Abstract
Background:
Fatigue and depression based on self-report and diagnosis are prevalent in patients with heart failure (HF), and adversely affect high rates of hospitalization and emergency department visits, which can impact use of medical services. The relationships of fatigue and depression to use of medical services in patients with preserved and reduced left ventricular ejection fraction (LVEF) may differ.
Purpose:
We examined the associations of diagnoses of fatigue and depression with use of medical services in patients with preserved and reduced LVEF, controlling for covariates.
Methods:
Data were collected on fatigue, depression, covariates, and use of medical services. Patients (N = 582) were divided into two groups based on LVEF (< 40%, reduced LVEF; ≥ 40%, preserved LVEF). Multiple linear regression analyses were used to analyze the data.
Results:
A diagnosis of fatigue was a significant factor associated with more use of medical services in the total sample (β = .18, p < .001, R2 = 54%) and patients with reduced (β = .13, p = .008, R2 = 54%) and also preserved LVEF (β = .21, p < .001, R2 = 54%), controlling for all covariates, but a diagnosis of depression was not.
Conclusions:
This study demonstrates the important roles of a diagnosis of fatigue in use of medical services. Thus, fatigue needs to be assessed, diagnosed, and managed effectively.
Keywords: depression, health services, heart failure, symptoms
Heart failure (HF) is a high-cost clinical condition. Costs associated with HF in the U. S. have increased from $23 billion in 2002 to $31 billion in 2012,1, 2 and they are estimated to reach approximately $70 billion by 2030.2 Use of medical services has been associated with high costs in this population. In the U.S., one factor contributing the total medical costs the most was inpatient costs (47%).3 In Europe, the main sources of the total costs in patients with HF were hospitalization (39%) and outpatient care (20%).4 In the study, approximately 31% of patients were admitted to hospitals unexpectedly, and 53% visited emergency departments at least once.4 In addition, all-cause or HF-related hospitalization were significantly associated with higher costs.4
Two factors that may affect use of medical services and costs through their effects on hospitalization are fatigue and depression, which are the most common and burdensome physical and psychological symptoms in patients with HF.5–8 Approximately 80% to 94% of patients with HF experience fatigue,5, 6 and reported that fatigue was one of the worst HF symptoms and less improved over time.9 Approximately 30% to 50% of patients with HF have depression or depressive symptoms7, 8 (hereafter the term depression will be used for both depression and depressive symptom). Both fatigue and depression based on self-report have been known to adversely affect hospitalization and mortality. Fatigue based on self-report and diagnosis have been significantly associated with higher number of hospitalizations and higher mortality risk scores.10, 11 In addition, depression based on self-report and diagnosis have been associated with shorter time to hospitalization, emergency department visit, or mortality,12 or higher number of hospitalization.11 Both in the U.S. and Europe, hospitalization has been the largest contributor to the medical costs in HF.3, 4 Thus, both fatigue and depression can impact high use of medical services. However, the relationships, especially based on diagnosis, have not been examined, controlling for typical covariates of high hospitalization, which can lead to high costs. Diagnosis of fatigue or depression can be identified by clinicians easily through medical records for effective management. Thus, it is valuable to examine the relationships of fatigue and depression based on diagnoses to use of medical services.
In addition to fatigue and depression, some demographic characteristics (e.g., age and gender)7, 13 and some clinical characteristics (e.g., body mass index [BMI], comorbidities, and New York Heart Association [NYHA] functional class)14, 15 are significantly associated with HF symptoms and/or depression. Some demographic characteristics (e.g., age, gender, and ethnicity),16, 17 clinical characteristics (e.g., comorbidities, left ventricular ejection fraction [LVEF], and medications),14, 17, 18 vital signs (e.g., blood pressure [BP] and heart rate),16 and laboratory tests (e.g., creatinine, sodium, hemoglobin, and troponin-1)17, 18 are associated with hospitalization or mortality. Thus, these factors can impact use of medical services through their adverse effects on HF symptoms, depression, and/or hospitalizations or mortality (Figure 1).
Figure 1.
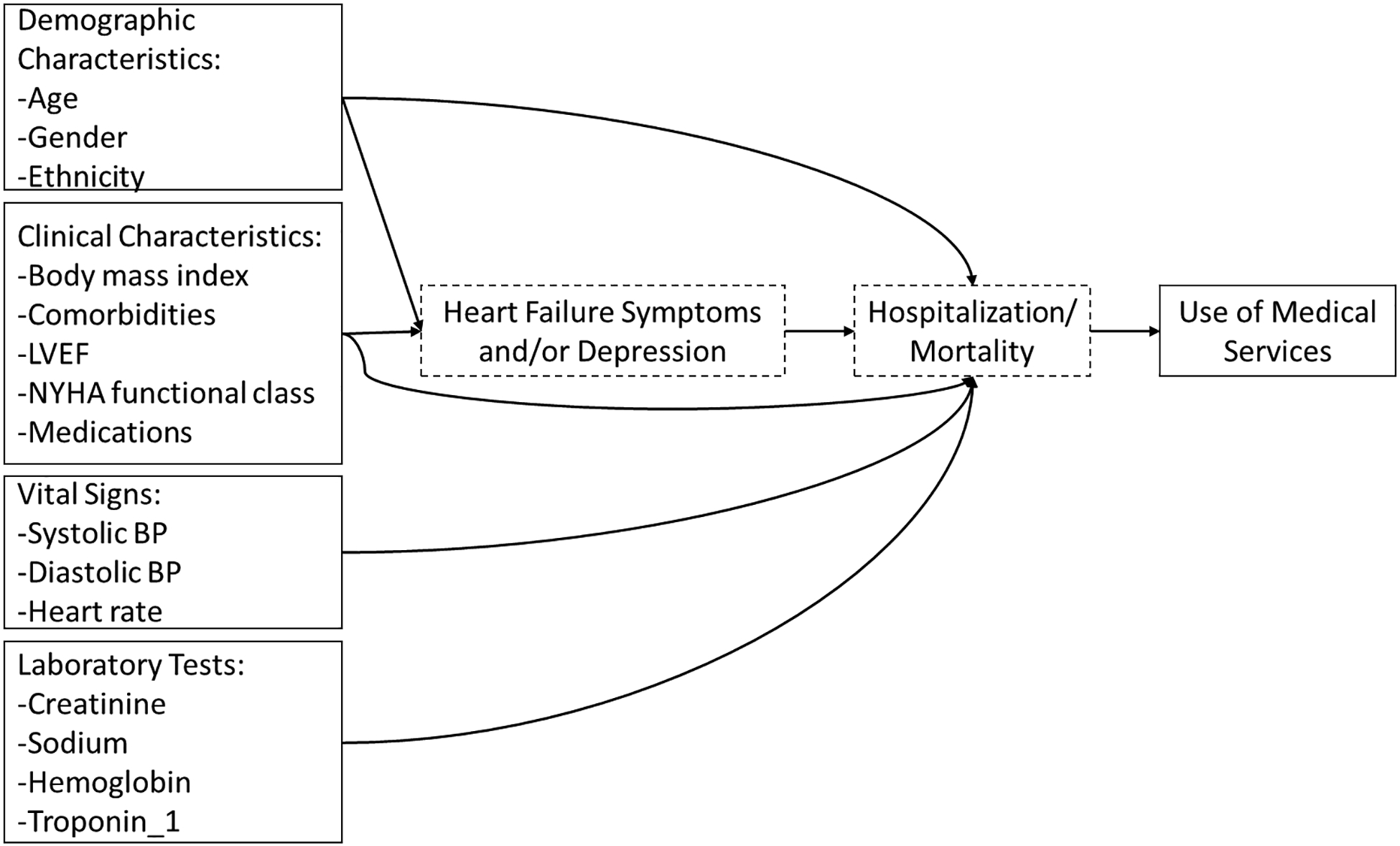
Theoretical Framework
LVEF, left ventricular ejection fraction. NYHA, New York Heart Association. BP, blood pressure. Only the direct relationships of demographic characteristics, clinical characteristics, vital signs, and laboratory tests, one heart failure symptom (i.e., fatigue), and depression to use of medical services were examined.
Therefore, the purpose of this study was to examine the associations of diagnoses of fatigue and depression with use of medical services in patients with HF, controlling for traditional covariates and also numbers of hospitalization and emergency department visit, and length of stay, which are the strongest contributors to high costs.
METHODS
Study design, Setting, and Procedure
The study design, setting, and procedure have been reported elsewhere.11 This was a cross-sectional, secondary analysis study using data from the Enterprise Data Warehouse of a university in the southern region of the U.S. The study was approved by the university’s Institutional Review Board. The Enterprise Data Warehouse team determined patients with HF as their primary or secondary diagnosis based on International Classification of Diseases (ICD)-9 codes (428–428.9) between January 1, 2010 and December 31, 2012. Then, the team retrieved data on all the study variables of those patients with HF. The research team received a 3-years of data on use of medical services based on Current Procedural Terminology (CPT) codes, fatigue (780.71 and 780.79) and depression (296.2–296.36 and 311) based on ICD-9 codes, demographic and clinical characteristics, vital signs, and laboratory tests from the medical record. The research conformed to the provisions of the Declaration of Helsinki as revised in Brazil 2013.
Measures
Use of medical services was assessed by counting the number of medical services used for the 3-year period based on CPT codes with no consideration of weight. Use of medical services included codes of the evaluation and management services, surgery services, radiology services, pathology and laboratory services, and medicine services. Use of medical services did not include number of hospitalizations, ED visits, and length of stay. Because there were multiple data for BMI, vital signs, laboratory tests, and clinical characteristics, a statistician who was one of the research team members calculated the mean of each of all the variables for the 3-year period, and the means were used for data analyses. Hospitalizations and ED visits were assessed by counting numbers of hospitalizations and ED visits for the 3-year time period, respectively. Length of stay was assessed by counting number of nights during the hospitalizations and ED visits for the 3-year time period.
Data Analysis
Initially, sample characteristics were summarized using means and standard deviations (SD) for continuous variables and frequencies and percents for categorical variables. t-test analyses were used to compare sample characteristics between HF patients with reduced and preserved LVEF, between HF patients with and without fatigue or depression. Multiple regression analyses with Enter method (all variables, including fatigue and depression, were entered into each model simultaneously) were used to determine factors associated with the number of all medical services use, including evaluation and management services, surgery services, radiology services, pathology and laboratory services, and medicine services, controlling for covariates, in the total sample and also two subgroups of patients with preserved LVEF and reduced LVEF. Although hospitalization rates between HF patients with preserved and reduced LVEF did not differ, factors associated with hospitalization rates in the groups differed.19 Inpatient costs considerably contributed to high costs, which implies the connection between hospitalization and more use of medical services. Thus, factors associated with use of medical services between HF patients with preserved and reduced LVEF may differ. Thus, the analyses were done in the total sample and in the two subgroups. Two-tailed tests were used, and a p < .05 was set up as significant. All data analyses were done using SPSS (24 version).20
RESULTS
Sample Characteristics
Demographic and clinical characteristics, vital signs, and laboratory tests are presented in Table 1. In the total sample, the mean age was 63.2 (± 14.4) years old, and approximately half of them were males (54.5%) and Caucasians (51.2%) (Table 1). In the sample, 48.5% had HF with reduced LVEF, 45.4% had a diagnosis of fatigue, and 26.3% had a diagnosis of depression. Patients with reduced LVEF were younger, and had lower BMI and systolic BP, lower levels of blood creatinine and sodium, higher levels of blood hemoglobin, less use of medical services, fewer number of hospitalizations, and shorter length of stay than HF patients with preserved LVEF. In addition, they were more frequently males, and had fewer comorbidities and diagnosis of depression than their counterparts. Patients with diagnosis of fatigue were older, and had lower BMI and lower systolic and diastolic BP, lower levels of blood monocytes and neutrophils, more use of medical services, more frequent hospitalizations, longer length of stay, and more frequent ED visits than patients without a diagnosis of fatigue. In addition, they were more frequently females, and had more comorbidities and diagnosis of depression than their counterparts. Patients with a diagnosis of depression had lower systolic and diastolic BP, higher LVEF, more use of medical services, more frequent hospitalizations, longer length of stay, and more frequent ED visits than patients without a diagnosis of depression. In addition, they were more likely females and Caucasian race, and had cancer, and fatigue than their counterparts.
Table 1.
Sample Characteristics
| Variable | Total (N = 582) |
Reduced LVEF (n = 282) |
Preserved LVEF (n = 300) |
No Fatigue (n = 318) |
Fatigue (n = 264) |
No Depression (n = 429) |
Depression (n =153) |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age, year | 63.2 ± 14.4 | 60.7 ± 14.0 | 65.6 ± 14.4† | 61.1 ± 14.0 | 65.7 ± 14.5† | 62.8 ± 14.0 | 64.4 ± 15.4 |
| BMI, kg/m2 | 31.0 ± 9.1 | 30.1 ± 8.8 | 31.9 ± 9.4* | 31.8 ± 10.0 | 30.2 ± 7.9* | 30.9 ± 8.8 | 31.5 ± 10.1 |
| SBP, mmHg | 135.4 ± 19.6 | 131.3 ± 19.6 | 139.2 ± 18.9† | 137.3 ± 20.9 | 133.1 ± 17.7* | 136.2 ± 20.7 | 133.0 ± 16.2* |
| DBP, mmHg | 78.8 ± 12.6 | 79.8 ± 12.9 | 77.9 ± 12.2 | 80.4 ± 13.9 | 76.9 ± 10.5* | 79.8 ± 13.2 | 76.1 ± 10.2† |
| Heart rate | 81.2 ± 12.3 | 82.1 ± 12.1 | 80.3 ± 12.4 | 81.9 ± 13.6 | 80.3 ± 10.4 | 81.4 ± 12.7 | 80.4 ± 11.0 |
| LVEF, % | 40.8 ± 15.1 | N/A | N/A | 40.4 ± 15.1 | 41.2 ± 15.0 | 39.7 ± 14.8 | 43.8 ± 15.4* |
| Troponin_1, ng/mL | .7 ± 3.2 | 1.0 ± 4.0 | .5 ± 2.2 | .9 ± 3.5 | .6 ± 3.0 | .7 ± 3.1 | .7 ± 3.7 |
| Albumin, g/dL | 3.1 ± .6 | 3.2 ± .6 | 3.1 ± .6 | 3.2 ± .6 | 3.1 ± .6 | 3.2 ± .6 | 3.2 ± .6 |
| Triglycerides, mg/dL | 128.0 ± 90.9 | 123.2 ± 101.3 | 132.5 ± 79.7 | 125.9 ± 82.7 | 130.5 ± 99.9 | 125.7 ± 93.1 | 134.4 ± 84.4 |
| Creatinine, mg/dL | 1.8 ± 1.6 | 1.6 ± 1.2 | 2.0 ± 1.9* | 1.8 ± 1.5 | 1.8 ± 1.8 | 1.9 ± 1.6 | 1.7 ± 1.7 |
| Sodium, mEq/L | 137.1 ± 2.9 | 136.8 ± 3.0 | 137.5 ± 2.8* | 137.0 ± 3.1 | 137.2 ± 2.7 | 137.1 ± 3.0 | 137.2 ± 2.8 |
| Hemoglobin, g/dL | 11.5 ± 1.8 | 11.8 ± 1.8 | 11.1 ± 1.7† | 11.5 ± 1.8 | 11.4 ± 1.7 | 11.5 ± 1.8 | 11.3 ± 1.6 |
| Monocytes, K/uL | .7 ± .3 | .7 ± .3 | .7 ± .3 | .7 ± .3 | .7 ± .2* | .7 ± .3 | .7 ± .3 |
| Neutrophils, K/uL | 5.7 ± 2.5 | 5.6 ± 2.3 | 5.7 ± 2.7 | 6.0 ± 2.7 | 5.3 ± 2.2† | 5.7 ± 2.5 | 5.6 ± 2.5 |
| Medical services use | 96.5 ± 84.3 | 83.3 ± 72.0 | 108.9 ± 92.8† | 66.1 ± 50.7 | 133.1 ± 100.6† | 86.3 ± 77.8 | 124.9 ± 94.9† |
| Hospitalization | 3.4 ± 3.2 | 3.0 ± 2.7 | 3.8 ± 3.6* | 2.6 ± 2.2 | 4.3 ± 4.0† | 3.1 ± 3.0 | 4.1 ± 3.7* |
| Length of Stay | 20.7 ± 26.4 | 17.8 ± 24.6 | 23.4 ± 27.6* | 16.0 ± 21.8 | 26.3 ± 30.0† | 18.2 ± 23.2 | 27.4 ± 33.0* |
| ED visit | 1.8 ± 3.4 | 1.5 ± 2.6 | 2.1 ± 4.0 | 1.3 ± 2.4 | 2.4 ± 4.2† | 1.6 ± 2.9 | 2.4 ± 4.4* |
| n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | |
| Gender (male) | 317(54.5) | 182(64.5) | 135(45.0)† | 185(58.2) | 132(50.0)* | 257 (59.9) | 60(39.2)† |
| Race (Caucasian) | 298(51.2) | 141(50.0) | 157(52.3) | 156(49.1) | 142(53.8) | 203(47.3) | 95(62.1)* |
| NYHA (II) | 480(82.5) | 238(84.4) | 242(80.7) | 263(82.7) | 217(82.2) | 349(81.4) | 131(85.6) |
| ACE inhibitors | 271(46.6) | 127(45.0) | 144(48.0) | 149(46.9) | 122(46.2) | 201(46.9) | 70(45.8) |
| Beta-blockers | 367(63.1) | 184(65.2) | 183(61.0) | 204(64.2) | 163(61.7) | 273(63.6) | 94(61.4) |
| Antidepressants | 241 (41.4) | 119 (42.2) | 122 (40.7) | 135(42.5) | 106(40.2) | 185 (43.1) | 56(36.6) |
| DM | 305(52.4) | 139(49.3) | 166(55.3) | 169(53.1) | 136(51.5) | 223(52.0) | 82(53.6) |
| MI | 223(38.3) | 119(42.2) | 104(34.7) | 112(35.2) | 111(42.0) | 171(39.9) | 52(34.0) |
| CPHD | 85(14.6) | 28(9.9) | 57(19.0)* | 42(13.2) | 43(16.3) | 62(14.5) | 23(15.0) |
| Renal disease | 389(66.8) | 175(62.1) | 214(71.3)* | 202(63.5) | 187(70.8) | 284(66.2) | 105(68.6) |
| CVD | 155(26.6) | 64(22.7) | 91(30.3)* | 66(20.8) | 89(33.7)† | 106(24.7) | 49(32.0) |
| Cancer | 145(24.9) | 66(23.4) | 79(26.3) | 54(17.0) | 91(34.5)† | 96(22.4) | 49(32.0)* |
| Mortality | 217 (37.3) | 104 (36.9) | 113 (37.7) | 116(36.5) | 101(38.3) | 160(37.3) | 57(37.3) |
| Depression | 153(26.3) | 63(22.3) | 90(30.0)* | 62 (19.5) | 91(34.5)† | N/A | N/A |
| Fatigue | 264 (45.4) | 122 (43.3) | 142 (47.3) | N/A | N/A | 173(40.3) | 91(59.5)† |
p < .05.
p < .001.
ACE, angiotensin converting enzyme inhibitor. BMI, body mass index. BP, blood pressure. CI, confidence interval. CPHD, Chronic pulmonary heart disease. CVD, Cerebrovascular Disease. DBP, diastolic blood pressure. DM, diabetes mellitus. ED visit, hospitalization, and mortality, number of emergency department visit, hospitalization, and death between 1/1/2010 and 12/31/2012. LVEF, left ventricular ejection fraction. MI, myocardial infarction. NYHA, New York Heart Association functional class. SBP, systolic blood pressure. SD, standard deviation. All lab tests were based on serum. Monocyte for patients without and with fatigue: 0.74 ± .29 vs. .68 ± .24.
Associations of Fatigue and Depression With Use of Medical Services
A diagnosis of fatigue was a significant factor associated with more use of medical services in the total sample (β = .18, p < .001) and patients with reduced LVEF (β = .13, p = .008) and also patients with preserved LVEF (β = .21, p < .001), controlling for all covariates. However, a diagnosis of depression was not a significant factor associated with more use of medical services (Table 2). In the total sample, all the variables explained 54% of the variance in use of medical services. In patients with reduced LVEF, the model explained 54% of the variance in use of medical services. In patients with preserved LVEF, the model explained 54% of the variance in use of medical services.
Table 2.
The Effects of Fatigue and Depression on Use of Medical Services in Patients with Reduced Ejection Fraction and Preserved Ejection Fraction
| Variable | Medical Use: Total Sample (N = 582) |
Medical Use: Patients with Reduced Ejection Fraction | Medical Use: Patients with Preserved Ejection Fraction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | t* | p value | 95% CI | B | β | t* | p value | 95% CI | B | β | t* | p value | 95% CI | |
| Fatigue | 30.55 | .18 | 5.602 | <.001 | 19.84, 41.26 | 19.33 | .13 | 2.672 | .008 | 25.90, 57.41 | 39.16 | .21 | 4.570 | <.001 | 22.29, 56.04 |
| Depression | 5.67 | .03 | .956 | .340 | −5.98, 17.31 | −.89 | <−.01 | −.113 | .910 | −16.45, 14.66 | 12.82 | .06 | 1.398 | .164 | 5–5.26, 30.91 |
| Age | −.28 | −.05 | −1.215 | .225 | −.73, .17 | −.36 | −.07 | −1.109 | .269 | −1.00, .28 | −.07 | −.01 | −.197 | .844 | −.77, .63 |
| Gender | −10.90 | −.06 | −1.917 | .056 | −22.07, .27 | −13.56 | −.09 | −1.789 | .075 | −28.49, 1.37 | −5.45 | −.03 | −.603 | .547 | −23.23, 12.33 |
| Race | 10.96 | .06 | 1.910 | .057 | −.31. 22.22 | 12.59 | .09 | 1.701 | .090 | −1.99, 27.18 | 11.68 | .06 | 1.276 | .203 | −6.34, 29.70 |
| BMI | −.25 | −.03 | −.796 | .427 | −.87, .37 | −.39 | −.05 | −.871 | .385 | −1.26, .49 | −.05 | <−.01 | −.102 | .919 | −.92, 1.02 |
| SBP | −.16 | −.04 | −.778 | .437 | −.56, .24 | −.16 | −.04 | −.552 | .581 | −.71, .40 | −.12 | −.02 | −.403 | .687 | −73, .48 |
| DBP | −.32 | −.05 | −.932 | .352 | −.98, .35 | −.32 | −.06 | −.679 | .498 | −1.25, .61 | −40 | −.05 | −.752 | .453 | −1.44, .64 |
| Heart rate | −.22 | −.03 | −.933 | .351 | −.70, .24 | −.31 | −.05 | −.944 | .346 | −.95, .33 | −.13 | −.02 | −.341 | .733 | −.86, .61 |
| LVEF | .26 | .05 | 1.338 | .182 | −.12, .65 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| NYHA | −4.55 | −.02 | −.736 | .462 | −16.69, 7.59 | −.21 | <−.01 | −.025 | .980 | −17.16, 16.73 | −4.43 | −.02 | −.465 | .642 | −23.19, 14.33 |
| ACEis | 5.54 | .03 | 1.060 | .290 | −4.73, 15.82 | 7.01 | .05 | 1.016 | .311 | −6.59, 20.61 | 2.68 | .01 | .325 | .745 | −13.56, 18.93 |
| Beta-blockers | −7.03 | −.04 | −1.288 | .198 | −17.74, 3.69 | −2.87 | −.02 | .395 | .693 | −17.15, 11.42 | −13.19 | −.07 | −1.535 | .126 | −30.11, 3.73 |
| Antidepressants | −7.36 | −.04 | −1.461 | .145 | −17.26, 2.54 | −1.58 | −.01 | −.247 | .805 | −14.21, 11.04 | −11.96 | −.06 | −1.458 | .146 | −28.11, 4.19 |
| DM | 6.59 | .04 | 1.194 | .233 | −4.25, 17.43 | 7.51 | .05 | 1.025 | .305 | −6.91, 21.93 | 5.21 | .03 | .601 | .549 | −11.86, 22.27 |
| MI | 12.34 | .07 | 2.231 | .026 | −3.25, 28.17 | 3.98 | .03 | .568 | .571 | −9.83, 17.79 | 20.66 | .11 | 2.314 | .021 | 3.08, 38.23 |
| CPHD | −2.48 | −.01 | −.340 | .734 | −16.81, 11.84 | −8.08 | −.03 | −.721 | .472 | −30.15, 13.99 | −.11 | <−.01 | −.011 | .992 | −21.11, 20.88 |
| Renal disease | 1.35 | .01 | .222 | .825 | −.12, 27.20 | 3.05 | .02 | .394 | .694 | −12.19, 18.29 | −.75 | <−.01 | −.075 | .940 | −20.38, 18.88 |
| CVD | −4.58 | −.02 | −.766 | .444 | −16.34, 7.18 | −3.82 | −.02 | −.465 | .643 | −19.99, 12.36 | −9.48 | −.05 | −1.020 | .309 | −27.78, 8.82 |
| Cancer | 41.02 | .21 | 6.479 | <.001 | 28.59, 53.46 | 35.47 | .21 | 4.227 | <.001 | 18.94, 52.00 | 48.38 | .23 | 4.787 | <.001 | 28.48, 68.28 |
| Troponin_1 | −.69 | −.03 | −.855 | .393 | −2.27, .90 | −.48 | −.03 | 566 | .572 | −2.13, 1.18 | −.94 | −.02 | −.502 | .616 | −4.63, 2.75 |
| Albumin | 27.52 | .21 | 5.953 | < .001 | 18.44, 36.60 | 27.80 | .24 | 4.608 | <.001 | 15.92, 36.68 | 29.62 | .21 | 23.943 | <.001 | 14.83, 44.41 |
| Triglycerides | .01 | .01 | .416 | .678 | −.05, .07 | .04 | .05 | 1.058 | .291 | −.03, .10 | <.01 | <.01 | .017 | .987 | −.10, .11 |
| Creatinine | 3.17 | .06 | 1.766 | .078 | −.36, 6.70 | 2.05 | .04 | .667 | .505 | −4.01, 8.11 | 4.40 | .09 | 1.762 | .079 | −.52, 9.32 |
| Sodium | .41 | .01 | .450 | .653 | −1.37, 2.18 | 1.74 | .07 | 1,484 | .139 | −.568, 4.04 | −.48 | −.02 | −.330 | .742 | −3.36, 2.40 |
| Hemoglobin | −5.94 | −.12 | −3.294 | .001 | −9.49, −2.40 | −5.03 | −.13 | −2.346 | .020 | −9.26, −.81 | −7.29 | −.13 | −2.299 | .022 | −13.53, −1.05 |
| Monocytes | −.28 | <−.01 | −.024 | .981 | −22.55, 22.00 | 6.49 | .02 | .388 | .698 | −26.44, 39.41 | −.513 | <−.01 | −.031 | .975 | −33.13, 32.10 |
| Neutrophils | −2.91 | −.09 | −2.321 | .021 | −5.37, −.45 | −3.55 | −.11 | −1.840 | .067 | −7.34, .25 | −2.75 | −.08 | −1.523 | .129 | −6.30, .803 |
| Hospitalization | 6.80 | .26 | 6.291 | <.001 | 4.68, 8.93 | 8.11 | .31 | 4.874 | <.001 | 11.39, .52 | 5.94 | .23 | 3.782 | <.001 | 2.85, 9.04 |
| ED visit | 3.54 | .14 | 4.218 | <.001 | 1.89, 5.19 | 3.61 | .13 | 2.523 | .012 | .79, 6.42 | 3.62 | .15 | 3.109 | .002 | 1.33, 5.91 |
| LOS | .69 | .22 | 5.253 | <.001 | .43, .95 | .65 | .22 | 3.769 | <.001 | .31, .99 | .70 | .21 | 3.309 | .001 | .28, 1.11 |
| Model | F = 20.651 p < .001 R2 = .538 |
F = 9.993 p < .001 R2 = .544 |
F = 10.499 p < .001 R2 = .539 |
||||||||||||
t* t statistics. Use of medical services = number of all medical service uses between 1/1/2010 and 12/31/2012. All lab tests were based on serum. ACEis = angiotensin converting enzyme inhibitor inhibitors. B = unstandardized B, β = standardized beta. BMI = body mass index. CPHD = chronic pulmonary heart disease. CVD = cerebrovascular disease. DBP = diastolic blood pressure. DM = diabetes mellitus. ED = emergency department. LVEF = left ventricular ejection fraction. MI = myocardial infarction. NYHA = New York Heart Association functional class. SBP = systolic blood pressure.
Discussion
The findings of this study demonstrate the important role of a diagnosis of fatigue in use of medical services in HF patients with reduced and also preserved LVEF. A diagnosis of fatigue was significantly associated with more use of medical services, even controlling for all typical covariates of demographic and clinical characteristics, vital signs, and laboratory tests, and also hospitalization, ED visit, and length of stay, which are major contributors for use of medical services in patients with HF. The beta coefficients, which indicates the strength of the effects of individual predictor variable on the outcome variable,21 of fatigue in the total sample and patients with preserved LVEF were comparable with those of cancer, length of stay, or ED visit, and comparable with or slightly lower than those of hospitalization. Factors associated with use of medical services in patients with preserved LVEF and reduced LVEF were very similar, and each model explained very similar amount of variance in use of medical services. Thus, in order to decrease use of medical services, diagnosis of fatigue should be considered in HF patients with both preserved and reduced LVEF. On the other hand, depression was not associated with use of medical services may be because of the strong relationships of several independent variables, including fatigue, hospitalization, emergency department visits, length of stay, and cancer, to use of medical services.
In the literature, fatigue is one of the most common and distressing HF symptoms, and up to 80% to 90% of patients with HF reported that they experienced fatigue.5, 6, 22 In the current study, approximately half of the patients (45.4%) had a diagnosis of fatigue. Both self-reported and a diagnosis of fatigue have been associated with high hospitalization rates. Several studies have shown that self-reported fatigue or increased self-reported fatigue was significantly associated with high rates of hospitalization or mortality in this population.10, 11, 23 In addition, a diagnosis of fatigue also has been associated with greater number of hospitalization, controlling for typical covariates that were included in the current study except hospitalization, ED visits, and length of stay.11 In both Europe and the U. S., hospitalization or inpatient costs have been associated with high costs.3, 4 In the current study, as expected, all hospitalization, ED visits, and length of stay were associated with higher use of medical services in both patients with preserved and reduced LVEF, controlling for all typical covariates. More importantly, a diagnosis of fatigue was also associated with higher use of medical services in both patients with preserved and reduced LVEF, controlling for all typical demographic and clinical characteristics and lab tests, and all hospitalization, ED visits, and length of stay. These findings imply that improvement in fatigue may reduce use of medical services. To improve fatigue, we need to assess and manage fatigue in both patients with preserved and reduced LVEF to reduce hospitalization rates, use of medical services, and, in turn, costs.
In addition, further research needs to be done to know whether prevalence of fatigue based on diagnosis and self-report matches each other. Because the current study was a secondary analysis, it was impossible to collect data on self-reported fatigue. The relationship between fatigue based on diagnosis and self-report rarely has been examined in patients with HF. However, mismatch among a diagnosis of depression, self-reported depressive symptoms, and the treatment has been well known. For example, the prevalence rate of depression based on diagnosis in medical records was 23.4%; prescription of antidepressants was 33%; and depressive symptoms based on self-report was 43.1%.24 The findings of the current study support the previous findings, with the prevalence rate of depression based on diagnosis was 26.3%, and prescription of antidepressants were 41.4%. There may be a possibility that prevalence of fatigue based on self-report and diagnosis differs, thus, it may be meaningful to examine the prevalence of fatigue based on self-report and also diagnosis in patients with HF at the same time. If a diagnosis of fatigue is recorded in the medical record of the patient, clinicians can be involved in management of fatigue to reduce hospitalization and use of medical services.
Despite high rates of prescriptions of HF medications (prescription of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and beta-blockers: ≥ 80%), the prevalence of self-reported fatigue (85%) and hospitalization rates (62% within one year) still remain high.25 Although fatigue is a common HF symptom, and many patients with HF report fatigue, many of them did not recognize fatigue as a HF symptom or concern, which can lead to delayed seeking treatment.22, 26 In addition, fatigue compared with dyspnea was less improved during hospitalization and also after discharge.9 Thus, more effective strategies are needed for patients and clinicians to assess and manage fatigue appropriately. Clinicians can help patients with HF assess HF symptoms, including fatigue, during the patient’s regular outpatient clinic visits or hospital admissions using a reliable and valid instrument, then diagnose fatigue adequately. For instance, the Symptom Status Questionnaire-Heart Failure14 is a reliable and valid instrument assessing seven common HF symptoms, including fatigue,14 and approximately less than five minutes are needed to fill it out.
Because fatigue has been very common in patients with HF and, compared with dyspnea, was less improved, more comprehensive and effective interventions are needed to improve fatigue. In the current study, although depression was not associated with use of medical services, depression and fatigue are commonly associated with each other14, 27–30 Thus, to manage fatigue effectively, it may be better to manage fatigue and depression using more comprehensive interventions. For instance, some additional treatment components that can deal with both fatigue and depression, such as meditation combined with self-management, may be beneficial. For example, in a HF study,31 meditation combined with psychoeducational component prevented worsening of HF symptoms and reduced depression at 6 months. In addition, mindful, compassionate meditation combined with self-management showed promising outcomes of reducing both HF symptoms and depressive symptoms.32 Meditation interventions also improved fatigue and/or depression in breast cancer patients.33, 34 In addition, a collaborative symptom and psychosocial care program provided by a team of a nurse, a social worker, and a cardiologist also improved depression and fatigue.35
This study has some limitations. Diagnoses of fatigue and depression and use of medical services were included if documented at any time of the 3-year period based on medical records. Thus, the cause and effect relationships could not be examined. In addition, rates of depression based on diagnosis and depressive symptoms based on questionnaires have differed.24 Thus, rates of depression based on diagnosis might differ from actual rates of depression. In addition, diagnosis of fatigue also might not reflect the actual fatigue status if health care providers did not record the diagnosis to the medical records. Some somatic/affective symptoms of depression may be overlapped with fatigue, which could impact the relationships. However, the sample represents both sexes and different races very well, which expand the generalizability. In addition, the findings of this study demonstrate the important roles of fatigue in use of medical services, controlling for all typical covariates of medical service uses.
Conclusions
This study demonstrates the important roles of a diagnosis of fatigue in use of medical services. Thus, fatigue needs to be assessed, diagnosed, and managed effectively. Further studies are needed to develop and test comprehensive interventions, which focus on both fatigue and depression because depression can impact fatigue, to improve these symptoms, and in turn, to reduce use of medical services in patients with HF.
Acknowledgments
The project described was supported by the Translational Research Institute (TRI), grant UL1TR000039 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Seongkum Heo, University of Arkansas for Medical Sciences, College of Nursing, Little Rock, US.
Jean McSweeney, University of Arkansas for Medical Sciences, College of Nursing, Little Rock, US.
Pao-Feng Tsai, University of Arkansas for Medical Sciences, College of Nursing, Little Rock, US.
Songthip Ounpraseuth, University of Arkansas for Medical Sciences, College of Public Health, Little Rock, US.
Debra K. Moser, University of Kentucky, College of Nursing, Lexington, US.
JinShil Kim, Gachon University, College of Nursing, Incheon, South Korea.
References
- 1.American Heart Association. 2002 heart and stroke statistical update. 2001.
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Echouffo-Tcheugui JB, Bishu KG, Fonarow GC and Egede LE. Trends in health care expenditure among US adults with heart failure: The Medical Expenditure Panel Survey 2002–2011. Am Heart J. 2017;186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farre N, Vela E, Cleries M, Bustins M, Cainzos-Achirica M, Enjuanes C, et al. Medical resource use and expenditure in patients with chronic heart failure: A population-based analysis of 88 195 patients. Eur J Heart Fail. 2016;18:1132–40. [DOI] [PubMed] [Google Scholar]
- 5.Zaharias E, Cataldo J, Mackin L and Howie-Esquivel J. Simple measures of function and symptoms in hospitalized heart failure patients predict short-term cardiac event-free survival. Nurs Res Pract. 2014;2014:815984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen DJ, Spruit MA, Uszko-Lencer NH, Schols JM and Wouters EF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14:735–43. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb SS, Khatta M, Friedmann E, Einbinder L, Katzen S, Baker B, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43:1542–9. [DOI] [PubMed] [Google Scholar]
- 8.Johnson TJ, Basu S, Pisani BA, Avery EF, Mendez JC, Calvin JE Jr., et al. Depression predicts repeated heart failure hospitalizations. Journal of cardiac failure. 2012;18:246–52. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Stevenson LW, Palardy M, Campbell PM, May CW, Lakdawala NK, et al. The worst symptom as defined by patients during heart failure hospitalization: Implications for response to therapy. Journal of cardiac failure. 2012;18:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink AM, Gonzalez RC, Lisowski T, Pini M, Fantuzzi G, Levy WC, et al. Fatigue, inflammation, and projected mortality in heart failure. Journal of cardiac failure. 2012;18:711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo S, McSweeney J, Tsai PF and Ounpraseuth S. Differing effects of fatigue and depression on hospitalizations in men and women with heart failure. Am J Crit Care. 2016;25:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu JR, Lennie TA, Dekker RL, Biddle MJ and Moser DK. Medication adherence, depressive symptoms, and cardiac event-free survival in patients with heart failure. Journal of cardiac failure. 2013;19:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo S, Doering LV, Widener J and Moser DK. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care. 2008;17:124–132. [PubMed] [Google Scholar]
- 14.Heo S, Moser DK, Pressler SJ, Dunbar SB, Mudd-Martin G and Lennie TA. Psychometric properties of the Symptom Status Questionnaire-Heart Failure. Journal of Cardiovascular Nursing. 2015;30:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eastwood JA, Moser DK, Riegel BJ, Albert NM, Pressler S, Chung ML, et al. Commonalities and differences in correlates of depressive symptoms in men and women with heart failure. Eur J Cardiovasc Nurs. 2012;11:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowder RS, Irons BK, Meyerrose G and Seifert CF. Factors associated with increased hospital utilization in patients with heart failure and preserved ejection fraction. Pharmacotherapy. 2010;30:646–53. [DOI] [PubMed] [Google Scholar]
- 17.Ross JS, Mulvey GK, Stauffer B, Patlolla V, Bernheim SM, Keenan PS, et al. Statistical models and patient predictors of readmission for heart failure: A systematic review. Arch Intern Med. 2008;168:1371–86. [DOI] [PubMed] [Google Scholar]
- 18.Ingle L, Rigby AS, Carroll S, Butterly R, King RF, Cooke CB, et al. Prognostic value of the 6 min walk test and self-perceived symptom severity in older patients with chronic heart failure. European Heart Journal. 2007;28:560–8. [DOI] [PubMed] [Google Scholar]
- 19.Mangla A, Kane J, Beaty E, Richardson D, Powell LH and Calvin JE, Jr. Comparison of predictors of heart failure-related hospitalization or death in patients with versus without preserved left ventricular ejection fraction. Am J Cardiol. 2013;112:1907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IBM SPSS Statistics for Windows, Version 24.0 [computer program]. Armonk, NY: IBM Corp; 2016. [Google Scholar]
- 21.Montgomery DC, Peck EA and Vining GG. Introduction to linear regression analysis 5th ed. Hoboken, NJ: A John Wiley & Sons, Inc.; 2012. [Google Scholar]
- 22.Jurgens CY, Hoke L, Byrnes J and Riegel B. Why do elders delay responding to heart failure symptoms? Nursing Research. 2009;58:274–82. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Moreno AC, Jhund PS, Macdonald MR, Petrie MC, Cleland JG, Bohm M, et al. Fatigue as a predictor of outcome in patients with heart failure: analysis of CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail. 2014;2:187–97. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez JA, Redwine LL, Rutledge TR, Dimsdale JE, Pung MA, Ziegler MG, et al. Depression ratings and antidepressant use among outpatient heart failure patients: Implications for the screening and treatment of depression. Int J Psychiatry Med. 2012;44:315–34. [DOI] [PubMed] [Google Scholar]
- 25.Zambroski CH, Moser DK, Bhat G and Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4:198–206. [DOI] [PubMed] [Google Scholar]
- 26.Reeder KM, Ercole PM, Peek GM and Smith CE. Symptom perceptions and self-care behaviors in patients who self-manage heart failure. Journal of Cardiovascular Nursing. 2015;30:E1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan X and Meng Z. The mutual association between depressive symptoms and dyspnea in Chinese patients with chronic heart failure. European Journal of Cardiovascular Nursing. 2015;14:310–6. [DOI] [PubMed] [Google Scholar]
- 28.Williams BA. The clinical epidemiology of fatigue in newly diagnosed heart failure. BMC Cardiovasc Disord. 2017;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang WR, Yu CY and Yeh SJ. Fatigue and its related factors in patients with chronic heart failure. J Clin Nurs. 2010;19:69–78. [DOI] [PubMed] [Google Scholar]
- 30.Mills PJ, Wilson K, Iqbal N, Iqbal F, Alvarez M, Pung MA, et al. Depressive symptoms and spiritual wellbeing in asymptomatic heart failure patients. J Behav Med. 2015;38:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan MJ, Wood L, Terry J, Brantley J, Charles A, McGee V, et al. The Support, Education, and Research in Chronic Heart Failure Study (SEARCH): A mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. American Heart Journal. 2009;157:84–90. [DOI] [PubMed] [Google Scholar]
- 32.Heo S, McSweeney J, Ounpraseuth S, Shaw-Devine A, Fier A and Moser DK. Testing a Holistic Meditation Intervention to Address Psychosocial Distress in Patients With Heart Failure: A Pilot Study. Journal of Cardiovascular Nursing. 2018;33:126–134. [DOI] [PubMed] [Google Scholar]
- 33.Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, et al. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer. 2015;121:1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garlick M, Wall K, Corwin D and Koopman C. Psycho-spiritual integrative therapy for women with primary breast cancer. J Clin Psychol Med Settings. 2011;18:78–90. [DOI] [PubMed] [Google Scholar]
- 35.Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, et al. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: The CASA randomized clinical trial. JAMA Intern Med. 2018;178:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]


