Abstract
Background:
Femoral-sided graft fixation in medial patellofemoral ligament (MPFL) reconstruction is commonly performed using an interference screw (IS). However, the IS method is associated with several clinical disadvantages that may be ameliorated by the use of suture anchors (SAs) for femoral fixation.
Purpose:
To compare the load to failure and stiffness of SAs versus an IS for the femoral fixation of a semitendinosus autograft in MPFL reconstruction.
Study Design:
Controlled laboratory study.
Methods:
Based on a priori power analysis, a total of 6 matched pairs of cadaveric knees were included. Specimens in each pair were randomly assigned to receive either SA or IS fixation. After an appropriate reconstruction procedure, the looped end of the MPFL graft was pulled laterally at a rate of 6 mm/s until construct failure. The best-fit slope of the load-displacement curve was then used to calculate the stiffness (N/mm) in a post hoc fashion. A paired t test was used to compare the mean load to failure and the mean stiffness between groups.
Results:
No significant difference in load to failure was observed between the IS and the SA fixation groups (294.0 ± 61.1 vs 250.0 ± 55.9; P = .352), although the mean stiffness was significantly higher in IS specimens (34.5 ± 9.6 vs 14.7 ± 1.2; P = .004). All IS reconstructions failed by graft pullout from the femoral tunnel, whereas 5 of the 6 SA reconstructions failed by anchor pullout.
Conclusion:
In this biomechanical study using a cadaveric model of MPFL reconstruction, SA femoral fixation was not significantly different from IS fixation in terms of load to failure. The mean load-to-failure values for both reconstruction techniques were greater than the literature-reported values for the native MPFL.
Clinical Relevance:
These results suggest that SAs are a biomechanically viable alternative for femoral-sided graft fixation in MPFL reconstruction.
Keywords: medial patellofemoral ligament, interference screw, suture anchor, femoral fixation, cadaveric, biomechanics
The medial patellofemoral ligament (MPFL) is the primary soft tissue restraint to lateral patellar dislocation.4,6,12 A variety of reconstructive procedures have been described to treat MPFL rupture, but there is a lack of consensus in terms of a gold standard technique for MPFL reconstruction.18,21 Several soft tissue grafts, both autograft and allograft, have been utilized.1,7,34 The fixation of the soft tissue graft to the patella has been performed using a number of different strategies, including transosseous tunnels, suture anchors (SAs), or an interference screws (IS).8,24,28 Multiple prior studies have reported the biomechanical properties of these fixation methods on the patellar side.13,19,22,26,27
Adequate graft fixation to the femur is also critical to preventing the laxity of the reconstructed MPFL. Femoral fixation is commonly performed using an IS placed at the MPFL insertion point defined by Schöttle et al.29 However, the IS method of femoral fixation has some possible disadvantages, including the potential for injury to the distal femoral physis, which can lead to iatrogenic growth alteration and ultimately result in angular growth deformities.15,16,20,30
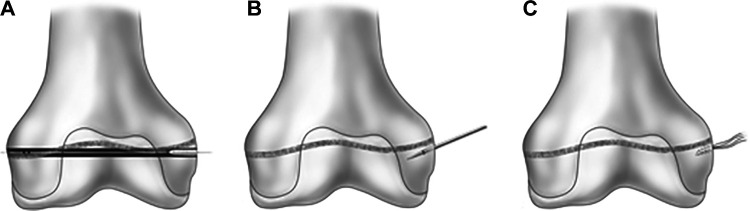
SA femoral fixation of the MPFL graft may be an appealing alternative to the IS method. In contrast to an IS, SAs allow the surgeon to avoid violating the curvilinear distal femoral physis without compromising the appropriate anatomic location of the MPFL femoral attachment (Figure 1). SAs may confer a greater ability to control the tension of the individual graft limbs and do not require reaming of bone tunnels, thereby eliminating the potential for tunnel-related adverse sequelae.
Figure 1.
(A) Insertion of an interference screw over the nitinol guide wire, violating the distal femoral physis. (B) A bone punch angled parallel to the medial aspect of the distal femoral physis. (C) A suture anchor placed anatomically at the MPFL femoral attachment site, avoiding the distal femoral physis.
Although SA fixation has demonstrated utility in patellar fixation, the potential role for an SA fixation method on the femoral side is not well-established. One clinical study reported good preliminary results after MPFL reconstruction with SA fixation on both the patellar and femoral sides,5 but the biomechanical viability of SA femoral fixation remains unclear. Thus, the aim of the present study was to compare the load to failure and stiffness of SAs versus an IS for the femoral fixation of a semitendinosus autograft in MPFL reconstruction. The null hypothesis was that there would be no difference in biomechanical properties between the SA and IS construct for the femoral fixation of an MPFL graft.
Methods
Six fresh-frozen matched pairs of male cadaveric knees (mean age, 52.8 years [range, 44-62 years]) were obtained from donations to the state anatomy board and thawed for 24 hours. A priori power analysis was conducted based on the results of the study by Joyner et al,14 who previously reported the mean load-to-failure values for IS (261.6 ± 67.8 N, 267.1 ± 88.5 N, 191.2 ± 82.7 N) and SA (120 ± 21.6 N) graft fixation in MPFL reconstruction. It was determined that 5 specimens per group would provide a power of 80% to detect a difference (α < .05) of 119.9 N in the mean load to failure. An additional specimen pair was procured to ensure that any unforeseen technical or specimen complications would not affect the sample size required to obtain adequate power.
The cadaveric specimens were assessed for study inclusion by the senior author (R.Y.H.), a fellowship-trained orthopaedic sports medicine surgeon. None of the specimens had a history of osteoporosis, trauma, or procedures to the knee. Specimens in each pair were randomized to SA or IS fixation. Semitendinosus autografts were harvested using the method described by Russ et al.26 The proximal aspect of the tendon graft was debrided of any excess muscle, and the 2 free limbs of each graft were arranged side by side to create a looped configuration. The femur was then isolated and stripped of all soft tissue attachments, with care taken to preserve the periosteum surrounding the medial epicondyle and associated structures. The looped end of the graft was aligned beside the native MPFL to determine the anatomically appropriate graft length. Finally, the native MPFL was carefully divided from its attachment site on the femur.
In SA reconstruction, the anatomic attachment of the MPFL on the femur was identified using fluoroscopy in the manner described by Schöttle et al.29 A 5.5-mm biocomposite SA with 2 embedded No. 2 FiberWire sutures (Arthrex) was inserted at the native MPFL femoral insertion point (Figure 2).
Figure 2.
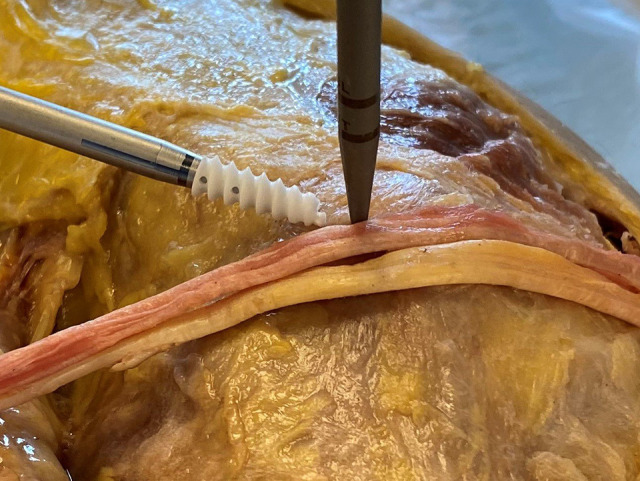
Medial femoral condyle of the left knee, 2-limb hamstring autograft with a bone punch, and a 5.5-mm biocomposite suture anchor at the Schöttle point.
A bone punch and a bone tap were used to create a pilot hole to place the anchor. The anchor was then inserted, and an appropriate purchase was confirmed by applying tension to the sutures. Each limb of the semitendinosus autograft was individually secured to the MPFL femoral insertion point using 3 running locked suture throws placed medially to laterally, and then laterally to medially, in a Krackow configuration (Figure 3). After SA fixation, the graft was trimmed to the appropriate length, and a supplemental figure-of-8 suture was placed posterior to the graft to provide an additional point of backup fixation to the periosteum (Figure 4).
Figure 3.
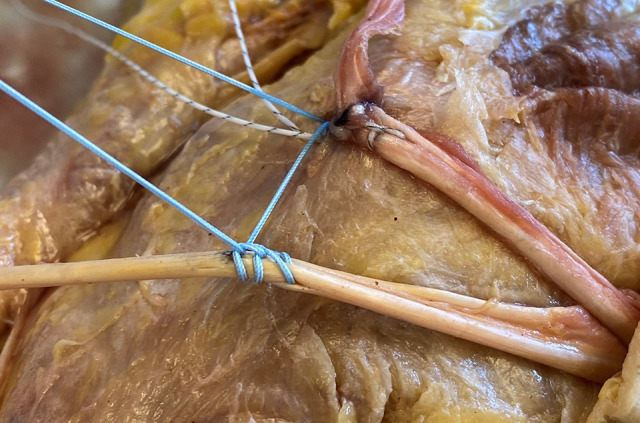
Medial femoral condyle of the left knee, 2-limb hamstring autograft with a 5.5-mm biocomposite suture anchor, with the proximal limb fixed and the distal limb not yet fixed.
Figure 4.
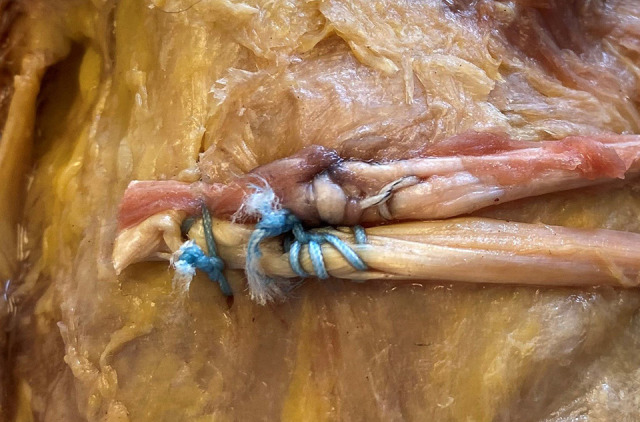
Medial femoral condyle of the left knee, femoral fixation of 2-limb hamstring autograft, with a 5.5-mm biocomposite suture anchor and a supplemental figure-of-8 suture placed posterior to the suture anchor.
In IS reconstruction, the anatomic femoral insertion of the MPFL was again identified.29 A Beath pin was introduced across the femur, parallel to the joint line and exiting at the lateral epicondyle. A reamer was then used to create a femoral tunnel that extended to the lateral cortex. The free ends of the suture attached to the semitendinosus autograft were placed in the eyelet of the Beath pin, and the graft was reduced into the femoral tunnel. Finally, a 6-mm biocomposite interference screw (Arthrex) was advanced over a nitinol wire to secure the graft within the tunnel (Figure 5).
Figure 5.
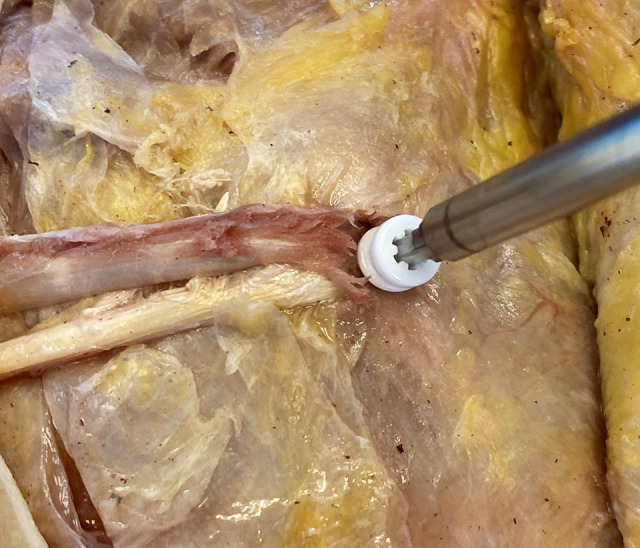
Medial femoral condyle of the right knee, femoral fixation of 2-limb hamstring autograft with a 6-mm biocomposite interference screw.
The femur was secured to the MTS Mini Bionix load frame (MTS Systems) and oriented parallel to the floor, with the looped end of the hamstring graft attached directly to the load cell (Figure 6).
Figure 6.

Biomechanical testing setup with the left femur secured to the MTS Mini Bionix load frame with the looped end of the 2-limb hamstring autograft attached to the load cell.
Each graft was preconditioned from 0 to 30 N for 10 cycles to reduce tissue hysteresis. The graft was then loaded parallel to the joint line to simulate the force vector of a lateral patellar dislocation. The graft was pulled at a rate of 6 mm/s until a sudden decrease in load was observed, which was defined as failure.26 The mode of failure as well as the load-to-failure value were recorded. The mean load to failure was compared between groups using paired t tests. The stiffness of each MPFL reconstruction was calculated in a post hoc fashion using the best-fit slope of the load-displacement curve (N/mm).
Results
Load to failure was not significantly different between the IS and SA femoral fixation groups (Table 1).
Table 1.
Comparison of IS and SA Femoral Fixationa
| Outcome | IS Group | SA Group | P Value |
|---|---|---|---|
| Load to failure (N), | 294.0 ± 61.1 | 250.0 ± 55.9 | .352 |
| Stiffness (N/mm), | 34.5 ± 9.6 | 14.7 ± 1.2 | .004b |
| Failure method | Graft pullout from femoral tunnel: n = 6 (100.0%) | Anchor pullout from femur: n = 5 (83.3%) Sutures torn from anchor: n = 1 (16.7%) |
N/A |
aValues are presented as mean ± SD. IS, interference screw; N/A, not applicable; SA, suture anchors.
bA statistically significant between-group difference was set at P < .05.
The mean stiffness was significantly higher in the IS group compared with the SA group. All IS reconstructions failed by the graft pulling out of the femoral tunnel. Five of the 6 SA reconstructions failed by the anchor pulling out of the femur. The remaining SA construct failed at the anchor-suture interface, with the sutures tearing from the fixation point on the anchor, while the anchor itself remained embedded in the bone.
Discussion
No significant difference in the mean load to failure was observed between the IS and SA femoral fixation methods, although the mean stiffness was higher in the IS group compared with the SA group. These findings may support the use of an SA construct over an IS construct because SAs demonstrate similar biomechanical strength while eliminating many of the clinical disadvantages associated with IS femoral fixation.
In contrast to SA fixation, IS fixation necessitates reaming a large tunnel across the femur, which may create the potential for long-term osteolysis, as has been reported after anterior cruciate ligament reconstructions and distal biceps tendon repairs.3,25,37 Reaming across the distal femur also puts the physis at risk, which is of particular consequence in skeletally immature patients. Previous studies have demonstrated that the MPFL femoral attachment is most commonly located just distal to the medial aspect of the distal femoral physis.11,31–33 Given the concave morphology of the distal femoral physis, a tunnel placed at the anatomic femoral attachment of the MPFL and drilled parallel to the joint line is in danger of violating the physis. A deliberate, nonanatomic tunnel malpositioning to avoid the physis is not an ideal compensatory strategy because tunnels placed more proximally can overload the patellofemoral compartment, while tunnels placed more distally can result in a loose, nonfunctional graft.9,10,35,36 Although it is possible to place an IS at an oblique trajectory that enters through the MPFL femoral attachment site and avoids the curvilinear morphology of the distal femoral physis, an SA fixation may be advantageous because it minimizes the risk of iatrogenic physis injury while avoiding the need to drill a tunnel through the intercondylar notch. Furthermore, SAs may confer a greater ability to control the tension of the individual graft limbs, which may be valuable given the emerging body of evidence, suggesting that double-bundle MPFL reconstruction provides superior outcomes compared with single-bundle reconstruction.18
The present study suggests that SA femoral fixation is biomechanically similar to IS fixation in terms of load to failure. These findings differ from those of Joyner et al,14 who examined the tensile strength of 5 reconstruction techniques and concluded that suspensory cortical fixation to the femur with IS fixation to the patella was the strongest configuration and suspensory cortical femoral fixation with SA patellar fixation was the weakest. In contrast to our reconstruction method, Joyner et al used a gracilis allograft with suspensory cortical fixation on the femoral side and an unspecified size of bioabsorbable SA on the patellar side. None of the 5 MPFL reconstruction techniques in that study included SA fixation on the femoral side. A separate biomechanical study by Russ et al26 investigating patellar graft fixation using SA and IS techniques found that IS fixation to the patella was significantly stronger than SA fixation in both stiffness and load to failure. Our results regarding the greater stiffness of the IS construct on the femoral side are consistent with those of Russ et al on the patellar side. However, we found no difference in the load to failure between IS and SA fixation to the femur. Variations in bony architecture have been shown to have a significant effect on the biomechanical performance of SAs.2 The bony structure of the patella, composed of predominantly cancellous bone, is inherently different from the cortical and cancellous architecture of the medial femoral condyle. The disparity between our load-to-failure results and those of Russ et al may be related to the inferior bony purchase of their patellar anchors compared with our SAs on the femoral side.
Both MPFL fixation constructs in the present study exceeded the load to failure of the native MPFL, suggesting that both an IS and SAs are biomechanically viable options for femoral fixation of the MPFL graft. Two previous studies have reported the tensile strength of the native MPFL to be 208 ± 90 N and 178 ± 46 N, respectively, with all ligament failures occurring by either midsubstance rupture or bony avulsion from the femur.17,23 The stiffness of the native MPFL is unknown, and no consensus exists with regard to the optimal stiffness of a reconstructed MPFL graft.
Finally, there were important differences between IS and SA femoral fixation methods with regard to the mode of failure. All IS specimens failed by graft pullout from the femoral tunnel, whereas 5 SA specimens failed by anchor pullout from the femur. The remaining SA specimen failed by the sutures tearing from their fixation point on the anchor. These differences in failure mechanism were expected, given the mechanical differences between the 2 fixation constructs. Securing the graft with an IS does not entail the direct fixation of the graft to the screw. The graft is placed into a tunnel and held in position by the force of static friction between the screw and surrounding bone. In contrast, SAs have an attachment site between the suture and the anchor itself. Thus, the 2 most likely points of failure are the bone-anchor and anchor-suture interface. The majority (5) of SA specimens (83.3%) failed at the bone-anchor interface, suggesting that our suturing method was sufficiently robust to withstand the laterally directed force applied to the graft and that the weakest point of the overall construct was the bony purchase obtained by the anchor.
Several limitations to our study design can be identified. The cadaveric tissue imperfectly mimics living tissue and represents only a time-zero snapshot that does not account for longitudinal healing potential or biological remodeling. This study did not include the native MPFL as a control group. Although biomechanical data exist in the literature regarding the tensile strength of the native MPFL, methodologic differences between studies may not permit definitive conclusions to be drawn from these comparisons. Additionally, the MPFL grafts in our study were biomechanically loaded in a laterally directed vector parallel to the joint line. Although this method is substantiated by prior biomechanical studies in the literature, it may not accurately represent the constellation of forces to which an MPFL reconstruction is subjected in vivo, particularly given that the patellar attachment of the graft was not present in our cadaveric model. This study exclusively reported load to failure and stiffness. We did not measure other biomechanical parameters, such as cyclic displacement, nor did we perform a comparative cost analysis of the 2 repair techniques. Also, the length of the looped portion of the MPFL graft was determined by the length of the native MPFL before dividing the ligament. Thus, the final working length of the looped graft was not standardized between individual specimens or between the IS and SA specimen groups. This may have confounded our stiffness calculations because the IS fixation technique requires the free limbs of the graft to be buried within a bone tunnel, thereby decreasing the working length of the looped graft in that group of specimens. As a result, the stiffness values reported in the study should be considered to represent only the stiffness of the femoral fixation itself and not the stiffness of the entire MPFL reconstruction construct.
Conclusion
In this biomechanical study, using a cadaveric model of MPFL reconstruction, SA femoral fixation was not significantly different from IS fixation in terms of load to failure. The mean load-to-failure values for both reconstruction techniques were greater than the literature-reported values for the native MPFL. Our results suggest that SAs are a biomechanically viable method of femoral fixation in the setting of MPFL reconstruction.
Acknowledgment
The authors thank Lyn Camire, MA, ELS, for her editorial assistance.
Footnotes
Final revision submitted September 25, 2020; accepted October 23, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: All screws, anchors, and suture material were donated by Arthrex. N.R.D. has received education payments from Arthrex, SeaPearl, and Smith & Nephew. B.G.P. has received royalties from Arthrex, DJO, DARCO, and Zimmer Biomet. R.Y.H. has received education payments and nonconsulting fees from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Avikainen VJ, Nikku RK, Seppanen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation: technique and preliminary results. Clin Orthop. 1993;297:12–16. [PubMed] [Google Scholar]
- 2. Barber FA, Herbert MA, Click JN. The ultimate strength of suture anchors. Arthroscopy. 1995;11(1):21–28. [DOI] [PubMed] [Google Scholar]
- 3. Bhullar R, Habib A, Zhang K, et al. Tunnel osteolysis post-ACL reconstruction: a systematic review examining select diagnostic modalities, treatment options, and rehabilitation protocols. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):524–533. [DOI] [PubMed] [Google Scholar]
- 4. Burks RT, Desio SM, Bachus KN, Tyson L, Springer K. Biomechanical evaluation of lateral patellar dislocations. Am J Knee Surg. 1998;11(1):24–31. [PubMed] [Google Scholar]
- 5. Calanna F, Pulici L, Carimati G, Quaglia A, Volpi P. Medial patello-femoral ligament (MPFL) reconstruction using suture anchors fixation: preliminary results. Muscles Ligaments Tendons J. 2016;6(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conlan T, Garth WP, Jr, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682–693. [DOI] [PubMed] [Google Scholar]
- 7. Deie M, Ochi M, Adachi N, Shibuya H, Nakamae A. Medial patellofemoral ligament reconstruction fixed with a cylindrical bone plug and a grafted semitendinosus tendon at the original femoral site for recurrent patellar dislocation. Am J Sports Med. 2011;39(1):140–145. [DOI] [PubMed] [Google Scholar]
- 8. Drez D, Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298–306. [DOI] [PubMed] [Google Scholar]
- 9. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am. J Sports Med. 2006;34(9):1478–1485. [DOI] [PubMed] [Google Scholar]
- 10. Elias JJ, Kelly MJ, Smith KE, Gall KA, Farr J. Dynamic simulation of the effects of graft fixation errors during medial patellofemoral ligament reconstruction. Orthop J Sports Med. 2016;4(9):2325967116665080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farrow LD, Alentado VJ, Abdulnabi Z, Gilmore A, Liu RW. The relationship of the medial patellofemoral ligament attachment to the distal femoral physis. Am J Sports Med. 2014;42(9):2214–2218. [DOI] [PubMed] [Google Scholar]
- 12. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop. 1998;349:174–182. [DOI] [PubMed] [Google Scholar]
- 13. He W, Yang YM, Liu M, Wang AY, Liu YJ. Reconstruction of the medial patellofemoral ligament using hamstring tendon graft with different methods: a biomechanical study. Chin Med Sci J. 2013;28(4):201–205. [DOI] [PubMed] [Google Scholar]
- 14. Joyner PW, Bruce J, Roth TS, et al. Biomechanical tensile strength analysis for medial patellofemoral ligament reconstruction. Knee. 2017;24(5):965–976. [DOI] [PubMed] [Google Scholar]
- 15. Kocher MS, Saxon HS, Hovis WD, Hawkins RJ. Management and complications of anterior cruciate ligament injuries in skeletally immature patients: survey of the Herodicus Society and The ACL Study Group. J Pediatr Orthop. 2002;22(4):452–457. [PubMed] [Google Scholar]
- 16. Koman JD, Sanders JO. Valgus deformity after reconstruction of the anterior cruciate ligament in a skeletally immature patient: a case report. J Bone Joint Surg Am. 1999;81(5):711–715. [DOI] [PubMed] [Google Scholar]
- 17. LaPrade MD, Kallenbach SL, Aman ZS, et al. Biomechanical evaluation of the medial stabilizers of the patella. Am J Sports Med. 2018;46(7):1575–1582. [DOI] [PubMed] [Google Scholar]
- 18. Lee DY, Park YJ, Song SY, Hwang SC, Park JS, Kang DG. Which technique is better for treating patellar dislocation? A systematic review and meta-analysis. Arthroscopy. 2018;34(11):3082–3093, e3081. [DOI] [PubMed] [Google Scholar]
- 19. Lenschow S, Schliemann B, Gestring J, Herbort M, Schulze M, Kosters C. Medial patellofemoral ligament reconstruction: fixation strength of 5 different techniques for graft fixation at the patella. Arthroscopy. 2013;29(4):766–773. [DOI] [PubMed] [Google Scholar]
- 20. Lipscomb AB, Anderson AF. Tears of the anterior cruciate ligament in adolescents. J Bone Joint Surg Am. 1986;68(1):19–28. [PubMed] [Google Scholar]
- 21. Liu JN, Steinhaus ME, Kalbian IL, et al. Patellar instability management: a survey of the international patellofemoral study group. Am J Sports Med. 2018;46(13):3299–3306. [DOI] [PubMed] [Google Scholar]
- 22. Mehta V, Mandala C, Akhter A. Cyclic testing of 3 medial patellofemoral ligament reconstruction techniques. Orthop J Sports Med. 2017;5(6):2325967117712685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mountney J, Senavongse W, Amis AA, Thomas NP. Tensile strength of the medial patellofemoral ligament before and after repair or reconstruction. J Bone Joint Surg Br. 2005;87(1):36–40. [PubMed] [Google Scholar]
- 24. Panagopoulos A, van Niekerk L, Triantafillopoulos IK. MPFL reconstruction for recurrent patella dislocation: a new surgical technique and a review of the literature. Int J Sports Med. 2008;29(5):359–365. [DOI] [PubMed] [Google Scholar]
- 25. Potapov A, Laflamme YG, Gagnon S, Canet F, Rouleau DM. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J Shoulder Elbow Surg. 2011;20(5):819–826. [DOI] [PubMed] [Google Scholar]
- 26. Russ SD, Tompkins M, Nuckley D, Macalena J. Biomechanical comparison of patellar fixation techniques in medial patellofemoral ligament reconstruction. Am J Sports Med. 2015;43(1):195–199. [DOI] [PubMed] [Google Scholar]
- 27. Russo F, Doan J, Chase DC, Farnsworth CL, Pennock AT. Medial patellofemoral ligament reconstruction: fixation technique biomechanics. J Knee Surg. 2016;29(4):303–309. [DOI] [PubMed] [Google Scholar]
- 28. Schöttle P, Schmeling A, Romero J, Weiler A. Anatomical reconstruction of the medial patellofemoral ligament using a free gracilis autograft. Arch Orthop Trauma Surg. 2009;129(3):305–309. [DOI] [PubMed] [Google Scholar]
- 29. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801–804. [DOI] [PubMed] [Google Scholar]
- 30. Shea KG, Belzer J, Apel PJ, Nilsson K, Grimm NL, Pfeiffer RP. Volumetric injury of the physis during single-bundle anterior cruciate ligament reconstruction in children: a 3-dimensional study using magnetic resonance imaging. Arthroscopy. 2009;25(12):1415–1422. [DOI] [PubMed] [Google Scholar]
- 31. Shea KG, Martinson WD, Cannamela PC, et al. Variation in the medial patellofemoral ligament origin in the skeletally immature knee: an anatomic study. Am J Sports Med. 2018;46(2):363–369. [DOI] [PubMed] [Google Scholar]
- 32. Shea KG, Polousky JD, Jacobs JC, Jr, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2014;34(8):808–813. [DOI] [PubMed] [Google Scholar]
- 33. Sochacki KR, Shea KG, Varshneya K, et al. Relationship of the medial patellofemoral ligament origin on the distal femur to the distal femoral physis: a systematic review. Am J Sports Med. 2021;49(1):261–266. [DOI] [PubMed] [Google Scholar]
- 34. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365–370. [DOI] [PubMed] [Google Scholar]
- 35. Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA. The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med. 2014;42(2):364–372. [DOI] [PubMed] [Google Scholar]
- 36. Stephen JM, Kittl C, Williams A, et al. Effect of medial patellofemoral ligament reconstruction method on patellofemoral contact pressures and kinematics. Am J Sports Med. 2016;44(5):1186–1194. [DOI] [PubMed] [Google Scholar]
- 37. Wilson TC, Kantaras A, Atay A, Johnson DL. Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med. 2004;32(2):543–549. [DOI] [PubMed] [Google Scholar]