Abstract
Background and Aims
Recent treatment guidelines for ulcerative colitis [UC] do not recommend long-term corticosteroid [CS] use. The present study aimed to capture the changes in CS use from 2006 to 2016 and to identify factors associated with long-term CS use after 2014, when the first two anti-tumour necrosis factor antibodies [infliximab and adalimumab] became available.
Methods
A retrospective study using the JMDC Claims Database included UC patients who initiated UC medications in any year from January 2006 to December 2016, or after January 2014, who were under continuous observation from 6 months before to 12 months after initiation. Patients with Crohn’s disease before initiation and those prescribed <8 days of CSs were excluded.
Results
Among 7907 UC patients who initiated UC medications within the study period, 1555 were prescribed CSs. The proportion of patients using CSs in each year decreased from 2011 as use of thiopurines and biologics increased. The proportion of patients with a starting dose ≥30 mg/day of CSs and patients continuing CSs for <90 days increased from 2011, reaching 49.1% and 41.0%, respectively, in 2016. However, even in 2016, 34.3% continued to use CSs for ≥180 days. Among 1230 patients with CS use after January 2014, low initial CS dose [<10 mg/day] was most strongly associated with long-term CS use [≥180 days].
Conclusions
CS use became more appropriate as use of thiopurine and biologics increased, although there were still many cases of inappropriate use. Long-term CS use was most strongly associated with low initial doses of CSs.
Keywords: Ulcerative colitis, corticosteroid, claims database
1. Introduction
Ulcerative colitis [UC] is a chronic inflammatory disorder of the colonic mucosa, characterized by alternating periods of remission and relapse.1 The estimated number of patients with UC in Japan was ~220 000 in 2014, with a prevalence rate of about 170 per 100 000 people. Although the prevalence of UC in Japan is less than half of that in the USA,2 it has increased in recent years.3,4 Based on changes in the incidence and prevalence observed in Europe and North America,5 the number of patients with UC in Japan is expected to increase further.
Corticosteroids [CSs] are effective for the induction of remission in UC.6,7 In the current treatment guidelines,4,8 CSs are recommended for patients with moderate-to-severe inflammation or symptoms. However, CSs are not effective for the maintenance of remission, and long-term use of CSs should be avoided because of the risk of adverse events or comorbidities such as impaired glucose tolerance and osteoporosis.4,9 The guidelines recommend the use oral prednisolone [PSL] at 30–40 mg/day for patients with moderate UC and intravenous PSL at 40–80 mg/day for those with severe UC followed by tapering and withdrawal.8 Furthermore, CS doses should be reduced to below the equivalent of PSL 10 mg/day within 3 months of starting CSs.10 However, many patients continue to use CSs for extended periods of time.11
In the past few decades, several medications with new modes of action have launched in succession and been prescribed to patients with CS-dependent and/or CS-resistant UC in Japan. One of these new medications, azathioprine, was approved for use in CS-dependent UC patients in 2007. Since 2010, anti-human tumour necrosis factor [TNF]-α monoclonal antibodies have been used for induction and maintenance of remission in patients with CS-dependent and CS-resistant moderate-to-severe UC [infliximab in 2010, followed by adalimumab in 2013 and golimumab in 2017]. In addition, an anti-human α4β7 integrin monoclonal antibody [vedolizumab] and Janus kinase inhibitor [tofacitinib] have also been used in these patients since 2018. While several studies have reported the efficacy of these molecular targeted agents in reducing and discontinuing the use of CSs,12–17 the impact of these new treatments on CS use in real-world practice has not been identified.
The present claims database study aimed to understand changes in use of CS, thiopurine and biologics for UC and the impact of use of thiopurine and biologics on appropriate CS use. We also aimed to examine differences in the background of patients with long-term and non-long-term CS use and to identify the factors associated with long-term CS use.
2. Materials and Methods
2.1. Study design and data source
This study used three different study designs, one for each of the three objectives. Objective A-1 was to determine whether the prescription of UC drugs has changed over time for patients diagnosed with UC via a descriptive cross-sectional study design. Objective A-2 was to confirm whether the proportion of CS prescriptions has changed over time for patients diagnosed with UC via a descriptive cohort study design. Objective B was to identify differences in the background of patients with long-term and non-long-term CS use via a retrospective cohort study design.
We used a large Japanese administrative claims database constructed by JMDC Inc.18 This database contains accumulated claims data of Japanese employees from medium to large companies and their family members. Data have been collected from health insurance societies since 2005 and the total number of individuals in the database was 5.6 million at the end of 2018. The database contains anonymized information such as the characteristics of patients [e.g. age, sex]; claims data for inpatients, outpatients and dispensing services; and clinical diagnoses coded using the International Classification of Diseases 10th revision [ICD-10]. In this database, patients can be continuously followed up, even if they were transferred to another hospital or visited multiple medical institutions. All available medical and prescription records were obtained from January 1, 2005 to March 31, 2018. The study period was from January 1, 2006 to December 31, 2016 for Objectives A-1 and A-2 or from January 1, 2014 [when infliximab and adalimumab became available] to December 31, 2016 for Objective B.
The JMDC database is anonymized and ethical review of subject research, as defined by the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan, is not required. Nonetheless, the study protocol was approved by the Ethics Review Committee of Mitsubishi Tanabe Pharma Corporation [No. H-19-027].
2.2. Study population
Patients with a confirmed diagnosis of UC were identified using the ICD-10 code K51, as in other studies.11,19 There were no exclusion criteria based on age.
For Objective A-1, UC patients with at least one prescription for a UC medication in any year from January 1, 2006 to December 31, 2016 were included. The index date was defined as January 1 of the year in which a prescription for any UC medication was provided. For Objective A-2, UC patients with at least one prescription for CSs in any year from January 1, 2006 to December 31, 2016 were included. The index date was defined as the date on which a CS was prescribed for the first time or again after discontinuation. For Objective B, UC patients with at least one prescription for CSs from January 1, 2014 to December 31, 2016 were included. The index date for objective B was the same as that for Objective A-2. Additionally, for all objectives, patients with an index date after the first UC diagnosis [no limitations on the period from the day of the first UC diagnosis to the index date] and with an 18-month observation period from 6 months before to 12 months after the index date were included.
For all objectives, patients with at least one confirmed diagnosis of Crohn’s disease [CD] [ICD-10 D518 or K50] within the period from 6 months before to 12 months after the index date were excluded. For Objectives A-2 and B, patients with a CS prescription duration of less than 8 days were excluded, because CSs for the treatment of UC are unlikely to be prescribed for less than 8 days in clinical practice.
The CS treatment duration was defined as the period from the date of CS initiation to the last date of CS treatment more than 90 days before the next CS prescription date. The last date of CS treatment was calculated as the CS prescription date plus the number of CS prescription days, and the CS initiation date was defined as the CS prescription date with more than 90 days of no CS prescription.
2.3. Endpoints
Primary endpoints of each objective were as follows: for Objective A-1, the number and proportion of patients who were prescribed each UC medication; for Objective A-2, the dose per day at initial CS use, duration of CS use and retreatment with CSs within 12 months; and for Objective B, differences in demographic and clinical background characteristics of patients with long-term and non-long-term CS use. Demographic characteristics included sex and age. Clinical characteristics included the CS dose per day during the observation period and at initial CS use, the type of medical institution that provided the initial CSs (university hospital, national/public hospital, clinic with <20 beds, and other hospital [hospital with ≥20 beds other than a university hospital or national/public hospital]), and use of thiopurine or biologics after the index date.
Secondary endpoints of the three objectives were demographic and clinical characteristics of all eligible patients. For Objective B, factors associated with long-term CS use were also examined.
The following UC medications were included in the analysis: 5-aminosalicylic acid [5-ASA] (salazosulfapyridine [oral, suppository], mesalazine [oral, suppository, enema]], CS (PSL [oral], PSL sodium succinate [injection]; only PSL was included because CSs other than PSL are rarely used for UC in Japan), thiopurine (azathioprine [oral], mercaptopurine hydrate [oral]), biologics [infliximab, adalimumab, golimumab, vedolizumab] and others (tofacitinib citrate [oral], tacrolimus hydrate [oral]). These drugs were identified in the database using the codes listed in Supplementary Table 1.
2.4. Statistical analysis
For Objective A, the data were expressed as median and interquartile range [IQR] for continuous variables [age, CS dose and duration], and as frequency and proportion for categorical variables. The Cochran–Armitage trend test was used to evaluate trends in the proportion of patients with use of each medication, initially prescribed 30 mg/day or more of CS, who received CSs for less than 90 days or more than one course of CSs within 12 months from 2011 [one year after infliximab was launched] onwards. The Jonckheere–Terpstra trend test was used to evaluate trends in CS dose at the first initiation and the longest CS prescription duration for each patient from 2011.
For Objective B, multiple logistic regression analysis was conducted to identify factors associated with long-term CS use. The dependent variable was either patients with long-term use or patients with non-long-term CS use. Patients who were prescribed CSs for 180 days or more were defined as long-term users, while those prescribed CSs for less than 180 days were defined as non-long-term users. The following explanatory variables were used: sex, age, CS dose on the index date, experience with re-prescription of CS, experience with thiopurine use during the follow-up period, experience with biologics use during the follow-up period, and the type of medical institute where patients were prescribed CSs on the index date. The following were used as reference values: female, less than 20 years old, CS dose of less than 10 mg/day on the index date, no re-prescription of CS, thiopurine use, biologics use, and clinics with fewer than 20 beds on the index date, respectively.
Statistical analysis was conducted using Statistical Analysis Software R [version 3.6.0]. All comparisons were performed using two-sided tests for statistical significance.
3.Results
3.1. Changes in UC medication use over time [Objective A-1]
Among 16 125 patients with at least one UC diagnosis from January 1, 2005 to March 31, 2018, 11 606 were prescribed at least one UC medication. No patients were prescribed vedolizumab because it was not approved during the study period. During any year from 2006 to 2016, 7907 were prescribed at least one UC medication and did not have a CD diagnosis from 6 months before to 12 months after the index date [Supplementary Figure 1a]. As the cumulative sample size of this database continually increased, the number of eligible patients also continued to increase over 11 years. Neither the demographic nor the clinical characteristics of patients changed over the 11 years [Table 1]. The median age ranged from 39.0 to 44.0 years and more than 60% of patients were male. Since 2008, nearly 80% of patients were treated at medical institutions that were neither university hospitals nor national/public hospitals.
Table 1.
Demographic and clinical characteristics of patients prescribed any UC medication [Objective A-1]
| Year | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients prescribed UC medicationsa, n | 121 | 146 | 168 | 278 | 501 | 945 | 1594 | 2346 | 3977 | 4534 | 6215 |
| Sex, male, n [%] | 76 [62.8] | 98 [67.1] | 111 [66.1] | 187 [67.3] | 309 [61.7] | 604 [63.9] | 1034 [64.9] | 1547 [65.9] | 2597 [65.3] | 2927 [64.6] | 3973 [63.9] |
| Age, years, median [IQR] | 40.0 [33.0, 47.0] | 39.0 [33.0, 46.5] | 39.0 [33.0, 45.0] | 39.0 [32.3, 46.0] | 40.0 [32.0, 47.0] | 41.0 [33.0, 48.0] | 42.0 [34.0, 49.0] | 42.0 [35.0, 50.0] | 43.0 [35.0, 51.0] | 43.0 [35.0, 51.0] | 44.0 [36.0, 52.0] |
| Medical institution visited during the follow up period, n [%] | |||||||||||
| University hospital | 19 [15.7] | 18 [12.3] | 19 [11.3] | 44 [15.8] | 85 [17.0] | 143 [15.1] | 219 [13.7] | 346 [14.7] | 543 [13.7] | 603 [13.3] | 952 [15.3] |
| National/public hospital | 31 [25.6] | 31 [21.2] | 30 [17.9] | 52 [18.7] | 79 [15.8] | 128 [13.5] | 228 [14.3] | 308 [13.1] | 529 [13.3] | 602 [13.3] | 807 [13.0] |
| Clinic [<20 beds] | 33 [27.3] | 47 [32.2] | 60 [35.7] | 98 [35.3] | 186 [37.1] | 337 [35.7] | 555 [34.8] | 846 [36.1] | 1460 [36.7] | 1729 [38.1] | 2358 [37.9] |
| Other hospitalb | 43 [35.5] | 61 [41.8] | 70 [41.7] | 103 [37.1] | 178 [35.5] | 377 [39.9] | 687 [43.1] | 978 [41.7] | 1670 [42.0] | 1861 [41.0] | 2445 [39.3] |
| Unknown | 4 [3.3] | 4 [2.7] | 4 [2.4] | 1 [0.4] | 0 | 0 | 0 | 3 [0.1] | 1 [0.0] | 2 [0.0] | 2 [0.0] |
Abbreviations: 5-ASA, 5-aminosalicylic acid; CS, corticosteroid; IQR, interquartile range; PSL, prednisolone; UC, ulcerative colitis.
aThe following UC medications were included in the analysis: 5-ASA (salazosulfapyridine [oral, suppository], mesalazine [oral, suppository, enema]), CS (PSL [oral], PSL sodium succinate [injection]), thiopurine (azathioprine [oral], mercaptopurine hydrate [oral]), biologics [infliximab, adalimumab, golimumab, vedolizumab] and others (tofacitinib citrate [oral], tacrolimus hydrate [oral]).
bHospital with ≥20 beds other than a university hospital or national/public hospital.
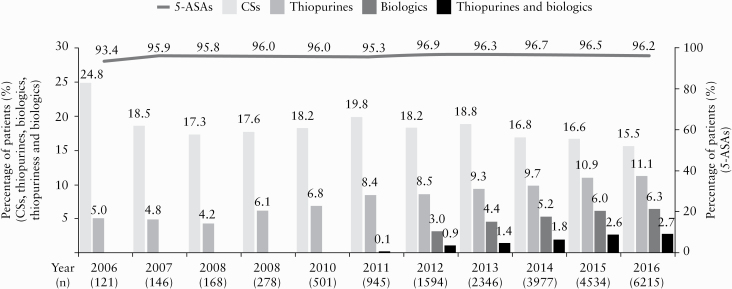
The proportion of patients who were prescribed 5-ASAs ranged from 93.4% to 96.9% between 2009 and 2016 [Figure 1]. The proportion of patients with CS use in each year was 24.8% in 2006, ranged from 17.3% to 19.8% in 2007–2011, and decreased significantly from 2011 onwards [p < 0.001]. Meanwhile, the proportion of patients with thiopurine and biologics use in each year increased significantly from 2011 onwards [both p < 0.001]. The proportion of patients with CS, thiopurine, biologics, and biologics in combination with thiopurine use in 2016 was 15.5%, 11.1%, 6.3% and 2.7%, respectively. Among the 6.3% of patients who used biologics in 2016, 4.1% used infliximab and 2.4% used adalimumab. Among the 2.7% of patients who used biologics in combination with thiopurines in 2016, 1.8% used infliximab and 0.9% used adalimumab.
Figure 1.
Change in UC medication use over time. The proportion of patients with CS use in each year decreased significantly and that with thiopurine and biologics use in each year increased significantly from 2011 onwards [Cochran–Armitage trend test, p < 0.001]. 5-ASA, 5-aminosalicylic acid; CS, corticosteroid; UC, ulcerative colitis.
3.2. Changes in CS medication use over time [Objective A-2]
Among 16 125 patients with UC, 1555 were prescribed at least one CS medication for more than 8 days during any year from 2006 to 2016 [Supplementary Figure 1b]. We counted patients who received re-treatment with CSs as separate patients; the total number of these patients was 2096. Although the number of eligible patients increased, the demographic and clinical characteristics did not change over the 11 years [Table 2], as was observed for patient characteristics in Objective A-1.
Table 2.
Demographic and clinical characteristics of patients prescribed CSs [Objective A-2]
| Year | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients prescribed CSs, n | 17 | 13 | 21 | 44 | 55 | 111 | 212 | 259 | 360 | 428 | 576 |
| Sex, male, n [%] | 13 [76.5] | 10 [76.9] | 17 [81.0] | 29 [65.9] | 31 [56.4] | 68 [61.3] | 148 [69.8] | 163 [62.9] | 237 [65.8] | 263 [61.4] | 364 [63.2] |
| Age, years, median [IQR] | 40.0 [29.0, 43.0] | 38.0 [29.0, 42.0] | 36.0 [31.0, 45.0] | 39.5 [30.8, 44.3] | 41.0 [31.5, 51.0] | 35.0 [28.0, 46.0] | 41.0 [31.8, 48.3] | 41.0 [32.0, 50.0] | 40.0 [31.0, 49.0] | 41.0 [30.0, 50.0] | 41.0 [30.0, 51.0] |
| Medical institution visited on the index date, n [%] | |||||||||||
| University hospital | 3 [17.6] | 1 [7.7] | 3 [14.3] | 15 [34.1] | 9 [16.4] | 14 [12.6] | 33 [15.6] | 42 [16.2] | 61 [16.9] | 67 [15.7] | 98 [17.0] |
| National/public hospital | 8 [47.1] | 3 [23.1] | 3 [14.3] | 10 [22.7] | 11 [20.0] | 19 [17.1] | 37 [17.5] | 39 [15.1] | 43 [11.9] | 58 [13.6] | 78 [13.5] |
| Clinic [<20 beds] | 5 [29.4] | 8 [61.5] | 10 [47.6] | 10 [22.7] | 17 [30.9] | 31 [27.9] | 47 [22.2] | 77 [29.7] | 112 [31.1] | 133 [31.1] | 184 [31.9] |
| Other hospitala | 1 [5.9] | 1 [7.7] | 5 [23.8] | 9 [20.5] | 18 [32.7] | 47 [42.3] | 95 [44.8] | 101 [39.0] | 144 [40.0] | 170 [39.7] | 216 [37.5] |
Abbreviations: CS, corticosteroid; IQR, interquartile range; UC, ulcerative colitis.
aHospital with ≥20 beds other than a university hospital or national/public hospital.
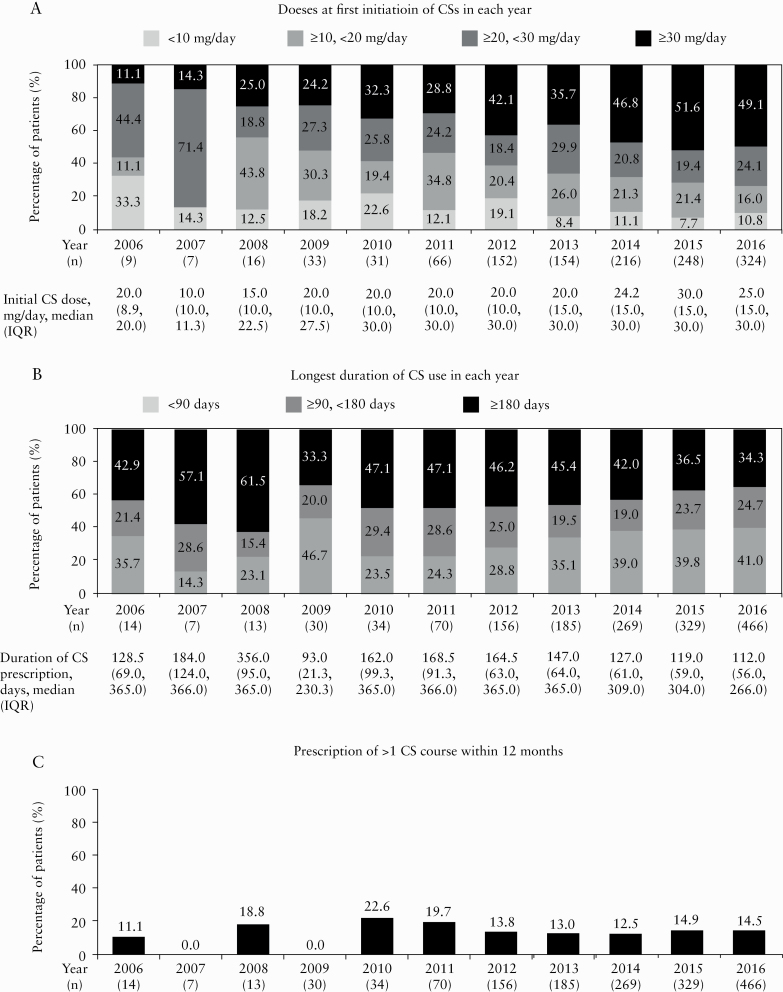
The CS dose at first initiation increased significantly from 2011 onwards [p = 0.002] [Figure 2a]. The proportion of patients initially prescribed 30 mg/day or more of CS, the dose recommended by the current guidelines, also increased significantly from 2011 onwards [p < 0.001], reaching 49.1% in 2016 [Figure 2a]. The longest CS prescription duration for each patient decreased significantly from 2011 onwards [p = 0.002] and the proportion of patients who received CSs for less than 90 days increased significantly from 2011 onwards [p < 0.001] [Figure 2b]. However, 59.0% and 34.3% continuously used CSs for 90 and 180 days or more, respectively, in 2016 [Figure 2b]. The proportion of patients who received more than one course of CSs in 12 months among patients prescribed CSs for the first time ranged from 12.5% to 19.7% between 2011 and 2016, with no significant changes observed over time [p = 0.824] [Figure 2c].
Figure 2.
Change in initial CS dose [a], the longest duration of CS use [b] and prescription of more than one CS course of CSs within 12 months [c] in each year. [a] The CS dose at first initiation and the proportion of patients initially prescribed 30 mg/day or more of CS increased significantly from 2011 onwards [Jonckheere–Terpstra trend test, p = 0.002, and Cochran–Armitage trend test, p < 0.001, respectively]. [b] The longest CS prescription duration for each patient decreased significantly [Jonckheere–Terpstra trend test, p = 0.002] and the proportion of patients who received CSs for less than 90 days increased significantly [Cochran–Armitage trend test, p < 0.001] from 2011 onwards. [c] The proportion of patients who received more than one course of CSs within 12 months among patients prescribed CSs for the first time in each year did not change significantly [Cochran–Armitage trend test, p = 0.824] from 2011 onwards. CS, corticosteroid; IQR, interquartile range.
3.3.Differences in background characteristics between patients with long-term and non-long-term CS use [Objective B]
Among the 14 035 patients with at least one UC diagnosis from January 1, 2014 to December 31, 2016, 1230 were prescribed at least one CS medication for more than 8 days for any CS prescription duration [Supplementary Figure 1c]. A total of 590 patients were prescribed long-term CS medications [long-term users] and 640 patients were prescribed non-long-term CS medications [non-long-term users] [Table 3].
Table 3.
Demographic and clinical characteristics of patients prescribed CSs since 2014 [Objective B]
| Duration of CSs | Long-term use [≥180 days] | Non-long-term use [<180 days] | |
|---|---|---|---|
| Number of patients prescribed CSs, n | 590 | 640 | |
| Duration of CS use, days, median [IQR] | 365.0 [294.0, 365.0] | 72.0 [41.8, 117.3] | |
| Sex | Male, n [%] | 368 [62.4] | 387 [60.5] |
| Female, n [%] | 222 [37.6] | 253 [39.5] | |
| Age, years | <20, n [%] | 42 [7.1]a | 61 [9.5]b |
| ≥20, <40, n [%] | 195 [33.1] | 257 [40.2] | |
| ≥40, <60, n [%] | 297 [50.3] | 285 [44.5] | |
| ≥60, n [%] | 56 [9.5] | 37 [5.8] | |
| CS dosec, mg/day, median [IQR] | 8.1 [5.0, 11.5] | 14.7 [10.0, 18.9] | |
| Initial CS dose, mg/day | <10, n [%] | 190 [32.2] | 70 [10.9] |
| ≥10, <20, n [%] | 139 [23.6] | 132 [20.6] | |
| ≥20, <30, n [%] | 107 [18.1] | 153 [23.9] | |
| ≥30, n [%] | 154 [26.1] | 285 [44.5] | |
| With re-administration of CSs, n [%] | 180 [30.5] | 209 [32.7] | |
| Thiopurine non-user, n [%] | 374 [63.4] | 487 [76.1] | |
| Biologics non-user, n [%] | 475 [80.5] | 559 [87.3] | |
| Infliximab non-user, n [%] | 523 [88.6] | 595 [93.0] | |
| Adalimumab non-user, n [%] | 537 [91.0] | 604 [94.4] | |
| Golimumab non-user, n [%] | 585 [99.2] | 640 [100.0] | |
| Medical institution visited on the index date, n [%] | |||
| University hospital | 102 [17.3] | 109 [17.0] | |
| National/public hospital | 111 [18.8] | 81 [12.7] | |
| Clinic [<20 beds] | 152 [25.8] | 184 [28.8] | |
| Other hospitald | 225 [38.1] | 266 [41.6] |
Abbreviations: CS, corticosteroid; IQR, interquartile range.
aSeventeen [2.9%] patients were aged <16 years.
bTwenty-two [3.4%] patients were aged <16 years.
cCS dose throughout the follow up period.
dHospital with ≥20 beds other than a university hospital or national/public hospital.
The proportion of patients aged 60 years or older and using low doses of CS was slightly higher among long-term users than among non-long-term users [Table 3]. A lower proportion of patients were prescribed 30 mg/day or more CSs at initiation among long-term users than among non-long-term users. Among long-term users, the proportion of patients treated with thiopurines and biologics, and treated at national/public hospitals was higher than among non-long-term users. The proportion of patients prescribed 30 mg/day or more CSs was higher in hospitals than in clinics (37.4% [79/211], 38.0% [73/192], 25.3% [85/336] and 41.1% [202/491] in university hospitals, national/public hospitals, clinics and other hospitals, respectively). Among patients who used thiopurines, the proportion prescribed 30 mg/day or more CSs (42.3% [156/369]) was higher than for other dose groups (15.2% [56/369] were prescribed less than 10 mg/day, 19.8% [73/369] were prescribed 10 to less than 20 mg/day, and 22.8% [84/369] were prescribed 20 to less than 30 mg/day).
The factors related to long-term use of CSs identified using multiple logistic regression analysis are shown in Table 4. Odds ratios were higher for patients aged 60 years or older vs those aged below 20 years old, an initial CS dose of less than 20 mg/day vs 30 mg/day or more, and initiation of CSs at a national/public hospital vs at a clinic. Odds ratios were lower for non-users of thiopurines or biologics vs users. In particular, use of less than 10 mg/day at initiation of CS was most strongly associated with long-term use.
Table 4.
Factors related to long-term use of CSs [results of multiple logistic regression analysis] [Objective B]
| Odds ratio [95% CI] | p value | ||
|---|---|---|---|
| Sex | Male | 1.22 [0.95, 1.58] | 0.117 |
| Female | Reference | ||
| Age, years | <20a | Reference | |
| ≥20, <40 | 1.10 [0.69, 1.76] | 0.699 | |
| ≥40, <60 | 1.42 [0.89, 2.25] | 0.142 | |
| ≥60 | 2.20 [1.19, 4.07] | 0.012 | |
| Initial CS dose, mg/day | <10 | 5.94 [4.15, 8.50] | <0.001 |
| ≥10, <20 | 2.23 [1.61, 3.11] | <0.001 | |
| ≥20, <30 | 1.32 [0.95, 1.83] | 0.102 | |
| ≥30 | Reference | ||
| With re-administration of CSs | 0.98 [0.75, 1.27] | 0.854 | |
| Without re-administration of CSs | Reference | ||
| Thiopurine non-user | 0.46 [0.35, 0.61] | <0.001 | |
| Thiopurine user | Reference | ||
| Biologics non-user | 0.57 [0.41, 0.80] | 0.001 | |
| Biologics user | Reference | ||
| Medical institution visited on the index date | University hospital | 1.06 [0.72, 1.55] | 0.767 |
| National/public | 1.68 [1.13, 2.50] | 0.010 | |
| Clinic [<20 beds] | Reference | ||
| Other hospitalb | 1.09 [0.80, 1.48] | 0.581 |
Abbreviations: CI, confidence interval; CS, corticosteroid.
aAmong the 103 [8.4%] patients aged <20 years, 39 [3.2%] were aged <16 years.
bHospital with ≥20 beds other than a university hospital or national/public hospital.
4. Discussion
This Japanese claims database study was conducted to capture the changes in CS use from 2006 to 2016 and the difference in characteristics of patients with long-term and non-long-term CS use, and to identify factors associated with long-term CS use in UC patients. Our findings showed that CSs were used more appropriately as the use of thiopurines and biologics increased; however, there were still many cases of inappropriate use. Long-term use of CSs was most strongly associated with a low initial dose of CSs [<10 mg/day].
UC is an idiopathic, chronic inflammatory bowel disease characterized by alternating periods of remission and relapse.1 It is important to induce and maintain remission6,7 to improve the quality of life for patients with UC. CSs are effective for the induction, but not the maintenance, of remission.20,21 Therefore, treatment guidelines recommend early assessment of the efficacy of CSs. Even if CSs are effective, patients should be switched to other medications to avoid long-term use of CSs, which is associated with the risk of side effects, complications and CS-dependent UC.4,8,10 In the present study, a significant decrease in the proportion of patients prescribed CSs in each year was observed from 2011, 1 year after infliximab was launched. The proportion of patients prescribed 30 mg/day or more CSs at the first initiation increased significantly from 2011. In addition, although the proportion of patients prescribed CSs for less than 90 days also increased significantly, those who received more than one course of CSs within 12 months did not change significantly from 2011. The proportion of patients using thiopurines and biologics increased significantly from 2011. These results suggest that the perceived risks associated with CS use increased after the release of guidelines in 2010,22 20168 and 20184 and the availability of biologics, leading to an increase in proper use of CSs. However, our results also showed the persistence of inappropriate CS use, including low starting doses [50.9%] and use for 90 days or more [59.0%]. Okayasu et al. investigated the use of CSs for remission induction therapy after a UC diagnosis from 2008 to 2014 using a Japanese claims database, and reported that although the rate of CS use tended to decrease every year after 2010, many patients continued long-term use [≥6 months] of CSs,11 which is consistent with the findings in this study. Studies in the UK have reported that 14.0% of UC patients [~29% of patients with a CS prescription] continue CS use for more than 3 months,23 and that 13.8% [~49% of patients with a CS prescription] have CS excess [i.e. exceeding 3 months] or dependency.24 These studies indicate that there is a proportion of patients with inappropriate CS use in the UK, as there was in our study, although different criteria were used to indicate inappropriate CS use. In the future, appropriate use of CSs should be further promoted.
In this study, an initial low dose of CSs was the most strongly factor associated with long-term CS use, while a shorter duration of CS use was observed in patients with an initial high dose of CSs of 30 mg/day or more. These results suggest that physicians who prescribed the high doses of CSs recommended by the guidelines may have been able to confirm the responsiveness of UC patients to CSs in a short period of time and select appropriate treatment options to achieve CS-free conditions for non-responders. In addition, our findings suggest that incomplete suppression of inflammation by low doses of CSs may lead to long-term use. However, CSs are not effective as maintenance therapy and use for more than 90 days is inappropriate. Older age was also significantly associated with long-term use of CSs. It is possible that physicians have long been prescribing low-dose CSs to elderly patients. Furthermore, physicians may be hesitant to prescribe biologics to elderly patients because of concerns about infections and other adverse effects, resulting in long-term use of CSs. Yet, the risk of infection associated with long-term use of CSs in the elderly may exceed that with biologics.25,26 However, because the number of elderly patients in this claims database was limited, further investigation is required.
Long-term CS use was negatively associated with non-users of thiopurines and biologics after the initial prescription of CSs. These results may suggest that among non-long-term users of CSs, a large number of patients responded well to CSs and did not originally require thiopurines or biologics. Okayasu et al. reported that more patients were prescribed a high dose of CSs [1500 mg or more in a 6-month period] at university hospitals and national/public hospitals, which have a large number of specialists treating UC patients, treat patients with relatively high disease activity and have a large management structure, compared to other types of medical institutions.11 In the present study, the prevalence of long-term users of CSs was significant and highest in national/public hospitals, but the overall trends according to type of medical institution were similar to those described by Okayasu et al.11 These results suggest that in addition to inappropriate practice [an initial low dose of CSs], long-term CS use is associated with difficult-to-treat patients [use of thiopurines or biologics and treatment in a large hospital with many specialists]. Unlike our study, which analysed factors related to long-term CS use without information on disease activity, Selinger et al. analysed factors associated with CS excess or dependency, including those for disease activity. The study reported that moderate/severe disease activity and thiopurine monotherapy were associated with a risk of CS excess or dependency and that treatment at a quality intervention centre decreased this risk.24
In this study, the proportion of patients who were initially prescribed 30 mg/day or more CSs tended to be higher in large hospitals. This appears to be inconsistent with the result that an initial low dose of CSs was strongly associated with long-term use of CSs and that large hospitals were a weakly related factor. However, the difference in disease activity among patients who visited large hospitals and clinics may have affected the results. Additionally, among patients with thiopurine use, the proportion prescribed an initial dose of 30 mg/day or more CSs tended to be higher than that of patients prescribed less than 30 mg/day. These findings suggest that specialists, for example, who are able to make full use of thiopurines, are likely to prescribe CSs according to the guidelines.
The Japanese guidelines recommend 5-ASAs as the first-line treatment for induction and maintenance therapy in patients with mild-to-moderate UC.4,8 In the present study, 5-ASAs were prescribed to over 90% of patients across the entire study period, with no changes observed over time. These results reflect the consistent importance of 5-ASAs in the treatment of UC.
The goal of maintenance therapy for UC is to maintain CS-free remission, as clinically and endoscopically defined in the ECCO Consensus Guideline.27 Several studies have reported the efficacy of thiopurines and biologics for the reduction and discontinuation of CSs in CS-dependent and/or CS-resistant UC.12–16,28 In addition, guidelines have recommended that patients with CS-dependent disease be treated with thiopurines and biologics, and patients with CS-resistant UC be treated with either biologics or a combination of biologics and thiopurines.8,29 However, the present study found that, despite the availability of thiopurine and biologics therapy, the proportion of thiopurine and biologics users was low among long-term users of CSs. After determining the efficacy of CSs, physicians should consider the switch to thiopurines or biologics as early as possible in CS-refractory patients to discontinue CS use. One of the factors that increased appropriate use of CSs in Japan was publication of the Japanese guideline. Thus, to achieve CS-free remission, it may also be important to educate physicians with the appropriate guidelines.
There were several limitations in this study. First, the JMDC claims database only covers the healthcare claims of currently employed workers and their families, and does not follow patients after retirement; therefore, there were only a few elderly patients in our study. However, considering the age distribution of patients with UC,30 the present results are considered to reflect the UC treatment conditions in Japanese clinical settings. Second, the number of eligible patients in the early years of the study period was small based on the number of individuals in the database. We investigated the changes that occurred in the early few years using only descriptive analyses; thus, additional analyses are needed to confirm our findings. Third, no information on disease severity was available in the database. Therefore, we were unable to investigate differences in disease severity, which probably influenced CS use. Fourth, when more than one disease code was recorded in the JMDC claims database, we were unable to determine for which disease the drug was used. Although patients with a CS prescription duration of less than 8 days were excluded from this study, patients who were prescribed CSs for the treatment of other diseases may have been included.
In conclusion, this study found that appropriate use of CSs increased in clinical settings in Japan in parallel with the increase in use of thiopurine and biologics, although there were still many cases of inappropriate CS use. Long-term use of CSs was most strongly associated with a low initial dose of CSs. Our findings and those of similar studies may provide useful information for further compliance with guidelines for the management of UC.
Funding
This work was supported by Mitsubishi Tanabe Pharma Corporation.
Conflict of Interest
K.M. received consulting fees from Mitsubishi Tanabe Pharma Corporation related to the submitted work. Additionally, K.M. has received grants and personal fees from Abbvie Inc., EA Pharma Co., Ltd, Kissei Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd, Nippon Kayaku Co., Ltd, Pfizer Inc., Takeda Pharmaceutical Co., Ltd and ZERIA Pharmaceutical Co., Ltd, and personal fees from Janssen Pharmaceutical K.K., JIMRO Co., Ltd and KYORIN Pharmaceutical Co., Ltd outside the submitted work. A.I. received consulting fees from Mitsubishi Tanabe Pharma Corporation related to the submitted work. Additionally, A.I. has received grants from Abbott Japan Inc., Abbvie G.K., Becton, Dickinson and Company, Creativ-Ceuticals Inc., Eli Lilly Japan K.K., Gilead Sciences K.K., Intuitive Surgical G.K., Milliman Inc., Pfizer Inc., Sanofi Pasteur Inc. and Terumo Corporation, and personal fees from Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd, CSL Behring Japan Inc., FUJIFILM Corporation, Sanofi K.K. and Takeda Pharmaceutical Co., Ltd outside the submitted work. N.S., Y.I. and M.G. are employees of Mitsubishi Tanabe Pharma Corporation. A.S. and K.I. are employees of Medilead, Inc., which was commissioned to perform this work by Mitsubishi Tanabe Pharma Corporation. T.H. received consulting fees from Mitsubishi Tanabe Pharma Corporation related to the submitted work. Additionally, T.H. has received research grants and lecture fees from Abbvie G.K., EA Pharma Co., Ltd, JIMRO Co., Ltd, KYORIN Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd, Pfizer Inc. and Takeda Pharmaceutical Co., Ltd, research grants from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd, Nippon Kayaku Co., Ltd and ZERIA Pharmaceutical Co., Ltd, lecture and consulting fees from Janssen Pharmaceutical K.K., consulting fees from Nichi-Iko Pharmaceutical Co., Ltd and conducted joint research with Alfresa Pharma Corporation outside the submitted work.
Author Contributions
K.M., A.I., N.S. and Y.I. made substantial contributions to the study conception, study design, study protocol, data interpretation, drafting of the paper or revising it critically for important intellectual content. M.G., A.S. and K.I. made substantial contributions to the study design, study protocol, analysis or data interpretation, and revising the draft critically for important intellectual content. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
Acknowledgments
We thank the JMDC for providing the claims database and for useful advice.
References
- 1. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 2. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol 2019;54:1070–7. [DOI] [PubMed] [Google Scholar]
- 3. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol 2009;44:659–65. [DOI] [PubMed] [Google Scholar]
- 4. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018;53:305–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 6. Baron JH, Connell AM, Kanaghinis TG, Lennard-Jones JE, Jones AF. Out-patient treatment of ulcerative colitis. Comparison between three doses of oral prednisone. Br Med J 1962;2:441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001;121:255–60. [DOI] [PubMed] [Google Scholar]
- 8. Diagnostic criteria and treatment guidelines for ulcerative colitis and Crohn’s disease, Supplemental volume, Annual report [in Japanese]. Research study of intractable inflammatory bowel disease by the Ministry of Health, Labour and Welfare research group [Suzuki group], 2016. [revised July 2017]. [http://www.ibdjapan.org/pdf/doc01.pdf] Accessed March 23, 2020. [Google Scholar]
- 9. Suzuki Y, Nawata H, Soen S, et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 2014;32:337–50. [DOI] [PubMed] [Google Scholar]
- 10. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis 2012;6:965–90. [DOI] [PubMed] [Google Scholar]
- 11. Okayasu M, Ogata H, Yoshiyama Y. Use of corticosteroids for remission induction therapy in patients with new-onset ulcerative colitis in real-world settings. J Mark Access Health Policy 2019;7:1565889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65.e1–3. [DOI] [PubMed] [Google Scholar]
- 14. Reinisch W, Colombel JF, Gibson PR, et al. Continuous clinical response is associated with a change of disease course in patients with moderate to severe ulcerative colitis treated with Golimumab. Inflamm Bowel Dis 2019;25:163–71. [DOI] [PubMed] [Google Scholar]
- 15. Feagan BG, Rutgeerts P, Sands BE, et al.; GEMINI 1 Study Group . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 16. Fukuda T, Naganuma M, Kanai T. Current new challenges in the management of ulcerative colitis. Intest Res 2019;17:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:496–7. [DOI] [PubMed] [Google Scholar]
- 18. Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol 2010;20:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kobayashi T, Udagawa E, Uda A, Hibi T, Hisamatsu T. Impact of immunomodulator use on treatment persistence in patients with ulcerative colitis: a claims database analysis. J Gastroenterol Hepatol 2020;35:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lennard-Jones JE, Misiewicz JJ, Connell AM, Baron JH, Jones FA. Prednisone as maintenance treatment for ulcerative colitis in remission. Lancet 1965;1:188–9. [DOI] [PubMed] [Google Scholar]
- 22. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology . Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23; quiz 524. [DOI] [PubMed] [Google Scholar]
- 23. Chhaya V, Saxena S, Cecil E, et al. Steroid dependency and trends in prescribing for inflammatory bowel disease - a 20-year national population-based study. Aliment Pharmacol Ther 2016;44:482–94. [DOI] [PubMed] [Google Scholar]
- 24. Selinger CP, Parkes GC, Bassi A, et al. Assessment of steroid use as a key performance indicator in inflammatory bowel disease-analysis of data from 2385 UK patients. Aliment Pharmacol Ther 2019;50:1009–18. [DOI] [PubMed] [Google Scholar]
- 25. Naganuma M, Kunisaki R, Yoshimura N, Takeuchi Y, Watanabe M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol 2013;48:595–600. [DOI] [PubMed] [Google Scholar]
- 26. Sturm A, Maaser C, Mendall M, et al. European Crohn’s and colitis organisation topical review on IBD in the elderly. J Crohns Colitis 2017;11:263–73. [DOI] [PubMed] [Google Scholar]
- 27. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012;6:991–1030. [DOI] [PubMed] [Google Scholar]
- 28. Chebli LA, Chaves LD, Pimentel FF, et al. Azathioprine maintains long-term steroid-free remission through 3 years in patients with steroid-dependent ulcerative colitis. Inflamm Bowel Dis 2010;16:613–9. [DOI] [PubMed] [Google Scholar]
- 29. Harbord M, Eliakim R, Bettenworth D, et al.; European Crohn’s and Colitis Organisation [ECCO] . Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017;11:769–784. [DOI] [PubMed] [Google Scholar]
- 30. 2019 Report on Public Health Administration and Services [in Japanese]. Japanese Ministry of Health Labor and Welfare, 2019. [https://www.nanbyou.or.jp/wp-content/uploads/upload_files/koufu20191.xlsx] Accessed February 10, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.