Abstract
Background and Aims
Real-life data on long-term disease activity in Crohn’s disease [CD] are scarce. Most studies describe disease course by using proxies, such as drug exposure, need for surgery or hospitalisations, and disease progression. We aimed to describe disease course by long-term disease activity and to identify distinctive disease activity patterns in the population-based IBD South Limburg cohort [IBDSL].
Methods
All CD patients in IBDSL with ≥10 years follow-up [n = 432] were included. Disease activity was defined for each yearly quarter by mucosal inflammation on endoscopy or imaging, hospitalisation, surgery, or treatment adjustment for increased symptoms. Six distinct disease activity clusters were defined. Subsequently, the associations between clinical characteristics and the patterns were assessed using multivariable logistic regression models.
Results
On average, patients experienced 5.44 (standard deviation [SD] 3.96) quarters of disease activity during the first 10 years after diagnosis. Notably, 28.2% of the patients were classified to a quiescent pattern [≤2 active quarters in 10 years], and 89.8% of those never received immunomodulators nor biologics. Surgery at diagnosis (odds ratio [OR] 2.99; 95% confidence interval [CI] 1.07–8.34) and higher age [OR 1.03; 95% CI 1.01–1.06] were positively associated with the quiescent pattern, whereas inverse associations were observed for ileocolonic location [OR 0.44; 95% CI 0.19–1.00], smoking [OR 0.43; 95% CI 0.24–0.76] and need for steroids <6 months [OR 0.24; 95% CI 0.11–0.52].
Conclusions
Considering long-term disease activity, 28.2% of CD patients were classified to a quiescent cluster. Given the complex risk-benefit balance of immunosuppressive drugs, our findings underline the importance of identifying better predictive markers to prevent both over-treatment and under-treatment.
Keywords: Disease course, Crohn’s disease, disease activity, quiescent disease, predictive markers
1. Introduction
Crohn’s disease [CD] is a chronic inflammatory disease characterised by intermittent periods of mucosal inflammation. Ongoing disease activity and recurrent flares predispose to structural bowel damage and an unfortunate disease outcome. Novel treatment options, strategies, and goals have been introduced in order to improve the long-term disease outcome. Previously we showed, in line with other population-based studies, that the exposure of patients to immunomodulators and biologics increased significantly over recent decades.1 Despite their efficacy, these drugs are associated with toxic side effects, [opportunistic] infections, and malignancies.2–4 Due to the complex risk-benefit balance of immunosuppressive drugs, there is an increasing need to distinguish patients at risk for an unfavourable outcome from patients who are prone to a more quiescent course, in order to prevent both undertreatment and overtreatment, respectively.5
In most cohorts, the severity of the disease course in CD is described by drug exposure, hospitalisation, surgery, and disease progression.6 However, most of these outcomes heavily depend on local treatment strategies and recommendations in guidelines. For example, Beaugerie et al. performed a large retrospective study in which patients were classified as ‘disabling disease’ when they were treated with more than two courses of steroids, required hospitalisation, needed immunomodulators, or needed surgery.7 In that study, the prevalence of disabling disease was 64.9–80.5% [1985–1999]. In our IBD South Limburg [IBDSL] cohort, during the most recent era [2006 until 2011], 70.8% of the patients would be classified as having a disabling disease merely based on the exposure to immunomodulators, whereas this would be only 30.6% in the first era [1991 until 1998].1 This underlines the time-dependency of certain outcomes and the need for a new classification of CD disease course severity, better reflecting the actual varying disease activity.
Since chronically active bowel inflammation or frequent flares precede unfavourable outcomes, analysing long-term disease activity patterns in large cohorts may contribute to a better classification of patients.8 Until now, only a limited number of studies have analysed population-based relapse rates and even less studies defined distinctive disease activity patterns.6,9–12 Solberg et al. asked patients in the IBSEN cohort to assign themselves to one of four pre-defined disease activity patterns: decreasing disease activity over time, increasing disease activity over time, chronic continuous disease activity, or chronic intermittent disease activity.11 Irrespective of the clinical relevance of this information, the survey-based approach employed in that study is subject to recall bias and reflects only subjective disease activity rather than overall disease activity. Moreover, a pattern of ‘quiescent disease’ was missing, although being of added value, supported by older findings of Jess et al. showing that 18.4% of the patients experienced no flares during the first 5 years after diagnosis.9
In the present study, we aimed to describe CD disease course by long-term disease activity, and to identify distinctive disease activity patterns in the IBDSL cohort. We also aimed to define early clinical predictors of the distinctive disease activity patterns.
2. Materials and Methods
2.1. Study design, data collection, and patient involvement
All CD patients included in the population-based IBDSL cohort were eligible for this study. This cohort was previously described in detail.13 In brief, all patients diagnosed with IBD between January 1991 and June 2011, being at least 18 years of age at diagnosis and living in the region of South Limburg, were included. IBD was diagnosed by certified gastroenterologists based on the combination of endoscopic, radiological, and/or histological findings. A multifaceted identification strategy, including hospitals, the nationwide Dutch pathology database [PALGA], and general practitioners, resulted in 93% completeness of our cohort. The remaining 7% of patients may have had an IBD diagnosis without meeting the aforementioned IBDSL inclusion criteria, may have been misdiagnosed, or may have attended Belgian or German hospitals. As health care provided in these neighbouring countries is comparable to that in The Netherlands, it is unlikely that this is associated with a specific IBD phenotype. Altogether, the risk for selection bias is limited. The IBDSL study design has been approved by the Ethics Committee of the Maastricht University Medical Centre [NL31636.068.10], is registered in ClinicalTrial.gov [NCT02130349], and meets the ethical standards of the revised version of the Declaration of Helsinki.14
CD patients in the IBDSL cohort were followed as from the date of diagnosis to the end of data collection [ie, October 2014] or to date of migration, death, or last outpatient clinic visit. For this study, only patients with a follow-up [FU] of at least 10 years were included [ie, date of diagnosis between 1991 and 2005]. Data on demographics, disease phenotype, complications [ie, fistula or stricture], medication use, hospitalisations, and surgery were collected from medical records using standardised case report forms. In addition, all endoscopy and imaging reports were analysed for disease activity.
2.2. Definitions
As from diagnosis, each yearly quarter was assessed for disease activity in all patients retrospectively, using the obtained case report forms. Disease activity was defined by either: i] active disease on endoscopy or imaging; ii] hospitalisation; iii] surgery; or iv] treatment adjustment due to symptoms. i] Active disease on endoscopy or imaging comprised all reports in which the gastroenterologist or radiologist who performed the endoscopy or judged the imaging, respectively, considered lesions to be due to active CD. ii] Hospitalisations were only taken into account when patients were admitted for exacerbations of IBD. Elective admissions for endoscopy, drug administration, and surgery, and admissions due to side effects of medication use, were excluded. iii] Surgical procedures were taken into account when the indication for surgery was either active luminal disease, active internal fistula, stricturing complications, or active perianal disease. Diagnostic procedures and procedures secondary to surgical complications were excluded. iv] Finally, a patient was considered as having disease activity when a treatment adjustment was made by the treating physician based on highly suggestive biochemical changes or symptoms alone. Changes solely due to trough levels or side effects were excluded. Also, the prescription of 5-aminosalicylate [5-ASA] drugs was not taken into account, since the effectiveness of these drugs in CD is barely supported by evidence.15
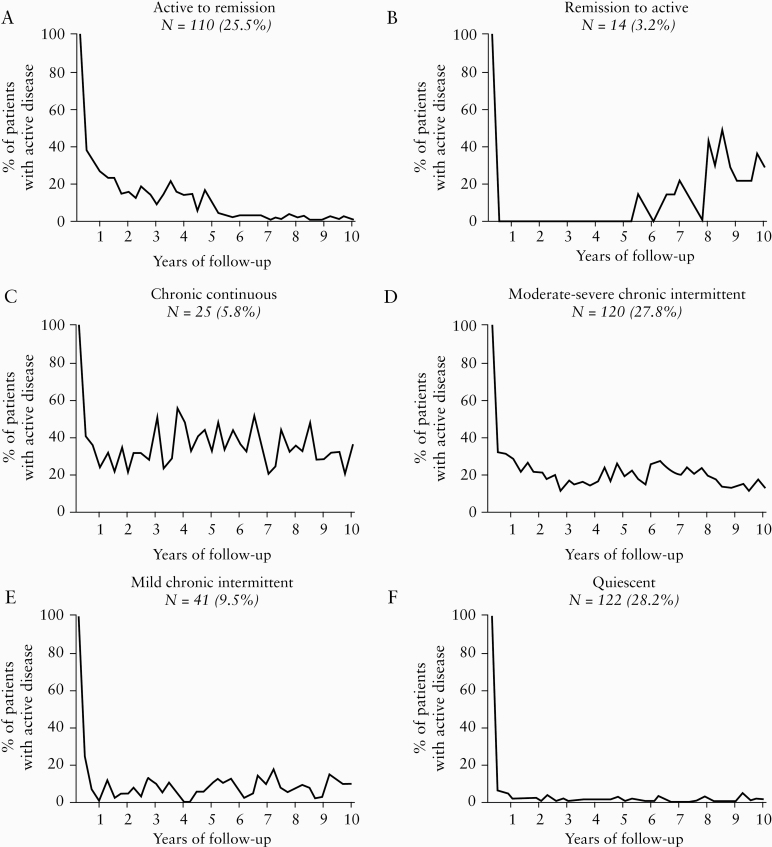
Subsequently, patterns were defined partly based on the four previously published survey-based disease activity patterns by Solberg et al.11 All definitions were discussed in expert meetings with multiple gastroenterologists from both academic and non-academic hospitals. The first pattern [Active to remission] was defined by >2 quarters of disease activity in first 5 years and <2 quarters of disease activity in second 5 years. The second pattern [Remission to active] was inversely defined by <2 quarters of disease activity in first 5 years and >2 quarters of disease activity in second 5 years. The third pattern [Chronic continuous] was defined by at least 1 quarter of disease activity per year in ≥8 years. Supported by a rough, unsupervised pilot analysis, the ‘Chronic intermittent’ pattern of Solberg et al. was divided into a ‘Mild-moderate’ pattern [≤5 quarters of disease activity] and ‘Severe’ intermittent pattern [>5 quarters of disease activity, but not meeting the criteria of the ‘Chronic continuous’ pattern], and a Quiescent pattern [≤2 quarters of disease activity in total] was added. Patients who could not be classified using mathematical formulas were reviewed by two independent researchers and assigned to one of the patterns. In case of disagreement, final assignment was determined during another expert meeting. Eventually, graphs for each pattern were generated by plotting the percentage of patients with active disease per yearly quarter of follow-up [Figure 1].
Figure 1.
Distribution of Crohn’s disease [CD] patients over six disease activity patterns and 10 years. Definitions: A: >2 quarters of disease activity in first 5 years, <2 quarters of disease activity in second 5 years. B: <2 quarters of disease activity in first 5 years, >2 quarters of disease activity in second 5 years. C: At least 1 quarter of disease activity per year in ≥8 years. D: Chronic intermittent pattern with >5 quarters of disease activity [but not fulfilling criteria of C]. E: Chronic intermittent pattern with ≤5 quarters of disease activity. F: ≤2 quarters of disease activity in total. n, number of patients.
2.3. Statistics
Baseline characteristics are presented as means with corresponding standard deviations [SD] for numerical variables and as number and percentage of patients [%] for categorical variables. For comparison between groups, the independent-samples t test, chi square test or Fisher’s exact test, where appropriate, were used for numerical and categorical variables, respectively.
A multivariable logistic regression model was used to analyse the association between baseline characteristics and the disease activity patterns. In order to increase sample size and power, the two most severe groups [ie, ‘Chronic continuous’ and ‘Moderate-severe chronic intermittent’] were combined as the ‘Most severe’ pattern. We compared the ‘Quiescent’ pattern with the ‘Most severe’ pattern, the ‘Quiescent’ pattern with all other patterns, and the ‘Most severe’ pattern with all other patterns. The following baseline variables were included in the model: age, gender, smoking status, disease location, disease behaviour, perianal disease, and upper gastrointestinal involvement [according to the Montreal classification16], and need for surgery at diagnosis. The latter was defined as surgery within a week after diagnosis and includes acute abdominal surgery when this was attributed to CD after histological evaluation.
Next, we compared outcome variables after 6 months of follow-up between the same groups, using multivariable logistic regression models adjusted for baseline variables with a p-value <0.1 and clinically relevant baseline variables [ie, disease location and behaviour according to the Montreal classification16]. The following outcome variables after 6 months of follow-up were included in the model: disease progression [Montreal B1 to B2/B3], need for surgery [baseline excluded], need for hospitalisation, need for systemic corticosteroids, need for immunomodulators, and need for biologics.
A partial least square discriminant analysis [PLS-DA] model was built to evaluate the predictive power of several subsets of variables in order to differentiate between the ‘Quiescent’, ‘Most severe’, and other patterns. In this model, the following three subsets of variables were tested: the Montreal classification alone [ie, age, disease localisation, disease behaviour, perianal disease, upper gastrointestinal involvement], all of the aforementioned baseline variables, and all baseline variables and outcome variables after 6 months. PLS models were double cross-validated for metaparameter estimation and to assess model reproducibility. In detail, the data was split in three parts: validation, training, and test. The training and test data were used to calibrate the prediction model by optimising the metaparameters [ie, the number of latent components] as described elsewhere.17 The validation data were used to determine the predictive power of the model in an unbiased fashion by predicting the class of samples that were not used to train the model. The overall procedure was repeated 10 times with different data split to assess variability and generalisability of the models and results [specificity, sensitivity and area under the receiver operating characteristic curve; [AUROC] were averaged over the repetition. Finally a permutation test was applied to obtain a measure of statistical significance of the predictive models and to ascertain that the model was not overfitting the data as described elsewhere.18
In general, two-sided p-values [p] lower than 0.05 were considered statistically significant. Statistical analyses were performed with SPSS [version 25.0, SPSS Inc., Chicago, IL, USA] to describe cohort characteristics. PLS-DA was performed in Matlab [version R2018a; Natick, MA, The MathWorks Inc.] using in-house written routines.
3. Results
In the population-based IBDSL cohort, 626 patients were diagnosed with CD between January 1991 and January 2005. Of these, 11 patients [1.8%] were lost to follow-up [LTFU] due to migration out of the region, and 31 patients [5.0%] were LTFU due to death. For the remaining patients, we decided to include only those with a completely documented follow-up [ie, outpatient clinic visits exceeding 10 years of follow-up] in order to guarantee full documentation of disease activity. Since end of data collection for each individual patient was determined by the last outpatient clinic visit, patients who visit the hospital only once a year or less could be considered LTFU in this calculation [n = 152].
Baseline characteristics of both patients with a complete and patients with incomplete 10-year follow-up are presented in Table 1. Patients who died or migrated during follow-up were excluded from the table. In general, patients who were LTFU for other reasons were more recently diagnosed [p <0.001] and less often had perianal disease at diagnosis [p = 0.026]. Eventually, 432 patients were eligible for further analysis. On average, patients experienced 5.44 [SD 3.96] quarters of disease activity during the first 10 years after diagnosis.
Table 1.
Baseline characteristics of CD patients diagnosed between 1991 and 2005 in the IBDSL cohort.
| Complete 10-year FU [n = 432] | LTFU [n = 152] | p-value | |
|---|---|---|---|
| Age at diagnosis, mean [SD] | 34.1 [13.6] | 37.0 [16.1] | 0.051 |
| Male, n [%] | 153 [35.4] | 62 [40.8] | 0.242 |
| Era of diagnosis, n [%] | <0.001 | ||
| 1991–1998 | 243 [56.2] | 55 [36.2] | |
| 1999–2005 | 189 [43.8] | 97 [63.8] | |
| Smoking at diagnosis, n [%] | 219 [55.6] | 58 [47.2] | 0.120 |
| NA: 38 [8.8] | NA: 29 [19.1] | ||
| Disease location at diagnosis, n [%] | 0.005 [0.921 if L4 excluded] | ||
| L1 | 210 [48.6] | 68 [44.7] | |
| L2 | 127 [29.4] | 45 [29.6] | |
| L3 | 92 [21.3] | 31 [20.4] | |
| L4 | 3 [0.7] | 8 [5.3%] | |
| Concomitant upper GI disease at diagnosis, n [%] | 39 [9] | 14 [9.2] | 1.000 |
| Disease behaviour at diagnosis, n [%] | 0.965 | ||
| B1 | 325 [75.2] | 115 [75.7] | |
| B2 | 67 [15.5] | 24 [15.8] | |
| B3 | 40 [9.3] | 13 [8.6] | |
| Perianal disease at diagnosis, n [%] | 43 [10] | 6 [3.9] | 0.026 |
Phenotype according to Montreal classification. Disease location of CD was defined as ileal involvement [L1], exclusive colonic involvement [L2], ileocolonic involvement [L3], or isolated upper gastrointestinal disease [L4]. Disease behaviour of CD was defined as non-stricturing/non-penetrating [B1], stricturing [B2], or penetrating [B3].
CD, Crohn’s disease; IBDSL, Inflammatory Bowel Disease South Limburg; GI, gastrointestinal; n, number of patients; SD, standard deviation; NA, not available; FU, follow-up; LTFU, lost-to-follow-up.
The distribution of patients over the six disease activity patterns is presented in Figure 1. In summary, patients were classified to either the ‘Active to remission’ [n = 110; 25.5%], ‘Remission to active’ [n = 14; 3.2%], ‘Chronic continuous’ [n = 25; 5.8%], ‘Moderate-severe chronic intermittent’ [n = 120; 27.8%], ‘Mild chronic intermittent’ [n = 41; 9.5%], and ‘Quiescent’ [n = 122; 28.2%] pattern. CD diagnosis was based on histopathological findings in 87.0% of the patients. Other baseline characteristics corresponding with all patterns are presented in Table 2. Of note, patients with the ‘Quiescent’ pattern were diagnosed most frequently in the first era [70.5%]. Group sizes are too small to analyse baseline characteristics in further detail. Disease outcome after 10 years of follow-up is presented in Table 3 for all patterns. Patients with a ‘Quiescent’ pattern experienced only 1.49 yearly quarters of disease activity. Disease progression from inflammatory to stricturing [4.2%] or penetrating disease [2.1%] in this group was rare. Notably, 89.8% of the patients with this pattern never received any immunomodulator or biologic. Also, even though the percentage of patients with surgery at diagnosis was the highest in patients with a ‘Quiescent’ pattern [23.8%], the mean number of surgical procedures after 10 years of follow-up [0.21; SD 0.41] is the lowest of all groups. It can also be appreciated from Table 3 that the total number of colorectal carcinomas [n = 1] and extra-intestinal malignancies [n = 20] in all groups was low during the first 10 years of follow-up.
Table 2.
Baseline characteristics of CD patients for each of the six patterns of disease activity.
| Active to remission [n = 110] | Remission to active [n = 14] | Chronic continuous [n = 25] | Moderate-severe chronic intermittent [n = 120] | Mild chronic intermittent [n = 41] | Quiescent [n = 122] | |
|---|---|---|---|---|---|---|
| Age at diagnosis, mean [SD] | 34.5 [13.7] | 32.5 [8.6] | 29.0 [12.3] | 30.8 [11.3] | 35.8 [15.0] | 37.6 [14.9] |
| Male, n [%] | 38 [34.5] | 10 [71.4] | 9 [36.0] | 37 [30.8] | 13 [31.7] | 46 [37.7] |
| Era of diagnosis, n [%] | ||||||
| 1991–1998 | 54 [49.1] | 11 [78.6] | 14 [56.0] | 61 [50.8] | 17 [41.5] | 86 [70.5] |
| 1999–2005 | 56 [50.9] | 3 [21.4] | 11 [44.0] | 59 [49.2] | 24 [58.5] | 36 [29.5] |
| Smoking at diagnosis, n [%] | 60 [54.5] | 9 [64.3] | 15 [60.0] | 73 [60.8] | 19 [46.3] | 43 [35.2] |
| NA: 8 [7.3] | NA: 0 [0.0] | NA: 0 [0.0] | NA: 7 [5.8] | NA: 3 [7.3] | NA: 20 [16.4] | |
| Disease location at diagnosis, n [%] | ||||||
| L1 | 46 [41.8] | 10 [71.4] | 12 [48.0] | 46 [38.7] | 21 [51.2] | 75 [61.5] |
| L2 | 37 [33.6] | 1 [7.1] | 8 [32.0] | 35 [29.4] | 14 [34.1] | 32 [26.2] |
| L3 | 27 [24.5] | 2 [14.3] | 5 [20.0] | 38 [31.7] | 6 [14.6] | 14 [11.5] |
| L4 | 0 [0] | 1 [7.1] | 0 [0.0] | 1 [0.8] | 0 [0] | 1 [0.8] |
| Concomitant upper GI disease at diagnosis, n [%] | 13 [11.8] | 3 [21.4] | 1 [4.0] | 10 [8.3] | 7 [17.1] | 5 [4.1] |
| Disease behaviour at diagnosis, n [%] | ||||||
| B1 | 76 [69.1] | 10 [71.4] | 20 [80.0] | 93 [77.5] | 30 [73.2] | 96 [78.7] |
| B2 | 17 [15.5] | 1 [7.1] | 4 [16.0] | 21 [17.5] | 6 [14.6] | 18 [14.8] |
| B3 | 17 [15.5] | 3 [21.4] | 1 [4.0] | 6 [5.0] | 5 [12.2] | 8 [6.6] |
| Perianal disease at diagnosis, n [%] | 13 [11.8] | 2 [14.3] | 2 [8.0] | 11 [9.2] | 6 [14.6] | 9 [7.4] |
| Surgery at diagnosis, n [%] | 8 [7.3] | 3 [21.4] | 2 [8.0] | 6 [5.0] | 2 [4.9] | 29 [23.8] |
Phenotype according to Montreal classification. Disease location of CD was defined as ileal involvement [L1], exclusive colonic involvement [L2], ileocolonic involvement [L3], or isolated upper gastrointestinal disease [L4]. Disease behaviour of CD was defined as non-stricturing/non-penetrating [B1], stricturing [B2], or penetrating [B3].
CD, Crohn’s disease; GI, gastrointestinal; n, number of patients; SD, standard deviation; NA, not available.
Table 3.
Disease outcome after 10 years of follow-up for each of the six patterns of disease activity.
| Active to remission [n = 110] | Remission to active [n = 14] | Chronic continuous [n = 25] | Moderate-severe chronic intermittent [n = 120] | Mild chronic intermittent [n = 41] | Quiescent [n = 122] | |
|---|---|---|---|---|---|---|
| Number of yearly quarters with disease activity, mean [SD] | 4.77 [1.63] | 4.79 [1.97] | 14.48 [3.04] | 8.74 [2.52] | 4.02 [0.82] | 1.49 [0.50] |
| Disease progression, inflammatory [B1] to stricturing [B2], n [%] | 16 [21.1] | 4 [40.0] | 10 [50.0] | 26 [28.0] | 2 [6.7] | 4 [4.2] |
| Disease progression, inflammatory [B1] to penetrating [B3], n [%] | 5 [6.6] | 0 [0.0] | 3 [15.0] | 8 [8.6] | 1 [3.3] | 2 [2.1] |
| Stricturing disease [B2], n [%] | 42 [38.2] | 8 [57.1] | 15 [60.0] | 53 [44.2] | 11 [26.8] | 26 [21.3] |
| Penetrating disease [B3], n [%] | 25 [22.7] | 3 [21.4] | 4 [16.0] | 17 [14.2] | 6 [14.6] | 10 [8.2] |
| Need for systemic corticosteroids, n [%] | 84 [76.4] | 7 [50.0] | 23 [92.0] | 108 [90.0] | 20 [48.8] | 28 [23.0] |
| Need for immunomodulators, n [%] | 71 [64.5] | 12 [85.7] | 25 [100] | 104 [86.7] | 21 [51.2] | 12 [9.8] |
| Need for biologics, n [%] | 15 [13.6] | 2 [14.3] | 16 [64.0] | 57 [47.5] | 11 [26.8] | 3 [2.5] |
| Number of hospitalisations, mean [SD] | 1.27 [1.53] | 1.14 [0.95] | 2.72 [2.26] | 1.67 [1.91] | 0.56 [0.84] | 0.33 [0.54] |
| Number of surgical procedures, mean [SD] | 0.50 [0.63] | 0.79 [0.89] | 1.00 [1.00] | 0.53 [0.69] | 0.29 [0.60] | 0.21 [0.41] |
| Development of CRC, n [%] | 0 [0.0] | 0 [0.0] | 0 [0.0] | 0 [0.0] | 0 [0.0] | 1 [0.8] |
| Development of extra-intestinal malignancy, n [%] | 5 [4.5] | 1 [7.1] | 1 [4.0] | 4 [3.3] | 2 [4.9] | 7 [5.7] |
Phenotype according to Montreal classification. Disease behaviour of CD was defined as non-stricturing/non-penetrating [B1], stricturing [B2], or penetrating [B3].
SD, standard deviation; CRC, colorectal cancer.
Results from the multivariable logistic regression analysis using all baseline variables are presented in Table 4. The ‘Quiescent’ pattern was compared with both the combination of Most severe patterns [ie, combination of ‘Chronic continuous’ and ‘Moderate-severe chronic intermittent’] as well as the combination of all other patterns. Significant positive associations were observed with surgery at diagnosis and an older age at diagnosis, whereas significant inverse associations were observed with an ileocolonic [L3] disease location and with smoking at diagnosis. When comparing the ‘Most severe’ patterns combined with the other patterns, only a younger age and active smoking at diagnosis were significantly associated.
Table 4.
Results from multivariable logistic regression analysis at baseline.
| Quiescent vs most severe, OR [95% CI] | p-value | Quiescent vs all other patients, OR [95% CI] | p-value | Most severe vs all other patients, OR [95% CI] | p-value | |
|---|---|---|---|---|---|---|
| Age at diagnosis, years | 1.03 [1.01–1.06] | 0.010* | 1.02 [1–1.04] | 0.093 | 0.97 [0.95–0.99] | 0.003* |
| Montreal location | ||||||
| L1 | REF | REF | REF | |||
| L2 | 0.79 [0.39–1.60] | 0.508 | 0.71 [0.39–1.30] | 0.268 | 1.04 [0.60–1.81] | 0.900 |
| L3 | 0.44 [0.19–1.00] | 0.050* | 0.44 [0.21–0.92] | 0.029* | 1.54 [0.86–2.74] | 0.144 |
| Montreal behaviour | ||||||
| B1 | REF | REF | REF | |||
| B2 | 0.70 [0.31–1.56] | 0.383 | 0.78 [0.39–1.57] | 0.487 | 1.16 [0.64–2.11] | 0.629 |
| B3 | 0.96 [0.26–3.46] | 0.944 | 0.44 [0.17–1.16] | 0.096 | 0.39 [0.15–1.02] | 0.054 |
| Perianal disease | 0.91 [0.35–2.36] | 0.912 | 0.77 [0.34–1.74] | 0.533 | 0.72 [0.35–1.46] | 0.357 |
| Upper GI involvement | 0.44 [0.12–1.64] | 0.221 | 0.33 [0.11–1.02] | 0.540 | 0.77 [0.34–1.72] | 0.517 |
| Gender, male | 1.04 [0.56–1.91] | 0.910 | 0.88 [0.52–1.48] | 0.625 | 0.97 [0.61–1.56] | 0.906 |
| Smoking at diagnosis | 0.43 [0.24–0.76] | 0.004* | 0.44 [0.27–0.73] | 0.001* | 1.63 [1.05–2.55] | 0.031* |
| Surgery at diagnosis | 2.99 [1.07–8.34] | 0.036* | 2.93 [1.34–6.39] | 0.007* | 0.52 [0.21–1.30] | 0.160 |
Phenotype according to Montreal classification, disease location was defined as ileal involvement [L1], exclusive colonic involvement [L2] or ileocolonic involvement [L3]. Disease behaviour was defined as non-stricturing/non-penetrating [B1], stricturing [B2], or penetrating [B3].
GI, gastrointestinal; OR, odds ratio; 95% CI, 95% confidence interval; REF, reference category.
*p-value below 0.05
Results from the multivariable logistic regression analysis using outcome variables after 6 months of follow-up (adjusted for [nearly] significant baseline variables) are presented in Table 5. From all the variables included, only the absence of the need for systemic corticosteroids was strongly associated with the ‘Quiescent’ pattern. No associations were found with early disease progression, need for immunomodulators or biologics, or the need for hospitalisation or surgery in the first 6 months after diagnosis.
Table 5.
Results from multivariable logistic regression analysis with outcome variables after 6 months of follow-up.
| Quiescent vs most severe, OR [95% CI] | p-value | Quiescent vs all other patients, OR [95% CI] | p-value | Most severe vs all other patients, OR [95% CI] | p-value | |
|---|---|---|---|---|---|---|
| Disease progression, inflammatory [B1] to stricturing [B2] | 0.09 [0.01–1.67] | 0.107 | 0.18 [0.02–1.84] | 0.148 | 1.33 [0.22–8.12] | 0.761 |
| Disease progression, inflammatory [B1] to penetrating [B3] | NA | NA | NA | |||
| Need for systemic corticosteroids | 0.24 [0.11–0.52] | 0.000* | 0.29 [0.14–0.57] | 0.000* | 1.98 [1.16–3.35] | 0.012* |
| Need for immunomodulators | 0.90 [0.29–2.78] | 0.858 | 0.84 [0.31–2.28] | 0.731 | 0.76 [0.37–1.55] | 0.445 |
| Need for biologics | 0.39 [0.03–5.14] | 0.473 | 0.62 [0.06–7.00] | 0.702 | 4.43 [0.66–29.7] | 0.126 |
| Need for hospitalisation | 1.20 [0.53–2.76] | 0.661 | 1.18 [0.59–2.37] | 0.639 | 0.98 [0.53–1.78] | 0.936 |
| Need for surgery [excluding at baseline] | 2.02 [0.59–6.94] | 0.266 | 1.83 [0.66–5.07] | 0.246 | 0.73 [0.28–1.89] | 0.512 |
Adjusted for age, disease location/behaviour according to the Montreal classification, smoking, and surgery at diagnosis. Phenotype according to Montreal classification, disease behaviour defined as non-stricturing/non-penetrating [B1], stricturing [B2], or penetrating [B3].
OR, odds ratio; 95% CI, 95% confidence interval; NA, not applicable.
*p-value below 0.05.
Results from the PLS-DA prediction models are presented in Table 6. Predictive power using the Montreal variables alone was moderate for all models [AUROC 0.68–0.72]. Adding other baseline variables and variables collected during the first 6 months of follow-up generated a slight improvement of the models, with an increase in AUROC ranging from 0.02 [Most severe vs all other patients] to 0.07 [Quiescent vs Most severe].
Table 6.
Results from PLS discriminant analysis for prediction of quiescent and severe patterns.
| Quiescent vs most severe | Quiescent vs all other patients | Most severe vs all other patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | AUROC | p-value | Sens | Spec | AUROC | p-value | Sens | Spec | AUROC | p-value | |
| Montreal classificationa | 0.678 | 0.638 | 0.715 | <0.001 | 0.678 | 0.594 | 0.697 | <0.001 | 0.610 | 0.656 | 0.683 | <0.001 |
| All baseline variablesb | 0.617 | 0.766 | 0.770 | <0.001 | 0.593 | 0.722 | 0.722 | <0.001 | 0.553 | 0.710 | 0.687 | <0.001 |
| Baseline variables and 6 month follow-up combinedc | 0.677 | 0.755 | 0.793 | <0.001 | 0.634 | 0.754 | 0.779 | <0.001 | 0.589 | 0.679 | 0.701 | <0.001 |
PLS, partial least square; Sens, sensitivity; Spec, specificity; AUROC, area under the receiver operating characteristic curve.
aAge, disease localisation, disease behaviour, perianal disease, upper gastrointestinal involvement.
bMontreal classification and gender, smoking status, and surgery at diagnosis.
cAll baseline variables and disease progression, need for resective surgery, need for prednisone, need for immunomodulators, need for biologics, and need for hospitalisation within the first 6 months after diagnosis.
4. Discussion
We defined disease course in CD patients in a real-life cohort based on analyses of disease activity in the first 10 years after diagnosis. Remarkably, 28.2% of the patients were classified to a quiescent pattern. Older age at diagnosis and surgery at baseline were positively associated with this pattern, whereas inverse associations were observed for ileocolonic disease location at diagnosis, active smoking at diagnosis, and treatment with steroids in the first 6 months. Predictive modelling showed that, despite the associations found, the disease course can be onlymoderately predicted using clinical parameters.
This is the first study assessing disease activity patterns in CD in a more recent era. We classified CD patients as ‘Active to remission’ [25.5%], ‘Remission to active’ [3.2%], ‘Chronic continuous’ [5.8%], ‘Moderate-severe chronic intermittent’ [27.8%], ‘Mild chronic intermittent’ [9.5%], or ‘Quiescent’ [28.2%]. The classification based on disease activity patterns of CD patients described in our study partly overlap with the limited number of previous studies, and underline the heterogeneity of the CD population. The first cohort in which distinctive patterns were described was the Olmsted cohort [1943–1982].10 They reported three different patterns, namely unremitting [12.6%], chronic intermittent [72.8%], and ‘cured’ [9.7%]; it was however unclear how these groups were defined. As previously described, Solberg et al. performed a survey-based study [IBSEN-cohort; 1990–2004] in which up to 51% of the patients experienced a chronic continuous or chronic intermittent activity pattern. Also, 10% of the patients did not relapse during 10 years of follow-up.11 Jess et al. [Denmark; 1962–2005] found that 24.5% of the patients experienced at least one flare every year [ie, chronic continuous] and 18.4% experienced no flares at all during a 5 year follow-up.9 In the past decade, no further studies have been performed.
The largest proportion of patients [28.2%] were classified to the ‘Quiescent’ pattern and experienced only 1.49 yearly quarters of disease activity on average during the total follow-up of 10 years. The majority of patients with this pattern [89.9%] were never exposed to immunosuppressives nor biologics, though 23.8% of the patients underwent surgery at diagnosis. A lower proportion of patients with a quiescent course was found in two other cohorts. As stated, 9.7% of the patients in the Olmsted cohort were considered to be ‘cured’ for 10 years [ie, no recurrence after resection or no disease activity on imaging for at least 10 years without disease progression] and 10% of the patients in the IBSEN cohort did not relapse during 10 years of follow-up. The EC-IBD European inception cohort assessed relapse-free survival after diagnosis and reported that 26.8% of the patients did not have any surgical or non-surgical recurrence within 10 years, which is in line with our results.19 Differences in definitions complicate direct comparisons, but most studies only used surgery and treatment adjustments as markers of disease activity.9,11,12,19 Since we also included imaging, endoscopy, and hospitalisations, our definition is more extensive and therefore the proportion of quiescent patients might even have been larger if less outcome measures were used. Remarkably, patients with the ‘Quiescent’ pattern were more often [70.5%] diagnosed in the first era [ie, 1991–1998]. Although the number of patients is too small to further analyse this finding, it may indicate that despite the introduction of biologics, disease activity patterns did not improve. This is supported by the observed lack of improvement of disease progression rates over time in other studies.1 Some may hypothesise that these quiescent patients may experience low-grade inflammation and therefore are at risk for disease progression, though we observed very low progression rates [6.3%] after 10 years of follow-up. Given the delicate risk/benefit balance of immunosuppressive therapy, especially in older patients, and the increasing exposure to biologics and immunosuppressives early after diagnosis, identification of this quiescent group at diagnosis is important to prevent overtreatment. However, we also found a ‘Remission to active’ pattern. Although this is a small proportion of our cohort, this indicates that some patients still relapse after a longer period of sustained remission. Continued monitoring therefore remains essential, also in patients with a predicted beneficial long-term outcome.
With regard to the Montreal classification, a higher age at diagnosis was associated with the quiescent pattern, whereas ileocolonic disease location was associated with the severe patterns. In line with our finding, Solberg et al. observed that a young age was associated with a chronic intermittent pattern.11 Other available studies on distinctive disease patterns do not report predictors.9,10 Previous analysis on relapse rates in our own cohort also showed an association between younger age and relapses.12 Ileocolonic disease location has not been linked to flares specifically, but it was found to be associated with disease progression and surgery compared with colonic disease.20–23 Ileal disease has been even more frequently linked to these negative outcomes.1,20–25 Complicated disease behaviour at diagnosis was not associated with either ‘Severe’ or ‘Quiescent’ disease in our study, although this has been linked to surgery, hospitalisations, and disabling disease as a composite score previously.1,7,22,23,25–28 This can be explained by the fact that most hospitalisations and surgical resections are a direct consequence of a complicated disease behaviour. Perianal disease at diagnosis was also not associated with any of the patterns, although this has been linked to disease progression, hospitalisations, and disabling disease previously.7,20,23,28 These contradictory results support our hypothesis that the current classification of patients, based upon the Montreal consensus alone, may not be sufficient for the classification of patients at diagnosis.
Smoking at diagnosis and the use of systemic corticosteroids in the first 6 months were associated with the ‘Most severe’ patterns and were negatively associated with the ‘Quiescent’ course in all analyses. Smoking has been widely accepted as a risk factor for negative outcomes in CD, and the association with disease activity was confirmed by a recent meta-analysis.29 However, Solberg et al. observed no differences in smoking status between their disease activity patterns.11 This may be due to differences in the definition of disease activity patterns. As in our study, the association between early corticosteroid use and disease activity was observed in their study.11 A limited number of population-based studies analysed the association of early prednisone use and subsequent negative outcomes. An association was seen with surgery24 and a ‘disabling disease’,30 but not with hospitalisations28 or disease progression.21,25 The association between early steroid use and subsequent disease activity or flares has not been reported by any population-based cohort study before.
Notably, surgery at baseline was strongly associated with the ‘Quiescent’ disease activity pattern. Most of these surgical procedures were ileocaecal resections [79.3%] and, of these, most were performed due to luminal inflammation without fistula or stenosis [31.0%] or as diagnostic procedure [48.0%]. Very limited population-based evidence on this topic is available, but our findings are supported by Golovics et al. who studied disease outcome after early resective surgery in Hungary. They observed significantly lower prednisone exposure, lower biologic use, and lower steroid dependency rates after a long period of follow-up.31 However, since a considerable proportion of our patients with surgery at baseline were classified to other patterns [42.0%], our findings should be interpreted with care. In particular, postoperative recurrence rates in an older population-based cohort were as high as 33% and 44% within 5 and 10 years after surgery, respectively, and a more recent meta-analysis reported that 24.2% and 35% of the patients require second surgery within 5 and 10 years, respectively.32,33 However, a retrospective study showed important differences between early and late surgery, by means of clinical recurrence in favour of early surgery.34 Another retrospective study comparing CD diagnoses during surgery for acute abdominal complaints, with CD diagnoses by conventional diagnostics, showed decreased use of steroids and immunosuppressants in the first group.35 Of note, the LIR!C trial recently showed that, in a highly selected population with pre-treated patients, surgery is a reasonable alternative for biologic therapy in consideration of recurrence rates, even in the long term.36,37 Thus early surgery may be beneficial in selected patient groups and should not be considered as a marker of a severe disease course by definition, despite the bias by indication in observational cohorts.
Using the Montreal variables alone, a limited predictive value for distinguishing the ‘Quiescent’ and the ’Most severe’ patterns from the other groups in our PLS discriminant analysis was found. If we combine the Montreal classification variables with the other baseline variables and with the variables collected during the first 6 months of follow-up in our prediction models, the AUROC remains stable for the prediction of the ‘Most severe’ patterns and increases by only 0.10 for the prediction of the ‘Quiescent’ pattern. Although prediction of patterns is essential in order to stratify patients at diagnosis, this seems not possible using available clinical parameters. Analysis of big data including baseline pathology, genetics, gut microbiota, and other molecular markers, would be of interest to improve the predictive models.
The major strength of this study is the real-world design with high case certainty, long-term follow-up, and a relatively large sample size. Hereby, we were able to define solid patterns which are likely to be representative for other IBD populations as well. Also, due to our prospective registration of newly diagnosed patients and extensive case report forms, reliable and detailed clinical characteristics and data on follow-up were available to use in our models. Another strength of our study is the well-defined criteria for disease activity. As noted, disease activity is mostly defined by surgical or non-surgical interventions, and we also include imaging, endoscopy, and hospitalisations.
Some limitations need to be addressed. Most importantly, a relatively large proportion of patients were considered LTFU due to the lack of outpatient clinic visits exceeding the 10 years of follow-up, as described earlier in the discussion. Patients in long-term remission without treatment only visit the hospital once a year or even less. This may lead to an under-representation of quiescent patients in our study, which is supported by the fact that out of the 152 patients within this LTFU group, 95 had a follow-up longer than 5 years and they experienced 2.88 active quarters during this follow-up, compared with 3.54 active quarters in the first 5 years of the included patients [p = 0.015]. Including these patients would possibly lead to bias, since this would be based on the assumption that no event has occurred, and most probably will not change our conclusion. Next, we were not able to include biochemical parameters [eg, calprotectin] in our definition for disease activity since these parameters were not available at the start of data collection. It should also be noted that in some population-based cohorts, patients may be misclassified and subsequently add to the number of ‘quiescent’ patients. However, our IBDSL cohort has stringent inclusion criteria at time of diagnosis, which limits the inclusion of non-IBD cases. Moreover, most of the patients with the ‘Quiescent’ pattern have histologically proven CD [82.0%] and, when only histologically proven CD cases are included in the analysis, still 26.6% of all patients are classified to the ‘Quiescent’ pattern. Furthermore, 70.5% of the patients had more than one endoscopic or radiological examination during follow-up, and all patients are considered CD cases for at least 10 years by gastroenterologists. Altogether, we believe it is safe to assume that the patients included in this study are true CD cases. As a final limitation, some patients may experience disease activity or even disease progression without attending a hospital and therefore may be missed in our analysis. However, our follow-up of 10 years captures a very long period and it is highly likely that if a patient develops disease progression due to long-term low-grade inflammation, he or she will show up at the hospital at some point during follow-up, in line with health care organisation in The Netherlands.
In conclusion, in this real-life cohort, we classified CD patients according to disease activity patterns. A substantial proportion of patients were classified to a quiescent disease pattern during the first 10 years after diagnosis, underlining the importance of patient stratification to prevent both undertreatment and overtreatment. Early clinical markers can only moderately predict patients’ disease course. Further studies are warranted to confirm our findings and to identify better [molecular] markers in order to select the optimal treatment strategy for every patient at diagnosis. Alternatively, novel treatment strategies with introduction of the most potent medication or even surgery at diagnosis, in combination with subsequent discontinuation and monitoring, are of interest to prevent delay in patients prone to a severe course and at the same time to protect patients with a favourable outcome from long-term exposure to potentially harmful drugs.
Data sharing statement
A data management plan for the IBDSL is made in collaboration with DataHub MUMC+ [www.portal.datahubmaastricht.nl]. DataHub works according to the FAIR principles. Metadata of IBDSL are available for interested researchers, and data are available after approval of research proposals by the IBDSL scientific committee [contact: m.pierik@mumc.nl].
Funding
This work was supported by the European Union Seventh Framework Programme [FP7/2012–2017] under grant agreement no. 305564, since the IBDSL cohort is involved in the Sysmed-IBD consortium which focuses on the identification and validation of biomarkers.
Conflict of Interest
AM reports grants from Netherlands Organisation for Health Research and Development, grants from Dutch Cancer Society, grants from Will Pharma S.A., grants from Allergan, grants from Grunenthal GmbH, grants from Pentax Europe GmbH, personal fees from Bayer, personal fees from Kyowa Kirin, personal fees from Takeda, all outside the submitted work. VMdS reports grants from European Commission, grants from ZONMW [Dutch national research fund], all outside the submitted work, DJ reports grants from Top Knowledge Institute [Well on Wheat], grants from Horizon 2020 DISCOvERIE, grants from NWO-CCC Partnership program [Carbokinetics], all outside the submitted work. MP reports grants and non-financial support from Falk Pharma, grants from European Commission, grants from ZONMW [Dutch national research fund], grants and non-financial support from Takeda, grants and non-financial support from Johnson and Johnson, grants and non-financial support from Abbvie, non-financial support from Ferring, non-financial support from Immunodiagnostics, non-financial support from MSD, all outside the submitted work. The other authors have nothing to disclose.
Author Contributions
DW, VMdS, and MP conceived of the study. DW, SJ, TH, MR, and LO collected the data. DW, FB, ES, DJ, and MP analysed and interpreted the data. DW and MP drafted the manuscript. FB, ES, DJ, and AM critically reviewed the data and first drafts. All authors critically reviewed and approved the final manuscript.
References
- 1. Jeuring SF, van den Heuvel TR, Liu LY, et al. Improvements in the long-term outcome of Crohn’s disease over the past two decades and the relation to changes in medical management: results from the population-based IBDSL cohort. Am J Gastroenterol 2017;112:325–36. [DOI] [PubMed] [Google Scholar]
- 2. Goldberg R, Irving PM. Toxicity and response to thiopurines in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2015;9:891–900. [DOI] [PubMed] [Google Scholar]
- 3. Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ 2017;357:j2505. [DOI] [PubMed] [Google Scholar]
- 4. van den Heuvel TR, Wintjens DS, Jeuring SF, et al. Inflammatory bowel disease, cancer and medication: cancer risk in the Dutch population-based IBDSL cohort. Int J Cancer 2016;139:1270–80. [DOI] [PubMed] [Google Scholar]
- 5. Gomollón F, Dignass A, Annese V, et al. ; ECCO . Third European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 7. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology 2006;130:650–6. [DOI] [PubMed] [Google Scholar]
- 8. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 9. Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis 2007;13:481–9. [DOI] [PubMed] [Google Scholar]
- 10. Gollop JH, Phillips SF, Melton LJ 3rd, Zinsmeister AR. Epidemiologic aspects of Crohn’s disease: a population based study in Olmsted County, Minnesota, 1943-1982. Gut 1988;29:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solberg IC, Vatn MH, Høie O, et al. ; IBSEN Study Group . Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5:1430–8. [DOI] [PubMed] [Google Scholar]
- 12. Romberg-Camps MJ, Hesselink-van de Kruijs MA, Schouten LJ, et al. Inflammatory bowel disease in South Limburg [The Netherlands] 1991‐2002: incidence, diagnostic delay, and seasonal variations in onset of symptoms. J Crohns Colitis 2009;3:115–24. [DOI] [PubMed] [Google Scholar]
- 13. van den Heuvel TR, Jonkers DM, Jeuring SF, et al. Cohort Profile: The Inflammatory Bowel Disease South Limburg cohort [IBDSL]. Int J Epidemiol 2017;46:e7. [DOI] [PubMed] [Google Scholar]
- 14. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310[]:2191–4. [DOI] [PubMed] [Google Scholar]
- 15. Lim WC, Wang Y, MacDonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev 2016;7:CD008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Filzmoser P, Liebmann B, Varmuza K. Repeated double cross validation. J Chemometrics 2009;23:160–71. [Google Scholar]
- 18. Szymańska E, Saccenti E, Smilde AK, Westerhuis JA. Double-check: validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012;8:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut 2006;55:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype ‐ results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol 2008;103:3082–93. [DOI] [PubMed] [Google Scholar]
- 21. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sjöberg D, Holmström T, Larsson M, et al. Incidence and clinical course of Crohn’s disease during the first year—results from the IBD Cohort of the Uppsala Region [ICURE] of Sweden 2005‐2009. J Crohns Colitis 2014;8:215–22. [DOI] [PubMed] [Google Scholar]
- 23. Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977-2009. Am J Gastroenterol 2012;107:579–88. [DOI] [PubMed] [Google Scholar]
- 24. Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn’s disease in a population-based cohort from Cardiff [1986-2003]: a study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200–6. [DOI] [PubMed] [Google Scholar]
- 25. Ng SC, Zeng Z, Niewiadomski O, et al. Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia. Gastroenterology 2016;150[]:86–95 e3; quiz e13-4. [DOI] [PubMed] [Google Scholar]
- 26. Burisch J, Pedersen N, Cukovic-Cavka S, et al. ; EpiCom Group . Initial disease course and treatment in an inflammatory bowel disease inception cohort in Europe: the ECCO-EpiCom cohort. Inflamm Bowel Dis 2014;20:36–46. [DOI] [PubMed] [Google Scholar]
- 27. Rönnblom A, Holmström T, Karlbom U, Tanghöj H, Thörn M, Sjöberg D. Clinical course of Crohn’s disease during the first 5 years. Results from a population-based cohort in Sweden [ICURE] diagnosed 2005-2009. Scand J Gastroenterol 2017;52:81–6. [DOI] [PubMed] [Google Scholar]
- 28. Golovics PA, Lakatos L, Mandel MD, et al. Prevalence and predictors of hospitalization in Crohn’s disease in a prospective population-based inception cohort from 2000-2012. World J Gastroenterol 2015;21:7272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther 2016;43:549–61. [DOI] [PubMed] [Google Scholar]
- 30. Niewiadomski O, Studd C, Hair C, et al. Prospective population-based cohort of inflammatory bowel disease in the biologics era: Disease course and predictors of severity. J Gastroenterol Hepatol 2015;30:1346–53. [DOI] [PubMed] [Google Scholar]
- 31. Golovics PA, Lakatos L, Nagy A, et al. Is early limited surgery associated with a more benign disease course in Crohn’s disease? World J Gastroenterol 2013;19:7701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol 2014;109:1739–48. [DOI] [PubMed] [Google Scholar]
- 33. Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg 2000;231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aratari A, Papi C, Leandro G, Viscido A, Capurso L, Caprilli R. Early versus late surgery for ileo-caecal Crohn’s disease. Aliment Pharmacol Ther 2007;26:1303–12. [DOI] [PubMed] [Google Scholar]
- 35. Latella G, Cocco A, Angelucci E, et al. Clinical course of Crohn’s disease first diagnosed at surgery for acute abdomen. Dig Liver Dis 2009;41:269–76. [DOI] [PubMed] [Google Scholar]
- 36. Ponsioen CY, de Groof EJ, Eshuis EJ, et al. ; LIR!C study group . Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol 2017;2:785–92. [DOI] [PubMed] [Google Scholar]
- 37. Stevens T, Haasnoot L, D’Haens G, et al. Reduced need for surgery and medical therapy after early ileocaecal resection for Crohn’s disease: long-term follow-up of the LIR!C trial [abstract]. J Crohns Colitis 2020;14:S003‐4. [Google Scholar]