Abstract
Heart failure (HF) with preserved ejection fraction (HFpEF) is a clinical condition characterized by large pathophysiology heterogeneity with lack of effective therapies as proven by the disappointing results generated by randomized controlled trials. The innovative therapeutic concept provided by sacubitril–valsartan, a molecule combining angiotensin receptor blocking agent and neprilysin inhibitor has suggested the hypothesis it would have led to a reduced risk of hospitalization for HF or death from cardiovascular causes among patients with HF and preserved ejection fraction. The PARAGON-HF (ClinicalTrials.gov number, NCT01920711) investigated HF subjects class II to IV HF, ejection fraction of 45% or higher, elevated level of natriuretic peptides, and structural heart disease to receive sacubitril–valsartan (target dose, 97 mg of sacubitril with 103 mg of valsartan twice daily) or valsartan (target dose, 160 mg twice daily). The trial missed the primary outcome of cardiovascular death and HF hospitalization (HFH) in the overall study population. A subgroup analysis addressed significant decrease of HFH in subjects with left ventricular ejection fraction below the median 57% value in the study. The data were consistent with previous post hoc analysis performed in studies where candesartan and spironolactone were investigated in HFpEF. Those results open the door to investigate angiotensin aldosterone and peptidases inhibition efficacy in the unexplored HF middle range ejection fraction, currently lacking of valid evidence.
Keywords: Heart failure with preserved ejection fraction, Sacubitril valsartan, Candesartan, Neutroptidases, Neutral endopeptidase, Heart failure hospitalization
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a common and increasingly prevalent clinical syndrome, broadly characterized by signs and symptoms of heart failure (HF), in the absence of a reduced left ventricular ejection fraction (LVEF).1
The HFpEF definition is based on the assumption that LVEF >45% is not only the common disease stem, but it is also the end product of a specific, still not fully understood, disease pathophysiology. The concept takes roots by the evidence that hypertension is the prominent landmark of HFpEF population, supporting the hypothesis that development of a sufficient number of individual abnormalities in cardiovascular reserve can promote the transition from asymptomatic hypertensive diastolic dysfunction to symptomatic HFpEF.2
Unfortunately, many other pathophysiological components of the syndrome have been identified in HFpEF subpopulations such as chronotropic incompetence, volume overload, systolic dysfunction, high body mass index, renal dysfunction, obstructive sleep apnoea etc.3 Each component maybe variably involved in HfpEF phenotype assembling, thus providing a composite and not predictable response to other established therapies successfully adopted for HF with reduced ejection fraction.
The burning (cumbersome) point of the multiform disease nature is addressed by the increasingly frequent diagnostic challenge in HFpEF represented by compensated, euvolaemic patients complaining for exertional dyspnoea in the absence of overt clinical, radiographic, or biomarker evidence of congestion. In these subjects, the decisive diagnostic tool to screen the cardiac cause of symptoms is the right heart catheterization. If the rest haemodynamic profile is normal, only the invasive exercise testing can detect the abnormal pulmonary pressure changes related to the patient symptoms.4
The comprehensive disease picture addresses HFpEF as a multifaceted condition that probably cannot be simply defined by the LVEF cut-off.
Consistently, the heterogeneous nature of the syndrome may explain why only a few pharmacologic, namely spironolactone, and non-pharmacologic interventions, i.e. implantable pulmonary artery pressure monitoring, showed to reduced HF hospitalizations (HFHs) as a second endpoint, in randomized controlled trials,5,6 while the other clinical trials, which, thus far, have used essentially a one-size-fits-all concept, didn’t succeed.
The Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction (PARAGON-HF), background and study hypothesis
The PARAGON-HF7 study has been designed to determine whether sacubitril/valsartan was superior to angiotensin receptor blockade alone in patients with chronic symptomatic HfpEF.
At time of study design four outcome trials investigating inhibitors of the renin–angiotensin aldosterone system (RAAS), including two distinct angiotensin receptor blocking agents (irbesartan and candesartan), did not meet their primary endpoints.8,9 Therefore, the PARAGON HF study challenged the sacubitril standalone strength to overcome the hurdle of antecedent failures.
It’s worth to remind that neutral endopeptidase (NEP) and angiotensin-converting enzyme (ACE) have similarities in their active structure sites10 and NEP inhibitors (like sacubitril), are a class of cardiovascular drug that provide contemporary NEP and ACE inhibition.
Simultaneous blockade with an ACE inhibitor or an angiotensin receptor blocking agent like valsartan and an inhibitor of NEP, not only interrupts the renin–angiotensin system but also increases the availability of bradykinin and, secondarily, nitric oxide and prostacyclin. Based on the last peculiar action, the overall hypotensive effect of the two sides block is consistently magnified.
It’s important to highlight NEP inhibitors used alone in HF with reduced ejection fraction (HfrEF) patients, without simultaneous inhibition of angiotensin II formation, induced systemic vasoconstriction rather than vasodilatory action, despite significant activation of the ANP system. This effect produced enhanced pressor response,11 and consequently, unfavourable consequences.
This short summary of the current knowledge focuses on evidence that the complex neurohormonal system controlling circulatory torrent pressure and volume is based on two axes sharing common action pathways, and operating upon interdependent control and balance.
In order to secure the PARAGON HF goal accomplishment a preliminary investigation was performed. The study ‘The angiotensin receptor neprilysin inhibitor LCZ696 in HFpEF: a phase 2 double-blind randomized controlled trial’ scouted the hypothesis sacubitril–valsartan was safe and effective in decreasing N-terminal pro brain natriuretic peptide (NT-proBNP) concentration at a greater extent than valsartan at 12 weeks.12
In the PARAGON study, the sacubitril–valsartan administration was followed by evident reduction of NT-proBNP at 4 weeks that reached statistical significance at 12 weeks, remaining sustained after 36 weeks. The natriuretic peptide decline was coupled with significant decrease of left atrium size, but the two most common echocardiographic ventricular indexes linked to diastolic impairment, the E/e′ and Lateral E′, didn’t show statistically significant change. Moreover, after 36 weeks, the NT-proBNP concentration in the valsartan study arm displayed a delayed decline abolishing the statistically significant difference with sacubitril–valsartan arm (Figure 1).
Figure 1.
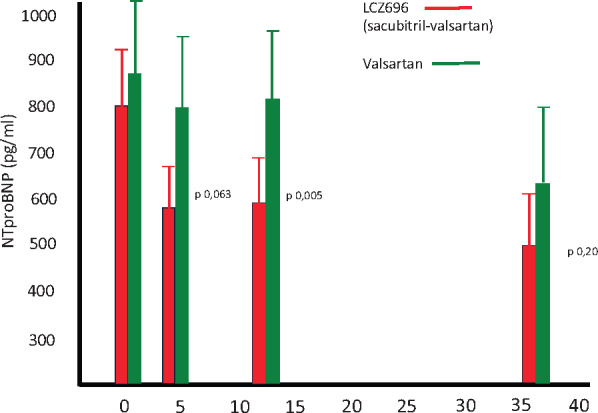
In PARAMAOUNT trial11 performed in heart failure with preserved ejection fraction subjects, sacubitril–valsartan administration was followed by reduction of NT pro-BNP after 4 weeks treatment that became statistically significant after 12 week of therapy and was sustained to 36 weeks, though the between group difference was no longer significant because valsartan treated group displayed a delayed NT-proBNP decline.
Therefore, in the valsartan treated arm, it would have been worth to measure atrial size after a further 10–12 weeks delay allowing comparison of changes in cardiac structure at time of maximum biological therapy effect in both arm.
On the basis of the PARAMOUNT12 findings, the PARAGON HF7 protocol was designed mirroring the running in phase and patient selection criteria of the antecedent research. The primary objective of the study was to compare in HFpEF (LVEF ≥ 45%) patients with New York Heart Association (NYHA) Class II–IV sacubitril–valsartan to valsartan, in reducing the rate of the composite endpoint of cardiovascular death and total (first and recurrent) HFHs. The lower LVEF 45% cut-off was motivated by the need to include a wide spectrum of patients for whom no proven therapy is available and to exclude subjects with any prior measurement of LVEF <45%, i.e. those with previous HFrEF occurrence.
The study population consisted of patients ≥55 years of age that, on top to LVEF entry criteria, had echocardiographic evidence of structural heart disease (left atrial enlargement or left ventricular hypertrophy) detected within 6 months prior to enrolment, symptomatic HF (NYHA Class II–IV) and HF symptoms requiring diuretic therapy for ≥30 days prior to Visit 1. In addition, patients had to have at least one of the following: (i) a hospitalization for HF within 9 months prior to enrolment, or (ii) an elevated NT-proBNP (>300 pg/mL for patients not in atrial fibrillation (AF) or >900 pg/mL for patients in AF at Visit 1).
The aim of the PARAGON HF study was to focus on a clearly symptomatic HF population. As in the I-PRESERVE study, prior HFH and elevated NT-proBNP were the strongest independent predictors of mortality and subsequent HFH,13 the study design valued recurrent HFHs as a component of primary endpoint.
In May 2015, further changes occurred in study entry criteria and two of them were targeted to reinforce the HF severity in the study population to be enrolled. The first change was to eliminate the simple intravenous diuretic treatment for HF lasting ≥12 h at a healthcare facility within 9 months prior to screening visit, as an enrolment criterion. The second was patients who entered the trial based on an HFH, to also had to have an elevated NT-proBNP (>200 pg/mL for patients in sinus rhythm or >600 pg/mL for patients in AF on the Visit 1 electrocardiogram).
The trial entry criteria changes were strictly focused in reinforcing the fact that the investigated HFpEF subjects had to have unequivocal HF signs and symptoms. The net effect led to restrict patient eligibility to enrolment, by excluding those HFpEF subjects without structural heart changes and clinical or biological (BNP–NT-proBNP) expression. Those study entry criteria led to intense patient selection and may explain the 5537 patients drop out from the eligibility phase to the randomization phase. We can argue the study investigated a not prevalent HFpEF subpopulation among the whole real world of HFpEF subjects and the goal was to target those whose symptoms were linked to persistent cardiac dysfunction.
The study sample size
The study sample size was calculated through simulations for the proportional rates model and the candesartan group of the CHARM-Preserved study8 involving patients with EF ≥45% provided the rates for statistical assumption.
On note, in the CHARM-Preserved trial overall mortality and cardiovascular mortality had over-imposable relative risk (RR) in the study arms [RR 1.03 vs. RR 1.00; confidence interval (CI) 0.87–1.21 vs. CI 0.82–1.22].
In PARAGON HF, the study sample size was defined assuming the target reduction in RR for the primary endpoint to be about 22%, which approximately corresponded to a reduction of 30% for HFH and a reduction of 10% for cardiovascular death.
For PARAGON HF study, with a one-sided alpha level of 0.0249, a total of 4300 patients were expected to provide more than 90% of power for the LWYY method (www.ema.europa.eu/en/documents/other/qualification-opinion-treatment-effect-measures-when-using-recurrent-event-endpoints-applicants_en.pdf). The assumption would have had required approximately 1721 primary events with sample size = 4300 and hazard ratio (HR) = 0.9 and RR = 0.7.
In December 2015, new amendments modified the sample size from 4300 to 4600, and the target number of endpoints from 1721 to 1847 which corresponded to a 25% reduction in recurrent HFHs and corresponding to an overall 19% reduction in the primary endpoint (cardiovascular deaths and total recurrent HFH).
Those changes were coupled with statistical stopping rules modification for superiority of sacubitril–valsartan over valsartan from one-sided P-value of <0.0001 for the primary endpoint to one-sided P-value of <0.001 for both the primary endpoint and cardiovascular death at the interim efficacy analysis.14
In the case sacubitril–valsartan wouldn’t have shown a benefit on cardiovascular death at the interim one, the study final analysis, to be performed in 2019, would have had the need to show superiority below a one-sided statistical significance level of 0.025. The overall statistical design change clearly entails a significant downgrading of the expected sacubitril standalone effectiveness.
What reason backed the revised version of the study statistical design?
It’s has to be highlighted in HFpEF studies the LVEF threshold to define ‘preserved’ ejection fraction varied significantly, ranging from ≥40 to ≥55%.
In 2015, Solomon et al.15 published (online published ahead of print 15 September 2015) the post hoc analysis performed on TOPCAT Spironolactone for HFpEF study population, analysing treatment effect modification for LVEF as a linear continuous variable. Treatment effects were assessed without further adjustment for covariates.
The study data addressed LVEF was able to influence the effect of spironolactone treatment, particularly for the primary outcome (first of either cardiovascular death, HFH, resuscitated sudden death, P 0.046) and for HFH (P 0.039), with higher estimated benefits of spironolactone at the lower end of LVEF spectrum with respect to the primary endpoint (LVEF 50%: HR 0.72, 95% CI 0.50–1.05; LVEF ≥60%: HR 0.97, 95% CI 0.76–1.23) and HFH (LVEF 50%: HR 0.76, 95% CI 0.46–1.27; LVEF ≥60%: HR 0.98, 95% CI 0.74–1.30). The size of benefit in the relatively impaired LVEF was even superior to the expected benefit in the overall study population.
On note, the positive spironolactone effect was detected in the HF sub-population that only lately the 2016 ESC-HF guidelines1 recognized as the distinct subgroup of HF middle range EF (HFmrEF).
In October 2017, a new post hoc analysis performed in 7598 patients enrolled in the CHARM Programme (HF across the spectrum of EF), was submitted to the European Journal of Heart Failure and subsequently published on line in February 2018. The analysis assessed characteristics, treatment effect and outcomes of candesartan according to LVEF as a continuous spline variable.
The analysis addressed for treatment effect, the incidence rates for the primary outcome for candesartan vs. placebo were 7.4 vs. 9.7 per 100 patient-years in HFmrEF (LVEF 40–49%, HR 0.76, 95% CI 0.61–0.96; P = 0.02), and 8.6 vs. 9.1 per 100 patient-years in HFpEF (HR 0.95, 95% CI 0.79–1.14; P = 0.57). For HFH, the incidence rate ratios were 0.48 in HFmrEF (95% CI 0.33–0.70; P < 0.001), and 0.78 in HFpEF (95% CI 0.59–1.03; P = 0.08).16
The PARAGON HF study results
In the Paragon HF,7 the primary endpoint of total HFHs or cardiovascular death was narrowly missed (HR 0.87, CI 0.75–1.01; P = 0.059). However, not surprisingly in the adjusted rate ratio for primary endpoint by subgroups LVEF (below 57% median) displayed a significant reduction of RR (HR 0.78, CI 0.64–0.95) with a benefit consistent with original study statistical hypothesis and with what was achieved by sacubitril–valsartan in HFrEF subjects enrolled in the PARADIGM HF trial (doi: 10.1056/NEJMoa1409077).
Both TOPCAT and CHARM Preserved investigated molecules that proved to be effective in targeting the renin–angiotensin system in HFrEF studies and sacubitril–valsartan on top to established ACE I-ARBs treatment in the same HF phenotype. By clearing the HFmrEF subpopulation in HFpEF studies any benefit in the active treatment arm would be subtracted.
The analysis provides the conclusion that simultaneous RAAS blockade with an angiotensin receptor-blocking agent like valsartan and an inhibitor of NEP, is able to restrain events only in HF subjects with structural damage of the left ventricle entailing impaired left ventricular systolic function, not differently by what was shown by spironolactone and candesartan, but sacubitril–valsartan added benefit on top of angiotensin blocking agent administration (Table 1).
Table 1.
Comparison of PARAGON-HF, CHARM-P, TOPCAT trials on left ventricular ejection fraction cut-offs and heart failure hospitalization outcomes
| CHARM-P8 (n. 3023) |
TOPCAT14 (n. 3445) |
PARAGON-HF7 (n. 4800) |
|
|---|---|---|---|
| Treatment arms | Candesartan vs. placebo | Spironolactone vs. placebo | Sacubitril/valsartan vs. valsartan |
| Key inclusion criteria |
LVEF > 40% NYHA functional class II–IV, prior CVH |
LVEF ≥ 45% >1 HF symptom, >1 HF sign, elevated NP, or HFH |
LVEF > 45% NYHA functional class II–IV, elevated NT-proBNP. Mildly elevated NT-proBNP if prior HFH, structural heart disease (LAE/LVH) |
| Endpoint | First of either CVD or HFH | First of either CVD, HFH, or RSD | CVD and total HFH (first and recurrent) |
|
Heart failure Hospitalizations In HFpEF trials Based on LVEF |
LVEF ≥50% HR 0.78, 95% CI 0.59–1.03; LVEF 40–49% HR 0.48 95% CI 0.33–0.70 |
LVEF ≥60%: HR 0.98, 95% CI 0.74–1.30 LVEF <50%: HR 0.76, 95% CI 0.46, 1.27 |
LVEF 57.6 + 7.8% HR 0.85: CI 0.72–1.00 LVEF <57%: HR 0.78: CI 0.64–0.95 |
CHARM-P, Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity-Preserved; CVD, cardiovascular death; CVH, cardiovascular hospitalization; HFH, heart failure hospitalization; HFpEF, heart failure with preserved ejection fraction; LAE, left atrial enlargement; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; NT pro-BNP, N-terminal pro brain natriuretic peptide; PARAGON-HF, Prospective Comparison of ARNI with ARB Global Outcomes in HF With Preserved Ejection Fraction; RSD, resuscitated sudden death; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist.
The sacubitril–valsartan effect in renal function and the outcome in heart failure patients with preserved ejection fraction
Among pre-specified subgroups that underwent analysis for the primary outcome in PARAGON HF study, the baseline estimated glomerular filtration rate <60 mL/min/1.73 m2 displayed a significant RR 0.79 (95% CI 0.66–0.95) vs. a not significant 1.01 (0.80–1.27) in 60 mL/min/1.73 m2. The data reinforce the relevance of renal function in HFpEF outcome, better preserved by sacubitril–valsartan as addressed by the inferior renal composite outcome, in treated arm, 33 (1.4%) and 64 (2.7%) in control arm, HR 0.50 (0.33–0.77).
The overall PARAGON HF study renal data are consistent with previous observations that highlight worsening of renal function induced by RAAS inhibition have an increased mortality risk in HFpEF patients.17
The sacubitril–valsartan effect and the gender difference
In the pre-specified subgroups analysis for the primary outcome, the female gender displayed significant better outcome (923/2479; RR 0.73, CI 0.59–0.90) in comparison to the male gender (980/2317; RR 1.03, CI 0.85–1.25). The result mirrors the data generated by the I-PRESERVE analysis in genders that addressed women had better overall prognosis. Though in presence of AF, renal dysfunction, stable angina pectoris, or advanced NYHA class symptoms, gender-related difference in risk of all-cause events was attenuated.18 Further PARAGON HF post hoc data investigations are expected to better qualify the relevance of the PARAGON HF analysis in the genders as well as in other subgroups.
Conclusions
The Paragon HF trial randomized 4800 patients to treatment with either sacubitril–valsartan or valsartan alone, comparing the two on a composite endpoint of cardiovascular death or HFH. The study had to clear a pretty high bar in a largely heterogeneous population, mostly because researchers do not completely understand the fuzzy pathophysiology of HFpEF.
However, beyond the early solid evidence in HFrEF patients, current study and precedent recent investigations on RAAS inhibitory therapies, address RAAS deactivation can work in reducing HF exacerbation in also those HF subjects bearing modest LVEF impairment.
Moreover, the simultaneous action of NEP inhibitor coupled with an angiotensin receptor blocking agent like valsartan, can add further clinical benefit in preventing HFH in this HF subpopulation.
Conflict of interest: none declared.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011;32:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah SJ. Innovative clinical trial designs for precision medicine in heart failure with preserved ejection fraction. J Cardiovasc Trasl Res 2017;10:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS; for the CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 6. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 7. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen H-D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON-HF Investigators and Committees . Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 8. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Östergren J; Charm Investigators . Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM Preserved Trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 9. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 10. Robl JA, Sun C-Q, Stevenson J, Ryono DE, Simpkins LM, Cimarusti MP, Dejneka T, Slusarchyk WA, Chao S, Stratton L, Misra RN, Bednarz MS, Asaad MM, Cheung HS, Abboa-Offei BE, Smith PL, Mathers PD, Fox M, Schaeffer TR, Seymour AA, Trippodo NC. Dual metalloprotease inhibitors: mercaptoacetyl-based fused heterocyclic dipeptide mimetics as inhibitors of angiotensin-converting enzyme and neutral endopeptidase. J Med Chem 1997;40:1570–1577. [DOI] [PubMed] [Google Scholar]
- 11. Weber MA. Vasopeptidase inhibitors. Lancet 2001;358:1525–1532. [DOI] [PubMed] [Google Scholar]
- 12. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators . The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- 13. Mckelvie RS, Komajda M, Mcmurray J, Zile M, Ptaszynska A, Donovan M, Carson P, Massie BM. Baseline plasma NT-proBNP and clinical characteristics: results from the irbesartan in heart failure with preserved ejection fraction trial. J Card Fail 2010;16:128–134. [DOI] [PubMed] [Google Scholar]
- 14. Solomon SD, Rizkala AR, Gong J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. J Am Coll Cardiol HF 2017;5:471–482. [DOI] [PubMed] [Google Scholar]
- 15. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016;37:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230–1239. [DOI] [PubMed] [Google Scholar]
- 17. Beldhuis IE, Streng KW, Ter Maaten JM, Voors AA, van der Meer P, Rossignol P, McMurray JJV, Damman K. Renin–angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction: a meta-analysis of published study data. Circ Heart Fail 2017;10:e003588.. [DOI] [PubMed] [Google Scholar]
- 18. Lang Cs Carson PE, Anand IS. Sex differences in clinical ejection fractions treatments on specific HFpEF phenotypes may identify subgroups that benefit. Latent class analysis of the I-PRESERVE data set identified a group of metabolic phenotype patients with HFpEF (high prevalence of diabetes, hyperlipidaemia and obesity) that benefited from irbesartan therapy. Circ Heart Fail 2012;5:571–578.22887722 [Google Scholar]


