Abstract
Objective
To evaluate the clinical value of high mobility group box-1 (HMGB1) expression levels in patients with gastric cancer.
Methods
Articles published from January 2000 to August 2022 were searched using PubMed, Google Scholar and Science Direct, Springer, Wiley and NIH to evaluate the clinicopathological significance of HMGB1 expression in gastric cancer.
Results
A total of 156 publications were selected, of which six studies, comprising 846 patients, met the criteria for inclusion in this study. Forest plots of clinicopathological characteristics indicated that HMGB1 expression was not associated with age (odds ratio (OR) = 1.07, 95% confidence interval (CI): 0.89–1.28), sex (OR = 0.90, 95% CI: 0.81–1.00), TNM (OR = 1.39, 95% CI: 0.82–2.37), N stage (OR = 1.42, 95% CI: 0.97–2.07), or tumor differentiation (OR = 0.96, 95% CI: 0.71–1.29), but was highly correlated with pT stage (OR = 1.56, 95% CI: 1.17–2.07). Funnel plots showed no significant publication bias in the included studies in terms of age, sex, TNM, pT stage, N stage, or tumor differentiation.
Conclusion
HMGB1 expression was significantly correlated with tumor pT stage, but not with age, sex, TNM stage, tumor N stage, tumor differentiation, or lymphatic metastasis in patients with GC.
Keywords: Gastric cancer, HMGB1, tumor stage, pT stage, Forest plot, prognosis
Introduction
Gastric cancer (GC) is one of the most common malignant cancers, accounting for 8% of newly diagnosed cancers and 10% of cancer deaths each year worldwide.1 Its early diagnosis and treatment are generally beneficial in terms of patient prognosis; however, most cases of GC are diagnosed at an advanced stage,2 highlighting the need for new diagnostic and prognostic biomarkers.
High mobility group box-1 (HMGB1) protein belongs to the HMG family of proteins, with two 80-amino acid DNA-binding domains (A-box and B-box) and an acidic carboxyl tail.3 HMGB1 is an inflammation-related protein that plays an important role in many diseases.4 Cytosolic HMGB1 was shown to attenuate tissue injury in inflammatory bowel disease and other complex inflammatory disorders through regulating cell autophagy and apoptosis.5 In addition, extracellular HMGB1 was involved in NLRP3 inflammasome activity and regulated interleukin-1β-associated sterile inflammation induced by multi-walled carbon nanotubes.6 Moreover, HMGB1 is a proinflammatory cytokine that may contribute to many inflammatory diseases, and which has also been reported to play paradoxical roles in promoting both cell survival and cell death by regulating multiple signaling pathways during tumor development.7 Recent studies have also identified a role for HMGB1 in cancers, including GC. Zhao et al.8 found that co-expression of the receptor for advanced glycation end products and HMGB1 was correlated with disease progression and a poor prognosis in patients with prostate cancer, while another study9 found that HMGB1 inhibited the anti-cancer activity of sunitinib by regulating TP53 autophagic degradation. Moreover, HMGB1-knockdown in bladder cancer was associated with a better response to radiotherapy and decreased autophagy.10 Song et al. also showed that HMGB1-knockdown suppressed cell proliferation and invasion and sensitized cells to apoptosis induced by oxaliplatin in MGC-803 gastric cancer cells.11 However, despite these studies, the clinical significance of HMGB1 in GC is still unclear and controversial.
In the present study, we conducted a meta-analysis to investigate the correlation between HMGB1 expression and progression of GC. We examined the correlations between HMGB1 expression and clinicopathological characteristics including tumor pT stage, age, sex, TNM stage, lymph node metastasis, and degree of tumor differentiation, to provide deeper insights into the clinical value of assessing HMGB1 expression in patients with GC.
Materials and Methods
Publication search strategy
We searched the PubMed, Google Scholar, Science Direct, Springer, Wiley, and NIH databases on 7 August 2020 for related studies published from January 2000 to August 2020 on. The following keywords were used: (high mobility group box 1 or HMGB1) and (gastric cancer or gastric carcinoma). The search was restricted to English language studies in humans. We also searched the reference lists of related publications as well as review articles to identify potentially relevant research. We first checked the abstracts and titles of the publications, followed by the full texts of the remaining articles, to confirm if they met the selection criteria. This study followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. The study did not register with PROSPERO, but this will be completed in future studies.
Inclusion and exclusion criteria
The inclusion criteria for the studies were as follows: (1) patients in the studies diagnosed with GC by pathological examination; (2) adequate data to calculate odds ratios (ORs); (3) correlation between HMGB1 expression level and prognosis, clinicopathological features, or patient characteristics analyzed; (4) displaying outcomes in the form of hazard ratios (HRs) with 95% confidence intervals (CI); and (5) full-text of the study available. The exclusion criteria were: (1) reviews, letters, and comments; (2) animal experiments, cancer cell studies, and other laboratory research; (3) articles in language other than English; (4) duplicated articles or data; and (5) insufficient data or information to obtain HRs.
Data extraction
The included studies were first evaluated by two independent investigators using the Newcastle–Ottawa Scale. Studies with a score ≥7 were considered high quality. The following data were extracted from the full text: name of first author, publication year, study period, country of patients, detection method, total number of cases, number of patients with high HMGB1 and low HMGB1, age, sex, TNM stage, tumor differentiation, pT stage, and nodal status.
Statistical analysis
HRs and corresponding 95% CIs were obtained by statistical analysis using Stata 15.0 software (Stata Corporation, College Station, TX, USA). Correlations between HMGB1 expression and clinicopathological features were analyzed. Significant heterogeneity was defined as a Q statistic P value <0.10 or I2 value >50%. A fixed-effects model was used if there was no heterogeneity among the included studies; otherwise, a random-effects model was used. Publication bias was evaluated by funnel plots and Egger’s test. A P value <0.05 was considered statistically significant.
Results
Search results and study characteristics
A total of 156 publications were initially identified. Of these, 35 were excluded due to duplication, and 34 were excluded for not being in English or because the full text was not available. Of the remaining 87 articles, 81 full-text articles were excluded because of a lack of sufficient data, not reporting GC, and missing sensitivity, specificity, accuracy, or correlation values. Six articles including 846 patients were therefore finally included for data extraction.12–17 All the included studies analyzed the clinicopathological value of HMGB1 in GC. The detailed search strategy is shown in Figure 1.
Figure 1.
Flow chart of selection of publications for inclusion in the study.
The six included references were published from 2010 to 2020 (Table 1). The total number of patients with GC was 846 (range 40–414). Five studies were performed in China and one in Egypt. HMGB1 was detected by immunohistochemical staining in four studies and by enzyme-linked immunosorbent assay in one study. Five studies reported the age distribution, sex, TNM, pT stage, and lymph node metastasis, and one study reported the tumor grade.
Table 1.
Clinical and histopathologic data of the patients.
| Study | Publication year | Country | Age | Sex | TNM | pT stage | N stage | Histologic grade | Detection method | Cases (n) | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤60 vs. >60 years | male/female | I + II/III + IV | T1 + T2/T3 + T4 | negative/positive | G3 + G4/G1 + G2 | ||||||
| Bao et al.12 | 2010 | China | H 15/18 | H 26/6 | H 12/20 | H 3/29 | H 11/21 | H 16/16 | IHC | 78 | 6 |
| L 29/16 | L 29/17 | L 23/23 | L 9/37 | L 23/23 | L 24/22 | ||||||
| Fang et al.13 | 2019 | China | H 98/217 | H 207/111 | H 126/167 | H 78/236 | H 102/207 | H 188/123 | N/A | 414 | 6 |
| L 35/59 | L 61/35 | L 46/50 | L 32/64 | L 29/67 | L 56/37 | ||||||
| Zhang et al.14 | 2014 | China | H 11/21 | H 24/8 | H 8/24 | H 4/28 | H 20 | N/A | IHC | 50 | 7 |
| L 5/13 | L 11/7 | L 6/12 | L 3/15 | L 12/6 | |||||||
| Zhang et al.15 | 2014 | China | H 10/27 | H 29/8 | H 13/24 | H 4/33 | H 7/30 | N/A | IHC | 88 | 6 |
| L 16/35 | L 36/15 | L 11/40 | L 7/44 | L 22/29 | |||||||
| He et al.16 | 2013 | China | H 45/36 | H 59/22 | H 17/64 | H 9/72 | H 19/62 | H 56/25 | IHC | 166 | 6 |
| L 39/46 | L 54/31 | L 41/44 | L 25/69 | L 44/41 | L 57/28 | ||||||
| Ghweil et al.17 | 2020 | Egypt | N/A | N/A | N/A | N/A | N/A | N/A | ELISA kits | 50 | 6 |
H: HMGB1 high expression; L: HMGB1 low expression; NOS, Newcastle–Ottawa Scale; N/A: not available; IHC, immunohistochemistry; ELISA, enzyme-linked immunosorbent assay.
Correlations between HMGB1 expression and clinicopathological characteristics
We analyzed the correlations between HMGB1 expression and clinicopathological characteristics in GC patients. HMGB1 expression was not significantly associated with age (OR = 1.07, 95% CI: 0.89–1.28, I2 = 25.8%; Figure 2), sex (OR = 0.90, 95% CI: 0.81–1.00, I2 = 0.00%; Figure 3), TNM (OR = 1.39, 95% CI: 0.82–2.37, P < 0.001, I2 = 79.6%; Figure 4), N stage (OR = 1.42, 95% CI: 0.97–2.07, P = 0.012, I2 = 68.9%; Figure 5), or histologic grade (OR = 0.96, 95% CI: 0.71–1.29, P = 0.022, I2 = 68.8%; Figure 6). However, expression level of HMGB1 was highly correlated with pT stage (OR = 1.56, 95% CI: 1.17–2.07, I2 = 0.0%; Figure 7).
Figure 2.
Forest plot evaluating association of HMGB1 expression with age.
RR, relative risk; CI, confidence interval.
Figure 3.
Forest plot evaluating association of HMGB1 expression with sex.
RR, relative risk; CI, confidence interval.
Figure 4.
Forest plot evaluating association of HMGB1 expression with TNM.
RR, relative risk; CI, confidence interval.
Figure 5.
Forest plot evaluating association of HMGB1 expression with N stage.
RR, relative risk; CI, confidence interval.
Figure 6.
Forest plot evaluating association of HMGB1 expression with histologic grade.
RR, relative risk; CI, confidence interval.
Figure 7.
Forest plot evaluating association of HMGB1 expression with pT stage.
RR, relative risk; CI, confidence interval.
Publication bias
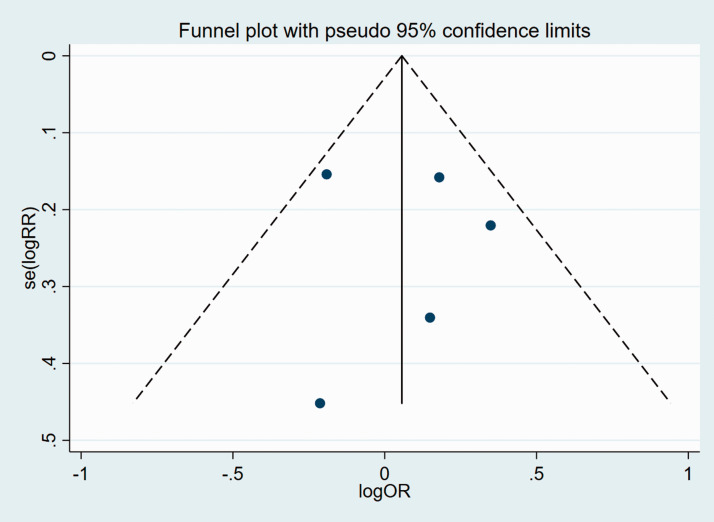
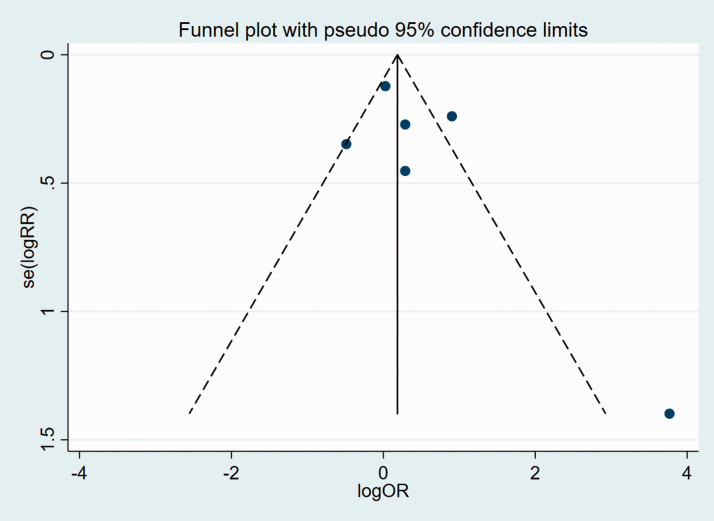
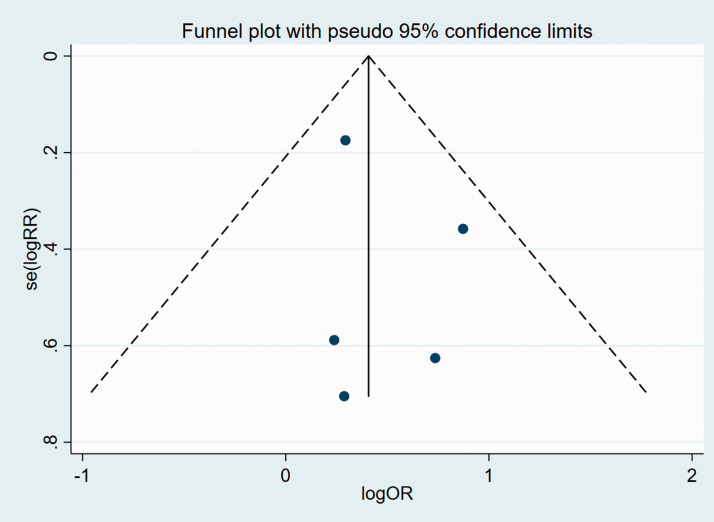
We subsequently analyzed publication bias of the included articles for all the clinicopathological characteristics, using funnel plots and Egger’s test. Funnel plots suggested that there was no significant publication bias in the meta-analysis in terms of age, sex, TNM, pT stage, N stage, or tumor differentiation (Figures 8–13).
Figure 8.
Funnel plot of studies used in the analysis of age.
se, standard error; RR, relative risk; OR, odds ratio.
Figure 9.
Funnel plot of studies used in the analysis of sex.
se, standard error; RR, relative risk; OR, odds ratio.
Figure 10.
Funnel plot of studies used in the analysis of TNM.
se, standard error; RR, relative risk; OR, odds ratio.
Figure 11.
Funnel plot of studies used in the analysis of N stage.
se, standard error; RR, relative risk; OR, odds ratio.
Figure 12.
Funnel plot of studies used in the analysis of histologic grade.
se, standard error; RR, relative risk; OR, odds ratio.
Figure 13.
Funnel plot of studies used in the analysis of pT stage.
se, standard error; RR, relative risk; OR, odds ratio.
Discussion
Despite surgical and medical developments, the prognosis of GC, especially metastatic GC, remains poor.18 A better understanding of the molecular mechanisms underlying GC is therefore necessary. The newly identified inflammation-related factor HMGB1 has recently been shown to play important roles in many diseases, including cancer development19; however, its role in GC is still unclear. In the present study, we conducted a meta-analysis to investigate the correlations between HMGB1 expression and clinical outcomes and prognosis in GC patients, based on six recent references. We demonstrated that HMGB1 was associated with depth of invasion in GC.
The role of HMGB1 in GC has been reported in several studies, both in vitro and in vivo. The release of HMGB1 mediated by autophagy promoted survival of GC cells in vitro.20 Overexpression of extracellular HMGB1 promoted epithelial–mesenchymal transition and increased cell migration/invasion, while HMGB1 knockdown significantly inhibited tumor growth.21 Another in vitro and in vivo study also revealed that HMGB1 knockdown suppressed cell growth and invasion in GC via the nuclear factor-κB pathway.15 In addition to the studies included in the current meta-analysis, Ghweil et al. demonstrated that higher serum levels of both serum amyloid A and HMGB1 reflected advanced tumor stage and higher tumor grade.17 He et al.16 also showed that HMGB1 expression was closely associated with TNM stage, pT stage, nodal status, tumor size, metastasis status, and poor prognosis in patients with GC. Moreover, rs1045411 in HMGB1 was associated with clinical outcomes in GC patients in China.22 However, several studies have found apparently conflicting results. Zhang et al.14 found no association between HMGB1 expression and clinicopathologic features, including TNM stage, metastatic lymph nodes, and overall survival in patients with GC, while another study12 found that overexpression of HMGB1 was positively associated with cancer-free survival in GC, suggesting that HMGB1 overexpression might be a marker of good prognosis. A meta-analysis was therefore required to clarify the role of HMGB1 in GC. The current meta-analysis demonstrated that HMGB1 expression was correlated with pT stage, but not with age, sex, TNM stage, N stage, tumor differentiation, or lymphatic metastasis.
In addition to GC, HMGB1 has also been associated with other cancers. Tumor-derived HMGB1 promoted the suppressive function of regulatory T cells in patients with head and neck cancer.23 HMGB1 was also considered to act as a novel tumor suppressor in pancreatic cancer.24 Lee et al.25 found that serum levels of HMGB1 were increased in a subset of colorectal carcinomas, suggesting that it might act as a supportive diagnostic marker for colorectal carcinomas. A previous meta-analysis26 showed that overexpression of HMGB1 was significantly associated with poorer overall and progression-free survival in patients with various types of cancer, including pancreatic cancer, hepatocellular carcinoma, nasopharyngeal carcinoma, colorectal cancer, head and neck cancer, and cervical carcinoma. Another meta-analysis27 found that HMGB1 expression was associated with TNM stages III–IV, and upregulated HMGB1 was also correlated with disease progression and the risk of disease progression in patients with non-small-cell lung cancer. However, there has been no previous meta-analysis of the role of HMGB1 in GC. The current meta-analysis thus confirmed an important role for HMGB1 in GC patients, and indicated that its expression might be correlated with tumor pT stage.
The present study also had some limitations. Notably, the number of references included in the meta-analysis was limited. Further clinical research is therefore needed to confirm the results.
In conclusion, we conducted a meta-analysis to investigate the role of HMGB1 in GC. HMGB1 expression was significantly correlated with tumor pT stage but not with age, sex, TNM stage, lymph node metastasis, or degree of tumor differentiation. This study might further our understanding of the association between HMGB1 expression and GC progression.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060521993312 for Value of HMGB1 expression for assessing gastric cancer severity: a systematic meta-analysis by Chunxiang Zhou and Qun Yang in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is funded by the project of “Study on the relationship and mechanism between ALDH2 gene polymorphism and CINV”, Department of science and technology of Hunan Province,Grant No.2020JJ4821. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Qun Yang https://orcid.org/0000-0001-5342-485X
References
- 1.Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016; 388: 2654–2664. [DOI] [PubMed] [Google Scholar]
- 2.Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018; 10: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng M, Tang Y, Li W, et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 2018; 49: 740–753.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson U, Yang H, Harris H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets 2018; 22: 263–277. [DOI] [PubMed] [Google Scholar]
- 5.Zhu X, Messer JS, Wang Y, et al. Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J Clin Invest 2015; 125: 1098–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessop F, Holian A. Extracellular HMGB1 regulates multi-walled carbon nanotube-induced inflammation in vivo. Nanotoxicology 2015; 9: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elia R, Simona M, Mauro P. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther 2015; 4: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao CB, Bao JM, Lu YJ, et al. Co-expression of RAGE and HMGB1 is associated with cancer progression and poor patient outcome of prostate cancer. Am J Cancer Res 2016; 5: 369–377. [PMC free article] [PubMed] [Google Scholar]
- 9.Luo P, Xu Z, Li G, et al. HMGB1 represses the anti-cancer activity of sunitinib by governing TP53 autophagic degradation via its nucleus-to-cytoplasm transport. Autophagy 2018; 14: 2155–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrivastava S, Mansure JJ, Almajed W, et al. The role of HMGB1 in radioresistance of bladder cancer. Mol Cancer Ther 2016; 15: 471–479. [DOI] [PubMed] [Google Scholar]
- 11.Song B, Song WG, Li ZJ, et al. Effect of HMGB1 silencing on cell proliferation, invasion and apoptosis of MGC-803 gastric cancer cells. Cell Biochem Funct 2012; 30: 11–17. [DOI] [PubMed] [Google Scholar]
- 12.Bao G, Qiao Q, Zhao H, et al. Prognostic value of HMGB1 overexpression in resectable gastric adenocarcinomas. World J Surg Oncol 2010; 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang J, Ge X, Xu W, et al. Bioinformatics analysis of the prognosis and biological significance of HMGB1, HMGB2, and HMGB3 in gastric cancer. J Cell Physiol 2019; 235: 3438–3446. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zhang R, Lu WW, et al. Clinical significance of HMGB1 expression in human gastric cancer. Int J Immunopathol Pharmacol 2014; 27: 543–551. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Kou YB, Zhu JS, et al. Knockdown of HMGB1 inhibits growth and invasion of gastric cancer cells through the NF-κB pathway in vitro and in vivo. Int J Oncol 2014; 44: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 16.He W, Tang B, Yang D, et al. Double-positive expression of high-mobility group box 1 and vascular endothelial growth factor C indicates a poorer prognosis in gastric cancer patients. World J Surg Oncol 2013; 11: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghweil AA, Osman HA, Hassan MH, et al. Validity of serum amyloid A and HMGB1 as biomarkers for early diagnosis of gastric cancer. Cancer Manag Res 2020; 12: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019; 14: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripathi A, Shrinet K, Kumar A. HMGB1 protein as a novel target for cancer. Toxicol Rep 2019; 6: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang QY, Wu LQ, Zhang T, et al. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol Rep 2015; 33: 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung HW, Jang S, Kim H, et al. Combined targeting of high-mobility group box-1 and interleukin-8 to control micrometastasis potential in gastric cancer. Int J Cancer 2015; 137: 1598–1609. [DOI] [PubMed] [Google Scholar]
- 22.Guoqiang B, Qu F, He L, et al. Prognostic significance of Tag SNP rs1045411 in HMGB1 of the aggressive gastric cancer in a Chinese population. PLoS One 2016; 11: e0154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wild CA, Brandau S, Lotfi R, et al. HMGB1 is overexpressed in tumor cells and promotes activity of regulatory T cells in patients with head and neck cancer. Oral Oncol 2012; 48: 409–416. [DOI] [PubMed] [Google Scholar]
- 24.Kang R, Xie Y, Zhang Q, et al. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res 2017; 27: 916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Song M, Shin N, et al. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS One 2012; 7: e34318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T, Zhang W, Yang G, et al. HMGB1 overexpression as a prognostic factor for survival in cancer: a meta-analysis and systematic review. Oncotarget 2016; 7: 50417–50427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Q, Xu J, Chen H, et al. Association between an elevated level of HMGB1 and non-small-cell lung cancer: a meta-analysis and literature review. Onco Targets Ther 2016; 9: 3917–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060521993312 for Value of HMGB1 expression for assessing gastric cancer severity: a systematic meta-analysis by Chunxiang Zhou and Qun Yang in Journal of International Medical Research