Abstract
Background
Paediatric data on the association between diagnostic delay and inflammatory bowel disease [IBD] complications are lacking. We aimed to determine the effect of diagnostic delay on stricturing/fistulising complications, surgery, and growth impairment in a large paediatric cohort, and to identify predictors of diagnostic delay.
Methods
We conducted a national, prospective, multicentre IBD inception cohort study including 1399 children. Diagnostic delay was defined as time from symptom onset to diagnosis >75th percentile. Multivariable proportional hazards [PH] regression was used to examine the association between diagnostic delay and stricturing/fistulising complications and surgery, and multivariable linear regression to examine the association between diagnostic delay and growth. Predictors of diagnostic delay were identified using Cox PH regression.
Results
Overall (64% Crohn’s disease [CD]; 36% ulcerative colitis/IBD unclassified [UC/IBD-U]; 57% male]), median time to diagnosis was 4.2 (interquartile range [IQR] 2.0–9.2) months. For the overall cohort, diagnostic delay was >9.2 months; in CD, >10.8 months and in UC/IBD-U, >6.6 months. In CD, diagnostic delay was associated with a 2.5-fold higher rate of strictures/internal fistulae (hazard ratio [HR] 2.53, 95% confidence interval [CI] 1.41–4.56). Every additional month of diagnostic delay was associated with a decrease in height-for-age z-score of 0.013 standard deviations [95% CI 0.005–0.021]. Associations persisted after adjusting for disease location and therapy. No independent association was observed between diagnostic delay and surgery in CD or UC/IBD-U. Diagnostic delay was more common in CD, particularly small bowel CD. Abdominal pain, including isolated abdominal pain in CD, was associated with diagnostic delay.
Conclusions
Diagnostic delay represents a risk factor for stricturing/internal fistulising complications and growth impairment in paediatric CD.
Podcast
This article has an associated podcast which can be accessed at https://academic.oup.com/ecco-jcc/pages/podcast
Keywords: Inflammatory bowel disease [IBD], fistula, stricture
1. Introduction
Canada has one of the highest rates of inflammatory bowel disease [IBD] in the world.1 It is projected that by the year 2030, the prevalence of IBD in Canada will approach 1% [981 per 100 000].2 About a fifth of IBD cases have their onset in the paediatric age range,3 and children are the patient subgroup predicted to experience the largest annual percentage increase in IBD prevalence over the coming decade.2 As these patients will live with IBD for their full lives, optimising their management and outcomes has critical implications for individual quality of life and health care costs. Furthermore, it has been shown that the period preceding IBD diagnosis is associated with increased societal costs,4 providing additional incentive to expedite IBD diagnosis.
In adults, several studies support an association between diagnostic delay and an increased risk of disease complications, particularly in Crohn’s disease [CD]. Such complications include stenosis, internal fistulae, and surgery.5–9 However, paediatric data on the topic of diagnostic delay, and particularly its association with disease complications, remain sparse. In a recent study using data from the Swiss IBD Cohort Study, children with diagnostic delay were not at increased risk of stricturing or fistulising complications or surgical resection over time. This was in contrast to the adults in this cohort.8 The reasons for the discrepancy between this paediatric population and the adult literature are unclear; they may relate to differences in sample size, follow-up duration, or the way in which diagnostic delay was defined.
Given the importance of diagnostic delay as a potentially actionable factor for optimising paediatric IBD outcomes, we aimed to characterise time to diagnosis and its predictors in a large, prospective, national, paediatric IBD inception cohort and to examine the relationship between diagnostic delay and clinically important outcomes, including stricturing and fistulising complications, growth, and surgery.
2. Materials and Methods
2.1. Study design and participants
The Canadian Children IBD Network [CIDsCaNN], a joint partnership with the Canadian Institutes of Health Research [CIHR] and the CH.I.L.D. Foundation, maintains a prospective, national, paediatric IBD inception cohort study. Children <17 years of age, newly diagnosed with IBD at participating paediatric academic centres across Canada, are enrolled at time of diagnosis and followed until the current date or transition to adult care [at 18 years of age]. Data are collected prospectively at each clinic visit using standardised case report forms and are entered into a research electronic data capture [REDCap] database, with central oversight provided by the data coordinating centre. Standard diagnostic work-up at participating centres includes upper endoscopy and ileocolonoscopy, as well as small bowel imaging with magnetic resonance enterography [MRE] in CD. Disease location is defined according to the paediatric Paris modification of the Montreal classification.10 At the baseline visit, data are systematically collected about presenting symptoms and signs with corresponding dates of onset. Patients and families are specifically questioned by treating physicians during interview about the date patients were ‘last well’, which is recorded. Children enrolled between January 2014 and January 2019 were included in this analysis.
2.2. Variables and outcomes
The following baseline factors were extracted for analysis: date of symptom onset [date ‘last well’], date of IBD diagnosis [diagnostic ileocolonoscopy], IBD type, age at diagnosis, sex, presenting gastrointestinal and extraintestinal symptoms and signs [outlined in Tables 1 and 2], disease location and behaviour, height for age z-scores [HAZ], weight for age z-scores [WAZ], clinical (weighted Paediatric CD Activity Index [wPCDAI] in CD, Paediatric UC Activity Index [PUCAI] in UC) and, endoscopic activity (Simple Endoscopic Score-CD [SES-CD] in CD, Mayo endoscopic sub-score in UC), serum markers (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], albumin), initial induction and maintenance therapy, and family history of IBD [first-degree relative]. Dates of subsequent clinical outcomes of interest, namely surgery and stricturing and/or internal fistulising complications in CD, were extracted as well. As previously described,11 clinical site directors, all paediatric gastroenterologists with a clinical focus in IBD, were responsible for approving IBD diagnostic labels as CD or UC as per standard clinical, endoscopic, and histological criteria. In CIDsCaNN, the designation of IBD-U [with the option to specify whether favouring CD or UC] is applied in the setting of colonic IBD with features suggestive of both UC and CD, anticipating that a clearer impression of CD or UC may be verified over time.
Table 1.
Patient and disease characteristics at diagnosis, overall and by diagnostic delay status [<75th percentile vs >75th percentile], CD subgroup.
| N [%] or median [IQR] | Overall [N = 898] | With diagnostic delaya [N = 224] | Without diagnostic delay [N = 674] | p-value |
|---|---|---|---|---|
| Patient demographics | ||||
| Male | 540 [60%] | 132 [59%] | 408 [61%] | 0.67 |
| Age at diagnosis [years] | 12.9 [10.8–14.8] | 13.2 [10.9–14.8] | 12.8 [10.7–14.8] | 0.48 |
| Family history of IBD | 149 [17%] | 36 [16%] | 113 [17%] | 0.81 |
| Presenting symptoms and signs | ||||
| Stool type | 0.019 | |||
| Formed, non-bloody | 169 [20%] | 53 [26%] | 116 [18%] | |
| Non-bloody diarrhoea | 261 [30%] | 59 [28%] | 202 [31%] | |
| Formed, bloody | 88 [10%] | 26 [13%] | 62 [9%] | |
| Bloody diarrhoea | 346 [40% | 69 [33%] | 277 [42%] | |
| Abdominal pain | 752 [87%] | 177 [86%] | 575 [88%] | 0.45 |
| Isolated abdominal pain | 140 [16%] | 46 [22%] | 94 [14%] | 0.007 |
| Perianal symptoms | 242 [28%] | 55 [27%] | 187 [29%] | 0.60 |
| Isolated perianal symptoms | 57 [7%] | 15 [7%] | 42 [6%] | 0.67 |
| Fever | 211 [26%] | 25 [13%] | 186 [30%] | <0.001 |
| Vomiting | 176 [22%] | 30 [15%] | 146 [24%] | 0.011 |
| Skin manifestations | 80 [10%] | 19 [10%] | 61 [10%] | 0.89 |
| Oral ulcers | 219 [27%] | 47 [24%] | 172 [28%] | 0.24 |
| Arthritis | 40 [5%] | 12 [6%] | 28 [5%] | 0.40 |
| Anaemia | 485 [60%] | 109 [55%] | 376 [61%] | 0.13 |
| Iron deficiency | 396 [49%] | 104 [53%] | 292 [48%] | 0.21 |
| Extraluminal manifestations at presentation [as per physician assessment] | ||||
| Perianal disease | 131 [16%] | 35 [17%] | 96 [16%] | 0.69 |
| Any extraintestinal manifestations | 122 [15%] | 29 [14%] | 93 [15%] | 0.72 |
| Skin involvement | 36 [4%] | 8 [4%] | 28 [4%] | 0.69 |
| Joint involvement | 66 [8%] | 17 [8%] | 49 [8%] | 0.88 |
| Anthropometrics at presentation | ||||
| Height for age z-score | -0.14 [-0.93-0.59] | -0.31 [-1.20-0.41] | -0.09 [-0.75-0.65] | 0.002 |
| Weight for age z-score | -0.63 [-1.50-0.30] | -0.76 [-1.75-0.36] | -0.57 [-1.43-0.30] | 0.08 |
| Reported linear growth impairment | 171 [21%] | 70 [36%] | 101 [16%] | <0.001 |
| Reported weight loss | 589 [73%] | 121 [61%] | 468 [76%] | <0.001 |
| Biochemistry at presentation | ||||
| CRP [x upper limit of normal] | 3.0 [1.2–6.6] | 2.2 [1.0–4.7] | 3.2 [1.2–7.1] | 0.03 |
| ESR [mm/h] | 36 [20–52] | 27 [15–44] | 38 [22–56] | <0.001 |
| Albumin [g/L] | 35 [30–39] | 36 [33–40] | 34 [29–39] | <0.001 |
| Disease characteristics at presentation | ||||
| wPCDAI | 55 [35–75] | 42 [25–62] | 58 [38–78] | <0.001 |
| Physician global assessment | <0.001 | |||
| None | 23 [3%] | 9 [4%] | 14 [2%] | |
| Mild | 219 [25%] | 76 [34%] | 143 [22%] | |
| Moderate | 411 [46%] | 99 [45%] | 312 [47%] | |
| Severe | 233 [26%] | 37 [17%] | 196 [29%] | |
| Location [Paris classification] | 0.027b | |||
| No L1-L3 macroscopic involvement | 31 [4%] | 10 [5%] | 21 [3%] | |
| L1 | 158 [18%] | 53 [25%] | 105 [17%] | |
| L2 | 229 [27%] | 55 [26%] | 174 [27%] | |
| L3 | 432 [51%] | 95 [44%] | 337 [53%] | |
| L4 | 516 [65%] | 130 [64%] | 386 [66%] | 0.74c |
| Isolated small bowel disease [L1 ± L4 or L4] | 191 [22%] | 64 [29%] | 127 [20%] | 0.002 |
| Small bowel disease [±colonic] [L1, L3 or L4b] | 620 [73%] | 157 [74%] | 463 [72%] | 0.72 |
| Disease behaviour | ||||
| B1 [inflammatory] | 803 [91%] | 199 [89%] | 604 [92%] | |
| B2 [stricturing] | 58 [7%] | 16 [7%] | 42 [6%] | |
| B3 [internal fistulising] | 7 [0.8%] | 4 [2%] | 3 [0.5%] | |
| B2B3 | 13 [1%] | 4 [2%] | 9 [1%] | |
| B2 and/or B3 | 78 [9%] | 24 [11%] | 54 [8%] | 0.21 |
| Simple endoscopic score-CD [SES-CD] | 15 [9–21] | 13 [6.5–19] | 16 [9–23] | <0.001 |
| Medical therapy | ||||
| Induction | ||||
| 5ASA/sulphasalazine | 89 [10%] | 16 [7%] | 73 [11%] | 0.12 |
| Systemic steroids | 276 [31%] | 54 [24%] | 222 [33%] | 0.016 |
| Exclusive enteral nutrition | 324 [36%] | 102 [46%] | 222 [33%] | <0.001 |
| Anti-TNF | 146 [16%] | 31 [14%] | 115 [17%] | 0.28 |
| Methotrexate | 20 [2%] | 6 [3%] | 14 [2%] | 0.60 |
| Antibiotics | 22 [2%] | 7 [3%] | 15 [2%] | 0.44 |
| Rectal therapy alone | 1 [0.1%] | 0 | 1 [0.2%] | 1.0 |
| Dietary modifications | 3 [0.3%] | 2 [1%] | 1 [0.2%] | 0.15 |
| Surgery | 2 [0.2%] | 0 | 2 [0.3%] | 1.0 |
| None | 13 [1%] | 4 [2%] | 9 [1%] | 0.75 |
IBD, inflammatory bowel disease; CD, Crohn’s disease; IQR, interquartile range; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; wPCDAI, weighted Paediatric Crohn’s Disease Activity Index; SES-CD, Simple Endoscopic Score; 5-ASA, 5-aminosalicylate; TNF, tumour necrosis factor.
aTime to diagnosis >75th percentile in CD subgroup [10.8 months].
bOverall comparison of all categories.
cComparison of L4.
Table 2.
Patient and disease characteristics at diagnosis, overall and by diagnostic delay status [<75th percentile vs. >75th percentile], UC/IBD-U subgroup.
| N [%] or median [IQR] | Overall [N = 501] | With diagnostic delaya [N = 124] | Without diagnostic delay [N = 377] | p-value |
|---|---|---|---|---|
| Patient demographics | ||||
| Male | 253 [50%] | 64 [52%] | 189 [50%] | 0.77 |
| Age at diagnosis [years] | 13.2 [10.4–15.5] | 13.4 [11.2–15.6] | 13.1 [10.0–15.4] | 0.28 |
| Family history of IBD | 81 [16%] | 18 [15%] | 63 [17%] | 0.56 |
| Presenting symptoms and signs | ||||
| Stool type | <0.001 | |||
| Formed, non-bloody | 4 [1%] | 2 [2%] | 2 [0.5%] | |
| Non-bloody diarrhoea | 25 [5%] | 12 [10%] | 13 [3.5%] | |
| Formed, bloody | 42 [8%] | 21 [17%] | 21 [6%] | |
| Bloody diarrhoea | 427 [86%] | 89 [72%] | 338 [90%] | |
| Abdominal pain | 409 [82%] | 99 [80%] | 310 [83%] | 0.44 |
| Isolated abdominal pain | 4 [1%] | 2 [2%] | 2 [0.5%] | 0.26 |
| Fever | 41 [3%] | 4 [4%] | 37 [12%] | 0.027 |
| Vomiting | 73 [18%] | 15 [15%] | 58 [18%] | 0.50 |
| Skin manifestations | 14 [3%] | 5 [5%] | 9 [3%] | 0.33 |
| Oral ulcers | 40 [10%] | 12 [12%] | 28 [9%] | 0.33 |
| Arthritis | 7 [2%] | 4 [4%] | 3 [1%] | 0.057 |
| Anaemia | 245 [59%] | 56 [57%] | 189 [60%] | 0.66 |
| Iron deficiency | 220 [53%] | 61 [62%] | 159 [50%] | 0.041 |
| Extraluminal manifestations at presentation [as per physician assessment] | ||||
| Any extraintestinal manifestation | 42 [9%] | 13 [12%] | 29 [8%] | 0.27 |
| Skin involvement | 4 [0.9%] | 0 | 4 [1%] | 0.58 |
| Joint involvement | 10 [2%] | 4 [4%] | 6 [2%] | 0.26 |
| Anthropometrics at presentation | ||||
| Height for age z-score | 0.17 [-0.54–0.84] | 0.09 [-0.67–0.96] | 0.19 [-0.50–0.82] | 0.56 |
| Weight for age z-score | 0.00 [-0.79-0.73] | 0.15 [-0.77-0.77] | -0.04 [-0.79-0.72] | 0.50 |
| Reported linear growth impairment | 15 [4%] | 5 [5%] | 10 [3%] | 0.36 |
| Reported weight loss | 267 [64%] | 45 [46%] | 222 [70%] | <0.001 |
| Biochemistry at presentation | ||||
| CRP [x upper limit of normal] | 0.7 [0.2–1.6] | 0.6 [0.2–1.2] | 0.7 [0.2–1.9] | 0.11 |
| ESR [mm/h] | 25 [12–42] | 18 [10–32] | 26 [14–44] | 0.008 |
| Albumin [g/L] | 38 [33–42] | 41 [35–44] | 37 [32–41] | <0.001 |
| Disease characteristics at presentation | ||||
| PUCAI | 50 [35–65] | 40 [25–55] | 55 [40–70] | <0.001 |
| Physician global assessment | <0.001 | |||
| None | 17 [4%] | 6 [5%] | 11 [3%] | |
| Mild | 111 [23%] | 41 [34%] | 70 [19%] | |
| Moderate | 198 [40%] | 48 [40%] | 150 [41%] | |
| Severe | 163 [33%] | 26 [21%] | 137 [37%] | |
| Location [Paris classification] | 0.071b | |||
| No macroscopic involvement | 10 [2%] | 4 [4%] | 6 [2%] | |
| E1 [proctitis] | 42 [9%] | 15 [13%] | 27 [7%] | |
| E2 [distal to splenic flexure] | 30 [6%] | 10 [9%] | 20 [5%] | |
| E3 [distal to hepatic flexure] | 58 [12%] | 15 [13%] | 43 [12%] | |
| E4 [proximal to hepatic flexure] | 339 [71%] | 69 [61%] | 270 [74%] | 0.009c |
| Extensive [E3-E4] | 397 [83%] | 84 [74%] | 313 [86%] | 0.006d |
| Mayo endoscopic subscore | 2 [2–3] | 2 [1–3] | 2 [2–3] | 0.018 |
| Medical therapy | ||||
| Induction | ||||
| 5ASA/sulphasalazine | 172 [35%] | 61 [49%] | 111 [30%] | <0.001 |
| Systemic steroids | 300 [60%] | 50 [40%] | 250 [67%] | <0.001 |
| Anti-TNF | 7 [1%] | 3 [2%] | 4 [1%] | 0.37 |
| Rectal therapy alone | 14 [3%] | 8 [6%] | 6 [2%] | 0.009 |
| None | 4 [1%] | 2 [2%] | 2 [0.5%] | 1.0 |
IBD, inflammatory bowel disease; CD, Crohn’s disease; IQR, interquartile range; UC/IBDU, ulcerative colitis, inflammatory bowel disease unclassified; PUCAI, Paediatric Ulcerative Colitis Activity Index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; 5-ASA, 5-aminosalicylate; TNF, tumour necrosis factor.
aTime to diagnosis >75th percentile in UC/IBD-U subgroup [6.6 months].
bOverall comparison of all categories.
cComparison of E4.
dComparison of E3-E4.
Time to diagnosis was defined as the interval between symptom onset [date ‘last well’] and diagnostic ileocolonoscopy. A prolonged time to diagnosis [‘diagnostic delay’] was defined as a time to diagnosis greater than the 75th percentile, as has been done previously [for subgroup analyses, the CD- and UC/IBD-U specific definitions of diagnostic delay were used].6,12,13 For the primary analyses, patients with IBD-U favouring CD were grouped with CD, and patients with IBD-U favouring UC and IBD-U were grouped with UC. In addition, we undertook analyses specific to the IBD-U population [including IBD-U, IBD-U favouring UC, and IBD-U favouring CD], presented in Supplementary materials, available as Supplementary data at ECCO-JCC online.
2.3. Statistical analysis
Continuous variables were summarised as medians with interquartile range [IQR] and categorical variables as frequencies with proportions. Patient and disease characteristics were compared between patients with and without diagnostic delay [separately for CD and UC], using the Mann‐Whitney U test for continuous variables and the chi square test for categorical variables [or Fisher’s exact test where less than five cell counts were expected]. The association between diagnostic delay and the development of stricturing/internal fistulising complications following diagnosis [in CD] and surgery [in CD and UC] was examined using univariate and multivariable [MVA] Cox proportional hazards [PH] regression [with date of diagnosis set as time zero]. The analysis of stricturing/fistulising complications was restricted to patients without complications [ie, with an inflammatory phenotype] at diagnosis. The association between time to diagnosis and HAZ at presentation was examined using univariate and MVA linear regression. Finally, IBD-related presenting symptoms and signs were tested for their relationship with time to diagnosis [in an attempt to identify predictors of diagnostic delay] using univariate and MVA Cox PH regression. In all cases, the variables included in MVA models were selected a priori based on clinical relevance. Results were expressed as unadjusted and adjusted hazard ratios [HRs] or beta coefficients, as appropriate, with 95% confidence intervals [CI]. Given the importance of disease location as a potential confounder, in addition to MVA analyses, sensitivity analyses restricted to patients with small bowel involvement were performed for the outcomes of stricturing/fistulising disease and growth.
Statistical significance was defined as a p-value <0.05. All statistical analyses were performed using SAS University Edition [version 3.4, SAS Institute, Cary, NC].
2.4. Ethics
The study was approved by the institutional ethics review board at each participating centre. Informed consent was obtained from all individual participants included in the cohort and their parents/legal guardians.
3. Results
Between January 2014 and January 2019, 1399 children were enrolled in the inception cohort and included in the analysis. Diagnostic labels included 881 [63%] CD, 435 [31%] UC, and 83 [6%] IBD-U [including 17 IBD-U favouring CD and 39 IBD-U favouring UC]. As outlined above, for subsequent analyses, UC and IBD-U were grouped together [N = 501], except for IBD-U favouring CD, which was grouped with CD [N = 898]. The median age at IBD diagnosis was 13.0 [10.7–15.0] years. The median follow-up duration was 2.7 [IQR 1.7–3.6] years; follow-up duration was similar in patients with and without diagnostic delay.
3.1. Baseline patient and disease characteristics
The median time from symptom onset to diagnosis for the overall cohort was 4.2 [2.0–9.2] months, with 19% of patients diagnosed more than 1 year after symptom onset. The median time to diagnosis was significantly longer in CD [4.9, IQR 2.3–10.8, months] than UC/IBD-U [3.1, IQR 1.5–6.6, months] [p <0.001]. The distribution of times to diagnosis for CD and UC/IBD-U is shown in Supplementary Figure 1, available as Supplementary data at ECCO-JCC online; 23% of CD patients were diagnosed more than 1 year after symptom onset, compared with 12% of UC/IBD-U patients [p <0.001]. Even after adjusting for patient age, sex, colonic involvement, and clinical activity at presentation, CD remained independently associated with a slower rate of diagnosis, compared with UC/IBD-U [HR 0.72, 95% CI 0.64–0.81, Supplementary Table 1, available as Supplementary data at ECCO-JCC online]. The median time to diagnosis in the 83 children with an IBD-U label was 4 [IQR 1.8–9.4] months, intermediate between CD and UC.
Differences in presenting symptoms and signs, disease characteristics, and therapy between patients with and without diagnostic delay are highlighted in Tables 1 and 2, for CD and UC/IBD-U, respectively. In both groups, bloody diarrhoea and more active disease, clinically [based on wPCDAI and PUCAI] and endoscopically, were associated with a faster diagnosis; in UC/IBD-U, patients with PUCAI ≥65 at presentation [severe colitis] had a shorter time to diagnosis [median 2, IQR 1–4.5, months] than those with PUCAI < 65 [median 4, IQR 2–7.6, months] [p <0.001]. In CD, isolated abdominal pain [without intestinal symptoms] was associated with a slower diagnosis. Disease location differed according to diagnostic delay status, with more isolated small bowel disease [in CD] and more limited, left-sided disease [in UC/IBD-U] in the diagnostic delay group. In both groups, no association was observed between diagnostic delay and any of age, sex, family history of IBD, or extraintestinal skin/joint/eye symptoms.
At presentation, there was no difference in the proportion of CD patients with complicated luminal disease [strictures or internal fistulae] between patients with and without diagnostic delay. In CD [but not UC], HAZ was lower at presentation in patients with delayed diagnosis; CD patients with linear growth impairment were more likely to have small bowel involvement [80% vs 73%, p = 0.044] and distal small bowel disease [L4b] specifically [26% vs 17%, p = 0.011].
Initial therapy differed by diagnostic delay status, with more exclusive enteral nutrition [EEN] in CD patients with, and more corticosteroids in CD patients without, a delayed diagnosis. EEN was more frequent in CD patients with small bowel involvement [44% vs 17%, p <0.001] but the association between diagnostic delay and EEN persisted in an analysis restricted to patients with small bowel disease. In UC/IBD-U, diagnostic delay was associated with more 5-aminosalicylic acid [5-ASA] use, whereas patients diagnosed more rapidly were more likely to receive corticosteroids and anti-tumour necrosis factor alpha [anti-TNFα].
Baseline patient and disease characteristics are summarised specifically for the IBD-U group in Supplementary Table 1.
3.2. Diagnostic delay in paediatric CD is associated with a higher rate of stricturing/internal fistulising complications over time
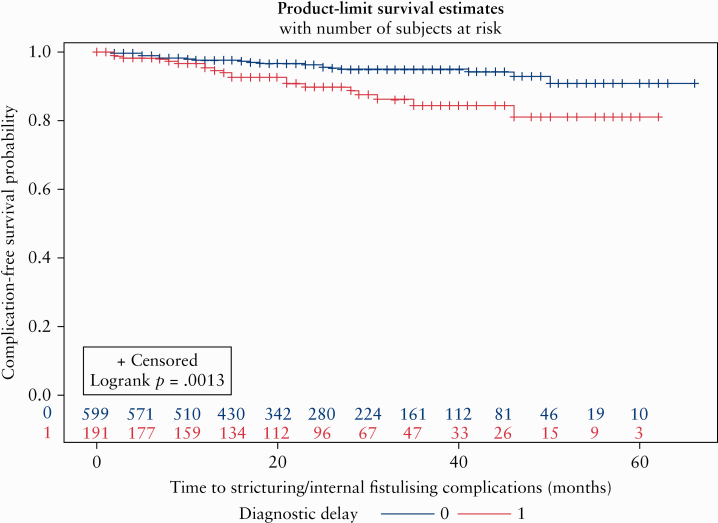
Of the 803 CD patients with inflammatory disease at presentation, 45 [5.6%] developed stricturing or internal fistulising complications over time; this included 10% [20/199] of patients with diagnostic delay compared with 4.1% [25/604] of patients without diagnostic delay [p = 0.001]. Among those who developed such complications, the median time to complication was 13 [7–25] months. All CD patients considered, by the end of follow-up, 20% of patients with diagnostic delay and 12% of those without delay had stricturing/internal fistulising complications [p = 0.003].
Figure 1 illustrates the faster rate of progression to stricturing/internal fistulising complications in CD patients with vs without diagnostic delay [log rank p = 0.001]. Even after adjusting for small bowel involvement and early anti-TNF use, diagnostic delay remained significantly associated with a more than 2-fold higher rate of stricturing/internal fistulising complications over time [HR 2.28, 95% CI 1.25–4.14] [Table 3]. Notably, small bowel involvement was also independently associated with an increased risk of stricturing/fistulising complications [HR 4.07, 95% CI 1.44–11.5]. Early anti-TNF use was associated with a numerically lower rate of complications, but this was not statistically significant. A sensitivity analysis restricted to patients with small bowel disease also yielded significant findings for diagnostic delay [HR 2.00, 95% CI 1.06–3.78] [Supplementary Table 2, available as Supplementary data at ECCO-JCC online].
Figure 1.
Kaplan‐Meier curve illustrating time to stricturing/internal fistulising complications in Crohn’s disease [CD] patients with inflammatory disease at presentation by diagnostic delay status [log rank p = 0.001].
Table 3.
Association between diagnostic delay and development of stricturing and/or internal fistulising complications among CD patients with inflammatory disease at presentation.
| Factors | Unadjusted hazard ratioa [95% CI] | p-value | Adjusted hazard ratio [95% CI] | p-value |
|---|---|---|---|---|
| Diagnostic delayb | 2.53 [1.41–4.56] | 0.002 | 2.28 [1.25–4.14] | 0.007 |
| Male | 0.59 [0.33–1.06] | 0.087 | 0.58 [0.32–1.06] | 0.077 |
| Age at diagnosis [years] | 1.11 [0.99–1.24] | 0.076 | 1.07 [0.94–1.20] | 0.29 |
| Small bowel involvementc | 4.45 [1.59–12.5] | 0.004 | 4.07 [1.44–11.5] | 0.008 |
| Upfront anti-TNF used | 0.26 [0.06–1.10] | 0.067 | 0.30 [0.07–1.25] | 0.098 |
CD, Crohn’s disease; HR, hazard ratio; CI, confidence interval; TNF, tumour necrosis factor.
aHR >1 indicates faster time to diagnosis; HR <1 indicates slower time to diagnosis.
bTime to diagnosis >75th percentile in CD subgroup [10.8 months].
cL1, L3 or L4b.
dAs first therapy.
3.3. Diagnostic delay in paediatric CD is associated with linear growth impairment at presentation
Table 4 shows the results of univariate and MVA linear regression examining the association between time to diagnosis and HAZ at presentation. After adjusting for patient demographics [age, sex] and small bowel involvement, a longer time to diagnosis remained significantly associated with lower HAZ [beta coefficient -0.013, 95% CI -0.022 to -0.005]. In other words, for every additional month of symptoms before diagnosis, HAZ decreased by 0.013 standard deviations. This association was preserved in a sensitivity analysis restricted to CD patients with small bowel involvement [Supplementary Table 3, available as Supplementary data at ECCO-JCC online].
Table 4.
Association between time to diagnosis and linear growth [HAZ at presentation] in CD patients.
| Factors | Unadjusted beta coefficient [95% CI] | p-value | Adjusted beta coefficient [95% CI] | p-value |
|---|---|---|---|---|
| Symptom duration [months] | -0.013 [-0.021, -0.005] | 0.001 | -0.013 [-0.022, -0.005] | 0.001 |
| Male | -0.075 [-0.237, 0.087] | 0.36 | -0.058 [-0.223-0.108] | 0.50 |
| Age at diagnosis [years] | 0.018 [-0.008–0.044] | 0.16 | 0.020 [-0.007–0.048] | 0.14 |
| Small bowel involvementa | 0.0009 [-0.182–0.184] | 0.99 | -0.017 [-0.201-0.167] | 0.86 |
HAZ, height for age z-score; CD, Crohn’s disease.
aL1, L3 or L4b.
3.4. Diagnostic delay is not associated with an increased risk of surgery in paediatric CD or UC/IBD-U
By the end of follow-up, 107 patients [7.1% overall] underwent surgery (76 CD [8.5% of the total CD cohort] and 31 UC/IBD-U [6.2% of the total UC/IBD-U cohort]). In CD, surgery occurred in the same proportion of patients with and without diagnostic delay [8%]. In UC/IBD-U, surgery was in fact more common in patients without diagnostic delay [7% compared with 2%, p = 0.048]. However, in adjusted analyses controlling for patient age, sex, and disease location [L1 vs other for CD, and pancolitis vs less extensive disease for UC/IBD-U], diagnostic delay was not independently associated with risk of surgery over time in either CD [HR 0.93, 95% CI 0.55–1.59] or UC/IBD-U [HR 0.41, 95% CI 0.12–1.34] [Tables 5 and 6, respectively]. This was also the case in an analysis restricted to CD patients with inflammatory disease at presentation and in a UC/IBD-U sensitivity analysis excluding patients presenting with severe colitis [PUCAI ≥65]. In CD, L1 location was independently associated with an increased risk of surgery [HR 1.71, 95% CI 1.03–2.86]. As shown in Supplementary Table 5, available as Supplementary data at ECCO-JCC online, diagnostic delay was not associated with an increased risk of surgery in the IBD-U group either.
Table 5.
Association between diagnostic delay and surgery in CD patients.
| Factors | Unadjusted hazard ratio [95% CI]a | p-value | Adjusted hazard ratio [95% CI] | p-value |
|---|---|---|---|---|
| Diagnostic delayb | 1.01 [0.60–1.70] | 0.97 | 0.93 [0.55–1.59] | 0.80 |
| Male | 0.92 [0.59–1.46] | 0.74 | 0.90 [0.57–1.44] | 0.67 |
| Age at diagnosis [years] | 1.04 [0.96–1.12] | 0.38 | 1.02 [0.94–1.11] | 0.55 |
| Distal ileal ± caecal [L1] | 1.74 [1.05–2.88] | 0.032 | 1.71 [1.03–2.86] | 0.040 |
| Upfront anti-TNF use2 | 1.35 [0.77–2.38] | 0.30 | 1.37 [0.77–2.42] | 0.28 |
CD, Crohn’s disease; TNF, tumour necrosis factor; CI, confidence nterval; HR, hazard ratio.
aHR >1 indicates faster time to diagnosis; HR <1 indicates slower time to diagnosis.
bTime to diagnosis >75th percentile in CD subgroup [10.8 months].
cAs first therapy.
Table 6.
Association between diagnostic delay and surgery in UC/IBD-U patients.
| Factorsa | Unadjusted hazard ratio [95% CI]c | p-value | Adjusted hazard ratio [95% CI] | p-value |
|---|---|---|---|---|
| Diagnostic delayb | 0.35 [0.11–1.16] | 0.085 | 0.41 [0.12–1.34] | 0.14 |
| Male | 1.70 [0.81–3.55] | 0.16 | 1.70 [0.81–3.55] | 0.16 |
| Age at diagnosis [years] | 1.01 [0.91–1.12] | 0.85 | 1.02 [0.92–1.12] | 0.78 |
| Pancolitis [E4] | 2.26 [0.87–5.90] | 0.094 | 2.14 [0.82–5.58] | 0.12 |
UC/IBDU, ulcerative colitis/inflammatory boweldisease unclassified; TNF, tumour necrosis factor; CI, confidence nterval; HR, hazard ratio.
aAnti-tumour necrosis factor-alpha [anti-TNF] use not included in model as virtually all UC/IBD-U patients who underwent surgery were first treated with anti-TNF [positioning anti-TNF use in the causal pathway].
bTime to diagnosis >75th percentile in UC/IBD-U subgroup [6.6 months].
cHR >1 indicates faster time to diagnosis; HR <1 indicates slower time to diagnosis.
3.5. Predictors of time to diagnosis in paediatric IBD
Last, we sought to identify predictors of time to diagnosis known at presentation [ie, before diagnostic endoscopy]. This analysis was therefore undertaken for the entire IBD cohort as a whole. Variables were selected for inclusion in the MVA model based on clinical relevance. As shown in Supplementary Table 4, available as Supplementary data at ECCO-JCC online, abdominal pain, iron deficiency, and linear growth impairment were independently associated with a longer time to diagnosis, and bloody diarrhoea, vomiting, and constitutional symptoms, such as fever and weight loss, were associated with a shorter time to diagnosis. More severe clinical activity was also associated with a shorter time to diagnosis. Of note, these associations remained significant when IBD type was added to the model.
4. Discussion
This is the largest study to date to examine the association between diagnostic delay and disease complications in paediatric IBD and the first paediatric study, to our knowledge, to report that diagnostic delay is independently associated with an increased risk of stricturing/internal fistulising complications over time in CD. We also observed an independent association between diagnostic delay and lower height in CD. We found no independent link, however, between diagnostic delay and risk of surgery in either CD or UC/IBD-U. In addition, we identified several patient and disease factors associated with diagnostic delay. CD was associated with a longer time to diagnosis than UC/IBD-U. In the CD subgroup, those with isolated small bowel disease were particularly at risk; in the UC/IBD-U subgroup, those with less extensive disease more often had diagnostic delay. Factors reflecting more severe disease activity, clinically, endoscopically, or biochemically, were associated with a shorter time to diagnosis. Among factors known at time of presentation, abdominal pain and linear growth impairment were associated with diagnostic delay, whereas intestinal and extraintestinal symptoms, such as bloody diarrhoea, fever, and weight loss, were associated with a faster diagnosis. This is likely due to their prompt recognition as worrisome by patients, caregivers, and referring physicians. Abdominal pain, particularly in isolation, is a relatively common and non-specific symptom, which may be attributed to functional aetiologies or organic entities besides IBD. This highlights the need for greater education about the possibility of new-onset IBD presenting in this subtle manner in children and the utility of objective biomarkers like faecal calprotectin in this setting. In an earlier study, we demonstrated that the biggest contributor to time to diagnosis is the interval between symptom onset and time of referral to a paediatric gastroenterologist.14 This suggests that educational interventions are best targeted at primary care physicians, including family doctors and general paediatricians.
Table 7 summarises adult and paediatric studies on diagnostic delay in IBD published over the past 20 years. As illustrated, numerous adult studies support an association between diagnostic delay and IBD complications, particularly bowel stenosis, internal fistulae, and surgical resection,5–9,13,15–21 as well as perianal fistulising disease,8 biologic use,21,22 and impaired quality of life.19 Conversely, paediatric data on the relationship between diagnostic delay and IBD complications are scant. Our findings are congruent with the adult literature; in our cohort, paediatric CD patients with diagnostic delay experienced stricturing/internal fistulising complications subsequent to diagnosis more than twice as often as children without diagnostic delay. Importantly, this association persisted after accounting for small bowel involvement and anti-TNF use. The Swiss IBD Cohort Study also recently investigated this association in a paediatric population. In examining 387 paediatric CD patients, Schoepfer et al. found that children with diagnostic delay were less likely to have disease complications at diagnosis, and no significant association was observed between diagnostic delay and the risk of complications over time.8 In contrast, we purposefully restricted our analysis to patients with inflammatory CD at presentation, to examine the influence of diagnostic delay on the subsequent risk of developing complications [rather than the effect of complications on presenting symptoms]. This factor, as well as our larger sample size, might account for our differing findings.
Table 7.
Summary of adult and paediatric studies investigating diagnostic delay in IBD.
| Author [year] Country | Study design | N IBD type | Time to diagnosis [months, median [IQR] unless otherwise specified] | Predictors of diagnostic delay | Diagnostic delay identified as a risk factor for disease complications? | ||
|---|---|---|---|---|---|---|---|
| Crohn’s disease [CD] | Ulcerative colitis [UC] | Overall | |||||
| Adult | |||||||
| Schoepfer [2019]8 Switzerland | National cohort study, prospective 2006 onward, retrospective prior to 2005 | 1163 CD | 6 [1–24] | Yes, diagnostic delay associated with a higher rate of stenosis, perianal fistulae, internal fistulae, any fistulae, surgical resection, fistula surgery at presentation; diagnostic delay associated with stenosis internal fistulae and any complication over time after diagnosis | |||
| Novacek [2019]28 Austria | Cross-sectional, multicentre | 830 CD 435 UC 21 IBD-U | 6 [2–23] | 3 [1–10] | CD [vs UC], older age, higher level education | ||
| Kang [2019]22 South Korea | Retrospective chart review, multicentre | 551 UC | 2.3 [?-6.4] | Age <60 y, smoking, misdiagnosis of haemorrhoids | Yes—diagnostic delay >24 months associated with anti-tumour necrosis factor-alpha use | ||
| Szanto [2018]21 Hungary | Prospective registry-based, single centre | 428 CD 483 UC | 25 | 55 | CD [vs UC] | Yes—diagnostic delay >1 y associated with surgery and biologic use [in CD and UC] | |
| Banerjee [2018]5 India | Retrospective analysis of prospective registry, single centre | 720 CD | 18 [28–30] | Yes—diagnostic delay >12 and >18 months associated with stenosis and surgery | |||
| Nguyen [2017]7 USA | Retrospective chart review, single centre | 110 CD 67 UC | 9.5 [4–26] | 3.1 [1–10] | CD [vs UC] Exclusive ileal location [in CD] Haematochezia associated with faster diagnosis [in CD] | Yes—diagnostic delay >26 months associated with increased overall complications and intestinal strictures in CD | |
| Lee [2017]17 South Korea | Retrospective chart review, single centre | 165 CD 130 UC | 6.2 | 2.4 | Perianal discomfort [in CD] | Yes—diagnostic delay associated with intestinal surgery in CD and UC | |
| Cantoro [2017]15 Italy | Prospective registry-based, multicentre | 1537 CD 1855 UC | 7.1 [1–26] | 2.0 [0–7] | 3.0 [0–13] | CD [vs UC], age >40 y | Yes—diagnostic delay >24 months associated with complicated disease at CD diagnosis |
| Zaharie [2016]9 Romania | National registry-based, multicentre | 478 CD 682 UC 36 IBD-U | 5 | 1 | CD [vs UC] Ileal location, active smoking, symptom onset during summer [in CD] Age <40 y [in UC] | Yes—diagnostic delay associated with bowel stenosis and IBD-related surgery | |
| Nahon [2016]6 France | Prospective database, multicentre | 497 CD | 5 [2–13] | Yes—diagnostic delay >13 months associated with shorter time to first major surgery | |||
| Pellino [2015]19 Italy | Cross-sectional, single centre | 361 CD | 11 [1–163] | Yes—diagnostic delay >18 months associated with poorer quality of life, risk of surgery and penetrating disease at presentation | |||
| Moon [2015]18 South Korea | Retrospective registry-based, multicentre | 1047 CD | Mean 16 [±33.1] | Age >40 y, upper gastrointestinal tract disease | Yes—diagnostic delay >18 months associated with penetrating disease at presentation | ||
| Li [2015]13 China | Retrospective chart review, single centre | 343 CD | 10 [2–34] | Age >40 y, education level, no family history of CD | Yes—diagnostic delay associated with surgery | ||
| Schoepfer [2013]20 Switzerland | National cohort study, prospective 2006 onward, retrospective prior to 2005 | 905 CD | 9 [3–24] | Female, ileal location, age ≥40 y at diagnosis | Yes –diagnostic delay associated with bowel stenosis and intestinal surgery | ||
| Goel [2013]16 India | Retrospective chart review, single centre | 223 CD | 24 [range 6–240] | Yes—diagnostic delay associated with complicated disease behaviour over time | |||
| Vavricka [2012]29 Switzerland | National cohort study, prospective 2006 onward, retrospective prior to 2005 | 932 CD 625 UC 34 IBD-U | 9 [3–24] | 4 [1–12] | Age <40 y, ileal location [in CD] NSAID use, male [in UC] | ||
| Paediatric | |||||||
| Schoepfer [2019]8 Switzerland | National cohort study, prospective 2006 onward, retrospective prior to 2005 | 387 CD | 3 [1–9] | No—diagnostic delay associated with a lower risk of stricture, internal fistulae. and surgery at diagnosis; no significant association between diagnostic delay and disease complications over time following diagnosis | |||
| Krishna [2019] USA23 | Retrospective chart review, single centre | 106 UC | 7.1 [3.2–21.5] wk [colectomy] 11.9 [5.9–25.7] wk [no colectomy] | No—time to diagnosis shorter in patients who underwent colectomy | |||
| El Mouzan [2019]12 Saudi Arabia | Retrospective chart review [2003–2012], prospective [2011–2018], multicentre | 240 CD 183 UC | 8 [4–24] | 5 [2.1–8.8] | CD Ileal location [in CD] <10 years at diagnosis [in UC] | ||
| Ricciuto [2018]14 Canada | Prospective cohort study, single centre | 65 CD 46 UC/IBD-U | 6.8 [2.9–12.5] | 2.4 [1.3–5.3] | 4.5 [2.1–8.8] | CD, small bowel disease Diarrhoea, blood per rectum, weight loss associated with faster diagnosis in unadjusted analysis | Yes—lower height for age z-score |
| Schoepfer [2017]32 Switzerland | National cohort study, prospective 2006 onward, retrospective prior to 2005 | 100 CD 75 UC | 4 [2–8] | 2 [1–7] | None identified | ||
| Buderus [2015]33 Germany & Austria | Registry-based, prospective and retrospective, multicentre | 616 CD 278 UC 64 IBD-U | 6.0 [9.6] <10 y 6.0 [8.4] ≥10 y | 3.6 [9.6] <10 y 3.6 [4.8] ≥10 y | |||
| Arcos-Machancoses [2014]31 Spain | Retrospective chart review, single centre | 31 CD 19 UC 3 IBD-U | 2.8 [1.1–5.5] | CD [vs UC], young age [trend only for both] | |||
| Timmer [2011]27 Germany & Austria | Registry-based, prospective and retrospective, multicentre | 1456 CD 817 UC 163 IBD-U | 4 [2–8] | CD [vs UC], young age [<6 y] Centre effect Ileal location [in CD] | Yes—growth failure | ||
| Sawczenko [2003]25 UK | Survey, multicentre | 431 CD 211 UC 86 IBD-U | 6 [3.6–12] | 4.8 [2.4–8.4] | 5 [range <1 mo-9 y] | CD [vs UC], young age Oral, perianal, jejunal [in CD] Rectal bleeding associated with faster diagnosis | Yes—short stature |
| Kugathasan [2003]30 USA | Prospective registry [voluntary reporting] | 129 CD 60 UC 10 IBD-U | Mean 4.5 | Mean 3 | CD [vs UC] | ||
| Spray [2001]26 UK | Retrospective chart review, single centre | 64 CD 41 UC 7 IBD-U | 10.8 [range 0.9–84] | 4.6 [0.5–36] | Symptoms other than diarrhoea | Yes—growth impairment | |
| Heineken [1999]24 USA | Retrospective chart review, single centre | 58 CD 24 UC 9 IBD-U | Mean 7.1 | Mean 6.7 | Small bowel [in CD] | Yes—growth failure | |
IBDU, inflammatory bowel disease unclassified; CD, Crohn’s disease; UC, ulcerative colitis; IQR, interquartile range; y, year; mo, month; wk, week.
As in the Swiss IBD Cohort Study, we found no association between diagnostic delay and surgery in CD. The apparent disconnect between the increased risk of stricturing/internal fistulising complications, but not surgery, in CD patients with diagnostic delay may relate to the potentially prolonged interval between radiographic/clinical diagnosis of stricturing/fistulising complications and surgical intervention, as well as our relatively short follow-up duration. However, this discordance might also relate to the early use of effective biologic agents. More generally, the frequently early use of biologics for paediatric IBD in the current era may also account for the fact that paediatric studies, thus far, have not supported an increased risk of surgery with diagnostic delay in CD, as has been the case in the adult literature. We did not find that diagnostic delay was associated with an increased risk of colectomy in UC/IBD-U either; in fact, in univariate analyses, colectomy was more frequent in patients without diagnostic delay. This may reflect the predilection of this group for more anatomically extensive and severe disease, as shown in Table 2. These findings are in keeping with those of a retrospective paediatric UC study, in which median time to diagnosis was shorter in patients who underwent colectomy compared with those who did not.23
In keeping with a number of earlier paediatric studies,14,24–27 we confirmed the association between diagnostic delay and linear growth impairment in paediatric CD. Notably, we showed that this association was not merely the result of the confounding effect of disease location; the association between diagnostic delay and lower HAZ was preserved in adjusted and sensitivity analyses controlling for small bowel involvement.
Table 7 summarises time to diagnosis in CD and UC in adult and paediatric studies; this illustrates that our findings are generally in keeping with previous paediatric studies, and that time to diagnosis tends to be longer in adults than in children. In addition, it demonstrates that CD, especially small bowel CD, is consistently associated with a longer time to diagnosis than UC, in both adults7,9,15,20,21,28,29 and children.12,14,24,25,27,30 We also found CD and isolated small bowel disease to be associated with diagnostic delay. This may well relate to the less frequent occurrence of overt intestinal symptoms, such as bloody diarrhoea, in the setting of small bowel CD. As shown in Table 7, the association between age and diagnostic delay has been conflicting in adult studies, but several paediatric studies have suggested that younger children are at increased risk of diagnostic delay.12,25,27,31 The absence of this finding in our cohort may reflect the decreasing age of IBD onset in Canada and thus greater awareness of IBD even in the youngest of children. In keeping with our findings, previous paediatric studies have suggested that symptoms other than diarrhoea are associated with diagnostic delay,25 whereas haematochezia is protective against diagnostic delay.25 A small number of studies have also reported an association between perianal symptoms/disease and diagnostic delay.17,25 Although we observed a significant relationship between perianal symptoms [as per patient report] in univariate analyses, this was not maintained in MVA and was not observed in the CD cohort separately. Perianal symptoms may therefore simply be associated with diagnostic delay because they function as a marker of CD.
Our study has numerous strengths. Chief among them are its large size, prospective nature, and standardised data collection methods. We also used several methods to address potential confounders, including disease location and medication use. There are some limitations as well, including the fact that the study is not truly population-based, as it includes children followed at tertiary paediatric centres. However, in Canada, it is conventional for paediatric IBD to be managed by paediatric gastroenterologists, the majority of whom practise at academic centres. It will also be important to undertake studies with longer follow-up duration, to determine whether the associations noted persist in the longer term. Although attempts were made in CIDsCaNN to enrol all consecutive paediatric IBD diagnoses over the study period, a small subset declined participation or were not captured, which may introduce selection bias; it is, however, reassuring that the full spectrum of disease severity was seen in both the CD and UC/IBD-U cohorts [as reflected in Tables 1 and 2]. An additional limitation is unavailability of data on parental social factors [education, income, etc.] and compliance with medical recommendations, both of which may be confounders [ie, may be associated with diagnostic delay and adverse outcomes]. We cannot rule this possibility out but, in a previous, smaller study, we found no association between family income [derived by linking postal codes to Canadian census data] and diagnostic delay.14 Last, ascertainment of true symptom onset is challenging and subject to possible recall bias. In this study, physicians prospectively questioned patients and parents, at first consultation, about timing of symptom onset. The prospective nature does not exclude the possibility of recall bias, but it helps to mitigate against it.
In summary, a substantial fraction of children newly diagnosed with IBD in Canada continue to experience prolonged delays between symptom onset and diagnosis. Diagnostic delay is an important modifiable factor in the management of IBD as it is associated with impaired patient outcomes, including delays in treatment initiation and an increased risk of stricturing/internal fistulising complications and linear growth impairment in paediatric CD. Interventions directed at minimising diagnostic delay, such as education about presenting symptoms and signs and improved access to care, are warranted.
Funding
This work was supported by grant 297862 from the Canadian Institutes of Health Research [CIHR] in partnership with the Children’s Intestinal and Liver Disease [Ch.I.L.D.] Foundation. EIB was supported by a New Investigator Award from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology, and Crohn’s and Colitis Canada. EIB was also supported by the Career Enhancement Program of the Canadian Child Health Clinician Scientist Program.
Potential competing interests
HH—advisory board: AbbVie, Janssen, Merck; research support: Janssen. KJ—advisory board: AbbVie, Janssen, Merck; speaker’s bureau: AbbVie Janssen; investigator-initiated research support: Janssen. AO—advisory board: AbbVie, Janssen, Shire; consultant: AbbVie, Janssen, Lilly; research site: AbbVie, Janssen, Takeda. JdB—advisory board: AbbVie, Janssen, Merck. WE-M—advisory board: AbbVie, Janssen, Merck. CD—advisory board: AbbVie, Janssen, Merck. MS—advisory board: AbbVie, Merck. KB—advisory board: AbbVie, Mead Johnson; pharmaceutical trials: AbbVie, Takeda, Janssen, Allergan, Pfizer; speaker: Mead Johnson; unrestricted educational grant: AbbVie. PJ—consultant: AbbVie, Janssen; advisory board: Ferring. EW—consultant: AbbVie, Janssen. MC—advisory board: AbbVie, Janssen. SL—advisory board: Janssen; speaker: AbbVie. JL—consulting, travel, and/or speaker fees and research support: AbbVie, Janssen, Nestlé Health Science, Merck, P&G, GSK, Illumina, Otsuka. TW—consultant: AbbVie, Ferring, Janssen, Merck; speaker: AbbVie, Ferring, Janssen, Merck, Nestle. AG—consultant: AbbVie, Merck, Janssen, Eli Lilly, Pfizer, Gilead, Roche, Takeda; speaker: AbbVie, Janssen, Shire; investigator-initiated research support: AbbVie. PC—consultant: AbbVie, Ferring, Janssen, Merck; speaker: AbbVie; research support: AbbVie.
Author Contributions
All authors contributed to acquisition of data, critical revision of the manuscript for important intellectual content, and approval of the final manuscript as submitted. PC, AG, TW, and AR contributed to the study concept and design, analysis and interpretation of data, statistical analysis, and drafting of the manuscript; PC provided study supervision.
The contents of this article were presented at the NASPGHAN Annual Meeting in Fort Lauderdale, USA, in 2018.
Supplementary Material
Acknowledgements
We acknowledge the assistance of Sophie Rossini with data extraction.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 2. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019;156:1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 3. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011;17:423–39. [DOI] [PubMed] [Google Scholar]
- 4. Vadstrup K, Alulis S, Borsi A, et al. Cost burden of Crohn’s disease and ulcerative colitis in the 10-year period before diagnosis ‐ a Danish register-based study from 2003–2015. Inflamm Bowel Dis 2019. doi: 10.1093/ibd/izz265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banerjee R, Pal P, Girish BG, Reddy DN. Risk factors for diagnostic delay in Crohn’s disease and their impact on long-term complications: how do they differ in a tuberculosis endemic region? Aliment Pharmacol Ther 2018;47:1367–74. [DOI] [PubMed] [Google Scholar]
- 6. Nahon S, Lahmek P, Paupard T, et al. Diagnostic delay is associated with a greater risk of early surgery in a French cohort of Crohn’s disease patients. Dig Dis Sci 2016;61:3278–84. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen VQ, Jiang D, Hoffman SN, et al. Impact of diagnostic delay and associated factors on clinical outcomes in a U.S. inflammatory bowel disease cohort. Inflamm Bowel Dis 2017;23:1825–31. [DOI] [PubMed] [Google Scholar]
- 8. Schoepfer A, Santos J, Fournier N, et al. Systematic analysis of the impact of diagnostic delay on bowel damage in paediatric versus adult onset Crohn’s disease. J Crohns Colitis 2019;13:1334–42. [DOI] [PubMed] [Google Scholar]
- 9. Zaharie R, Tantau A, Zaharie F, et al. ; IBDPROSPECT Study Group. Diagnostic delay in Romanian patients with inflammatory bowel disease: risk factors and impact on the disease course and need for surgery. J Crohns Colitis 2016;10:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 11. Dhaliwal J, Walters TD, Mack DR, et al. Phenotypic variation in paediatric inflammatory bowel disease by age: a multicentre prospective inception cohort study of the Canadian children IBD network. J Crohns Colitis 2020;14:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El Mouzan MI, AlSaleem BI, Hasosah MY, et al. Diagnostic delay of pediatric inflammatory bowel disease in Saudi Arabia. Saudi J Gastroenterol 2019;25:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Ren J, Wang G, et al. Diagnostic delay in Crohn’s disease is associated with increased rate of abdominal surgery: a retrospective study in Chinese patients. Dig Liver Dis 2015;47:544–8. [DOI] [PubMed] [Google Scholar]
- 14. Ricciuto A, Fish JR, Tomalty DE, et al. Diagnostic delay in Canadian children with inflammatory bowel disease is more common in Crohn’s disease and associated with decreased height. Arch Dis Child 2018;103:319–26. [DOI] [PubMed] [Google Scholar]
- 15. Cantoro L, Di Sabatino A, Papi C, et al. The time course of diagnostic delay in inflammatory bowel disease over the last sixty years: an Italian multicentre study. J Crohns Colitis 2017;11:975–80. [DOI] [PubMed] [Google Scholar]
- 16. Goel A, Dutta AK, Pulimood AB, Eapen A, Chacko A. Clinical profile and predictors of disease behavior and surgery in Indian patients with Crohn’s disease. Indian J Gastroenterol 2013;32:184–9. [DOI] [PubMed] [Google Scholar]
- 17. Lee DW, Koo JS, Choe JW, et al. Diagnostic delay in inflammatory bowel disease increases the risk of intestinal surgery. World J Gastroenterol 2017;23:6474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moon CM, Jung SA, Kim SE, et al. ; CONNECT study group. Clinical factors and disease course related to diagnostic delay in Korean Crohn’s disease patients: results from the CONNECT study. PLoS One 2015;10:e0144390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pellino G, Sciaudone G, Selvaggi F, Riegler G. Delayed diagnosis is influenced by the clinical pattern of Crohn’s disease and affects treatment outcomes and quality of life in the long term: a cross-sectional study of 361 patients in Southern Italy. Eur J Gastroenterol Hepatol 2015;27:175–81. [DOI] [PubMed] [Google Scholar]
- 20. Schoepfer AM, Dehlavi MA, Fournier N, et al. ; IBD Cohort Study Group. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol 2013;108:1744–53; quiz 1754. [DOI] [PubMed] [Google Scholar]
- 21. Szántó K, Nyári T, Bálint A, et al. Biological therapy and surgery rates in inflammatory bowel diseases ‐ data analysis of almost 1000 patients from a Hungarian tertiary IBD center. PLoS One 2018;13:e0200824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang HS, Koo JS, Lee KM, et al. Two-year delay in ulcerative colitis diagnosis is associated with anti-tumor necrosis factor alpha use. World J Gastroenterol 2019;25:989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krishna M, Britto S, Qian J, Ihekweazu F, Rodriguez JR, Kellermayer R. Diagnostic delay and colectomy risk in pediatric ulcerative colitis. J Pediatr Surg 2020;55:403–5. [DOI] [PubMed] [Google Scholar]
- 24. Heikenen JB, Werlin SL, Brown CW, Balint JP. Presenting symptoms and diagnostic lag in children with inflammatory bowel disease. Inflamm Bowel Dis 1999;5:158–60. [DOI] [PubMed] [Google Scholar]
- 25. Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child 2003;88:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spray C, Debelle GD, Murphy MS. Current diagnosis, management and morbidity in paediatric inflammatory bowel disease. Acta Paediatr 2001;90:400–5. [PubMed] [Google Scholar]
- 27. Timmer A, Behrens R, Buderus S, et al. ; CEDATA-GPGE Study Group. Childhood onset inflammatory bowel disease: predictors of delayed diagnosis from the CEDATA German-language pediatric inflammatory bowel disease registry. J Pediatr 2011;158:467–73.e2. [DOI] [PubMed] [Google Scholar]
- 28. Novacek G, Gröchenig HP, Haas T, et al. ; Austrian IBD Study Group [ATISG]. Diagnostic delay in patients with inflammatory bowel disease in Austria. Wien Klin Wochenschr 2019;131:104–12. [DOI] [PubMed] [Google Scholar]
- 29. Vavricka SR, Spigaglia SM, Rogler G, et al. ; Swiss IBD Cohort Study Group. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:496–505. [DOI] [PubMed] [Google Scholar]
- 30. Kugathasan S, Judd RH, Hoffmann RG, et al. ; Wisconsin Pediatric Inflammatory Bowel Disease Alliance. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr 2003;143:525–31. [DOI] [PubMed] [Google Scholar]
- 31. Arcos-Machancoses JV, Donat-Aliaga E, Polo-Miquel B, Masip-Simó E, Ribes-Koninckx C, Pereda-Pérez A. [Description and study of risk factors for the diagnostic delay of pediatric inflammatory bowel disease]. An Pediatr [Barc] 2015;82:247–54. [DOI] [PubMed] [Google Scholar]
- 32. Schoepfer AM, Vavricka S, Safroneeva E, et al. Systematic evaluation of diagnostic delay in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2017;64:245–7. [DOI] [PubMed] [Google Scholar]
- 33. Buderus S, Scholz D, Behrens R, et al. ; CEDATA-GPGE Study Group. Inflammatory bowel disease in pediatric patients: characteristics of newly diagnosed patients from the CEDATA-GPGE Registry. Dtsch Arztebl Int 2015;112:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.