodies in sera collected from unprimed animals 2 weeks following the boost showed that the presence of either RSV insert in the rB/HPIV3 vector was associated with a decrease in HPIV3-neutralizing antibodies, compared to the empty vector (Fig. 1D, lanes 3 and 5 versus lane 1), consistent with the decreased replication associated with the insert noted above. The presence of the DS-Cav1 insert did not detectably reduce HPIV3 immunogenicity in primed versus unprimed animals (Fig. 1D, lane 4 versus lane 3), but the DS-Cav1/B3TMCT insert resulted in a significant decrease in HPIV3 immunogenicity in primed versus unprimed animals (Fig. 1D, lane 6 versus lane 5), consistent with its restriction noted above.
Serum RSV-PRNTs induced by priming and boosting were measured by assays with or without added guinea pig complement (Fig. 1E and F). The addition of complement can confer steric hindrance and viral lysis activity to antibodies, resulting in enhanced detection of RSV-specific antibodies that might otherwise have poor neutralizing activity. The neutralization assay in the absence of added complement is a more stringent assay for “high-quality” antibodies that directly neutralize RSV (13, 30, 31). In the case of RSV, previous results showed that these appear to represent mainly pre-F epitopes and probably are of particular importance for vaccine development (30, 31).
The initial priming infection with wt RSV induced high serum RSV-PRNTs measured in the presence of complement and lower RSV-PRNTs measured in the absence of complement. Specifically, the sera collected from groups A to D 6 weeks following priming had mean serum RSV-PRNTs within the range of 10.6 log2 PRNT (1:1,552) to 11.2 log2 PRNT (1:2,353) measured with complement (Fig. 1E) and 4.5 log2 PRNT (1:23) to 5.2 log2 PRNT (1:37) measured without complement (Fig. 1F).
Boosting with wt RSV increased the mean serum RSV-PRNT measured with complement (Fig. 1E, lane 7 versus lane 8) by 3-fold, to a post-boost mean titer of 12.3 log2 units (1:5,043), and increased the mean serum RSV-PRNT measured without complement (Fig. 1F, lane 7 versus lane 8) also by 3-fold, to a post-boost mean titer of 6.6 log2 PRNT (1:97).
Boosting with the DS-Cav1 and DS-Cav1/B3TMCT vectors increased the mean serum RSV-PRNT measured with complement by 8- and 6-fold, respectively, to remarkably high post-boost mean titers of 13.8 log2 units (1:14,263) and 13.7 log2 units (1:13,308), respectively (Fig. 1E, lane 3 versus lane 4 for DS-Cav1 and lane 5 versus lane 6 for DS-Cav1/B3TMCT). Pre- and post-boost mean serum RSV-PRNTs measured without complement increased by 9- and 18-fold, respectively, to post-boost mean titers of 8.3 log2 units (1:315) and 9.3 log2 units (1:630), respectively (Fig. 1F, lane 3 versus lane 4 for DS-Cav1 vector and lane 5 versus lane 6 for DS-Cav1/B3TMCT vector).
The six animals per group used for post-boost serology also were challenged 2 days following serum collection by i.n. infection with 106 PFU of wt RSV per animal, NTs and lungs were collected 3 days postchallenge, and challenge RSV titers were determined by immunoplaque assay (see Materials and Methods) (data not shown). Animals that were not primed with RSV and were boosted with empty rB/HPIV3 vector (group E) and thus had no RSV-specific immunity had approximately 5.0 log10 PFU/g tissue of challenge RSV in the NTs and lungs. All of the hamsters in the other groups, which had been primed and/or boosted with wt RSV and/or rB/HPIV3 expressing RSV F, had no detectable infectious challenge RSV in the NTs and lungs (data not shown). Thus, all of the combinations of priming and/or boosting were highly protective in this semipermissive model for RSV replication and could not be distinguished based on protective efficacy.
In summary, when rB/HPIV3 vectors were used for the booster immunization, there was substantial vector replication and robust increases in serum RSV-PRNT, resulting in very high titers. In contrast, when wt RSV was used as a boost, its replication was highly restricted and the increase in RSV-PRNT was significantly lower. Furthermore, the fold increase in serum RSV-PRNT induced by the rB/HPIV3 vectors was greater for the high-quality RSV neutralizing serum antibodies measured without complement.
Comparison of booster immunizations in AGMs.
Booster immunizations also were evaluated in AGMs that, as part of other experiments outside the present report, had previously been primed by a single RSV infection by the combined i.n. and intratracheal (i.t.) routes (106 PFU per site). In those previous experiments, replication of RSV following the primary infection had been confirmed by RSV plaque titration of nasopharyngeal (NP) swab samples and tracheal lavage (TL) samples (data not shown). The viruses used in those primary immunizations were various attenuated derivatives of RSV strain A2 or the wt strain recombinant RSV (rRSV) A/Maryland/001/11 (which, like strain A2, is from subgroup A; see Materials and Methods). The priming RSVs are listed in Tables 1 to 3 and described below. Most of these viruses have advanced to clinical trials; in those cases, the ClinicalTrials.gov registration numbers are provided. Note that we treated all of these viruses as being equivalent with respect to priming, although we did distribute the animals that received the various viruses evenly among boosting groups.
TABLE 1.
Group assignments for AGMs that were boosted ∼2 months following priming (experiment 1)
| Animal | Sexa | Priming RSV | Boosting virus | Pre-boosting serum RSV-neutralizing titer (log2 PRNT)b | Post-boosting serum RSV-neutralizing titer (log2 PRNT)c |
|---|---|---|---|---|---|
| 9131 | M | 6120/F1G2/ΔNS1 | RSV 276 | 4 | 12.8 |
| 9128 | F | 6120/F1G2/ΔNS1 | RSV 276 | 8.1 | 13.6 |
| 9147 | M | 6120/ΔNS1 | RSV 276 | 6.5 | 11.2 |
| 9136 | F | 6120/ΔNS1 | RSV 276 | 8.8 | 13 |
| 9143 | F | A/Maryland/001/11 | RSV 276 | 10.3 | 12.4 |
| 9141 | M | A/Maryland/001/11 | RSV 276 | 10.8 | 11.9 |
| 9084 | M | 6120/F1G2/ΔNS1 | DS-Cav1/B3TMCT | 6.9 | 15.5 |
| 9135 | F | 6120/F1G2/ΔNS1 | DS-Cav1/B3TMCT | 5.5 | 15.3 |
| 9140 | M | 6120/ΔNS1 | DS-Cav1/B3TMCT | 7.8 | 13.1 |
| 9116 | F | 6120/ΔNS1 | DS-Cav1/B3TMCT | 7.4 | 13.8 |
| 9161 | M | A/Maryland/001/11 | DS-Cav1/B3TMCT | 10.2 | 14.6 |
| 9173 | F | A/Maryland/001/11 | DS-Cav1/B3TMCT | 11 | 14.6 |
M, male; F, female.
Sera were collected 2 weeks before boosting and were analyzed with the RSV PRN assay with added complement. Values are for individual animals. The mean pre-boosting serum RSV-neutralizing titers were 8.1 log2 PRNT for both groups.
Sera were collected 2 weeks after boosting and were analyzed with the RSV PRN assay with added complement. Values are for individual animals.
TABLE 2.
Group assignments for AGMs that were boosted ∼6 months following priming (experiment 2)
| Animal | Sexa | Priming RSV | Boosting virus | Pre-boosting serum RSV-neutralizing titer (log2 PRNT)b | Post-boosting serum RSV-neutralizing titer (log2 PRNT)c |
|---|---|---|---|---|---|
| 8926 | M | D46/NS2/N/ΔM2-2-HindIII | RSV 276 | 7.1 | 11.4 |
| 8983 | M | 6120/ΔNS2/1030s | RSV 276 | 8 | 11.4 |
| 8974 | F | 6120/F1BBG2/ΔNS1 | RSV 276 | 6.9 | 13.3 |
| 8951 | M | 6120/ΔNS1 | RSV 276 | 3.3 | 12 |
| 8960 | F | 6120/ΔNS1 | RSV 276 | 5.2 | 13.3 |
| 8918 | F | RSV 276 | RSV 276 | 5.7 | 12.3 |
| 8938 | F | D46/NS2/N/ΔM2-2-HindIII | DS-Cav1 | 6.7 | 14.8 |
| 8911 | M | D46/NS2/N/ΔM2-2-HindIII | DS-Cav1 | 4.3 | 16.2 |
| 8928 | F | 6120/ΔNS2/1030s | DS-Cav1 | 7.5 | 13.4 |
| 8994 | M | 6120/F1BBG2/ΔNS1 | DS-Cav1 | 4.5 | 14.3 |
| 8966 | M | 6120/F1BBG2/ΔNS1 | DS-Cav1 | 5.1 | 16.1 |
| 8992 | F | 6120/ΔNS1 | DS-Cav1 | 6.6 | 14.5 |
| 8913 | M | RSV 276 | DS-Cav1 | 7.3 | 13.6 |
| 9041 | F | D46/NS2/N/ΔM2-2-HindIII | DS-Cav1/B3TMCT | 7.1 | 14.9 |
| 9045 | M | 6120/ΔNS2/1030s | DS-Cav1/B3TMCT | 8.5 | 14.1 |
| 8940 | F | 6120/ΔNS2/1030s | DS-Cav1/B3TMCT | 6.7 | 12.9 |
| 8922 | F | 6120/F1BBG2/ΔNS1 | DS-Cav1/B3TMCT | 5.1 | 22.3 |
| 8904 | M | 6120/ΔNS1 | DS-Cav1/B3TMCT | 7.1 | 15.2 |
| 8902 | M | RSV 276 | DS-Cav1/B3TMCT | 3.7 | 14.4 |
| 8952 | M | RSV 276 | DS-Cav1/B3TMCT | 4.7 | 15.5 |
M, male; F, female.
Sera were collected 35 days before boosting and were analyzed with the RSV PRN assay with added complement. Values are for individual animals. The mean pre-boosting serum RSV-neutralizing titers were 6 log2 PRNT for the first two groups and 6.1 log2 PRNT for the third group.
Sera were collected 2 weeks after boosting and were analyzed with the RSV PRN assay with added complement. Values are for individual animals.
TABLE 3.
Group assignments for AGMs that were boosted ∼15 months following priming (experiment 3)
| Animal | Sexa | Priming RSV | Boosting virus | Pre-boosting serum RSV-neutralizing titer (log2 PRNT)b | Post-boosting serum RSV-neutralizing titer (log2 PRNT)c |
|---|---|---|---|---|---|
| 8903 | M | RSV 276 | RSV 276 | 6.2 | 14 |
| 8986 | F | RSV 276 | RSV 276 | 4.6 | 11.6 |
| 8900 | M | RSV 276 | DS-Cav1/B3TMCT | 5.2 | 15.9 |
| 9025 | F | RSV 276 | DS-Cav1/B3TMCT | 4.2 | 13.9 |
M, male; F, female.
Sera were collected 2 weeks before boosting and were analyzed with the RSV PRN assay with added complement. Values are for individual animals. The mean pre-boosting serum RSV-neutralizing titers were 5.4 log2 PRNT for the first group and 4.7 log2 PRNT for the second group.
Sera were collected 2 weeks after boosting and were analyzed with the RSV PRN assay with added complement. Values are for individual animals.
In three separate experiments (AGM experiments 1 to 3), the previously primed AGMs were given a single booster infection by the combined i.n. and i.t. routes with an attenuated RSV (106 PFU per site) or DS-Cav1/B3TMCT vector (106 TCID50 per site). In experiment 2, additional RSV-primed AGMs received DS-Cav1 vector (106 TCID50 per site). In all cases, the booster RSV was a live-attenuated vaccine candidate called RSV 276 (ClinicalTrials.gov registration numbers NCT03422237 and NCT03916185), which is a version of RSV strain A2 attenuated by deletion of most of the M2-2 ORF. RSV 276 differs by two noncoding nucleotides from the previously described MEDI/ΔM2-2 vaccine candidate that was highly attenuated in infants and young children (ClinicalTrials.gov registration number NCT01459198) (22).
For 12 animals (AGM experiment 1) (Fig. 2 and Table 1), the time interval between prime and boost was ∼2 months (specifically 51 days, which equals 2 months minus 9 days). For 20 other animals (AGM experiment 2) (Fig. 3 and Table 2), the time interval between prime and boost was ∼6 months (specifically 189 days, which equals 6 months plus 9 days). For the remaining 4 animals (AGM experiment 3) (Fig. 4 and Table 3), the time interval was ∼15 months (specifically 443 days, which equals 15 months minus 7 days).
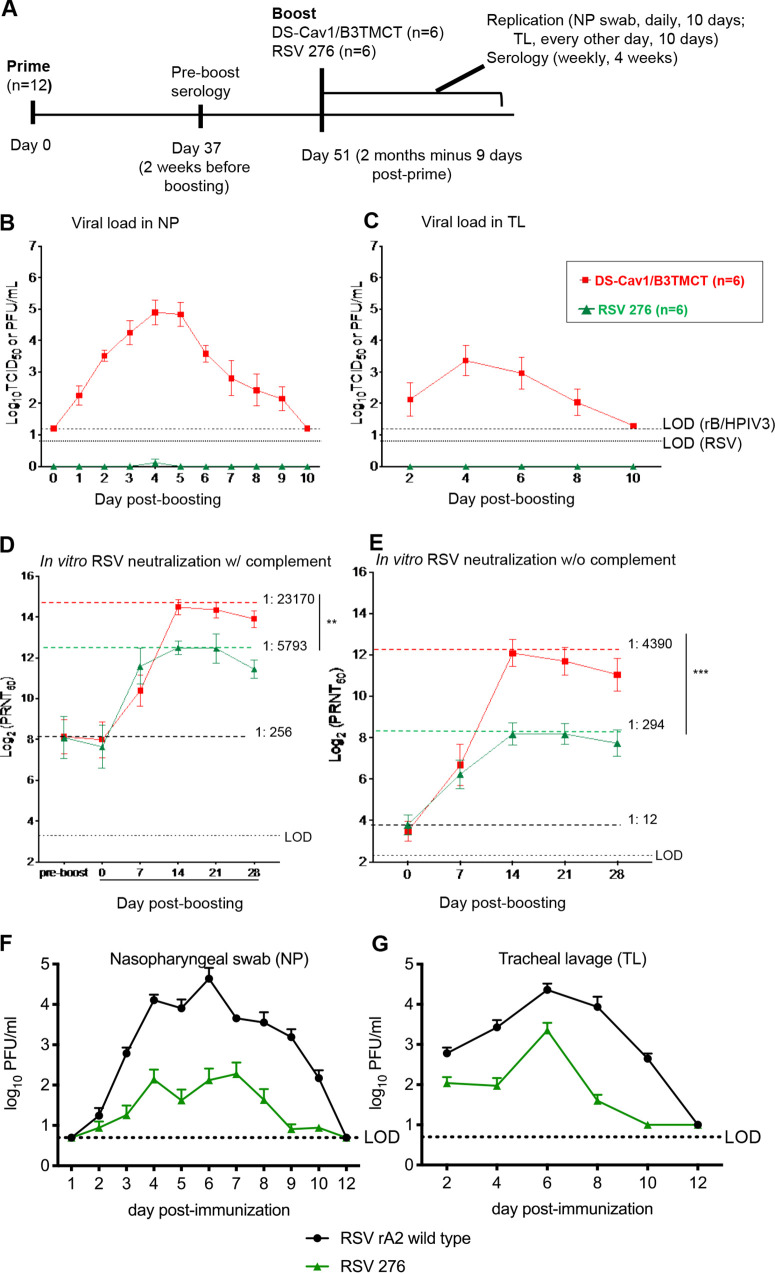
FIG 2.
AGM experiment 1: viral replication and serum RSV-PRNTs in AGMs when the interval between priming and boosting was ∼2 months (2 months minus 9 days). (A) Study design. Twelve AGMs were previously administered a primary infection with one of three RSVs (Table 1) by the combined i.n. and i.t. routes. Sera were collected on day 37 (2 weeks before boosting), and RSV-PRNTs were measured in the presence of complement. The AGMs were organized into two groups of 6 animals each that were balanced with regard to the day 37 RSV-PRNTs, the identity of the priming virus, and the sex ratio (Table 1). On day 51 (2 months minus 9 days) following priming, the groups were boosted with RSV 276 or DS-Cav1/B3TMCT vector by the combined i.n. and i.t. routes. Virus shedding was monitored on days 1 to 10 post-boost with NP and TL samples and virus titration. Sera were collected on days 7, 14, 21, and 28 post-boosting. (B and C) Viral titers in the NP (B) and TL (C) samples shown as means, with brackets indicating standard errors of means (SEMs), and LODs shown as dashed lines (vectors) and dotted lines (RSV). (D) Serum RSV-PRNTs at day 37 postpriming and days 0, 7, 14, 21, and 28 post-boosting, assayed in the presence of complement. (E) Serum RSV-PRNT at days 0, 7, 14, 21, and 28 post-boosting, assayed without complement. Panels D and E are annotated to show the mean serum RSV-PRNTs for the combined two groups at the time of boosting (black dashed lines, with mean arithmetic values shown); in addition, dashed colored lines indicate the highest mean serum RSV-PRNT for each group, with the arithmetic values shown. Mean serum RSV-PRNTs are shown with brackets indicating SEMs. Peak mean titers of two groups were compared by Student's t test. **, 0.001 < P < 0.01; ***, 0.0001 < P < 0.001. (F and G) Replication of RSV 276 and wt rRSV in RSV-seronegative AGMs. RSV-seronegative AGMs were infected by the combined i.n. and i.t. routes with 106 PFU of RSV 276 (n = 8) or wt rRSV (n = 4) in a 1-ml inoculum per site. NP (F) and TL (G) samples were collected daily and every second day, respectively, for 10 days and on day 12. Viral titers were determined by immunoplaque assay and are shown as group means for each time point. Brackets indicate the SEMs, and the LODs are shown as dotted lines. The 8 animals infected with RSV 276 here are the ones shown in Tables 2 and 3 that were subsequently boosted in AGM experiments 2 and 3.
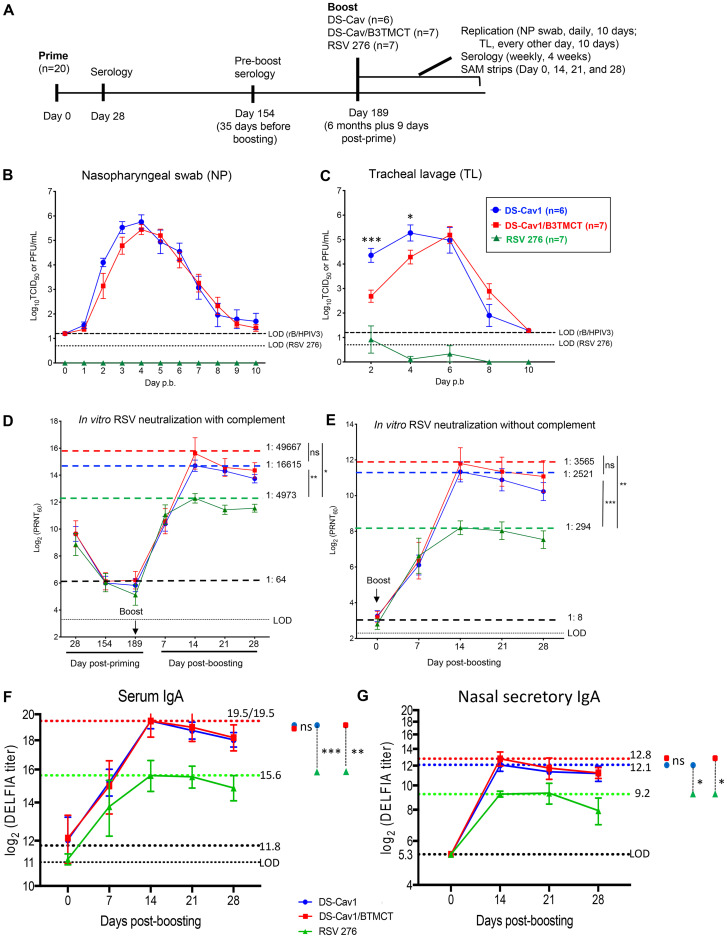
FIG 3.
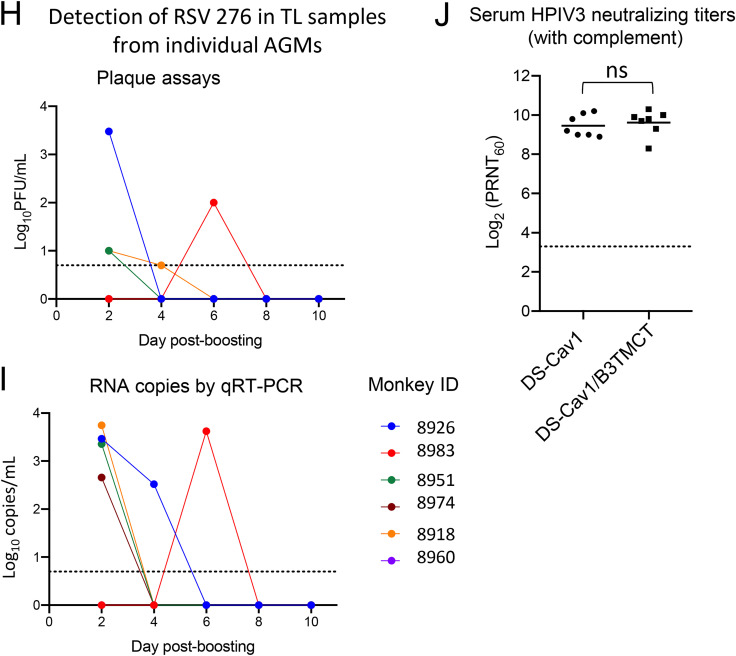
AGM experiment 2: viral replication and serum RSV-PRNTs in AGMs when the interval between priming and boosting was ∼6 months (6 months plus 9 days). (A) Study design. Twenty AGMs were previously administered a primary infection with one of five attenuated RSVs (Table 2) by the combined i.n. and i.t. routes. Sera were collected on days 28 and 154 (the latter being 35 days before boosting), and RSV-PRNTs were measured in the presence of complement. The AGMs were organized into three groups (n = 6, 7, and 7) that were balanced with regard to day 154 serum RSV-PRNTs, the identity of the priming virus, and the sex ratio (Table 2). On day 189 (6 months plus 9 days) postpriming, the groups were boosted with one of three viruses, i.e., (i) RSV 276 (n = 6), (ii) DS-Cav1 vector (n = 7), or (iii) DS-Cav1/B3TMCT vector (n = 7). Viral shedding and serum RSV-PRNTs were monitored as described in the legend to Fig. 2. Nasal mucosal lining fluid was collected for IgA analysis using SAM strips on days 0, 14, 21, and 28 post-boosting (see Materials and Methods for details). (B and C) Viral titers in the NP (B) and TL (C) samples shown as means, with brackets indicating SEMs, and LODs shown as dashed lines (vectors) and dotted lines (RSV). (D) Serum RSV-PRNTs at 28, 154, and 189 days postpriming, and 7, 14, 21, and 28 days post-boosting, assayed with complement. (E) RSV-PRNTs at 0, 7, 14, 21, and 28 days post-boosting, assayed without complement, shown as means, with brackets indicating SEMs. Panels D and E are annotated to show the mean serum RSV-PRNT for the combined three groups at the time of boost (black dashed lines, with mean arithmetic values given); in addition, dashed colored lines indicate the highest mean RSV-PRNT for each group, with arithmetic values given. (F and G) Serum and nasal mucosal IgA responses in AGMs. IgA antibody titers were measured in serum samples (F) collected on days 0, 7, 14, 21, and 28 post-boosting and nasal mucosal lining fluid samples (G) collected on days 0, 14, 21, and 28 post-boosting (note that one of the day 28 SAM specimens in the DS-Cav1/BTMCT group did not provide sufficient volume; therefore, the day 28 mean titer for the DS-Cav1/BTMCT vector was calculated for 6 animals only). IgA titers were measured by binding to purified RSV DS-Cav1 F protein in a DELFIA TRF assay. The antibody titers are given as the log2 dilution of serum or mucosal specimen yielding 400 fluorescence units, shown as means, with brackets indicating SEMs. Mean antibody titers for the combined three groups at the time of boost (day 0) are indicated by the larger black dotted lines; the titers of all SAM samples on day 0 were below the LOD (5.3 log2 units. The LODs are indicated by the smaller black dotted line. The dashed colored lines indicate the highest mean antibody titer for each group. Peak mean titers of three groups were compared pairwise by Student's t test (GraphPad Prism). *, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, 0.0001 < P < 0.001; ns, not significant (P > 0.05). (H and I) Replication of RSV 276 in the TL samples from AGMs primed with attenuated RSV ∼6 months earlier. The titers of RSV 276 in TL samples on days 2, 4, 6, 8, and 10 postboosting were quantified by immunoplaque assay (H) and RT-qPCR assays (I) specific for both positive- and negative-sense RSV M gene sequences. Each color represents an individual monkey. The LOD is indicated with a dotted line. (J) Serum HPIV3-PRNTs induced by DS-Cav1 and DS-Cav1/B3TMCT vectors in AGMs primed with live-attenuated RSV 6 months earlier. Sera collected 2 weeks after boosting with DS-Cav1 and DS-Cav1/B3TMCT vectors were analyzed by the 60% PRNT assay for HPIV3 with complement. The dots indicate individual animals; group means are indicated with short horizontal lines. The dotted line indicates the LOD. Mean serum HPIV3-PRNTs of two groups were compared by Student's t test. ns, not significant (P > 0.05).
FIG 4.
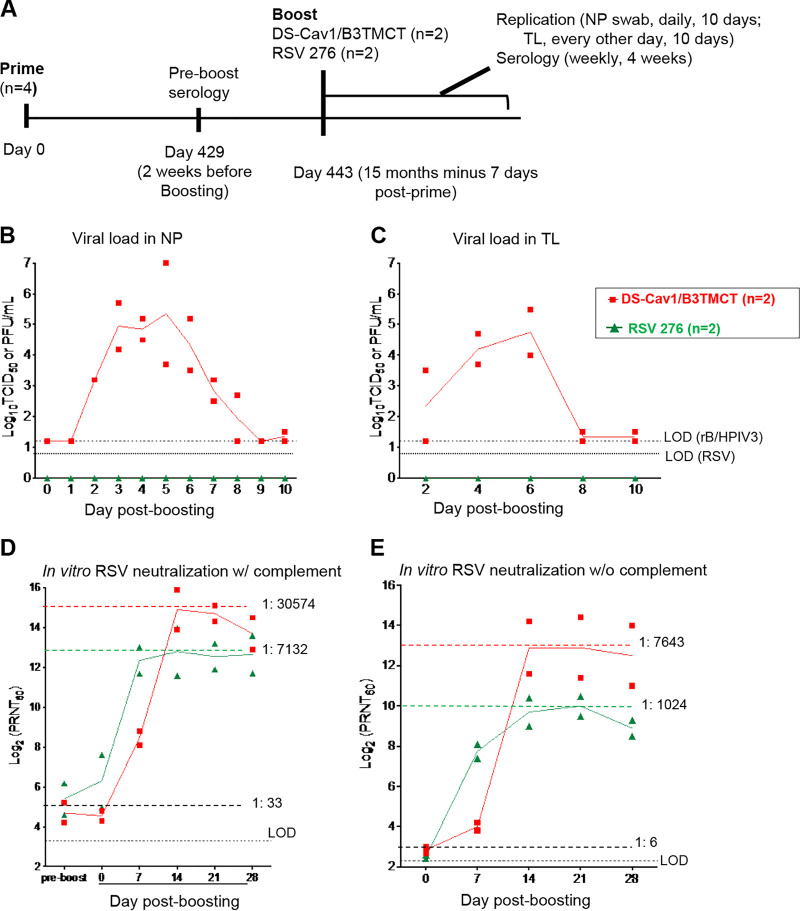
AGM experiment 3: viral replication and serum RSV-PRNTs in AGMs when the interval between priming and boosting was ∼15 months (15 months minus 7 days). (A) Study design. Four AGMs were previously administered a primary infection with the live-attenuated vaccine candidate RSV 276 (Table 3). Sera were collected on day 429 (2 weeks before boosting) and RSV-PRNTs were measured with complement. The AGMs were distributed into two groups of 2 animals each, such that the groups had similar individual and mean RSV-PRNTs based on the day 429 sera (Table 3). On day 443 (15 months minus 7 days) postpriming, sera were collected and the two groups were boosted with DS-Cav1/B3TMCT vector (n = 2) or RSV 276 (n = 2) administered i.n. and i.t. at dose of 106 TCID50 (DS-Cav1/B3TMCT vector) or106 PFU (RSV 276) per site. Viral replication and serological responses were monitored as described in the legends to Fig. 2 and 3. (B and C) Viral titers in the NP (B) and TL (C) samples shown as means, with LODs shown as dashed lines. Symbols indicate titers for individual animals, and the lines indicates mean values. (D) Serum RSV-PRNTs at day 429 postpriming and days 0, 7, 14, 21, and 28 post-boosting, assayed with complement. (E) Serum RSV-PRNTs at days 0, 7, 14, 21, and 28 post-boosting, assayed without complement. Panels D and E are annotated to show the mean serum RSV-PRNTs for the combined two groups at the time of boosting (black dashed lines, with the mean arithmetic values shown); in addition, panels D and E are annotated with dashed colored lines that indicate the highest mean serum RSV-PRNT for each group, with the arithmetic values shown.
In each of the three experiments, replication of each boosting virus was monitored by the collection of NP and TL samples daily and every other day, respectively, for 10 consecutive days following the boost, and virus titers were determined by immunoplaque assay (RSV) or limiting dilution (rB/HPIV3 vectors) (see Materials and Methods). Sera were collected on ≥1 days prior to the day of the boost (depending on the experiment, as described below), on the day of the boost, and every 7 days thereafter for 4 consecutive weeks, and PRNTs were determined.
AGM experiment 1: booster immunization of AGMs ∼2 months (2 months minus 9 days) following priming with RSV.
Twelve AGMs were available that had previously received a single priming immunization with one of three different RSVs (Table 1), i.e., (i) RSV 6120/ΔNS1, an NS1 gene deletion mutant (ClinicalTrials.gov registration number NCT03596801); (ii) RSV 6120/F1G2/ΔNS1, a derivative of the preceding virus in which the RSV F and G genes were moved to the first and second genome positions in order to increase their expression (ClinicalTrials.gov registration number NCT03596801); or (iii) the subgroup A wt strain rRSV A/Maryland/001/11 (ClinicalTrials.gov registration number NCT03624790) (see Materials and Methods). On day 37 postpriming (2 weeks before boosting), sera were collected and analyzed to measure RSV-PRNT in the presence of complement and to confirm HPIV3 seronegativity. The 12 AGMs were redistributed into two groups of 6 animals each, organized so that the two groups had similar numbers of animals with high, medium, or low day 37 serum RSV-PRNTs, had essentially identical group mean RSV-PRNTs, and were balanced with regard to the identities of the priming viruses and sex ratio (Table 1).
On day 51 (2 months minus 9 days) postpriming, one group was boosted with RSV 276 and the other with DS-Cav1/B3TMCT vector. NP, TL, and serum specimens were collected to evaluate virus shedding and immunogenicity (Fig. 2B to E). Shedding of RSV 276 was detected only in trace amounts on a single day in the NP samples and was not detected in the TL samples. It was evident that the very low level of shedding of RSV 276 was due to restriction by RSV-specific immunity in the RSV-primed animals because, in a previous experiment, infection of 4 RSV-seronegative AGMs with RSV 276 resulted in substantial viral shedding over a period of 10 days, with the highest peak mean titers approaching 3.0 log10 PFU/ml in NP and TL samples (Fig. 2F and G). In contrast, shedding of DS-Cav1/B3TMCT vector in the RSV-primed animals was substantial over 10 days in both the NP and TL samples, with peak mean titers of approximately 5.0 log10 TCID50/ml in the NP samples and 3.4 log10 TCID50/ml in the TL samples (Fig. 2B and C).
The mean pre-boost serum RSV-PRNT for all 12 animals measured with complement was 1:256 (Fig. 2D). Boosting with RSV 276 and the DS-Cav1/B3TMCT vector increased the serum RSV-PRNTs measured with complement by 22-fold and 91-fold over the pre-boost titer to peak mean titers of 1:5,793 and 1:23,170, respectively (occurring on day 14 post-boost), which were significantly different (Fig. 2D). The peak mean postboost serum RSV-PRNT for the vector was 4-fold greater than that for RSV 276.
Measured in the absence of complement, the mean pre-boost serum RSV-PRNT for all 12 animals was 1:12 (Fig. 2E). Boosting with RSV 276 and the DS-Cav1/B3TMCT vector increased the titer by 25-fold and 366-fold, respectively, to peak mean titers of 1:294 and 1:4,390, respectively (occurring on day 14 post-boost) (Fig. 2E), which were significantly different. The peak mean post-boost serum RSV-PRNT for the vector was 15-fold greater than that for RSV 276. Thus, the vector had a stronger boosting effect than RSV 276, particularly for high-quality neutralizing antibodies detected without complement.
AGM experiment 2: booster immunization of AGMs ∼6 months (6 months plus 9 days) following priming with RSV.
Twenty other AGMs were available that had previously received a single priming immunization with one of five different RSVs (Table 2), i.e., (i) RSV D46/NS2/N/ΔM2-2-HindIII, a M2-2 deletion mutant (ClinicalTrials.gov registration numbers NCT03099291 and NCT03102034); (ii) RSV 6120/ΔNS2/1030s, a NS2 deletion mutant with a genetically stabilized temperature sensitivity (ts) mutation in the L polymerase (ClinicalTrials.gov registration numbers NCT03387137 and NCT03916185); (iii) RSV 6120/ΔNS1, an NS1 deletion mutant (ClinicalTrials.gov registration number NCT03596801) that also was one of the priming viruses in experiment 1 but in different AGMs; (iv) RSV 6120/F1BBG2/ΔNS1, a version of the preceding 6120/ΔNS1 mutant in which the F gene was codon optimized for increased translation and the F and G genes were moved to the first and second genome positions, respectively, for increased expression; or (v) RSV 276 (described above; ClinicalTrials.gov registration numbers NCT03422237 and NCT03916185), which also is the attenuated ΔM2-2 RSV used in the boosts, as already noted.
Following the primary immunization, sera were collected on days 28 and 154 and analyzed to measure RSV-PRNTs in the presence of complement, as well as to confirm HPIV3 seronegativity (1 animal, AG 8960 [Table 2], was found to be HPIV3 seropositive and therefore was assigned to be boosted with RSV rather than a rB/HPIV3 vector). The 20 AGMs were redistributed into three groups (n = 6, 7, and 7) organized to balance the day 154 serum RSV-PRNTs, the identities of the priming viruses, and the sex ratio (Table 2). On day 189 (6 months plus 9 days) postpriming, the three groups were boosted with RSV 276, DS-Cav1 vector, or DS-Cav1/B3TMCT vector. NP, TL, and serum specimens were collected to evaluate virus shedding and immunogenicity (Fig. 3).
Shedding of RSV 276 was undetectable in the NP samples by immunoplaque assay (Fig. 3B), whereas there was low, sporadic detection of RSV 276 in the TL samples (Fig. 3C). Evaluation of TL specimens for a number of animals by reverse transcription-quantitative PCR (RT-qPCR) (that detected both positive- and negative-sense RSV M RNA) was more sensitive, yielding a greater number of samples positive for RSV RNA, with titers up to 450 to 5,500 RNA molecules/ml (Fig. 3H and I). In contrast, shedding of the DS-Cav1 and DS-Cav1/B3TMCT vectors was detected abundantly by limiting dilution assay in the NP and TL samples over a period of 8 to 10 days, with similar peak mean titers of approximately 5.5 log10 TCID50/ml in the NP samples and 5.0 log10 TCID50/ml in the TL samples. The DS-Cav1/B3TMCT vector appeared to replicate slightly more slowly than the DS-Cav1 vector, but this difference was significant only on days 2 and 4 in the TL samples (Fig. 3C). The two vectors eventually reached similar peak NP and TL sample titers (Fig. 3B and C).
When measured by neutralization assays with complement (Fig. 3D), the mean serum RSV-PRNT in the 20 AGMs induced by the primary immunization with the attenuated RSVs approached 10 log2 units (1:1,024) at day 28 (Fig. 3D) and then decreased to 1:64 on day 189 when the boost was given. Boosting with RSV 276, DS-Cav1 vector, and DS-Cav1/B3TMCT vector increased the peak mean serum RSV-PRNTs measured with complement by 78-fold, 260-fold, and 776-fold, respectively, to peak mean titers of 1:4,973, 1:16,615, and 1:49,667, respectively (occurring 14 days post-boost) (Fig. 3D), which were significantly different for each vector versus RSV 276. The peak mean post-boost serum RSV-PRNTs for the DS-Cav1 and DS-Cav1/B3TMCT vectors were 3-fold and 10-fold higher, respectively, than that for RSV 276. The DS-Cav1/B3TMCT vector appeared to be slightly more immunogenic than the DS-Cav1 vector despite its slightly slower replication noted above, which might be due to the B3TMCT modification, but the difference was not statistically significant (Fig. 3D).
When measured by the plaque-reduction neutralization (PRN) assay without complement (Fig. 3E), the mean pre-boost serum RSV-PRNT in the 20 AGMs was 1:8. Boosting with RSV 276, DS-Cav1 vector, and DS-Cav1/B3TMCT vector increased the peak mean serum RSV-PRNT by 37-fold, 315-fold, and 446-fold, respectively, to titers of 1:294, 1:2,521, and 1:3,565, respectively (occurring 14 days post-boost), which were significantly different for each vector versus RSV 276. The peak mean post-boost serum RSV-PRNTs for the DS-Cav1 and DS-Cav1/B3TMCT vectors were 9-fold and 12-fold higher, respectively, than that for RSV 276.
The ability of boosts with the DS-Cav1 and DS-Cav1/B3TMCT vectors to induce serum HPIV3-neutralizing antibodies was compared using HPIV3 neutralization assays with added complement (Fig. 3J). Note that the animals were seronegative for HPIV3 before the boosts, and thus these inoculations were primary immunizations with respect to HPIV3. The two vectors induced very similar peak mean serum HPIV3-PRNTs (1:724 and 1:776, respectively, occurring on day 14 post-inoculation), indicating that they were essentially equally immunogenic for HPIV3. This is in agreement with the observation that the two vectors replicated to similar peak titers in AGMs (Fig. 3B and C).
We also compared the ability of the boosts with the DS-Cav1 and DS-Cav1/B3TMCT vectors versus RSV 276 to induce serum and mucosal IgA antibodies that bind RSV DS-Cav1 F protein (Fig. 3F and G). We established a highly sensitive dissociation-enhanced lanthanide fluorescence immunoassay (DELFIA) time-resolved fluorescence (TRF) immunoassay to detect monkey IgA binding to purified recombinant RSV DS-Cav1 F protein as antigen. The IgA titers are expressed as the log2 dilution yielding 400 fluorescence units in the DELFIA TRF assay. Boosting with the two vectors induced strong serum IgA responses; peak mean titers following boosts with the two vectors were identical (19.5 log2 units) and were about 16-fold higher than those induced by boosting with RSV 276 (15.6 log2 units). The peak serum IgA response was detected at 14 days post-boost, coinciding with the peak serum RSV-neutralizing antibody response. Since the respiratory mucosal antibody response is considered to be particularly effective in limiting RSV infection, we also evaluated the nasal mucosal IgA response to the boosts. Nasal mucosal lining fluid was collected using absorptive membranes (synthetic adsorptive matrix [SAM] strips). This method provides relatively concentrated mucosal samples, suitable for detecting IgA (Fig. 3G). In all groups, the peaks of the mucosal IgA responses were at day 14 following the boosts (Fig. 3G), coinciding with the peaks of the serum IgA (Fig. 3F) and the serum RSV-neutralizing antibodies (Fig. 3D and E). Again, the DS-Cav1 and DS-Cav1/B3TMCT vectors induced similarly strong responses (peak mean titers of 12.1 and 12.8 log2 units, respectively), whereas the response to RSV 276 was about 8-fold lower (peak mean titer of 9.2 log2 units). Thus, the DS-Cav1 and DS-Cav1/B3TMCT vectors significantly exceeded RSV 276 in their ability to boost the serum and mucosal IgA antibody responses to the RSV pre-F protein.
AGM experiment 3: booster immunization of AGMs ∼15 months (15 months minus 7 days) following priming with RSV.
Four other AGMs were available that had previously received a single primary immunization with RSV 276 (Table 3). On day 429 following the primary infection (and 2 weeks before boosting), sera were collected and analyzed to determine RSV-PRNTs in the presence of complement and to confirm HPIV3 seronegativity. The AGMs were distributed into two groups (n = 2 each) that were balanced with regard to serum RSV-PRNTs and sex ratios (Table 3). On day 443 (15 months minus 7 days) postpriming, the two groups were boosted with RSV 276 or DS-Cav1/B3TMCT vector, and viral replication and serological responses were monitored as described above.
Replication of RSV 276 following the ∼15-month interval was highly restricted, with no virus detected by immunoplaque assay of the NP and TL samples, whereas replication of the DS-Cav1/B3TMCT vector was robust (Fig. 4B and C). The mean pre-boost serum RSV-PRNT for the four animals measured with complement was 1:33 (Fig. 4D). Boosting with RSV 276 and DS-Cav1/B3TMCT vector increased the peak mean serum RSV-PRNTs measured in the presence of complement by 216-fold and 926-fold, respectively, to titers of 1:7,132 and 1:30,574 (occurring on day 14) (Fig. 4D). The peak mean post-boost serum RSV-PRNT for the vector was 4-fold higher than that for RSV 276.
The mean pre-boost serum RSV-PRNT for the four animals measured without complement was 1:6 (Fig. 4E). Boosting with RSV 276 and the DS-Cav1/B3TMCT vector increased the peak mean serum RSV-PRNTs by 171-fold and 1,274-fold, respectively, to titers of 1:1,024 and 1:7,643, respectively (occurring on day 14) (Fig. 4E). The peak mean post-boost serum RSV-PRNT for the vector was 7-fold higher than that for RSV 276.
RSV serum antibodies suppressed DS-Cav1/B3TMCT vector replication in vitro.
Because the replication of the DS-Cav1/B3TMCT vector in hamsters was significantly reduced in the NTs by RSV-specific immunity from the primary infection (Fig. 1B to D), we investigated whether replication of this vector in vitro could be inhibited by RSV-neutralizing antibodies. LLC-MK2 cells were infected with the empty rB/HPIV3 vector or with the DS-Cav1 or DS-Cav1/B3TMCT vector at a multiplicity of infection (MOI) of 0.01 and then incubated with medium containing 10% heat-inactivated (56°C for 30 min) serum from uninfected hamsters or from hamsters infected with wt RSV or rB/HPIV3 (from the experiment in Fig. 1), in the absence of added complement (Fig. 5). A low MOI was used to allow multiple rounds of replication, so that any antibody-mediated inhibition should be detected. Replication of the vectors in the presence of added hamster sera was monitored by taking a small aliquot of culture medium supernatant daily for 3 consecutive days for virus titration.
FIG 5.
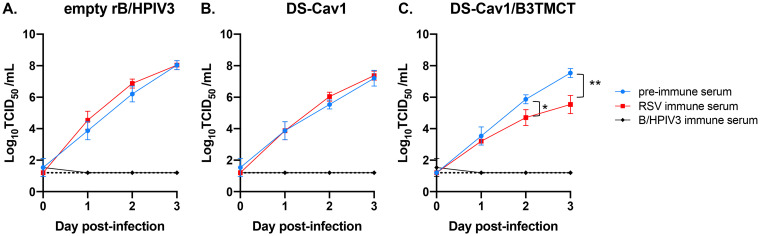
Multicycle replication of rB/HPIV3 vectors in vitro in the presence or absence of RSV-neutralizing antibodies. LLC-MK2 cells were infected with empty rB/HPIV3 vector (A), DS-Cav1 vector (B), or DS-Cav1/B3TMCT vector (C) at an MOI of 0.01 TCID50 per cell. After adsorption for 1 h, cells were washed three times with cell culture medium and then incubated, in the absence of added complement, with culture medium containing 10% of one of the following sera (which had been previously heated at 56°C for 30 min): preimmune hamster serum (blue) or pooled sera from hamsters infected with wt RSV (red) or with empty rB/HPIV3 vector (black). The hamster sera were from the experiment in Fig. 1. The different treatments were performed in triplicate. An aliquot of medium was taken daily for 3 consecutive days after infection and flash-frozen, and viral titers were determined. The significance of difference between the replication in the presence of the preimmune serum (blue) and RSV immune serum (red) was determined by Student’s t tests. *, P < 0.05; **, P < 0.01.
As expected, rB/HPIV3-immune serum completely inhibited the replication of all three vector constructs (Fig. 5A to C, black curve), while the preimmune serum, collected prior to the primary immunization, did not affect the replication of any construct (Fig. 5A to C, blue curve). RSV-immune serum had no effect on the replication of empty rB/HPIV3 vector (Fig. 5A, red curve) or DS-Cav1 vector (Fig. 5B, red curve), but it significantly reduced the replication of the DS-Cav1/B3TMCT vector by days 2 and 3, the latter by 100-fold (Fig. 5C, red curve). These results indicated that RSV-specific serum antibodies inhibited replication of the DS-Cav1/B3TMCT vector but not the DS-Cav1 vector.
DISCUSSION
Live-attenuated RSV candidates under development include ones with gene deletions, temperature sensitivity mutations, and gene rearrangements (34; reviewed in reference 35). Replication-competent vectors include human PIV3 and PIV1 (31, 32, 36), murine PIV1 (Sendai virus) (37), and murine pneumonia virus (38) engineered to express RSV F protein from an added gene. Live vectors expressing RSV G protein are also being developed (39, 40).
Among the many potential approaches to developing a pediatric RSV vaccine, two approaches described in the present report are (i) attenuated derivatives of RSV and (ii) rB/HPIV3 expressing the RSV F protein from an added gene (see the introduction). Both approaches have provided promising candidates presently under preclinical and clinical evaluation for primary immunization, but their usefulness for booster immunization was less clear. In previous pediatric clinical studies, live-attenuated RSVs as vaccine candidates were inefficient (at least with regard to inducing increases in RSV-specific serum antibodies) in booster immunizations administered in successive doses over several months. For example, when the live-attenuated RSV called MEDI-559 was given to infants and young children in three successive doses at 2-month intervals, the second and third doses generally were poorly infectious and inefficient in boosting serum antibody responses in subjects who had a “take” with a previous dose (41). Similar findings were made when the live-attenuated RSV cpts248/404 was given to infants and young children in two successive doses at an interval of 4 to 6 weeks (42). Similar findings have been observed with attenuated versions of PIV3 (which has broad similarities to RSV in vaccinology) given in successive doses at intervals of up to 6 months (18, 43–45). Thus, in a “homologous” prime-boost (i.e., successive doses of the same vaccine virus), attenuated RSV (or PIV3) usually is infectious and substantially immunogenic for serum virus-neutralizing antibodies only for the first take, at least within a time interval of ≤6 months. Incidentally, the situation is different when the secondary infection is with wt RSV rather than an attenuated RSV. Specifically, in vaccine trials in which subjects received an attenuated RSV during the summer and had natural exposure to community wt RSV during the following winter, there were 20- to 40-fold boosts in serum RSV-PRNTs (22). Thus, the poor immunogenicity of repeat doses of attenuated RSVs is largely due to their attenuation.
As an alternative, in the present study we investigated a “heterologous” prime-boost strategy. With this strategy, a primary immunization with RSV was boosted by a secondary immunization with rB/HPIV3 expressing RSV F protein from an added gene. Each of the experiments employed the DS-Cav1/B3TMCT vector, which expresses RSV F containing the DS-Cav1 mutations (which substantially stabilize the pre-F conformation) together with the B3TMCT modification (which mediates efficient packaging of RSV F into the vector virion). Some experiments also employed the DS-Cav1 vector, which expresses RSV F containing the DS-Cav1 mutations alone. We hypothesized that a PIV vector might be more effective than RSV in boosting RSV-specific immunity because, while replication and antigen expression by an attenuated RSV are highly restricted by preexisting RSV-specific immunity, a PIV vector is antigenically unrelated to RSV and would be anticipated to be unrestricted by RSV-specific immunity. However, there were possible caveats. While the rB/HPIV3 vector itself is antigenically distinct from RSV, it does express an RSV antigen, in this case the F protein. This might be targeted by RSV-specific immunity, including RSV-specific serum antibodies, as measured in the present study, as well as other effectors that historically have been less well characterized, including RSV-specific mucosal antibodies and cellular immunity. With regard to RSV-specific antibodies, binding to RSV F expressed by the vector probably would not directly block vector infection and spread because the RSV F in this study was largely nonfunctional for fusion due to the DS-Cav1 mutations and thus presumably would have minimal or no contribution to vector replication and spread. However, the expression of RSV F in vector-infected cells might target those cells for destruction by cytotoxic T cells or antibody-dependent cell-mediated cytotoxicity. In this regard, a previous study provided indirect evidence that cellular immunity induced by PIV infection in hamsters conferred resistance to challenge, and it showed that this resistance waned by 4 months following immunization (46). In addition, as noted, the DS-Cav1/B3TMCT vector used in all of experiments in the present study expressed the B3TMCT version of RSV F protein, which was efficiently packaged in the vector virion, and this might target the vector virion for destruction (e.g., by opsonization). Binding of antibodies to packaged RSV F might also create steric hindrance that interferes with the vector HN and F proteins during attachment and entry. It also was possible that antibody responses to RSV F might be suppressed by preexisting F-specific antibodies.
We first compared homologous and heterologous boosting in hamsters. When hamsters were given a primary infection with wt RSV and a homologous booster infection with wt RSV ∼6 weeks later, there was no detectable infectious RSV in harvested NT and lung tissue, consistent with the expectation that RSV-specific immunity would severely restrict replication of an RSV boost. However, there was a significant, ∼3-fold increase in serum RSV-PRNTs measured with or without added complement, suggesting that some infection and antigen expression had occurred (we did not formally show that the increases in serum RSV-PRNTs in this experiment were dependent on RSV replication, but that typically is the case [47]). In hamsters boosted with the empty rB/HPIV3 vector, there was no difference in virus titers in the NTs and lungs between RSV-primed animals and unprimed animals, showing that RSV-specific immunity indeed did not restrict the rB/HPIV3 backbone. In contrast, the replication of the DS-Cav1 and DS-Cav1/B3TMCT vectors exhibited a modest amount of restriction in RSV-primed animals versus unprimed animals, although the difference was significant only in the case of the DS-Cav1/B3TMCT vector in the NTs. This suggests that expression of the unpackaged version of the RSV F protein resulted in a marginal restriction of the vectors by RSV-specific immunity, and expression of the packaged version of RSV F protein resulted in a somewhat greater restriction. This was associated with a significant decrease in the induction of serum HPIV3-neutralizing antibodies by the DS-Cav1/B3TMCT vector (but not the DS-Cav1 vector) in RSV-primed versus unprimed hamsters, providing another indication of reduced DS-Cav1/B3TMCT vector replication. In the RSV-primed hamsters, the DS-Cav1 and DS-Cav1/B3TMCT vectors induced increases in serum RSV-PRNTs assayed with complement of 8- and 6-fold, respectively, versus 3-fold for wt RSV; when assayed without complement, the increases were 9-and 18-fold, respectively, versus 3-fold. The resulting peak mean post-boost serum RSV-PRNTs assayed with and without complement were significantly greater for the vectors than for wt RSV. Thus, these results in hamsters showed that (i) homologous boosts with wt RSV were highly restricted and modestly immunogenic; (ii) the empty rB/HPIV3 vector was unrestricted by RSV-specific immunity; (iii) expression of RSV F by rB/HPIV3 conferred a low level of sensitivity to restriction by RSV-specific immunity, which became significant when RSV F was packaged into the vector virion; and (iv) boosts with the vectors were significantly more immunogenic than with wt RSV.
Boosting also was evaluated in AGMs that previously had a primary RSV infection. Three different time intervals between the primary RSV infection and the boost were evaluated, ∼2, ∼6, and ∼15 months. It was anticipated that pre-boost host immunity might diminish with increasing intervals, which might affect the efficiency of the boost. Indeed, the mean pre-boost serum RSV-PRNTs diminished progressively in the ∼2-, ∼6-, and ∼15-month groups, with respective titers of 1:256, 1:64, and 1:33 measured with complement and 1:12, 1:8, and 1:6 measured without complement. It is reasonable to suppose that other immune effectors induced by the primary RSV infection, such as mucosal antibodies and cellular immunity, also diminished with time.
The replication of the RSV 276 boost was highly and nearly equally restricted following each time interval, with only sporadic traces of shed infectious virus. Thus, there was no difference between the time intervals in the ability to strongly restrict this attenuated RSV. Although shedding of infectious RSV was highly restricted, there presumably was some viral infection and antigen expression leading to the observed secondary immune response. This is supported by the RT-qPCR assay performed in experiment 2, which detected shedding of viral nucleic acid, possibly including progeny virus that was neutralized by secretory antibodies and not detected by an infectivity assay.
In contrast to RSV, the DS-Cav1/B3TMCT vector replicated robustly. The peak mean titers of vector in the NP and TL samples were very similar for experiments 2 and 3, ranging from 4.8 to 5.4 log10 TCID50/ml. This level of shedding of DS-Cav1/B3TMCT vector was similar to that observed previously in RSV- and HPIV3-seronegative rhesus macaques (31), suggesting that there was little or no restriction of the vector by preexisting immunity to RSV following the ∼6-month and ∼15-month time intervals. In contrast, for experiment 1 (∼2-month time interval), the titers of shed DS-Cav1/B3TMCT vector were lower by approximately 0.5 log10 TCID50/ml in the NP samples and by 1.4 to 1.8 log10 TCID50/ml in the TL samples (Table 4). This might indicate that expression of RSV F by this vector, and the packaging of RSV F into the vector virion, conferred a moderate amount of sensitivity to restriction by RSV-specific immunity following the shortest interval, similar to the restriction of the DS-Cav1/B3TMCT vector in RSV-primed versus unprimed hamsters. Since cellular immunity induced by a primary infection may persist only for several months, as noted above for the hamster model, this might have contributed to the restriction of the DS-Cav1/B3TMCT vector observed at ∼2 months but not at ∼6 and ∼15 months. In any event, the restriction following the ∼2-month interval was small and not statistically significant.
TABLE 4.
Preboost serum RSV-neutralizing titers in AGMs boosted with DS-Cav1/B3TMCT and peak mean titers of post-boosting replication and RSV-neutralizing antibodies in 2-month, 6-month, and 15-month study cohorts
| Study cohort | Pre-boosting serum RSV-neutralizing titer (log2 PRNT) (mean ± SEM)a | Peak titer in NP samples (TCID50/ml) (mean ± SEM)b | Peak titer in TL samples (TCID50/ml) (mean ± SEM)b | Post-boosting serum RSV-neutralizing titer with complement (log2 PRNT) (mean)c | Post-boosting serum RSV-neutralizing titer without complement (log2 PRNT) (mean)c |
|---|---|---|---|---|---|
| ∼2 mo | 8.1 ± 2.1 | 4.9 ± 1.0 | 3.4 ± 1.2 | 14.5 | 12.9 |
| ∼6 mo | 6.1 ± 1.7 | 5.4 ± 0.5 | 5.2 ± 0.9 | 15.6 | 11.8 |
| ∼15 mo | 4.7 ± 0.7 | 5.4 ± 2.3 | 4.8 ± 1.1 | 14.9 | 12.1 |
Sera were collected 2 weeks (∼2-month and ∼15-month studies) or 35 days (∼6-month study) before boosting and were analyzed by the RSV PRN assay with added complement.
NP and TL samples were collected daily and every second day, respectively, for 10 days post-boost and titrated by limiting dilution. Means were calculated for each day, and the peak mean titer SEM for each virus is shown.
Sera were collected 2 weeks following boosting and analyzed by the RSV PRN assay with or without complement, as indicated.
To investigate possible restriction in AGMs associated with packaging of RSV F protein into the vector virion, the post-boost replication of the DS-Cav1/B3TMCT vector (which efficiently packages RSV F) was compared to that of the DS-Cav1 vector (which is very inefficient for packaging) in AGM experiment 2 (∼6-month interval following priming). The replication of the DS-Cav1/B3TMCT vector hypothetically might have been reduced, compared to the DS-Cav1 vector, by (i) interference in vector replication due to packaging the RSV F protein into the vector virion and/or (ii) restriction due to RSV-specific immunity targeted to the packaged RSV F protein, as already noted. The kinetics of replication of the DS-Cav1/B3TMCT vector were slightly slower than those of the DS-Cav1 vector, but the difference was modest and was significant only for days 2 and 4 in the TL samples, and the peak titers for the two vectors were similar. We did find that RSV-specific hamster antiserum inhibited the replication of the DS-Cav1/B3TMCT vector and not the DS-Cav1 or empty vector in cell culture (in the absence of complement). In this in vitro setting, the inhibition presumably was due to the binding of antibodies to RSV F packaged in the vector virions and might not fully recapitulate the mechanism(s) that might operate in vivo. In any event, while restriction of the DS-Cav1/B3TMCT vector due to the packaging of RSV F into the vector virion is plausible, any effect apparently was minor when the boost was administered ∼6 months following the primary immunization. This could be further investigated following shorter intervals when additional RSV-primed AGMs become available.
Boosting with RSV 276 following intervals of ∼2, ∼6, and ∼15 months resulted in increases in peak mean serum RSV-PRNTs measured with complement of 22-fold, 78-fold, and 216-fold, respectively, resulting in peak mean titers of 1:5,793, 1:4,973, and 1:7,132, respectively. When measured without complement, the respective increases in peak mean serum RSV-PRNT were 25-fold, 37-fold, and 171-fold, resulting in peak mean titers of 1:294, 1:294, and 1:1,024. Thus, the post-boost serum RSV-PRNTs were generally similar for the different time intervals except that the titers following the ∼15-month interval were slightly higher (1-fold higher with complement and 3.5-fold higher without complement), compared to the other intervals. This might indicate that waning immunity at ∼15 months permitted a slightly increased secondary response to RSV, but the differences were small and this experiment involved only 4 animals.
For the DS-Cav1/B3TMCT vector, boosting following intervals of ∼2, ∼6, and ∼15 months resulted in increases in peak mean serum RSV-PRNTs measured with complement of 91-fold, 776-fold, and 926-fold, respectively, resulting in peak mean post-boost serum RSV-PRNTs of 1:23,170, 1:49,667, and 1:30,574, respectively. When measured without complement, the respective increases were 366-fold, 446-fold, and 1,274-fold, resulting in peak mean post-boost serum RSV-PRNTs of 1:4,390, 1:3,565, and 1:7,643. Thus, the post-boost titers were generally similar for the different time intervals, with differences that were no greater than 2-fold and were not consistent for any interval.
Previous studies in RSV-seronegative hamsters and AGMs showed that the DS-Cav1/B3TMCT vector (with efficient packaging of RSV F) could be significantly more immunogenic than the DS-Cav1 vector (with very inefficient packaging of RSV F) for the induction of antibodies that neutralize RSV in vitro in the absence of complement (30, 31). In AGM experiment 2, boosting with the DS-Cav1/B3TMCT vector versus the DS-Cav1 vector induced a 3-fold greater serum RSV-PRNT measured with complement and a 1.4-fold greater titer measured without complement. In the hamster study, boosting with the DS-Cav1/B3TMCT vector versus the DS-Cav1 vector was greater only for the serum RSV-PRNT measured without complement and only 2-fold. Thus, boosting with the DS-Cav1/B3TMCT vector appeared to be somewhat more immunogenic than the DS-Cav1 vector, but the differences were small.
In the AGM experiments, the peak mean post-boost serum RSV-PRNTs for the vectors were exceptionally high, up to 1:49,667 and 1:7,643 when assayed with and without complement, respectively. This raises the possibility that in some instances the full magnitude of boosting with the vectors might have been blunted due to limitations in the magnitude of the immune response. The titers for the vectors were higher than those for RSV 276 when assayed with complement (3- to 10-fold), and the difference was even greater when assayed without complement (7- to 15-fold). Both vectors also induced significantly greater responses of serum and mucosal IgA antibodies, compared to RSV 276, with little difference between the two vectors. The greater immunogenicity of the boosts by the vectors, compared to RSV, likely reflects both their higher level of replication (and resulting greater antigen expression) and the effects of the DS-Cav1 and B3TMCT modifications to the RSV F protein. In particular, we previously showed that the DS-Cav1 and B3TMCT modifications each preferentially induce antibodies that neutralize RSV in vitro without added complement (13, 30–32).
As noted above, boosts with an attenuated RSV in young infants and children in published clinical studies resulted in minimal or no increases in serum RSV-PRNTs, whereas in the present study boosts with RSV 276 in AGMs resulted in significant increases in serum RSV-PRNTs. The reason why an attenuated RSV provided a significant boost in AGMs but not in humans is not known. It may be that the semipermissive nature of RSV infection in monkeys resulted in a reduced primary immune response that was less restrictive and more readily boosted than in humans. Also, boosting involving i.n./i.t. administration in AGMs may be more immunogenic than the i.n. drops given to young children, because the i.t. administration in particular likely delivers the inoculum deeper into the respiratory tract. Another potential explanation is that the mature immune systems of the AGMs might have been more competent for a secondary response.
We note that the AGM studies had several limitations. The number of AGMs available for experiment 3 in particular was small. For all experiments, we did not have animals available to include unprimed controls. Also, there were insufficient animals to include control groups boosted with vector expressing RSV F protein without pre-F stabilization and the B3TMCT modification. Thus, we could not distinguish between the contributions to the immunogenicity of the vectors of increased replication versus pre-F stabilization and packaging. (We chose to evaluate a vector expressing RSV F protein with these modifications, rather than unmodified RSV F, because the former is a lead vaccine candidate and the latter is not.) The available AGMs had been primed with seven different viruses, whereas it would have been preferable to have employed a single priming virus, but we had to use what was available. It would have been preferable to have performed the boosts in experiments 1 to 3 in parallel rather than separately, but that number of animals could not be accommodated at the same time. Parenthetically, we did not perform post-boost challenges with wt RSV because our experience is that even a single infection/immunization with RSV in AGMs confers a high level of protection, and these AGMs had received two immunizations, resulting in very high levels of immunity, so that wt RSV challenge would not have been a useful way to compare levels of protective immunity (which indeed proved to be the case in the hamster study, in which all animals with RSV-specific immunization were completely protected). While limitations exist, these experiments involved primary and booster infections with attenuated RSV and rB/HPIV3 vectors that included lead vaccine candidates, and thus they involved realistic, relevant priming and boosting in the most permissive nonhuman primate model available.
This study demonstrated superior immunogenicity conferred by a heterologous prime-boost strategy in which the rB/HPIV3 vector expressing RSV pre-F was used as a booster following a primary immunization with attenuated RSV. Other vectored vaccines, such as ones based on human or murine PIV1 or murine pneumonia virus, could also be used. The choice of boost virus would depend on the age of the recipient at the time of booster vaccination. Like RSV, HPIV3 infects subjects very early in life, usually within first year, but the children remain susceptible to reinfections due to incomplete protection achieved by the natural infection (48). The booster dose will ideally be administered as early as <6 months of age, when most of the children would be HPIV3 naive. We speculate that, even if a few vaccinees would have experienced a natural HPIV3 infection, they would still have some level of susceptibility to the HPIV3 booster virus replication. However, if preexisting HPIV3 immunity would significantly limit rB/HPIV3 booster replication and immunogenicity, a different HPIV that epidemiologically infects subjects relatively later, such as HPIV1 expressing RSV pre-F (32), could be used as a booster instead.
In conclusion, because immune responses to RSV are reduced during infancy, it could be important to boost RSV-specific immunity following primary immunization with a pediatric RSV vaccine. In this study, boosting with an attenuated PIV vector expressing RSV F with DS-Cav1 and B3TMCT modifications was substantially more immunogenic than boosting with an attenuated RSV, particularly for high-quality RSV-neutralizing antibodies that neutralize in vitro without added complement. The results were similar whether the interval between priming and boosting was ∼2, ∼6, or ∼15 months. This suggests that a PIV vector could be effective in boosting during the same vaccination season or, alternatively, during the following year. In addition, a PIV-vectored RSV vaccine may be well suited for primary immunization of young infants who have passive serum RSV-neutralizing antibodies from maternal transfer, including following maternal immunization, or from passive antibody immunoprophylaxis, including the use of antibody engineered for increased half-life. These passive antibodies might restrict an attenuated RSV but not a PIV-vectored RSV vaccine. The use of an attenuated PIV3 vector also provides immunization against HPIV3, which is second only to RSV as an agent of severe acute pediatric respiratory disease.
MATERIALS AND METHODS
Viruses.
The RSVs in this study were recombinant wt or attenuated versions of the subgroup A strain A2 (GenBank accession number KT992094) prepared using reverse genetics (49). The attenuated RSVs are described in Results. One additional RSV was rRSV A/Maryland/001/11, which is a recombinant version of the wt subgroup A strain RSV A/Maryland/001/11 that was isolated from a nasal wash collected in 2011 from an adult patient with acute respiratory illness (R. Karron and U. J. Buchholz, unpublished results). The F and G proteins of the Maryland/001/11 strain have 97% and 86% amino acid sequence identity, respectively, to strain A2. The rB/HPIV3 constructs were made in previous work (31) and express modified versions of the F protein of RSV strain A2 (GenBank accession number KT992094) from an added gene in the second gene position. The vector constructs were (i) rB/HPIV3, which is the empty vector; (ii) rB/HPIV3/DS-Cav1 (abbreviated as DS-Cav1 vector), which expresses RSV F protein with increased stability in the pre-F conformation due to the DS and Cav1 mutations (12); and (iii) rB/HPIV3/DS-Cav1/B3TMCT (abbreviated as DS-Cav1/B3TMCT vector), which expresses RSV F with the DS-Cav1 mutations and with its TM and CT domains replaced by those of BPIV3 F (30, 31). In addition, as described previously (31), the RSV F ORF used in the vector constructs had been modified by codon optimization (GenScript, Piscataway, NJ) and had been further modified by two amino acid substitutions called HEK (K66E and Q101P), which make F identical at the amino acid level to an early passage of RSV strain A2.
RSVs and rB/HPIV3 vectors were grown at 32°C in Vero and LLC-MK2 cells, respectively. The complete genome sequences of all viruses were confirmed by automated Sanger sequencing analysis of uncloned RT-PCR products to be free of adventitious mutations detectable above background (except for ∼30 and ∼120 nucleotides at the 3′ and 5′ ends, respectively, which include the primer binding sites and were not sequenced).
Titration of infectious virus and serum antibodies.
Titers of RSV preparations were determined by plaque assays in Vero cells with immunostaining using a mixture of three F-specific monoclonal antibodies, as described previously (23); titers are reported as log10 PFU per milliliter or gram. Titers of rB/HPIV3 vectors were determined by limiting dilution in LLC-MK2 cells, with virus-positive wells detected by hemadsorption with guinea pig erythrocytes, as described previously (50); titers are reported as log10 TCID50 per milliliter or gram.
Serum RSV- or HPIV3-neutralizing antibody titers were measured by 60% PRN assays on Vero cells using RSV or HPIV3 expressing green fluorescent protein (GFP) (28). The assays were performed in the presence (RSV and HPIV3) or absence (RSV) of 5% added guinea pig complement (Cedarlane, Burlington, NC) as noted. The 60% PRNTs are reported in log2 units and/or arithmetic values. Prior to primary infection, animals were confirmed to be RSV or HPIV3 seronegative by a PRN assay with complement or a hemagglutination inhibition assay using guinea pig erythrocytes, respectively (51, 52).
Animal studies.
Animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the NIAID Animal Care and Use Committee.
A single prime-boost experiment was performed with Syrian Golden hamsters, as described in the legend to Fig. 1 and the accompanying results. Three prime-boost experiments were performed in AGMs (AGM experiments 1 to 3) (Tables 1 to 3 and Fig. 2 to 4). The primary AGM infections with RSV were performed in previous studies using a number of different RSVs, which mostly were attenuated derivatives of RSV strain A2 and are described in Results and Tables 1 to 3. In those studies, RSV was administered by the combined i.n. and i.t. routes at a dose of 106 PFU per site in 1 ml of L-15 medium. Booster infections with RSV or the DS-Cav1/B3TMCT or DS-Cav1 vector in AGMs in the present study were administered in the same way, by the combined i.n. and i.t. routes at a dose of 106 PFU per site (RSV) or 106 TCID50 per site (vectors) in 1 ml of L-15 medium. Following inoculation, NP and TL samples were collected daily and every other day, respectively, for 10 days and were flash-frozen on dry ice and stored at –80°C until titration. Sera were collected prior to inoculation on the day of the boost and every 7 days thereafter for 4 consecutive weeks.
In AGM experiment 2, nasal mucosal lining fluid was sampled on the day of boosting and on days 14, 21, and 28 post-boosting using SAM strips (Nasosorption FXi; Hunt Developments, UK). SAM strips were gently placed on the nasal mucosa of the lower turbinate of one nostril, held in place for 30 s, and placed in a microtube prefilled with 300 μl of phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA) and 0.1% Tween 20. Samples were flash-frozen on dry ice and kept at –80°C until use. Mucosal and serum IgA titers were measured by DELFIA, as described below.
RSV RNA copy numbers in AGM TL samples were quantified by a RT-qPCR assay that was specific for both positive- and negative-sense RSV M gene sequences (forward primer, 5′-GCAAATATGGAAACATACGTGAACAA-3′; reverse primer, 5-GGCACCCATATTGTAAGTGATGCA-3′; TaqMan minor groove binder (MGB) probe, 5′-CTTCACGAAGGCTCCACATA-3′). Frozen AGM TL samples were briefly thawed in a 37°C water bath and immediately kept on ice. After clarification by centrifugation at 800 × g for 5 min at 4°C, the total RNA in TL samples was extracted using the QIAamp viral RNA minikit (Qiagen). cDNA was generated by RT using the Applied Biosystems RT kit (Thermo Fisher Scientific) with random hexamer primers and was analyzed by the M-specific qPCR assay. For comparison, a standard curve was generated using a DNA plasmid encoding the full-length genome of the wt RSV A2 strain (i.e., 6120) analyzed by the same qPCR assay. The copy number of reverse-transcribed RSV RNA in TL samples was interpolated from the standard curve. Statistical analyses were performed using GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA).
Detection of IgA antibodies using a DELFIA.
Serum and mucosal IgA antibodies were detected by DELFIA. Briefly, the recombinant RSV DS-Cav1 F protein was expressed in HEK293 cells, purified as described previously (12), and the concentration was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific). Ninety-six-well DELFIA yellow plates (Perkin Elmer, Waltham MA) were coated with 100 ng/well of purified RSV DS-Cav1 F protein diluted in 100 μl of carbonate-bicarbonate buffer (Millipore Sigma, St. Louis MO) and incubated at 4°C overnight. All washes in the assay were performed once with 200 μl/well of wash buffer (1× DELFIA wash concentrate; Perkin Elmer). After overnight antigen coating, plates were washed and incubated with 100 μl/well of blocking solution (PBS containing 0.1% Tween 20 and 3% BSA) at 37°C for 1 h. Plates were washed and 100 μl of serum samples in 4-fold dilution series in blocking solution were added and incubated at 4°C overnight. Following a wash, 100 μl/well of anti-monkey IgA-biotin conjugate (Alpha Diagnostic, San Antonio, TX) diluted 1:5,000 in blocking solution was added and incubated at 37°C for 1 h. Plates were washed and 100 μl/well of europium-conjugated streptavidin diluted 1:2,000 in DELFIA assay buffer (Perkin Elmer) was added. After incubation at 37°C for 1 h, plates were washed and incubated with 100 μl/well of DELFIA enhancement solution (Perkin Elmer) at room temperature for 20 min, with gentle shaking. Fluorescence was measured using a europium-specific TRF program (excitation at 340 nm and emission at 615 nm) in a Synergy Neo 2 plate reader (BioTek, Winooski, VT). The blocking solution was used to generate blank values, and an average of 24 blank values plus 3 standard deviations was used as a cutoff value (corresponding to 400 fluorescence units). Test sample dilutions corresponding to 400 fluorescence units were interpolated from sigmoidal standard curves using GraphPad Prism version 8 and were expressed as log2 values.
ACKNOWLEDGMENTS
We thank Barney S. Graham, Tracy J. Ruckwardt, and Man Chen (Vaccine Research Center, NIAID, NIH) for generously providing the RSV F DS-Cav1 expression plasmids used to produce the recombinant RSV F for IgA immunoassays and Cindy Luongo (Laboratory of Infectious Diseases, NIAID, NIH) for assistance with the RSV 276 prime-boost study in AGMs.
This research was supported by the Intramural Research Program of the NIAID, NIH, and also received support from a Cooperative Research and Development Agreement between NIAID, NIH, and Sanofi Pasteur, Inc.
B.L., P.L.C., U.J.B., and S.M. are coinventors on a patent application for the development of PIV3-vectored bivalent vaccines against RSV and HPIV3. P.L.C. and U.J.B. are also coinventors on patent applications for the development of various attenuated versions of RSV for use as pediatric vaccines.
REFERENCES
- 1.Miller EK, Gebretsadik T, Carroll KN, Dupont WD, Mohamed YA, Morin LL, Heil L, Minton PA, Woodward K, Liu Z, Hartert TV, Williams JV. 2013. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J 32:950–955. doi: 10.1097/INF.0b013e31829b7e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang DA, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lazaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccalà G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida LM, Yu H, Zar HJ, Campbell H, Nair H. 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chirikov VV, Simoes EAF, Kuznik A, Kwon Y, Botteman M. 2020. Economic burden trajectories in commercially insured US infants with respiratory syncytial virus infection. J Infect Dis 221:1244–1255. doi: 10.1093/infdis/jiz160. [DOI] [PubMed] [Google Scholar]
- 5.Simoes EAF, Chirikov V, Botteman M, Kwon Y, Kuznik A. 2020. Long-term assessment of healthcare utilization 5 years after respiratory syncytial virus infection in US infants. J Infect Dis 221:1256–1270. doi: 10.1093/infdis/jiz278. [DOI] [PubMed] [Google Scholar]
- 6.IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 7.Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. 2011. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med 17:1132–1135. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 8.McLellan JS, Chen M, Chang JS, Yang Y, Kim A, Graham BS, Kwong PD. 2010. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J Virol 84:12236–12244. doi: 10.1128/JVI.01579-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A 108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham BS. 2017. Vaccine development for respiratory syncytial virus. Curr Opin Virol 23:107–112. doi: 10.1016/j.coviro.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang GY, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko SY, Todd JP, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, Yang L, McLellan JS, Graham BS, Kwong PD, Schaap-Nutt A, Collins PL, Munir S. 2015. Enhanced neutralizing antibody response induced by respiratory syncytial virus prefusion F protein expressed by a vaccine candidate. J Virol 89:9499–9510. doi: 10.1128/JVI.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 15.Schneider-Ohrum K, Cayatte C, Bennett AS, Rajani GM, McTamney P, Nacel K, Hostetler L, Cheng L, Ren K, O'Day T, Prince GA, McCarthy MP. 2017. Immunization with low doses of recombinant postfusion or prefusion respiratory syncytial virus F primes for vaccine-enhanced disease in the cotton rat model independently of the presence of a Th1-biasing (GLA-SE) or Th2-biasing (alum) adjuvant. J Virol 91:e02180-16. doi: 10.1128/JVI.02180-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connors M, Collins PL, Firestone CY, Sotnikov AV, Waitze A, Davis AR, Hung PP, Chanock RM, Murphy BR. 1992. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine 10:475–484. doi: 10.1016/0264-410X(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 17.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, O'Shea AF, Gruber WC, Murphy BR. 2007. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 25:7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein DI, Malkin E, Abughali N, Falloon J, Yi T, Dubovsky F. 2012. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr Infect Dis J 31:109–114. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 19.Karron RA, Luongo C, Mateo JS, Wanionek K, Collins PL, Buchholz UJ. 2020. Safety and immunogenicity of the respiratory syncytial virus vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-seronegative children. J Infect Dis 222:82–91. doi: 10.1093/infdis/jiz408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchholz UJ, Cunningham CK, Muresan P, Gnanashanmugam D, Sato P, Siberry GK, Rexroad V, Valentine M, Perlowski C, Schappell E, Thumar B, Luongo C, Barr E, Aziz M, Yogev R, Spector SA, Collins PL, McFarland EJ, Karron RA. 2018. Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis 217:1338–1346. doi: 10.1093/infdis/jiy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarland EJ, Karron RA, Muresan P, Cunningham CK, Valentine ME, Perlowski C, Thumar B, Gnanashanmugam D, Siberry GK, Schappell E, Barr E, Rexroad V, Yogev R, Spector SA, Aziz M, Patel N, Cielo M, Luongo C, Collins PL, Buchholz UJ. 2018. Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J Infect Dis 217:1347–1355. doi: 10.1093/infdis/jiy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, Buchholz UJ. 2015. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med 7:312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luongo C, Winter CC, Collins PL, Buchholz UJ. 2013. Respiratory syncytial virus modified by deletions of the NS2 gene and amino acid S1313 of the L polymerase protein is a temperature-sensitive, live-attenuated vaccine candidate that is phenotypically stable at physiological temperature. J Virol 87:1985–1996. doi: 10.1128/JVI.02769-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luongo C, Winter CC, Collins PL, Buchholz UJ. 2012. Increased genetic and phenotypic stability of a promising live-attenuated respiratory syncytial virus vaccine candidate by reverse genetics. J Virol 86:10792–10804. doi: 10.1128/JVI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng MN, Whitehead SS, Bermingham A, St Claire M, Elkins WR, Murphy BR, Collins PL. 2000. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J Virol 74:9317–9321. doi: 10.1128/jvi.74.19.9317-9321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehead SS, Bukreyev A, Teng MN, Firestone CY, St Claire M, Elkins WR, Collins PL, Murphy BR. 1999. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol 73:3438–3442. doi: 10.1128/JVI.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karron RA, Buchholz UJ, Collins PL. 2013. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang B, Munir S, Amaro-Carambot E, Surman S, Mackow N, Yang L, Buchholz UJ, Collins PL, Schaap-Nutt A. 2014. Chimeric bovine/human parainfluenza virus type 3 expressing respiratory syncytial virus (RSV) F glycoprotein: effect of insert position on expression, replication, immunogenicity, stability, and protection against RSV infection. J Virol 88:4237–4250. doi: 10.1128/JVI.03481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackow N, Amaro-Carambot E, Liang B, Surman S, Lingemann M, Yang L, Collins PL, Munir S. 2015. Attenuated human parainfluenza virus type 1 (HPIV1) expressing the fusion glycoprotein of human respiratory syncytial virus (RSV) as a bivalent HPIV1/RSV vaccine. J Virol 89:10319–10332. doi: 10.1128/JVI.01380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang B, Ngwuta JO, Herbert R, Swerczek J, Dorward DW, Amaro-Carambot E, Mackow N, Kabatova B, Lingemann M, Surman S, Yang L, Chen M, Moin SM, Kumar A, McLellan JS, Kwong PD, Graham BS, Schaap-Nutt A, Collins PL, Munir S. 2016. Packaging and prefusion stabilization separately and additively increase the quantity and quality of respiratory syncytial virus (RSV)-neutralizing antibodies induced by an RSV fusion protein expressed by a parainfluenza virus vector. J Virol 90:10022–10038. doi: 10.1128/JVI.01196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang B, Ngwuta JO, Surman S, Kabatova B, Liu X, Lingemann M, Liu X, Yang L, Herbert R, Swerczek J, Chen M, Moin SM, Kumar A, McLellan JS, Kwong PD, Graham BS, Collins PL, Munir S. 2017. Improved prefusion stability, optimized codon usage, and augmented virion packaging enhance the immunogenicity of respiratory syncytial virus fusion protein in a vectored-vaccine candidate. J Virol 91:e00189-17. doi: 10.1128/JVI.00189-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Liang B, Ngwuta J, Liu X, Surman S, Lingemann M, Kwong PD, Graham BS, Collins PL, Munir S. 2017. Attenuated human parainfluenza virus type 1 expressing the respiratory syncytial virus (RSV) fusion (F) glycoprotein from an added gene: effects of prefusion stabilization and packaging of RSV F. J Virol 91:e01101-17. doi: 10.1128/JVI.01101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt AC, McAuliffe JM, Murphy BR, Collins PL. 2001. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J Virol 75:4594–4603. doi: 10.1128/JVI.75.10.4594-4603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stobart CC, Rostad CA, Ke Z, Dillard RS, Hampton CM, Strauss JD, Yi H, Hotard AL, Meng J, Pickles RJ, Sakamoto K, Lee S, Currier MG, Moin SM, Graham BS, Boukhvalova MS, Gilbert BE, Blanco JC, Piedra PA, Wright ER, Moore ML. 2016. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat Commun 7:13916. doi: 10.1038/ncomms13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, Mejias A, Karron RA, Simoes EA, Knezevic I, Ramilo O, Piedra PA, Chu HY, Falsey AR, Nair H, Kragten-Tabatabaie L, Greenough A, Baraldi E, Papadopoulos NG, Vekemans J, Polack FP, Powell M, Satav A, Walsh EE, Stein RT, Graham BS, Bont LJ. 2018. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 18:e295–e311. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Liang B, Liu X, Amaro-Carambot E, Surman S, Kwong PD, Graham BS, Collins PL, Munir S. 2020. Human parainfluenza virus type 3 expressing the respiratory syncytial virus pre-fusion F protein modified for virion packaging yields protective intranasal vaccine candidates. PLoS One 15:e0228572. doi: 10.1371/journal.pone.0228572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell CJ, Hurwitz JL. 2016. Sendai virus as a backbone for vaccines against RSV and other human paramyxoviruses. Expert Rev Vaccines 15:189–200. doi: 10.1586/14760584.2016.1114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brock LG, Liu X, Liang B, Lingemann M, Liu X, Herbert R, Hackenberg AD, Buchholz UJ, Collins PL, Munir S. 2018. Murine pneumonia virus expressing the fusion glycoprotein of human respiratory syncytial virus from an added gene is highly attenuated and immunogenic in rhesus macaques. J Virol 92:e00723-18. doi: 10.1128/JVI.00723-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Phan S, DiStefano DJ, Citron MP, Callahan CL, Indrawati L, Dubey SA, Heidecker GJ, Govindarajan D, Liang X, He B, Espeseth AS. 2017. A single-dose recombinant parainfluenza virus 5-vectored vaccine expressing respiratory syncytial virus (RSV) F or G protein protected cotton rats and African green monkeys from RSV challenge. J Virol 91:e00066-17. doi: 10.1128/JVI.00066-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang B, Kabatova B, Kabat J, Dorward DW, Liu X, Surman S, Liu X, Moseman AP, Buchholz UJ, Collins PL, Munir S. 2019. Effects of alterations to the CX3C motif and secreted form of human respiratory syncytial virus (RSV) G protein on immune responses to a parainfluenza virus vector expressing the RSV G protein. J Virol 93:e02043-18. doi: 10.1128/JVI.02043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malkin E, Yogev R, Abughali N, Sliman J, Wang CK, Zuo F, Yang CF, Eickhoff M, Esser MT, Tang RS, Dubovsky F. 2013. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One 8:e77104. doi: 10.1371/journal.pone.0077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, Halburnt LL, Reed GW, Whitehead SS, Anderson EL, Wittek AE, Casey R, Eichelberger M, Thumar B, Randolph VB, Udem SA, Chanock RM, Murphy BR. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis 182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein DI, Falloon J, Yi T. 2011. A randomized, double-blind, placebo-controlled, phase 1/2a study of the safety and immunogenicity of a live, attenuated human parainfluenza virus type 3 vaccine in healthy infants. Vaccine 29:7042–7048. doi: 10.1016/j.vaccine.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 44.Karron RA, Casey R, Thumar B, Surman S, Murphy BR, Collins PL, Schmidt AC. 2011. The cDNA-derived investigational human parainfluenza virus type 3 vaccine rcp45 is well tolerated, infectious, and immunogenic in infants and young children. Pediatr Infect Dis J 30:e186–e191. doi: 10.1097/INF.0b013e31822ea24f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Englund JA, Karron RA, Cunningham CK, Larussa P, Melvin A, Yogev R, Handelsman E, Siberry GK, Thumar B, Schappell E, Bull CV, Chu HY, Schaap-Nutt A, Buchholz U, Collins PL, Schmidt AC. 2013. Safety and infectivity of two doses of live-attenuated recombinant cold-passaged human parainfluenza type 3 virus vaccine rHPIV3cp45 in HPIV3-seronegative young children. Vaccine 31:5706–5712. doi: 10.1016/j.vaccine.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao T, Davoodi F, Cho CJ, Skiadopoulos MH, Durbin AP, Collins PL, Murphy BR. 2000. A live attenuated recombinant chimeric parainfluenza virus (PIV) candidate vaccine containing the hemagglutinin-neuraminidase and fusion glycoproteins of PIV1 and the remaining proteins from PIV3 induces resistance to PIV1 even in animals immune to PIV3. Vaccine 18:1359–1366. doi: 10.1016/s0264-410x(99)00406-5. [DOI] [PubMed] [Google Scholar]
- 47.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt AC, Schaap-Nutt A, Bartlett EJ, Schomacker H, Boonyaratanakornkit J, Karron RA, Collins PL. 2011. Progress in the development of human parainfluenza virus vaccines. Expert Rev Respir Med 5:515–526. doi: 10.1586/ers.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5' proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci U S A 92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durbin AP, McAuliffe JM, Collins PL, Murphy BR. 1999. Mutations in the C, D, and V open reading frames of human parainfluenza virus type 3 attenuate replication in rodents and primates. Virology 261:319–330. doi: 10.1006/viro.1999.9878. [DOI] [PubMed] [Google Scholar]
- 51.Coates HV, Alling DW, Chanock RM. 1966. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol 83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 52.van Wyke Coelingh KL, Winter CC, Tierney EL, London WT, Murphy BR. 1988. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis 157:655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]