Abstract
Alzheimer’s disease (AD) is characterized by progressive cognitive decline and the presence of aggregates of amyloid beta (plaques) and hyperphosphorylated tau (tangles). Early diagnosis through neuropsychological testing is difficult due to comorbidity of symptoms between AD and other types of dementia. As a result, there is a need to identify the range of behavioral phenotypes expressed in AD. In the present study, we utilized a transgenic rat (TgF344-AD) model that bears the mutated amyloid precursor protein as well as presenilin-1 genes, resulting in progressive plaque and tangle pathogenesis throughout the cortex. We tested young adult male and female TgF344-AD rats in a spatial memory task in the Morris water maze, and for anxiety-like behavior in the elevated plus-maze. Results indicated that regardless of sex, TgF344-AD rats exhibited increased anxiety-like behavior in the elevated plus-maze, which occurred without significant deficits in spatial memory. Together, these results indicate that enhanced anxiety-like behavior represents an early-stage behavioral marker in the TgF344-AD rat model.
Keywords: dementia, transgenic model, anxiety, defensive behavior, spatial behavior, memory
1. Introduction
Alzheimer’s disease (AD) is a progressive and degenerative brain disorder that leads to profound cognitive impairments and memory decline (Eustache et al., 2006; Selkoe, 2001). Brain abnormalities in AD unequivocally include accumulation of the protein fragment Aβ “plaques”, neurofibrillary aggregates of the microtubule associated hyperphosphorylated tau protein “tangles” and cell loss, all of which occur throughout the limbic system including the hippocampus, prefrontal cortex and amygdala (Aggleton et al., 2016; Braak & Braak, 1991; Kromer Vogt et al., 1990; Thal et al., 2002; Van Hoesen et al., 2000). Fundamental to AD is the incorrect processing of amyloid precursor protein, which results in misfolded neurotoxic forms of Aβ plaques, and has been proposed to be foundational to other pathophysiological events such as hyperphosphorylated tau, neuronal injury, cell loss and activation of microglia (Selkoe, 2001). The strongest support for the “amyloid cascade hypothesis” comes from human genetic evidence in which mutations of amyloid precursor protein or presenilins 1 or 2 produce Aβ plaque formation and early-onset AD (Musiek & Holtzman, 2015).
Although AD is typically viewed as a disorder of memory, a majority of patients develop neuropsychiatric symptoms, which have been shown to precede and develop alongside the expression of cognitive difficulties (Geda et al., 2013; Jost & Grossberg, 1996; Lyketsos & Olin, 2002; Tayeb et al., 2014). For instance, epidemiologic work demonstrates that individuals prone to psychological distress or anxiety are more likely to be diagnosed with AD than age-matched controls and exhibit more rapid rates of cognitive decline (Geda & Roberts, 2008; Mega et al., 1996; Wilson et al., 2003). Research using transgenic (Tg+) rodent models of AD with mutations to amyloid precursor protein or tau, report perturbations in central stress signaling and increased anxiety-like behavior (Dong et al., 2014; Guo et al., 2012; Touma et al., 2004). However, many of these studies examined anxiety-like behavior at advanced stages of AD pathogenesis, involving extensive cerebral plaque and tangle burden, whereas tests at earlier stages produced conflicting results. For instance, experiments utilizing standard tasks such as the elevated plus-maze (EPM) generally fail to identify anxiety-like behavior in Tg+ animals at pre-amyloid stages (Boon et al., 2010; Galeano et al., 2014; Lalonde et al., 2002; Lee et al., 2004). Thus, the linkage between early stage AD pathology and the neuropsychiatric symptoms observed in pre-clinical AD patients, has been inconsistently represented in animal models of the disease.
One factor contributing to this inconsistency may be that most Tg+ models do not exhibit the full spectrum of AD pathology (Casadesus et al., 2010), which may limit the utility of characterizing their behavioral phenotypes. Although Tg+ models have proven invaluable for elucidating the pathophysiological mechanisms of AD (Epis et al., 2010; Jucker, 2010), the ability to detect subtle behavioral deficits in animal models of AD may be hindered without full spectrum pathophysiology. For example, many studies use Tg+ animals that do not develop neurofibrillary tangles and resultant cell loss (Do Carmo & Cuello, 2013; Sabbagh et al., 2013). Tangle formation is implicated in synaptic dysfunction (Jackson et al., 2017), as well as spatial memory deficits and cell loss (Fu et al., 2017). Furthermore, Aβ and hyperphosphorylated tau co-localize in AD neurons (Fein et al., 2008; Smith, et al., 1995), and their interaction may progressively damage synaptic structure and function (Manczak & Reddy, 2013). In addition, Pascoal and colleagues (2017) recently found that the interaction between Aβ and hyperphosphorylated tau drives metabolic decline in patients with AD. Consequently, models that do not display both amyloid and tau dysfunction may display attenuated behavioral features. Recently, a Tg+ rat model was developed (TgF344-AD) that bears the mutated human amyloid precursor protein as well as presenilin-1 genes resulting in characteristic plaque pathogenesis throughout brain areas associated with the disease (Cohen et al., 2013). Importantly, the TgF344-AD rat also mimics robust tangle pathology and neuroinflammation that progress across a developmental time course resulting in substantial cell loss. Nevertheless, we are aware of only one study that has investigated the behavioral consequences of pathology in the TgF344-AD rat model (see Cohen et al., 2013).
While a large body of rodent studies have investigated the relationship between AD pathogeneses and memory loss, few have examined the impact of pathology on anxiety-like behavior, particularly at pre-clinical stages. In the present study, we evaluated spatial memory and navigation by TgF344-AD rats in three standard variants of the Morris water task, and assessed anxiety-like behavior in the EPM. Testing was conducted in young adult male and female TgF344-AD rats between 4 and 6 months of age at which time cerebral plaque burden is minimal (Cohen et al., 2013). We report heightened anxiety-like behavior by TgF344-AD male and female rats, but also report the absence of significant spatial learning and memory deficits in the Morris water task. Thus, our findings indicate that enhanced anxiety-like behavior represents an early behavioral marker in TgF344-AD rats.
2. Materials and Methods
2.1. Subjects
Subjects were the AD rat model, TgF344-AD (Cohen et al., 2013), that were originally generated on a Fischer 344 background by co-injecting rat pronuclei with two human genes driven by the mouse prion promoter: “Swedish” mutant human amyloid precursor protein (APPsw) and Δ exon 9 mutant human presenilin-1 (PS1ΔE9). These Tg+ rats display a progressive increase in the characteristic AD clinical-pathology associated with the human condition, including plaques, tangles and cognitive impairment (Cohen et al., 2013; Tsai et al., 2014).
Wild-type (WT; male = 10; female = 10) and transgenic (Tg+; male = 12; female = 12) rats were tested between 4.29 and 6.52 months of age. For Experiments 1 – 4, ten groups of TgF344-AD littermates (male = 8; female = 8) were obtained at 2-3 months from the Rat Resource and Research Center at the University of Missouri, and 3 month old wild-type controls (male = 6; female = 6) were obtained from Harlan laboratories (Indianapolis, IN). For Experiment 4, three groups of naïve littermates, including TgF344-AD (male = 4; female = 4) and wild-type rats (male = 4; female = 4), were obtained from the Logan Hall breeding colony at the University of New Mexico. To confirm transgene expression, tissue samples from all rats included in these studies were analyzed using qPCR by the Rat Resource and Research Center (Fig. 1A). All rats were housed as transgenic or wildtype pairs and maintained under controlled temperature (21 ± 2 °C) and illumination (12 h light/dark cycle, lights off at 09:00 a.m.) with free access to food and water. The housing conditions and care of the rats were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). All testing procedures conducted on rats in these experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of New Mexico. All efforts were made to minimize any animal pain and/or suffering.
Figure 1.
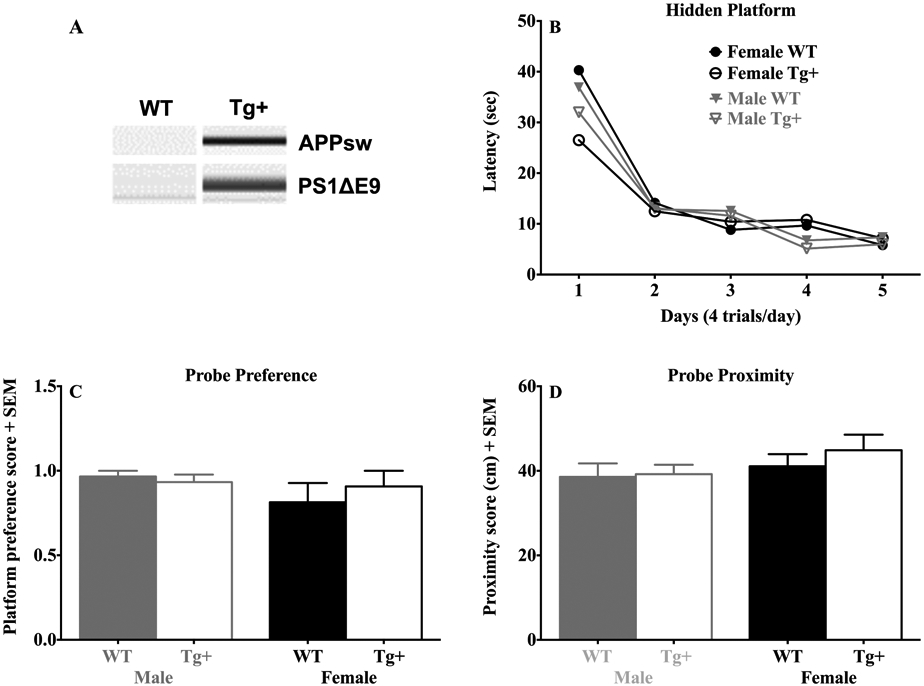
Effects of sex and genotype in the hidden platform navigation and probe test. (A) qPCR results from APPsw and PS1ΔE9 reactions. (B) Latency (seconds) to find the platform during the 5-day training period. (C) The platform quadrant preference during the 30 second probe test. (D) Swim proximity (cm) to the platform location during the probe test. Data are expressed as mean + SEM. Note that Tg+ and WT rats exhibited similar performance on hidden platform training and probe testing.
2.2. Experiment 1: Morris Water Task – Hidden Platform
The swimming pool was a 150 cm diameter and 48 cm high round white tub positioned 48 cm above the floor. The pool was filled to a depth of 35 cm with 21–22°C water. The water was made opaque by the addition of ~500 ml of powdered white paint. A clear Plexiglas platform with a square top (16 cm x 16 cm) was placed in the pool such that the top of the platform was 1 cm below the surface of the water. A video camera located above the center of the swimming pool recorded the rat’s behavior for subsequent analyses. The swimming pool was located in a test room that included many cues, including counters, cupboards and posters.
The purpose of the hidden platform variant of the Morris water task was to assess the rat’s ability to learn the fixed spatial relationship between the room cues and the hidden platform position (Clark & Taube, 2009; Clark et al., 2013; 2015; Harker & Whishaw, 2002). Thus, WT (male = 6; female = 6) and Tg+ (male = 8; female = 8) rats were trained for 5 consecutive days (4 trials/day) with each test trial consisting of placing a rat by hand into the water facing the wall of the pool at one of 4 pseudorandomly selected starting positions along the pool perimeter (north, south, east, and west). Rats were allowed to swim until they found the platform or until 60 sec elapsed. If a rat found the platform in less than 60 sec, it was permitted to remain on the platform for 10 sec. If after 60 sec the rat failed to find the platform, it was guided to the platform and permitted to remain there for 10 sec. At the end of the trial, the rat was returned to a holding cage, and approximately 10–20 min elapsed, during which time the remaining rats in the cohort were tested before beginning the next trial. At the end of the 4 trials, rats were returned to their home cages and the same procedure was repeated the next day. Swim paths were tracked (10 frames/sec) using the Manual Tracking plugin in Fiji (https://imagej.nih.gov/ij/). Each training trial was measured for duration (seconds) using a stopwatch. The mean escape latencies for each animal were calculated for each day (every 4 trials).
After 5 days of training in the hidden platform task, a 30 sec probe trial was conducted with the platform removed from the swimming pool at the beginning of the 6th day. Rats were released from the side of the pool opposite of the platform. From the tracked swim paths, a path proximity score was calculated by measuring the distance (in cm) between the rat’s position in the pool and the platform location (Gallagher et al., 1993; Tomas Pereira & Burwell, 2015). A distance measure was made 10 times per second and averaged across the probe trial. In addition, a platform preference score was calculated by measuring the duration of time spent swimming in two pie shaped quadrants centered over the trained platform location and the 180° opposite location. The platform preference was measured by subtracting the swim time in the opposite quadrant from the platform quadrant, and dividing by the sum of the total swim time in the two quadrants. A platform preference score can range between −1 to +1; thus, a score of 0 would indicate no preference between the two quadrants, while a score above 0 would indicate a preference for the platform quadrant. For display purposes, color plots were produced showing the percent swim time in each region of the swimming pool by dividing the maze up into a 125 × 125 matrix (each matrix bin = ~1.3 cm) and computing the percentage of time spent in each bin.
2.3. Experiment 2: Morris Water Task – Matching-to-Place
Following the hidden platform task and probe test, the same rats were tested in a matching-to-place variant of the Morris water task. This variant was composed of testing rats in 2 trials per day for 5 consecutive days, but with the hidden platform moved to a new location each day (Harker & Whishaw, 2002). The starting position for a given subject remained the same for both trials. Again, 4 start positions were selected pseudorandomly and were equally distributed among the subjects. As in the hidden platform task, rats were placed by hand into the water facing the wall of the pool at 1 of the 4 starting positions. In contrast to the hidden platform variant, rats were required to swim until they found the platform. Once on the platform, rats remained there for 10 sec. After the first trial, they were placed in a holding cage for 20 sec before beginning the second trial. Each training trial was measured for duration (sec) using a stopwatch.
2.4. Experiment 3: Morris Water Task – Cued Platform
Following the matching-to-place task, the same rats were trained in a cued platform variant of the Morris water task. This test examined the ability of rats to swim toward a cue directly associated with the platform location, i.e., taxon or beacon navigation (Clark & Taube, 2009; Clark et al., 2013; Morris, 1984). The platform was moved to a novel location and a visual cue was placed above the platform. The visual cue was a circular black ball (10 cm in diameter) mounted on a metal rod attached to the platform, and was clearly visible from any point in the pool. Rats were trained for a single day consisting of 12 consecutive trials. Again, a trial consisted of placing a rat by hand into the water facing the wall of the pool at 1 of the 4 starting positions (trial length 60 sec; time on platform 10 sec). Each training trial was measured for duration (sec) using a stopwatch. Escape latency for each animal was averaged the 12 training trials.
2.5. Experiment 4: Elevated Plus-Maze
Following cued platform training, the same rats were tested for anxiety-like behavior in the EPM (Handley and Mithani, 1984; Pellow et al., 1985) using previously published protocols (Pentkowski et al., 2009). In addition, a second naïve cohort of WT (male = 4; female = 4) and Tg+ (male = 4; female = 4) rats were tested in the EPM. The EPM apparatus consisted of 4 Plexiglas arms arranged in a cross, elevated 75 cm above the floor. Each arm was 10 cm wide and 50 cm long, and each arm was joined at the center by a 10 cm square platform. The 2 opposite “open” arms contained no walls, while the 2 opposite “closed” arms contained 40 cm tall opaque sides. Subjects were individually placed in the center arm of the apparatus facing 1 of the 2 closed arms. Tests were 5 min in duration and were conducted under red light. All test trials were recorded and were later analyzed using the behavioral analysis software ANY-maze (Stoelting Co., Wood Dale, IL, USA); animal movements were automatically tracked using the midline as the frame of reference. Behavioral measures included the number of open- and closed-arm entries, measured as movement from one marked section of the apparatus to another, the proportion of time (duration) spent in the closed versus open arms, and total distance traveled in meters.
2.6. Statistical Analysis
A repeated measures analysis of variance (ANOVA) was performed on dependent measures from the hidden platform and matching-to-place tasks with day as the within-subject variable and sex and genotype as between subject variables. Violations of sphericity were corrected using the Greenhouse-Geisser method. A one-way ANOVA was performed on dependent measures collected in the cued platform task, hidden platform probe test and the EPM, with sex and genotype as dependent variables. Significant interactions were followed by Post-hoc Newman-Keuls tests to make pair-wise comparisons. Alpha (α) was set at 0.05 for all comparisons.
3. Results
3.1. Experiment 1: Morris Water Task – Hidden Platform
Figure 1B plots the swim latencies of rats in WT and Tg+ groups during training in the hidden platform task. Overall, rats from both groups and sexes showed reduced swim latencies across training, as indicated by a significant day effect (F(corrected df: 1.9, 45.9) = 55.5, p < 0.001). Further, Tg+ and WT rats displayed numerically similar mean swim latencies throughout the 5 day training period, suggesting that Tg+ rats could learn the platform position at a comparable level to WT rats. This observation is supported by a non-significant ANOVA for group (p = 0.47) and a non-significant group by day interaction (p = 0.31). The ANOVA failed to show a significant effect for sex (p = 0.47), but the sex by day interaction approached significance (p = 0.09). This latter observation is likely related to sex differences in mean swim latency on Day 4 (see Fig. 1B). Nevertheless, by Day 5, rats from each group demonstrated equivalent performance. Finally, there was no significant interaction between genotype, sex, and day (p = 0.95).
Twenty-four hours after the final training day, rats were tested in a 30 sec probe trial in which the platform was removed from the pool. Figure 2 shows heat maps representing the percentage of time in each location of the pool collapsed across each animal in the Tg+ and WT groups. In general, rats from each group spent a disproportionate amount of time searching near the trained platform location. This observation is supported by similar group measures of platform preference (Fig. 1C) and swim proximity (Fig. 1D). Indeed, an ANOVA failed to reveal significant effects for group (Platform Preference: p = 0.71; Proximity: p = 0.50) or sex (Platform Preference: p = 0.29; Proximity: p = 0.23). In addition, the ANOVA failed to show significant group by sex interactions (Platform Preference: p = 0.45; Proximity: p = 0.64). Taken together, these observations suggest that Tg+ rats were able to retain the platform’s previous location at a level comparable to WT control rats.
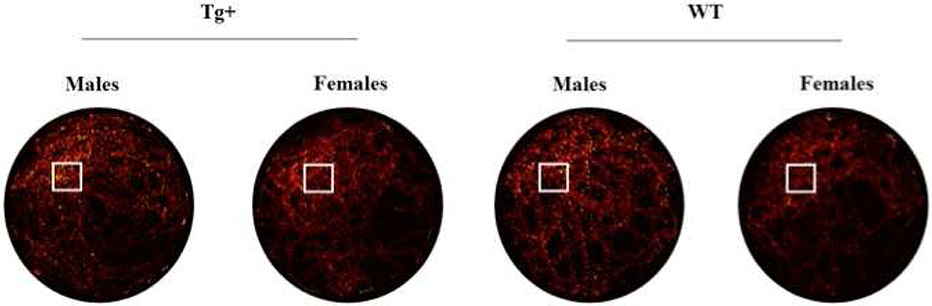
Figure 2.
Color maps collapsed across all animals in each group showing the percent time in each location of the swimming pool during the no-platform probe test. Lighter colors indicate regions of the pool where a large amount of time was spent, while darker colors indicate regions of the pool where a relatively low amount of time was spent. The platform location is marked by a white box. Note that Tg+ and WT rats spent a disproportionate amount of their swim time where the platform was located during training.
3.2. Experiment 2: Morris Water Task – Matching-to-Place
Performance on the matching-to-place variant of the water task evaluates the ability to learn a novel platform location in 1 trial (Harker & Whishaw, 2002; Whishaw, 1985). Thus, swim behavior by normal rats is characterized by elevated trial 1 latencies, in which rats search the previously reinforced platform location, followed by a significant reduction in trial 2 latencies. Figure 3A shows the swim latencies for Tg+ and WT rats for trial 1 and 2 for each daily platform position in the matching-to-place task as well as the averaged platform position across the 5 days of testing. Rats in both groups demonstrated elevated latencies in trail 1 followed by a comparable reduction in latencies during trial 2. Confirming this observation, repeated measures ANOVAs for latency revealed significant effects of trial for tests conducted on the 10 training trials across the five platform locations (F(corrected df: 3.1, 74.0) = 7.48, p < 0.001), and on the latency averaged across the platform locations (F(1, 24) = 55.7, p < 0.001). However, the ANOVAs failed to indicate significant group (p = 0.54) or sex differences (p = 0.15). Further the ANOVAs did not show group by trial (non-averaged: p = 0.38; averaged: p = 0.25), sex by trial (non-averaged: p = 0.83; averaged: p = 0.64), or group, sex and trial interactions (non-averaged: p = 0.12; averaged: p = 0.47).
Figure 3.
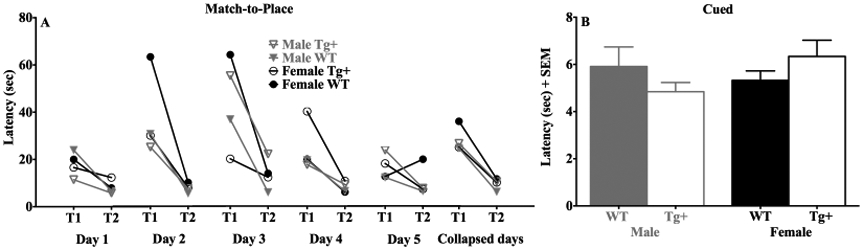
Effects of sex and genotype on match-to-place and cued navigation. (A) Latency (seconds) by Tg+ and WT groups over the 2 trials for each platform location and averaged across platform locations in the matching-to-place task. (B) Latency (seconds) by Tg+ and WT group in the cued platform task. Note that Tg+ and WT rats exhibited similar performance on both tasks.
Lastly, for each animal’s daily performance, we calculated a learning index which represents the normalized difference in swim latencies between trial 1 and trial 2 for each platform location (Trial 1 – Trial 2/ Trial 1 + Trial 2). Thus, a higher learning index measure would indicate lower latencies in trial 2, suggesting rapid learning of the new platform location. Table 1 reports the learning index (Mean ± SEM) as a function of trial 1 and trial 2 across the different platform positions, and averaged across each position. On average, learning index measures were slightly higher for WT animals across the first three locations (i.e., immediately following the stable platform protocol of Experiment 1), suggesting a greater change in swim latencies between trial 1 and trial 2 by the WT group. Nevertheless, despite these mean trends, a repeated measures ANOVA failed to detect significant group (p = 0.09), day (p = 0.14), or sex (p = 0.51) effects. The ANOVA also failed to detect group by day (p = 0.09), sex by day (p = 0.61), or group by sex by day interactions (p = 0.91). It is notable, however, that the group and group by day interactions approached significance (both p = 0.09), which suggests mildly less rapid learning by Tg+ rats over the first few days of the task.
Table 1.
Learning index measures (Mean ± SEM) plotted for Matching-to-Place performance for each day of the task.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| WT Female | 0.36 ± 0.11 | 0.63 ± 0.14 | 0.50 ± 0.27 | 0.41 ± 0.12 | −0.04 ± 0.15 |
| WT Male | 0.52 ± 0.10 | 0.45 ± 0.16 | 0.67 ± 0.07 | 0.35 ± 0.15 | 0.20 ± 0.15 |
| Tg+ Female | 0.17 ± 0.13 | 0.34 ± 0.18 | 0.25 ± 0.15 | 0.42 ± 0.12 | 0.27 ± 0.09 |
| Tg+ Male | 0.23 ± 0.11 | 0.36 ± 0.14 | 0.30 ± 0.19 | 0.27 ± 0.10 | 0.36 ± 0.15 |
3.3. Experiment 3: Morris Water Task – Cued Platform
This cued platform version of the water maze task was used to assess whether rats could navigate using a landmark directly associated with the platform. Figure 3B illustrates that Tg+ and WT rats demonstrated similar swim latencies across a block of 12 training trials. An ANOVA conducted on swim latencies failed to show a significant effect of group (p = 0.97), sex (p = 0.46) or group by sex interaction (p = 0.10). Thus, both TgF344-AD and control groups learned to approach the cued platform similarly.
3.4. Experiment 4: Elevated Plus-Maze
Figure 4 illustrates the effects of sex and genotype on anxiety-like behavior in the EPM. The ANOVAs for the duration of time spent in the open arms (Fig 4A) revealed a sex by genotype interaction [F(1,38)=5.64, p<0.05], and a main effect of genotype [F(1,38)=33.75, p<0.00001]; there was not a main effect of sex (p=0.91). Post-hoc analyses indicated that male and female Tg+ rats exhibited reduced open arm exploration compared to both matched sex and non-matched sex WT controls (Newman-Keuls, p<0.05 in each case); between sex comparisons did not reveal any differences between WT rats or Tg+ rats for open arm duration (Newman-Keuls, p>0.05 in each case). There was no sex by genotype interaction for the number of open arm entries (Fig 4B; p=0.13) or total distance traveled (Fig 4C; p=0.22). Collectively these results indicate that regardless of sex, Tg+ rats expressed heightened anxiety-like behavior compared to WT controls.
Figure 4.
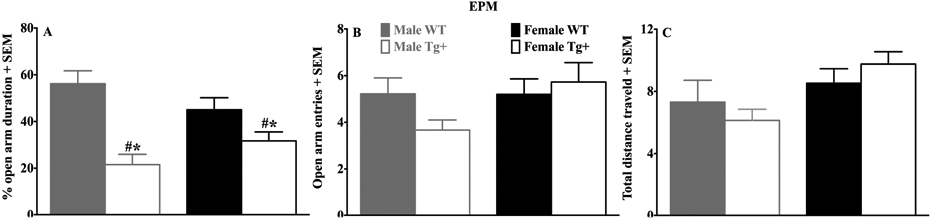
Effects of sex and genotype on anxiety-like defensive behavior during EPM testing. (A) The percentage of time spent in the open arms, (B) the number of open arm entries and (C) total distance traveled during a 5 min test session. Data are expressed as mean + SEM. Note that Tg+ rats exhibited reduced open-arm exploration compared to both matched sex and non-matched sex WT controls (Newman-Keuls, p<0.05 in each case). There were no group or sex differences in the number of open-arm entries or total distance traveled (meters). Wild-type (WT) controls; Transgenic TgF344-AD (Tg+); Asterisk (*) represents a difference compared to matched sex WT controls; Pound (#) represents a difference compared to non-matched sex WT controls.
4. Discussion
To our knowledge this is the first in-vivo study to characterize the TgF344-AD rat strain for unconditioned anxiety-like behaviors using the EPM, and spatial memory deficits using the Morris water task. Results indicate that regardless of sex, TgF344-AD rats exhibit increased anxiety-like behavior in the EPM model (Handley and Mithani, 1984; Pellow et al., 1985). The robust decrease in open-arm exploration detected in Tg+ rats (Fig 4A) was not due to a lack of open arm exploration or deficits in locomotor activity, respectively, as there were no differences in the number of open-arm entries (Fig 4B) or total distance traveled (Fig 4C). The heightened anxiety-like behavior seen in the Tg+ rats occurred without any measurable deficits in spatial learning in the Morris water task (Fig. 1, 2, and 3). Importantly, although we did not directly measure neuropathology we detected the anxiogenic-like phenotype at a time-point prior to the development of characteristic AD-related pathology (e.g., plaques, tangles and cognitive impairments) that occur in TgF344-AD rats (Cohen et al., 2013), suggesting that enhanced anxiety-like behavior represents an early stage behavioral marker that precedes the progression to late stage clinical pathology. These results provide additional translational validity for the TgF344-AD model, and highlight the need for future studies characterizing both early- and late-stage pathology in this Tg+ rat strain.
The present results suggest that the TgF344-AD strain represents a unique rat model for probing emotional components of AD. Indeed, AD patients exhibit alterations in mood including anxiety and depression that often precede or present concomitantly with initial signs of mild cognitive impairment (MCI) or memory loss (Feldman et al., 2004; Gabryelewicz et al., 2004; Panza et al., 2010). Previous studies examining anxiety-like behavior in various Tg+ models of AD have reported inconsistent effects. For instance, enhanced anxiety-like behavior is evident in APP/hAβ/PS1 (EPM & light-dark test, Guo et al., 2012), Tg2576 (light-dark test; Dong et al., 2012), PDAPP-J20 (open-field test; Beauquis et al., 2014), APPSWE (EPM; Bedrosian et al., 2011) and TgAPP (EPM; Lee et al., 2004) mice, while reduced anxiety-like behavior is evident in Tg2576 (EPM; Lalonde et al., 2003; Ognibene et al., 2005; Gil-Bea et al., 2007), Tg6799 (EPM; Jawhar et al., 2012) and EC-APP (EPM; Harris et al., 2010) mice; others report no differences in TgCRND8 (open field test & EPM; Touma et al., 2004) or Tg2576 (EPM; Arendash et al., 2001) mice, or in McGill-R-Thy1-APP (EPM; Galeano et al., 2014) rats. These discrepant findings may be due to the Tg+ or knock-in model examined, or may result from general behavioral disinhibition (i.e., increases in locomotor activity) that appears as reduced anxiety-like behavior (Lalonde et al., 2003; Ognibene et al., 2005; Gil-Bea et al., 2007; Roberson et al., 2007). Importantly, and consistent with the report by Cohen et al (2013), we did not detect differences in locomotor activity (Fig 4C) at this early time point, mitigating general behavioral disinhibition as an explanation for enhanced anxiety-like behavior in TgF344-AD rats.
The neurobiological mechanisms underlying the anxiogenic-like phenotype in TgF344-AD rats likely involve altered stress systems, particularly corticotropin-releasing factor (CRF). CRF is a 41 amino-acid neuropeptide that mediates the autonomic, behavioral, immune and endocrine responses to stress, via actions at two G protein-coupled receptor subtypes termed CRF1 and CRF2 (Spiess et al., 1981; Vale et al., 1981). Regarding endocrine function, TgCRND8 (Touma et al., 2004) and Tg2576 (Dong et al., 2008) mice exhibit hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis (e.g., elevated corticosterone), and APP/hAβ/PS1 Tg+ mice that display an anxiogenic-like phenotype express higher levels of CRF in key stress-related circuits (e.g., BNST, PVN; Guo et al., 2012). The perturbations in the APP/hAβ/PS1 mouse model are eliminated in Tg+ mice that are also heterozygous for crf1 and activation of ventral hippocampal CRF1 increases anxiety-like behavior in the EPM (Pentkowski et al., 2009), suggesting that the ventral hippocampal CRF1 systems underlie the anxiogenic-like phenotype in TgF344-AD rats and APP/hAβ/PS1 mice. Interestingly, the partial loss of crf1 does not eliminate the cognitive deficits in the APP/hAβ/PS1 mouse model, suggesting that elevated CRF1 signaling may account for enhanced anxiety-like behavior at early stages in TgF344-AD rats before the onset of late stage Aβ plaque and tangle formation that results in memory and cognitive impairments (Cohen et al., 2013).
Patients with AD exhibit stress system abnormalities including elevated levels of cortisol (Davis et al., 1986; Hartmann et al., 1997; Huang et al., 2009), as well as reduced CRF immunoreactive cells and upregulated CRF receptors (De Souza et al., 1986; Whitehouse et al., 1987; Pomara et al., 1989). Interestingly, these increases in cortisol occur at the MCI stage of AD (Lupien et al., 1998; Csernansky et al., 2006; Lind et al., 2007; Huang et al., 2009). Similarly, a growing body of evidence from genetic mouse models suggest that increased activation of the HPA axis often precedes plaque formation (Dong et al., 2008; Guo et al., 2012; Rothman et al., 2012; Hebda-Bauer et al., 2013; Brureau et al., 2013). Subsequently, late stage amyloid-associated neuropathology characterized by memory and cognitive impairments, involves CRF1-dependent Aβ- (Dong et al., 2004, 2012, 2014; Kang et al., 2007; Justice et al., 2015; Zang et al., 2016) and phosphorylated tau-induced (Rissman et al., 2007, 2012; Carroll et al., 2011) pathological alterations, particularly in the hippocampus. Collectively, these data highlight the critical role of CRF1 in modulating both early- and late-stage AD clinical pathology, and underscore the need for future studies examining these stress systems in the TgF344-AD model. Results from these studies may provide novel insight into the pathophysiology of AD.
The findings from the water task (Experiment 1, 2, and 3) expand on previous work investigating learning and memory, and navigation (Cohen et al., 2013). While Cohen et al. (2013) used the Barnes maze to evaluate spatial performance by TgF344-AD rats, the present work evaluated spatial learning and memory in three well characterized variants of the Morris water task, including the hidden platform (Experiment 1), matching-to-place (Experiment 2), and cued navigation tests (Experiment 3; Clark et al., 2009; 2013; Harker & Whishaw, 2002; Lehmann et al., 2007). Previous work has shown that accurate performance in the hidden platform and matching-to-place tasks is sensitive to lesions of the limbic system (Harker et al., 2002), all of which have been linked to pathological changes at the earliest stages of AD (Aggleton et al., 2016; Braak & Braak, 1991; Thal et al., 2002). Indeed, Cohen et al. (2013) reported increases in pre-plaque pathology (e.g., soluble Aβ, phosphorylated Tau and neuroinflammation) in limbic cortical and hippocampal regions in young adult TgF344-AD rats. However, despite the sensitivity of the Morris water tasks used in the present work, we failed to identify significant group differences in acquisition and retention of spatial performance. Further, as indicated by comparable group performance in Experiment 3, we determined that TgF344-AD animals could acquire a simple strategy of navigating toward a marked goal location. It is important to make note that Cohen et al (2013) reported trends toward impairments by young Tg+ rats (6 months of age) in reversal learning in the Barnes maze (i.e., the escape box was moved during each test session). Although the present study failed to observe significant deficits in a reversal variant (matching-to-place) of the Morris water task, we did detect subtle differences in one measure of learning in this task (see Table 1). One important distinction between studies is that Cohen et at used a Barnes maze with 20 escape holes along the perimeter of the open field. This feature may have ultimately placed a greater load on spatial discrimination and cognitive flexibility, resulting in greater sensitivity to detect navigational errors. Indeed, Cohen et al report that Tg+ animals made appreciably greater numbers of errors in the reversal phase of the Barnes maze testing, even at 6 months of age. These results strongly suggest that the TgF344-AD model displays intact spatial behavior at 4-5 months of age, and supports the observations by Cohen et al (2013) that TgF344-AD rats expresses limited cognitive deficits early in development.
In summary, the results of the present study point to the general conclusion that enhanced anxiety-like behavior exhibited by the TgF344-AD rat represents an early-stage behavioral marker that precedes the progression to impairments in learning and memory. We suggest that this pattern of behaviors provides additional translational validity for the TgF344-AD model, and future work should be directed toward understanding the neurobiological mechanisms of the anxiogenic-like phenotype in the TgF344-AD model.
Highlights.
Enhanced anxiety in the TgF344-AD transgenic rat model of Alzheimer’s disease.
TgF344-AD rats exhibit no significant deficits in the Morris water task at early time points.
Anxiety as a pre-clinical behavioral phenotype in the TgF344-AD rat strain.
Acknowledgments:
This research was supported by grant support from a Grice Faculty Research Enhancement Award to B.J.C., an NIGMS (P30GM103400) to B.J.C., and an NM-INBRE to B.J.C. The authors thank Dr. Derek Hamilton for technical support.
Footnotes
The authors do not have potential conflicts of interest including financial, personal, or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) their work. The author’s institution does not have contracts relating to this research through which it or any other organization may stand to gain financially now or in the future.
The authors verify that the Institutional Animal Care and Use Committee at the University of New Mexico approved all procedures for the studies reported here.
The current manuscript has not been published previously or is under consideration elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Pralus A, Nelson AJ, Hornberger M (2016). Thalamic pathology and memory loss in early Alzheimer’s disease: moving the focus from the medial temporal lobe to Papez circuit. Brain, 139, 1877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM (2001). Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res, 891, 42–53. [DOI] [PubMed] [Google Scholar]
- Beauquis J, Vinuesa A, Pomilio C, Pavía P, Galván V, Saravia F (2014). Neuronal and glial alterations, increased anxiety, and cognitive impairment before hippocampal amyloid deposition in PDAPP mice, model of Alzheimer's disease. Hippocampus, 24, 257–269. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Herring KL, Weil ZM, Nelson RJ (2011). Altered temporal patterns of anxiety in aged and amyloid precursor protein (APP) transgenic mice. PNAS, 108, 11686–11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon WC, Van Den Buuse M, Wegener N, Martin S, Chua HK, Bush AI, Masters CL, Adlard PA, Li QX (2010). Behavioural phenotype of APPC100.V717F transgenic mice over-expressing a mutant Abeta-bearing fragment is associated with reduced NMDA receptor density. Behav Brain Res, 209, 27–35. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1991). Neuropathological staging of Alzheimer-related changes. Acta Neuropathol, 82, 239–59. [DOI] [PubMed] [Google Scholar]
- Brureau A, Zussy C, Delair B, Ogier C, Ixart G, Maurice T, Givalois L (2013). Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer's disease rat model. Neurobiol Aging, 34, 1426–1439. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, Trojanowski JQ (2011). Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neursci, 31, 14436–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Arendash G, Laferla F, McDonald M (2010). Animal models of Alzheimer's disease. Int J Alzheimers Dis, 2010: 606357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Taube JS (2009). Deficits in landmark navigation and path integration after lesions of the interpeduncular nucleus. Behav Neurosci, 123, 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Hong NS, Bettenson DJ, Woolford J, Horwood L, McDonald RJ (2015). Maintained directional navigation across environments in the Morris water task is dependent on vestibular cues. Journal of Experimental Psychology: Animal Learning and Cognition, 41, 301–308. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Rice JP, Akers KG, Candelaria-Cook FT, Taube JS, Hamilton DA (2013). Lesions of the dorsal tegmental nuclei disrupt control of navigation by distal landmarks in cued, directional, and place variants of the Morris water task. Behav Neurosci, 127, 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T (2013). A Transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Ab, and frank neuronal loss. J Neurosci, 33, 6245–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC (2006). Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry, 163, 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathé AA, Johns CA, Horvath TB (1986). Cortisol and Alzheimer’s disease, I: Basal studies. Am J Psychiatry, 143, 300–305. [DOI] [PubMed] [Google Scholar]
- Delacourte A, Sergeant N, Champain D, Wattez A, Maurage CA, Lebert F, Pasquier F, David JP (2002). Nonoverlapping but synergetic tau and APP pathologies in sporadic Alzheimer’s disease. Neurology, 59(3), 398–407. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Whitehouse PJ, Kuhar MJ, Price DL, Vale WW (1986). Reciprocal changes in corticotropin-releasing factor CRF-like immunoreactivity and CRF receptors in cerebral cortex of Alzheimer's disease. Nature, 319, 593–595. [DOI] [PubMed] [Google Scholar]
- Do Carmo S, Cuello AC (2013). Modeling Alzheimer’s disease in transgenic rats. Mol Neurodegener, 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG (2004). Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience, 127, 601–609. [DOI] [PubMed] [Google Scholar]
- Dong H, Murphy KM, Meng L, Montalvo-Ortiz J, Zeng Z, Kolber BJ, Zhang S, Muglia LJ, Csernansky JG (2012). Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer's disease. J Alzheimers Dis, 28, 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wang S, Zeng Z, Li F, Montalvo-Ortiz J, Tucker C, Akhtar S, Shi J, Meltzer HY, Rice KC, Csemansky JG (2014). Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid- beta and behavior in Tg2576 mice. Psychopharmacology (Berl) 231, 4711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H, Scheltens P, Scarpini E, Hermann N, Mesenbrink P, Mancione L, Tekin S, Lane R, Ferris S (2004). Behavioral symptoms in mild cognitive impairment. Neurology, 62, 1199–1201. [DOI] [PubMed] [Google Scholar]
- Epis R, Gardoni F, Marcello E, Genazzani A, Canonico PL, Di Luca M (2010). Searching for new animal models of Alzheimer′ s disease. European J Pharmacology, 626(1), 57–63. [DOI] [PubMed] [Google Scholar]
- Eustache F, Giffard B, Rauchs G, Chételat G, Piolino P, Desgranges B (2006). Alzheimer’s disease and human memory. Rev. Neurol. (Paris), 162, 929–939. [DOI] [PubMed] [Google Scholar]
- Fein JA, Sokolow S, Miller CA, Vinters HV, Yang F, Cole GM, Gylys KH (2008). Co-localization of amyloid beta and tau pathology in Alzheimer's disease synaptosomes. American J Pathology, 172(6), 1683–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Rodriguez GA, Herman M, Emrani S, Nahmani E, Barrett G, Figueroa HY, Goldberg E, Hussaini SA, Duff KE (2017). Tau pathology induces excitatory neuron loss, grid cell dysfunction, and spatial memory deficits reminiscent of early Alzheimer’s disease. Neuron, 93(3), 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryelewicz T, Styczynska M, Pfeffer A, Wasiak B, Barczak A, Luczywek E, Androsiuk W, Barcikowska M (2004). Prevalence of major and minor depression in elderly persons with mild cognitive impairment—MADRS factor analysis. Int . Geriatr Psychiatry, 19, 1168–1172. [DOI] [PubMed] [Google Scholar]
- Galeano P, Martino Adami PV, Do Carmo S, Blanco E, Rotondaro C, Capani F, Castaño EM, Cuello AC, Morelli L (2014). Longitudinal analysis of the behavioral phenotype in a novel transgenic rat model of early stages of Alzheimer’s disease. Front Behav Neurosci, 8, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M (1993). Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci, 107, 618–626. [DOI] [PubMed] [Google Scholar]
- Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. (2013). Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement, 9, 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, Smith GE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA (2008). Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry, 65, 1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Bea FJ, Aisa B, Schliebs R, Ramírez MJ (2007). Increase of locomotor activity underlying the behavioral disinhibition in tg2576 mice. Behav Neurosci, 121, 340–344. [DOI] [PubMed] [Google Scholar]
- Guo Q, Zheng H, Justice NJ (2012). Central CRF system perturbation in an Alz- heimer’s disease knockin mouse model. Neurobiol Aging, 33, 2678–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SL, Mithani S (1984). Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of 'fear'-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol, 327, 1–5. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ (2002). Impaired spatial performance in rats with retrosplenial lesions: importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci, 22, 1155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Halabisky B, Lo I, Thwin MT, Yu GQ, Bredesen DE, Masliah E, Mucke L (2010). Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer's disease are independent of caspase cleavage of the amyloid precursor protein. J Neurosci, 30, 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I (1997). Twenty-four hour cortisol release profiles in patients with Alzheimer's and Parkinson's disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging, 18, 285–289. [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Simmons TA, Sugg A, Ural E, Stewart JA, Beals JL, Wei Q, Watson SJ, Akil H (2013). 3xTg-AD mice exhibit an activated central stress axis during early-stage pathology. J Alzheimers Dis, 33, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC (2009). Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci, 16, 1283–1286. [DOI] [PubMed] [Google Scholar]
- Jackson JS, Witton J, Johnson JD, Ahmed Z, Ward M, Randall AD, Hutton ML, Isaac JT, O’Neill MJ, Ashby MC (2017). Altered Synapse Stability in the Early Stages of Tauopathy. Cell Reports, 18(13), 3063–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O (2012). Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging, 196, e29–40. [DOI] [PubMed] [Google Scholar]
- Jost BC, Grossberg GT (1996). The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc, 44, 1078–81. [DOI] [PubMed] [Google Scholar]
- Jucker M (2010). The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nature medicine, 16(11), 1210–1214. [DOI] [PubMed] [Google Scholar]
- Justice NJ, Huang L, Tian J-B, Cole A, Pruski M, Hunt AJ Jr, Flores R, Zhu MX, Arenkiel BR, Zheng H (2015). Posttraumatic stress disorder-like induction elevates β-amyloid levels, which directly actives corticotropin-releasing factor neurons to exacerbate stress responses. J Neurosci, 35, 2612–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM (2007). Acute stress increases interstitial fluid amyloid-β via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci USA, 104, 10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer Vogt LJ, Hyman BT, Van Hoesen GW, Damasio AR (1990). Pathological alterations in the amygdala in Alzheimer’s disease. Neuroscience, 37, 377–85. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Dumont M, Fukuchi K, Strazielle C (2002). Transgenic mice expressing the human C99 terminal fragment of βAPP: Effects on spatial learning, exploration, anxiety, and motor coordination. Exp Gerontol, 37, 1401–1412. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Lews TL, Strazielle C, Kim H, Fukuchi K (2003). Transgenic mice expressing the betaAPP695SWE mutation: Effects on exploratory activity, anxiety, and motor coordination. Brain Res, 977, 38–45. [DOI] [PubMed] [Google Scholar]
- Lee KW, Lee SH, Kim H, Song JS, Yang SD, Paik SG, Han PL (2004). Progressive cognitive impairment and anxiety induction in the absence of plaque deposition in C57BL/6 inbred mice expressing transgenic amyloid precursor protein. J. Neurosci Res, 76, 572–580. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Clark BJ, Whishaw IQ (2007). Similar development of cued and learned home bases in control and hippocampal-damaged rats in an Open Field exploratory task. Hippocampus 17, 370–380. [DOI] [PubMed] [Google Scholar]
- Lind K, Edman A, Nordlund A, Olsson T, Wallin A (2007). Increased saliva cortisol awakening response in patients with mild cognitive impairment. Dement Geriatr Cogn Disord, 24, 389–395. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ (1998). Cortisol levels during hu- man aging predict hippocampal atrophy and memory deficits. Nat Neurosci, 1, 69–73. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Olin J (2002). Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry, 52, 243–252. [DOI] [PubMed] [Google Scholar]
- Manczak M, Reddy PH (2013). Abnormal interaction of oligomeric amyloid-β with phosphorylated tau: implications to synaptic dysfunction and neuronal damage. Journal of Alzheimer's Disease, 36(2), 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T, Gornbein J (1996). The spectrum of behavioral changes I Alzheimer’s disease. Neurology, 46, 130–5. [DOI] [PubMed] [Google Scholar]
- Morris R (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods, 11, 47–60. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM (2015). Three dimensions of the amyloid hypothesis: time, space and “wingmen”. Nat Neurosci, 18, 800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011). Guide for the care and use of laboratory animals: eighth edition. Washington, DC: The National Academies Press. [Google Scholar]
- Ognibene E, Middei S, Daniele S, Adriani W, Ghirardi O, Caprioli A, Laviola G (2005). Aspects of spatial memory and behavioral disinhibition in Tg2576 transgenic mice as a model of Alzheimer's disease. Behav Bbrain Res, 156, 225–232. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G, Seripa D, Pilotto A, Capurso A, Solfrizzi V (2010). Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J. Geriatr Psychiatry, 18, 98–116. [DOI] [PubMed] [Google Scholar]
- Pascoal TA, Mathotaarachchi S, Mohades S, Benedet AL, Chung CO, Shin M, Wang S, Beaudry T, Kang MS, Soucy JP, Labbe A, Gauthier S, Rosa-Neto P (2017). Amyloid-β and hyperphosphorylated tau synergy drives metabolic decline in preclinical Alzheimer’s disease. Molecular Psychiatry, 22(2), 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M (1985). M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Litvin Y, Blanchard DC, Vasconcellos A, King LB, Blanchard RJ (2009). Effects of acidic-astressin and ovine-CRF microinfusions into the ventral hippocampus on defensive behaviors in rats. Horm Behav, 56, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N, Singh RR, Deptula D, LeWitt PA, Bissette G, Stanley M, Nemeroff CB (1989). CSF corticotropin-releasing factor (CRF) in Alzheimer's disease: its relationship to severity of dementia and monoamine metabolites. Biol Psychiatry, 26, 500–504. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Lee KF, Vale W, Sawchenko PE (2007). Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J Neurosci, 27, 6552–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Staup MA, Lee AR, Justice NJ, Rice KC, Vale W, Sawchenko PE (2012). Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation. PNAS, 109, 6277–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L (2007). Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science, 316, 750–754. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, Mattson MP (2012). 3xTgAD mice exhibit altered behavior and elevated Aβ after chronic mild social stress. Neurobiol Aging, 830, e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh JJ, Kinney JW, Cummings JL (2013). Alzheimer’s disease biomarkers in animal models: closing the translational gap. Am. J. Neurodegener Dis, 2, 108–20. [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ (2001). Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev, 81, 741–66. [DOI] [PubMed] [Google Scholar]
- Smith MA, Siedlak SL, Richey PL, Mulvihill P, Ghiso J, Frangione B, Tagliavini F, Giaccone G, Bugiani O, Praprotnik D Kalaria RN, Perry G (1995). Tau protein directly interacts with the amyloid β-protein precursor: implications for Alzheimer's disease. Nature Medicine, 1(4), 365–369. [DOI] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, Vale W (1981). Primary structure of corticotropin-releasing factor from ovine hypothalamus. PNAS, 78, 6517–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeb HO, Murray ED, Price BH (2014). Neuropsychiatric symptoms of dementia. In Dickerson BC & Atri A (Ed.), Dementia: Comprehensive Principles and Practice (pp. 508–527). New York, NY: Oxford University Press. [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H (2002). Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology, 58, 1791–800. [DOI] [PubMed] [Google Scholar]
- Tomas Pereira I, Burwell RD (2015). Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci, 129, 533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C, Ambree O, Gortz N, Keyvani K, Lewejohann L, Palme R, Paulus W, Schwarze-Eicker K, Sachser N (2004). Age- and sex-dependent development of adrenocortical hyperactivity in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging, 25, 893–904. [DOI] [PubMed] [Google Scholar]
- Tsai Y, Lu B, Ljubimov AV, Girman S, Ross-Cisneros FN, Sadun AA, Svendsen N, Cohen RM, Wang S (2014). Ocular changes in TgF344-AD rat model of Alzheimer’s disease. Invest Ophthalmol Vis Sci, 55, 4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 213, 1394–1397. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R (2000). The parahippocampal gyrus in Alzheimer’s disease. Clinical and preclinical neuroanatomical correlates. Ann NY Acad Sci, 911, 254–74. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ (1985). Formation of a place learning-set by the rat: a new paradigm for neurobehavioral studies. Physiol Behav, 35, 139–43. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Vale WW, Zweig RM, Singer HS, Mayeux R, Kuhar MJ, Price DL, De Souza EB (1987). Reductions in corticotropin releasing factor-like immunoreactivity in cerebral cortex in Alzheimer's disease, Parkinson's disease, and progressive supranuclear palsy. Neurology, 37, 905–909. [DOI] [PubMed] [Google Scholar]
- Wilson RA, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA (2003). Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology, 61, 1479–1489. [DOI] [PubMed] [Google Scholar]
- Zang C, Kuo C-C, Moghadam SH, Monte L, Campbell SN, Rice KC, Sawchenko PE, Masliah E, Rissman RA (2016). Corticotropin-releasing factor receptor-1 antagonism mitigates beta amyloid pathology and cognitive and synaptic deficits in a mouse model of Alzheimer’s disease. Alzheimers Dement, 12, 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]