Abstract
Background
Methimazole (MMI) is used to treat hyperthyroidism in Graves’ disease. It is rare to encounter patients in whom hyperthyroidism cannot be controlled using high doses of MMI.
Case presentation: A 21-year-old woman was referred to our hospital because of MMI-resistant Graves’ disease. Although her MMI dose had been increased to 120 mg/day, her serum thyroid hormone concentration was too high to be measured. Additional therapy with lithium carbonate, and then with dexamethasone and inorganic iodine, was initiated. After 14 days, the patient’s serum thyroid hormone concentration normalized, while she was taking 150 mg/day MMI, 800 mg/day lithium carbonate, 6 mg/day dexamethasone and 306 mg/day inorganic iodine, and total thyroidectomy was then performed. The patient was discharged 8 days after the thyroidectomy and experienced no major complications.
Conclusions
We have presented a rare case of Graves’ disease that was resistant to high-dose MMI. Combination therapy of MMI with lithium carbonate, dexamethasone and inorganic iodine may represent a therapeutic option for the preoperative preparation of patients with MMI-resistant Graves’ disease.
Keywords: Dexamethasone, inorganic iodine, lithium carbonate, methimazole, Graves’ disease, hyperthyroidism, drug resistance
Background
Graves’ disease is the most common cause of thyrotoxicosis, and the lifetime risk of developing this disease is approximately 3% and 0.5% for women and men, respectively.1 Graves’ disease is caused by autoimmunity targeting thyroid-stimulating hormone (TSH) receptors in the thyroid glands, which induce thyrotoxicosis and a diffuse goitre.1,2 The principal means of managing Graves’ disease is the inhibition of excess thyroid hormone production using anti-thyroid drugs, radioiodine ablation or thyroidectomy.1,2 Among these treatment modalities, an anti-thyroid agent, methimazole (MMI), is the most widely used option for the initial treatment of Graves’ disease, because of its efficacy, convenience and low cost.3 However, thyroidectomy should be considered for patients with large thyroid glands, especially when anti-thyroid agents are not effective or tolerated. However, the patient’s hyperthyroidism should be adequately controlled before they undergo thyroidectomy; otherwise, the risk of thyroid storm during the perioperative period is higher.4,5 Furthermore, no definitive guidelines exist regarding the preoperative management for patients with Graves’ disease with severe hyperthyroidism because of resistance to MMI.
We recently encountered a rare case of Graves’ disease with resistance to extremely high doses of MMI. A previous clinical study showed that a high dose of MMI (120 mg/day) reduces thyroid hormone concentration more rapidly than standard doses of MMI (30 to 40 mg/day) in patients with hyperthyroidism. However, such a high dose of MMI may increase the risks of severe adverse effects, such as skin rashes and agranulocytosis.6 Nevertheless, a case report suggested that 150 mg/day MMI was effective at controlling hyperthyroidism in a patient with Graves’ disease who was resistant to standard doses of MMI.7 Although we carefully increased the doses of MMI (to 120 mg/day) administered to the patient we describe, her serum thyroid hormone concentration remained too high to be measured . However, she became euthyroid when combination therapy of 150 mg/day MMI with lithium carbonate, dexamethasone and inorganic iodine was instituted, and total thyroidectomy was then performed. The patient experienced no major complications and was discharged from the hospital post-operatively.
Case presentation
A 14-year-old girl initially presented with symptoms of general fatigue, palpitations, and excessive sweating. The patient did not have any significant medical history and had never consumed tobacco or alcohol. However, her grandmother had Graves’ disease. The patient was diagnosed as having Graves’ disease on the basis of the following laboratory findings: positivity for TSH receptor autoantibody (TRAb) (first generation assay, 47.8% [normal range, <10%]), high free triiodothyronine (T3) (39.86 pmol/L [normal range, 3.54–6.62 pmol/L]) and free thyroxine (T4) (57.14 pmol/L [normal range, 11.58–21.88 pmol/L]) concentrations, and low TSH concentration (<0.005 μIU/mL [normal range, 0.50–5.00 μIU/mL]). Fifteen mg/day of MMI was administered as the initial treatment. The patient became euthyroid after 3 months and her thyroid hormone concentration remained stable thereafter, while she administered 5 to 10 mg/day MMI. The patient wished to undergo definitive therapy for Graves’ disease, using radioiodine ablation or thyroidectomy, but she was not able to do so at the time because of financial constraints. Therefore, MMI treatment was continued. When the patient was 19 years old, her serum thyroid hormone concentration began to increase, and her attending physician increased the MMI dose to 40 mg/day and recommended the patient to undergo radioiodine ablation or thyroidectomy to control her hyperthyroidism. However, the patient declined the attending physician’s recommendation because the financial constraints remained. When the patient was 21 years old, her hyperthyroidism became uncontrollable, even at a dose of 90 mg/day MMI, which is above the standard dose range.3 She also required hospitalisation for 2 weeks because of severe insomnia. The hospital staff confirmed that the patient took her medication as prescribed during her hospitalisation. However, the patient’s thyroid hormone concentration did not improve. Her TRAb titre had not been assessed since her first measurement. The patient was referred to our hospital for further treatment of her hyperthyroidism.
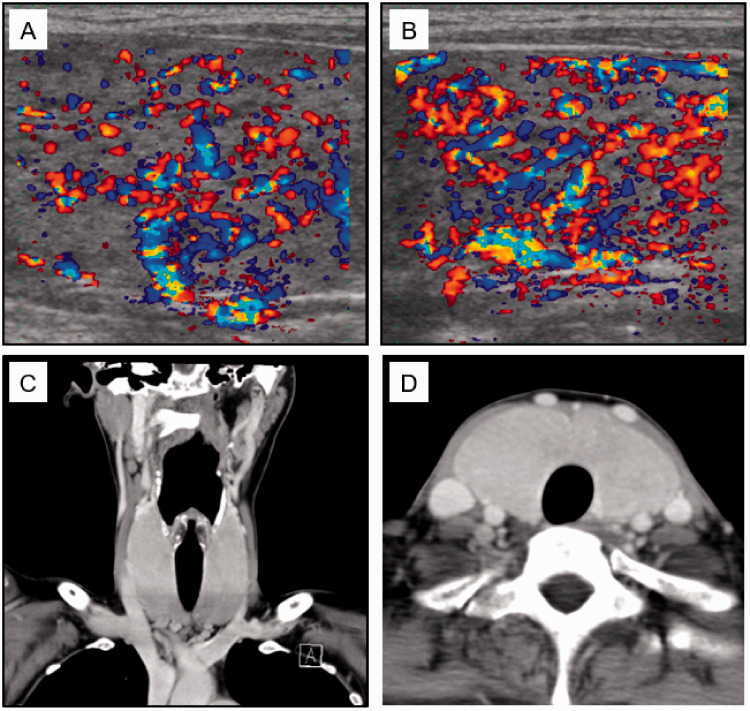
Clinical course: The patient’s height, body mass, and body mass index were 165 cm, 50 kg, and 18.4 kg/m2, respectively. Physical examination revealed that she had characteristic signs of hyperthyroidism, including diffuse large goitre with bruit, tremors, sweating, and sinus tachycardia (106 beats/minute); however, no ophthalmopathy was observed. Blood tests confirmed hyperthyroidism (free T3, 24.78 pmol/L; free T4, 42.99 pmol/L, TSH <0.005 μIU/mL) and there was a moderate TRAb titre (second generation assay, 5.6 IU/L [normal range, <1.0 IU/L]).7 Ultrasound and computed tomography scans showed a large thyroid gland with high blood flow, but no internal nodule was identified (Figure 1). All these findings were compatible with Graves’ disease. We did not consider changing the anti-thyroid therapy from MMI to propylthiouracil (PTU), because MMI is more effective than PTU for the control of hyperthyroidism.8,9
Figure 1.
Images of the ultrasound (A, B) and computed tomography (C, D) scans of the patient’s thyroid glands. A and B: Colour-flow Doppler images of the right and left lobe, respectively. C and D: Coronal and transverse sections, respectively, showing both thyroid lobes and the isthmus. Thyroid gland size was determined on the ultrasonographic images (thickness × width): right lobe, 24.2 × 22.8 mm; left lobe, 23.3 × 22.1 mm; isthmus, 8-mm thickness. The length of each thyroid lobe could not be measured.
First, we evaluated the patient’s drug compliance. We asked the patient’s family to monitor her drug compliance without notifying the patient, and they confirmed that the patient did take her medication and that the number of empty tablet containers matched the prescription. Therefore, we judged that she was resistant to high doses of MMI and that thyroidectomy would be necessary to treat her hyperthyroidism effectively. Next, we cooperated with medical social workers to seek financial support for the patient, and we were able to overcome her financial constraints regarding surgery.
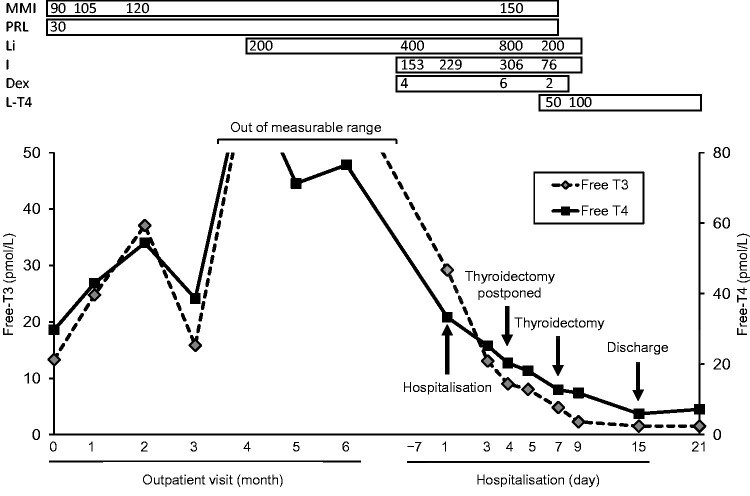
Hyperthyroidism should be adequately controlled before thyroidectomy is performed;4,5 therefore, we treated the patient’s disease with 120 mg/day MMI,7 which is a much higher dose than the standard dose range for MMI,3 but her serum thyroid hormone concentration remained too high to be measured (Figure 2). Therefore, we initiated additional therapy with 200 mg/day lithium carbonate, followed by 800 mg/day lithium carbonate,11 4 mg/day dexamethasone12 and 153 mg/day inorganic iodine.12 However, the patient’s thyroid hormone concentration remained high; therefore, the thyroidectomy was postponed. After further intensifying the drug therapy to 150 mg/day MMI,7 800 mg/day lithium carbonate, 6 mg/day dexamethasone and 306 mg/day inorganic iodine, the patient’s serum thyroid hormone concentration normalized. Therefore, total thyroidectomy was performed 7 days after hospitalisation. The patient’s thyroid gland weighed 86 g, which is approximately six-to-seven times heavier than a normal thyroid gland.13 Histopathological assessment of the excised thyroid gland showed diffuse follicular hyperplasia without autonomous thyroid nodules, which is compatible with Graves’ disease. Hormone replacement therapy with levothyroxine was initiated after the surgery, and the MMI, lithium carbonate, dexamethasone and inorganic iodine were tapered off. No surgical complications developed, except for moderate hypocalcaemia that lasted a few days. The patient was discharged 8 days after thyroidectomy.
Figure 2.
Clinical course of the patient in our hospital. The values shown are in mg/day for MMI, PRL, Li, I and Dex; and μg/day for L-T4.
MMI, methimazole; PRL, propranolol; Li, lithium carbonate, I, inorganic iodine; Dex, dexamethasone; L-T4, levothyroxine.
Discussion
Here, we report a rare case of Graves’ disease that was resistant to an extremely high dose (150 mg/day) of MMI. Some cases of Graves’ disease occur in which high doses of anti-thyroid agents, such as 150 mg/day of MMI or 60 to 90 mg/day of carbimazole (equivalent to 36 to 54 mg/day MMI), are needed to control the hyperthyroidism.7,15–19 However, to the best of our knowledge, this is the first case of Graves’ disease to be reported that showed no response to a very high dose of MMI and in which the patient’s uncontrolled hyperthyroidism was corrected by the addition of lithium carbonate, dexamethasone and inorganic iodine to the therapeutic regimen. For the present case, we had to increase the dose of MMI beyond the standard range to control the patient’s hyperthyroidism prior to thyroidectomy.3 However, a previous clinical study showed that a very high dose of MMI is associated with a higher risk of severe adverse effects.6 In this study, patients with hyperthyroidism were treated with 120 mg/day MMI, and eight of the 25 experienced severe adverse effects. Skin rash was the most common adverse effect, but two patients showed agranulocytosis.6 In addition, other clinical studies have shown that high doses of MMI are associated with higher risks of adverse effects.20–22 Therefore, very high doses of MMI should not be considered to represent a standard treatment option for Graves’ disease; instead, this treatment strategy should only be reserved for the very few patients for whom other treatment options are not available.
MMI inhibits thyroid hormone synthesis by preventing the iodination of tyrosine residues in thyroglobulin by thyroid peroxidase.3 Although some previous cases of Graves’ disease have been reported that involved resistance to standard doses of MMI, the underlying mechanisms of this resistance remain unclear.7,15–19 For several years after the onset of the disease, the patient’s hyperthyroidism was well controlled using relatively low doses of MMI, which excluded the possibility that the resistance to MMI was caused by genetic factors. After rapid and efficient absorption from the intestine,23 MMI is metabolized in the liver24 and is principally excreted through the bile and urine.25 A previous clinical study showed that the peak plasma concentration and half-life of MMI after oral administration are comparable in healthy individuals and patients with hyperthyroidism.23 In addition, the patient reported herein did not show any symptoms or signs of comorbid gastrointestinal or liver disease, which may have affected the pharmacokinetics of MMI. Therefore, it is unlikely that the MMI resistance documented herein was the result of defects in the absorption, metabolism or excretion of MMI. After its absorption, MMI accumulates at high concentrations in the thyroid gland, to levels approximately two-to-five times higher than those in the plasma.26 Therefore, it is probable that the concentration of MMI in the thyroid gland, rather than in the circulation, has the largest impact on its anti-thyroid effect.3 Indeed, in a patient who needed a high dose (150 mg/day) of MMI to control their hyperthyroidism, the concentration of MMI in their thyroid tissue was lower than that in the thyroid of MMI-sensitive patients.7
Given that large thyroids are associated with resistance to MMI in patients with Graves’ disease,27 the substantial thyroid gland hypertrophy may have caused impaired uptake or greater metabolism and excretion of MMI in the present case. Another possibility is atypical iodine intake, which affects the absorption and oxidation of MMI in the thyroid gland.28 Because the Japanese diet has been reported to contain many iodine-rich foods, such as seaweed and seafood,29 high dietary intake of iodine may have affected the sensitivity of the patient to MMI. However, because the plasma and intra-thyroidal MMI concentrations and urinary iodine excretion were not evaluated in the patient, we cannot comment on these possible causes of the MMI resistance in the present case. However, the evaluation of these factors should be considered in future cases of MMI resistance to elucidate the underlying mechanisms. Finally, poor drug compliance should be suspected initially in patients with Graves’ disease whose disease is resistant to MMI. We confirmed that the patient’s drug compliance was high, on the basis of a report from her family. In addition, the hospital staff confirmed that the patient took her medication as prescribed during her hospitalisation for severe insomnia, although the evaluation of drug compliance was not the principal aim of this period of hospitalisation. Furthermore, the patient’s circulating thyroid hormone concentration rapidly decreased after the initiation of lithium carbonate, inorganic iodine and corticosteroid therapy, which suggests that the patient did take these medications. Therefore, low drug compliance was unlikely to be the explanation for the MMI resistance in the present case.
The present patient responded to treatment with lithium carbonate, inorganic iodine and corticosteroid, in addition to MMI. Lithium carbonate and inorganic iodine ameliorate hyperthyroidism by inhibiting the release of thyroid hormone from the thyroid gland,30,31 and corticosteroids principally work by suppressing the conversion of T4 to T3 in peripheral tissues,32 whereas MMI blocks thyroid hormone synthesis within the thyroid gland.3 Therefore, we speculate that these drugs were effective at controlling the hyperthyroidism in the present patient because their mechanisms of action differ to that of MMI. Although lithium carbonate is not recommended as a first-line therapy for Graves’ disease because of a relatively high risk of adverse effects, several clinical studies have shown that lithium carbonate represents an alternative therapy for patients who do not tolerate MMI or PTU.11,24,33 We found that the addition of lithium carbonate (200 mg/day) to basal MMI treatment reduced the thyroid hormone concentration of the present patient. Because anti-thyroid effects of lithium carbonate have been reported at doses of 600 to 900 mg/day,11,33,34 we increased her lithium carbonate dose to 800 mg/day. However, inorganic iodine and corticosteroid were simultaneously administered; therefore, we do not know whether the escalation of the dose of lithium carbonate was the key change in therapy in the present case. Combination therapy of MMI with corticosteroid and inorganic iodine is a strategy that is commonly used for the management of thyroid storm; 8 mg/day dexamethasone and up to 200 mg/day inorganic iodine are recommended in the guidelines for the management of thyroid storm published by the Japan Thyroid Association and the Japan Endocrine Society.12 However, the optimal doses of these drugs for use in MMI-resistant Graves’ disease remain unclear. We found that the combination of inorganic iodine, which was initiated at 153 mg/day and increased to 306 mg/day, and dexamethasone, which was initiated at 4 mg/day and increased to 6 mg/day, normalized the very high thyroid hormone concentration of the patient within 14 days.
In conclusion, we encountered a rare case of Graves’ disease with resistance to an extremely high dose of MMI. We found that combination therapy with lithium carbonate, inorganic iodine and corticosteroid is an effective alternative means of preparing MMI-resistant patients with Graves’ disease for surgery.
Ethics statement
The patient provided her written consent for her treatment publication of the findings of this case study. We have de-identified all the patient’s details in this case report to comply with the CAse REport (CARE) guidelines.14 The approval of the Institutional Review Body was not required because of the nature of this study (a case report).
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: YM received financial support from Boehringer Ingelheim JP Co., Ltd. and Ono Pharmaceutical Co., Ltd.
Author contributions: YM treated the patient, and wrote, revised and finalized the paper. MH, MT, HK, MO, TF, YT, and SY supervised the treatment of the patient, contributed to the discussion and revised the manuscript. All the authors read and approved the final version of the manuscript.
ORCID iD: Yusaku Mori https://orcid.org/0000-0002-1734-0605
References
- 1.Smith TJ, Hegedüs L. Graves’ Disease. N Engl J Med 2016; 375: 1552–1565. [DOI] [PubMed] [Google Scholar]
- 2.Burch HB, Cooper DS. Management of Graves Disease: A Review. JAMA 2015; 314: 2544–2554. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DS. Antithyroid drugs. N Engl J Med 2005; 352: 905–917. [DOI] [PubMed] [Google Scholar]
- 4.Langley RW, Burch HB. Perioperative management of the thyrotoxic patient. Endocrinol Metab Clin North Am 2003; 32: 519–534. [DOI] [PubMed] [Google Scholar]
- 5.Piantanida E. Preoperative management in patients with Graves’ disease. Gland Surg 2017; 6: 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiberg JJ, Nuttall FQ. Methimazole toxicity from high doses. Ann Intern Med 1972; 77: 414–416. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Okuda J, Akamizu T, et al. A hyperthyroid patient with Graves’ disease who was strongly resistant to methimazole: investigation on possible mechanisms of the resistance. Endocr J 1995; 42: 697–704. [DOI] [PubMed] [Google Scholar]
- 8.Hiromatsu Y, Eguchi H, Matsuo Y, et al. Role of a new bioassay for thyroid-stimulating antibodies (aequorin TSAb) in Graves’ ophthalmopathy. Endocr J 2020; 67: 347–352. [DOI] [PubMed] [Google Scholar]
- 9.Nagasaka A, Hidaka H. Effect of antithyroid agents 6-propyl-2-thiouracil and 1-methyl-2-mercaptoimidazole on human thyroid iodine peroxidase. J Clin Endocrinol Metab 1976; 43: 152–158. [DOI] [PubMed] [Google Scholar]
- 10.Okamura K, Ikenoue H, Shiroozu A, et al. Reevaluation of the effects of methylmercaptoimidazole and propylthiouracil in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab 1987; 65: 719–723. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus JH, Richards AR, Addison GM, et al. Treatment of thyrotoxicosis with lithium carbonate. Lancet 1974; 2: 1160–1163. [DOI] [PubMed] [Google Scholar]
- 12.Satoh T, Isozaki O, Suzuki A, et al. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society (First edition). Endocr J 2016; 63: 1025–1064. [DOI] [PubMed] [Google Scholar]
- 13.Berghout A, Wiersinga WM, Smits NJ, et al. Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area. Clin Endocrinol (Oxf) 1987; 26: 273–280. [DOI] [PubMed] [Google Scholar]
- 14.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep 2013; 2013: bcr2013201554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winsa B, Rastad J, Larsson E, et al. Total thyroidectomy in therapy-resistant Graves’ disease. Surgery 1994; 116: 1068–1075. [PubMed] [Google Scholar]
- 16.Jude EB, Dale J, Kumar S, et al. Treatment of thyrotoxicosis resistant to carbimazole with corticosteroids. Postgrad Med J 1996; 72: 489–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey CK, Raza M, Dhiraaj S, et al. Rapid preparation of severe uncontrolled thyrotoxicosis due to Graves’ disease with Iopanoic acid–a case report. Can J Anaesth 2004; 51: 38–40. [DOI] [PubMed] [Google Scholar]
- 18.Sebastián-Ochoa A, Quesada-Charneco M, Fernández-García D, et al. Dramatic response to cholestyramine in a patient with Graves' disease resistant to conventional therapy. Thyroid 2008; 18: 1115–1117. [DOI] [PubMed] [Google Scholar]
- 19.Saleem T, Sheikh A, Masood Q. Resistant thyrotoxicosis in a patient with Graves disease: a case report. J Thyroid Res 2011; 2011: 649084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner MC, Romaldini JH, Bromberg N, et al. Adverse effects related to thionamide drugs and their dose regimen. Am J Med Sci 1989; 297: 216–219. [DOI] [PubMed] [Google Scholar]
- 21.Takata K, Kubota S, Fukata S, et al. Methimazole-induced agranulocytosis in patients with Graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid 2009; 19: 559–563. [DOI] [PubMed] [Google Scholar]
- 22.Wang MT, Lee WJ, Huang TY, et al. Antithyroid drug-related hepatotoxicity in hyperthyroidism patients: a population-based cohort study. Br J Clin Pharmacol 2014; 78: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamura Y, Shigemasa C, Tatsuhara T. Pharmacokinetics of methimazole in normal subjects and hyperthyroid patients. Endocrinol Jpn 1986; 33: 605–615. [DOI] [PubMed] [Google Scholar]
- 24.Heidari R, Niknahad H, Jamshidzadeh A, et al. An overview on the proposed mechanisms of antithyroid drugs-induced liver injury. Adv Pharm Bull 2015; 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark SM, Saade GR, Snodgrass WR, et al . Pharmacokinetics and pharmacotherapy of thionamides in pregnancy. Ther Drug Monit 2006; 28: 477–483. [DOI] [PubMed] [Google Scholar]
- 26.Burch HB, Cooper DS. ANNIVERSARY REVIEW: Antithyroid drug therapy: 70 years later. Eur J Endocrinol 2018; 179: R261–R274. [DOI] [PubMed] [Google Scholar]
- 27.Benker G, Vitti P, Kahaly G, et al. Response to methimazole in Graves’ disease. The European Multicenter Study Group. Clin Endocrinol (Oxf) 1995; 43: 257–263. [DOI] [PubMed] [Google Scholar]
- 28.Marchant B, Papapetrou PD, Alexander WD. Relation between thyroid iodine content and the accumulation and oxidation of [35-S] Methimazole in the rat. Endocrinology 1975; 97: 154–161. [DOI] [PubMed] [Google Scholar]
- 29.Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res 2011; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff J, Chaikoff IL. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology 1949; 45: 504. [DOI] [PubMed] [Google Scholar]
- 31.Berens SC, Bernstein RS, Robbins J, et al. Antithyroid effects of lithium. J Clin Invest 1970; 49: 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chopra IJ, Williams DE, Orgiazzi J, et al. Opposite effects of dexamethasone on serum concentrations of 3,3',5'-triiodothyronine (reverse T3) and 3,3'5-triiodothyronine (T3). J Clin Endocrinol Metab 1975; 41: 911–920. [DOI] [PubMed] [Google Scholar]
- 33.Akin F, Yaylali GF, Bastemir M. The use of lithium carbonate in the preparation for definitive therapy in hyperthyroid patients. Med Princ Pract 2008; 17: 167–170. [DOI] [PubMed] [Google Scholar]
- 34.Zheng R, Liu K, Chen K, et al. Lithium Carbonate in the Treatment of Graves’ Disease with ATD-Induced Hepatic Injury or Leukopenia. Int J Endocrinol 2015; 2015: 694023. [DOI] [PMC free article] [PubMed] [Google Scholar]