Abstract
Limiting opportunities for captive non-human primates (NHPs) to express species-specific social behaviors may disrupt the adaptive drive for social companionship and may lead to increases in coping behaviors and inactivity. While captive NHPs show improved welfare when moving to pair-housing from single-housing, the impact of daily separation of pair-mates, as is implemented in intermittent pair-housing, is not fully understood. We compared behavioral indices of welfare exhibited by adult female rhesus macaques (Macaca mulatta) in two conditions: (1) intermittent pair-housing, involving daily overnight separation of pair-mates, and (2) continuous pair-housing, involving little separation of pair-mates. A within-subjects study design tested two groups of females experiencing both pairing conditions in an alternate order, switching either from continuous to intermittent pair-housing, or from intermittent to continuous pair-housing. Behavioral observations, recording activity state, self-directed, abnormal, and social behaviors, were conducted at midday when all females were paired, and in the afternoon when intermittent pairs were separated. Females exhibited higher levels of inactivity and self-directed behavior when separated due to intermittent pair-housing in comparison to continuous pair-housing. In addition, intermittently paired females showed higher levels of grooming and other types of affiliation when paired, than during the same time frame when they were continuously paired. These results suggest that females in the continuous presence of a social partner experience improved levels of activity and do not need to elevate levels of behavioral coping mechanisms (e.g., self-scratching, increased affiliation) as they receive the benefits associated with social companionship consistently throughout the day. Overall, this study provides the first evidence that continuous pair-housing affords better welfare than intermittent pair-housing in adult female rhesus macaques. Pair-housing options, such as continuous pairing, that reduce reliance on behavioral coping mechanisms and promote adaptive social behavior throughout the entirety of the day should be prioritized over husbandry care scheduled for convenience.
Keywords: social interaction, activity, pair-housing, behavioral management, heterogeneous environment
INTRODUCTION
Social interactions and relationships are fundamentally important to primates, providing individuals with a range of fitness and health benefits (Majolo & Huang, 2018; Ostner & Schülke, 2018; Silk, 2007; Snyder-Mackler et al., 2020). For captive non-human primates (NHPs), social housing is the most effective form of environmental enrichment (Lutz & Novak, 2005), improving physiological, behavioral, and psychological measures of welfare (Olsson & Westlund, 2007). Federal law and regulatory agencies accrediting and inspecting research facilities have increased the emphasis for implementing social housing, as captive NHPs have also demonstrated improved health in social environments (Directive of the European Parliament, 2010; National Research Council, 2011; Office of Laboratory Animal Welfare, 2019; United States Department of Agriculture, 2013). Indoor-housed NHPs, particularly macaque species, are often pair-housed where two individuals inhabit connected adjacent cages (Baker et al., 2012b). Ideally, pair-mates would be continuously pair-housed, having complete physical access to their pair-mate with very little separation, but this presents a problem for some research objectives (e.g., urine sample collection). Intermittent pair-housing, involving the temporary daily or weekly separations of pair-mates that can last 12 or more hours (often occurring overnight), has been developed to simultaneously allow some social contact and facilitate research protocols.
Temporary separations due to intermittent pair-housing, however, may still negatively impact NHP welfare due to the reduced opportunity to receive the benefits social contact provides. In a study of adult female rhesus macaques (Macaca mulatta), higher urinary cortisol concentration (a measure of stress physiology) was related to the interaction of intermittent pair-housing and a poor pair relationship quality (Hannibal et al., 2018). By contrast, in the same species, no differences in activity, abnormal, or anxiety-related behaviors were seen between continuous and intermittent pair-housing conditions while pair-mates were together (Baker et al., 2012a, 2012b); however, these behavioral measures were not assessed while animals were separated. Pair-housed adult female rhesus macaques strongly prefer to be in close proximity with their pair-mate, particularly overnight (Eaton, Kelley, Axthelm, Iliff‐Sizemore, & Shiigi, 1994). Co-sleeping behavior is suggested to have evolved in response to ecological pressures such as predation (Capellini, Barton, McNamara, Preston, & Nunn, 2008a; Capellini, Nunn, McNamara, Preston, & Barton, 2008b) and low ambient temperature (Gilbert et al., 2009). While captivity obviously removes these pressures, NHPs instinctively seek out social partners to sleep with during the night (e.g., Mochida & Nishikawa, 2014) and the prevention of such behavior could impair welfare. Therefore, evaluating measures of welfare during the time pair-mates spend apart is critical, as intermittently paired NHPs are separated for up to three-quarters of the day, including overnight.
Generally, captive management programs utilize a multi-faceted approach to make robust assessments of NHP well-being, including measures of abnormal behavior, other coping behaviors (e.g., elevated affiliation or aggression), stress physiology, and immune system activation (Hannibal, Bliss-Moreau, Vandeleest, McCowan, & Capitanio, 2017). Increases in these measures may indicate poor welfare (Broom, 1986; Mason, 1991), and are often linked to anxiety and stress (Moberg & Mench, 2000). Measurement of behavior provides a non-invasive and cost-effective means for assessing welfare (Reamer, Tooze, Coulson, & Semple, 2010). Studies on NHPs have found that rates of self-directed behaviors (SDBs; e.g., self-scratching and self-grooming) can act as an indicator of anxiety (Maestripieri, Schino, Aureli, & Troisi, 1992; Troisi, 2002). SDBs are known to increase in response to stressors (Aureli, 1992) or administration of anxiogenic drugs (Schino, Perretta, Taglioni, Monaco, & Troisi, 1996), and decrease in response to environmental enrichment (Blois-Heulin & Jubin, 2004; Carder & Semple, 2008) or anxiolytic drugs (Schino, Troisi, Perretta, & Monaco, 1991). Stress and anxiety have also been implicated in the development and maintenance of abnormal behavior in captive environments (Novak, Meyer, Lutz, & Tiefenbacher, 2006). Captive NHPs may increase abnormal behavior in response to stressors (e.g., Mallapur et al., 2005), or decrease these behaviors in less stressful environments (e.g., Kitchen & Martin, 1996).
NHPs may cope with stress and anxiety induced by captivity through affiliation with conspecifics. Terry (1970) found evidence that affiliative behaviors, such as grooming, mitigate anxiety and stress. In NHPs, the receipt of grooming is associated with reductions in SDBs (Schino, Scucchi, Maestripieri, & Turillazzi, 1988), heart rate (Aureli, Preston, & de Waal, 1999; Boccia, Reite, & Laudenslager, 1989), and fecal glucocorticoids (Gust, Gordon, Hambright, & Wilson, 1993), while giving grooming is also associated with reductions in SDBs (Aureli & Yates, 2010) and fecal glucocorticoids (Shutt, MacLarnon, Heistermann, & Semple, 2007). Merely the presence of conspecifics has a social buffering effect for these animals in response to adverse husbandry events (Gilbert & Baker, 2011) and unfamiliar environments (Gerber, Anzenberger, & Schnell, 2002). Lastly, social contact gives animals the opportunity to express other species-specific behaviors and reduce inactivity (Schapiro, Bloomsmith, Suarez, & Porter, 1996), which could otherwise result in poor welfare (Fureix & Meagher, 2015).
Here we investigate behavioral indices of welfare in intermittent and continuous pair-housed adult female rhesus macaques (Macaca mulatta), quantifying time spent inactive, exhibiting abnormal behavior, exhibiting affiliative behavior, and bouts of self-directed behavior. We predicted that inactivity, abnormal behavior, and self-directed behavior would be highest during the intermittent pair-housing phase, when pair-mates were separated. Additionally, affiliative behaviors were predicted to be higher during the intermittent pair-housing phase.
MATERIALS AND METHODS
Study site and ethics statement
This study was conducted between March and May 2015 at the California National Primate Research Center (CNPRC) in Davis, California, United States of America. The CNPRC is a biomedical research institution accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AALAC) and is regularly inspected by United States Department of Agriculture (USDA) and National Institute of Health Office of Laboratory Animal Welfare (NIH OLAW). Aspects of NHP management and research use followed applicable United States federal regulations, the Guide for Care and Use of Laboratory Animals of the National Research Council (2011), and United States Department of Agriculture’s Animal Welfare regulations (2013). Research adhered to protocols approved by the University of California, Davis Institutional Animal Care and Use Committee. Additionally, the research followed the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates.
Study subjects
Subjects were 24 adult female rhesus macaques, born and reared at the CNPRC. Females were raised in either 0.2-hectare outdoor enclosures (i.e., field cage) each containing up to 180 monkeys of all age and sex classes (N= 17 individuals), or in approximately 43.7 m2 outdoor enclosures (i.e., corn crib) each containing up to 30 monkeys of all age and sex classes (N= 7 individuals). Age of the subjects ranged from 4.9 to 10.9 years (mean ± standard deviation: 6.5 ± 1.7 years). Females weighed 4.7 to 8.7 kg (6.5 ± 1.2 kg) and were not pregnant. Prior to study participation, animals were housed indoors for at least four months (18.2 ± 18.1 months) and paired together for more than three months (9.9 ± 6.1 months) in their initial pairing condition. Study subjects were housed with one other female either initially as a continuous (N= 7 pairs, 12 study animals) or intermittent (N= 6 pairs, 12 study animals) pair; two females were paired with pair-mates not included in the study as they did not meet our subject selection criteria for rearing history (i.e., mother-reared in an outdoor social group during the first year of life). Two of six pairs that were initially intermittently paired had a previous relationship with each other (e.g., outdoor cage mates); five of seven pairs that were initially continuously paired had previous relationships, where four of these pairs were from the same matriline as their pair-mate.
Housing
The CNPRC and other primate centers throughout the United States utilize intermittent and continuous pair-housing to provide indoor-housed NHPs with contact socialization with conspecifics. During the current study, intermittent pair-housed rhesus macaques were separated before afternoon feeding (about 14:00) and reunited the next day after morning feeding (by 08:00); these animals thus experienced about six hours of physical contact per day with their pair-mate. In contrast, continuously pair-housed animals were paired for over 18 hours daily and experienced separation only during feeding times to eliminate the likelihood of food aggression and facilitate urine collection for another project (Hannibal et al., 2018). Overnight separation for subjects in the intermittent contact phase, while the continuous pairs remained together overnight, allowed the behavioral effects of this difference to be tested.
Each subject was housed in a stainless-steel cage (0.80 m high, floor space of 0.4 m2). These cages sat on a two-tiered rack so that two pairs of cages were stacked one on top of the other. The cages of paired subjects were adjoined by a square opening (0.3m by 0.3m) that was equipped with a sliding solid steel partition which was used to separate pair mates when necessary and prevented physical and direct visual contact. Each cage was sanitized daily and was equipped with a metal reflective mirror, chew toy, forage board, and a metal perch. Animals were fed nutritionally complete biscuits and a forage mixture (i.e., rice, split peas, and oats) twice a day by CNPRC animal care staff, prior to 08:00 hours and after 14:00 hours. Fresh water was available ad libitum. Additional facility enrichment (e.g., puzzle tubes filled with fruit, coconuts, novel forage, and alfalfa cubes) was provided once every two weeks. Animals experienced a 12-hour light:dark cycle, with light onset at 06:00. Room temperature was maintained between 18–29° C and relative humidity between 30–70%. Subjects were housed in rooms containing 25 to 77 animals (48.5 ± 20.6 animals) at the beginning of the study period with visual, auditory, and olfactory access to other monkeys from a variety of age/sex classes.
Experimental study design
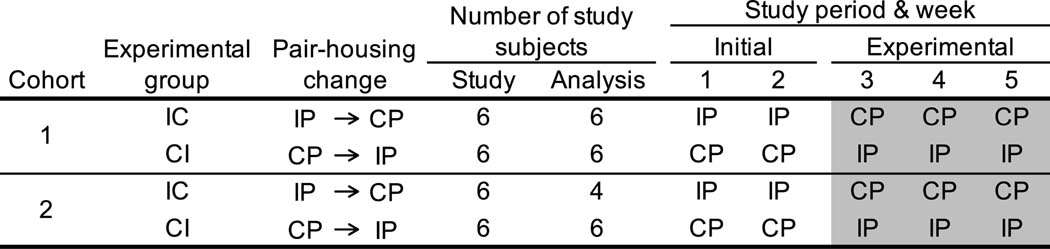
Subjects were studied for five weeks, over which they were observed in their initial pairing condition at the beginning of the study for two weeks and then in their alternative pairing condition for three weeks (Figure 1). Changes to pair-housing condition occurred due to the experimental manipulation of the present study as females were not enrolled in a concurrent project. Each pair of females was classified into one of two experimental groups: females in the CI experimental group were initially continuously (C) housed and then intermittently (I) housed. Conversely, IC experimental group animals began in intermittent pair-housing and then were switched to continuous pair-housing (Figure 1; Table 1). Pairs were split into two cohorts of 12 subjects each, balanced by experimental group, to accommodate data collection for all animals. Cohort one was studied March 23 to April 24, 2015 and Cohort two was studied April 27 to May 29, 2015. Behavior during the initial pairing condition was observed for each pair on weekdays for 9 to 10 data collection days and for the alternative pairing condition for 14 to 15 days.
Figure 1:
Experimental design. Each female in the study experienced intermittent (IP) and continuous (CP) pair-housing throughout the study. Females were assigned to an experimental group (IC, CI) depending on which pair-housing condition they began in.
Table 1:
Behavioral and model variable categories analyzed, with sub-categories where applicable. The sampling method for each behavior (1/0, event) and type of model variable (factor, covariate) is indicated in bold and parentheses. Definitions were derived from Hannibal et al. (2018).
| Category | Sub-category | Definition |
|---|---|---|
| Behavioral definitions | ||
| Inactivity (1/0) | Passive sitting or standing, awake, or asleep. | |
| Abnormal behavior (1/0) | Appetitive (e.g., regurgitation), over-groom (e.g., hair plucking own body), motor (e.g., pacing), non-injurious self-directed (e.g., eye poke), and self-injurious (i.e., self-bite). | |
| Affiliative behavior (1/0) | Proximity | Within less than one body diameter (in sitting position) for more than 5 seconds. |
| Coalition behavior | Recruit attempts, recruit successes, co-threat neighbor, and co-threat observer. | |
| Grooming | Giving grooming, receiving grooming, and attempting to groom pair mate. | |
| Other affiliative behavior | Huddle, play, anogenital exploration, mount, present body, present rump, and reconcile. | |
| Self-directed behavior (event) | Self-scratch, self-groom, yawing, body shaking, and tooth grinding. | |
| Model variable definitions | ||
| Predictor variables | Current condition (factor) | Current pair-housing condition (continuous or intermittent) |
| Observation period (factor) | Time of day observation occurred (midday or afternoon) | |
| Experimental group (factor) | Pair began as intermittent and then experimentally changed to continuous (IC), or began as continuous and then experimentally changed to intermittent (CI) | |
| Control variables | Weight (covariate) | Weight in kilograms (kg) determined at most recent health assessment prior to the study. |
| Age (covariate) | Age in years at the beginning of the study. | |
| Dominant (factor) | Whether the animal received the greatest proportion of status signaling behaviors (move away, turn away, silent bared teeth) displayed between pair-mates over the entire study (yes or no). | |
| Time spent indoors (covariate) | Total time in months that the subject was living in indoor housing prior to the study. | |
| Previous relationship (factor) | Whether the pair-mates were familiar with each other prior to pairing (yes or no). | |
| Duration paired (covariate) | Total time in months that the subject was living with current pair-mate prior to the study. | |
Data collection
Behavioral data were collected by LC during observations of both animals in a pair during the two different pair-housing conditions. Each pair was observed for eight-minute behavioral observations that took place four times a day during two observation periods, twice around midday (between 11:15 to 13:45; i.e., midday observation period), and twice in the late afternoon (between 15:30 and 18:00; i.e., afternoon observation period) after intermittent pairs were separated for the day. During the initial pairing condition, 3.6 to 4.3 hours (4.0 ± 0.3 hours) per animal of behavioral data were collected, and during the alternative pairing condition 7.3 to 8.0 hours (7.7 ± 0.3 hours) of data were collected; in total, 303.6 hours of behavioral data were collected from all animals observed. Pair observation order was randomized to account for variation in behavior depending on time of day and to minimize the potential for disruptions due to CNPRC daily activity.
Behavioral data were recorded using fields and forms created with HanDBase Desktop (Version 4.00 Build 5; DDH Software, Wellington, Florida, USA), and collected on a Samsung Galaxy Tab 3 Lite (Samsung Electronics America, Inc., Ridgefield Park, New Jersey, USA) loaded with the HanDBase application for Android (DDH Software, Wellington, Florida, USA).
Behaviors of interest (Table 1) were coded using either one-zero sampling (1/0) or all occurrences (i.e., event) sampling during a 20 second sample interval (Altmann, 1974). Proximity was coded concurrently with other behaviors. Other behaviors (e.g., locomotion, foraging) relating to activity budget were recorded, but not included in analyses. All behaviors except for SDB were recorded using 1/0 sampling. All bouts of SDB were recorded using event sampling and a new entry was made if separate bouts of the behavior occurred within the same interval. A break in a bout was defined if three or more seconds passed without the behavior occurring (Gilbert et al., 2011). Disruptions were recorded if the behavior of the focal subjects was interrupted due to noise outside of the room or CNPRC personnel entering the room.
Data analyses
One pair from the IC experimental group required temporary separation due to injuries received due to an intra-pair conflict part way through the study; therefore, the behavioral data of these two females were not included in data analyses. Poor focal observations were identified if observations were disrupted by an unusual event (e.g., research technician entering room, broken water spigot). These observations (N = 4) and all data collected on pairing condition transition days (day of pairing condition change from initial to alternative; N = 2 days) were eliminated from the data set.
Behavioral data were pooled by each observation period (i.e., midday and afternoon) per day per animal. SDBs were quantified as total bouts observed per observation period, and inactivity, affiliative and abnormal behavior reflected the total number of intervals these behaviors were observed per observation period. Inactivity, SDB, and abnormal behavior were assessed using data collected during both observation periods. Affiliative behaviors could only be assessed during the midday observation period when all individuals had access to their pair-mate.
To assess whether housing condition affected NHP welfare, we fit four generalized linear mixed models (GLMMs) with inactivity, SDB, abnormal behavior, and affiliation as the response variables with a negative binomial error structure using Maximum Likelihood (Bolker et al., 2009). We included interactions between the current pair-housing condition (i.e., current condition) and observation period (i.e., midday when all pairs were paired, afternoon when intermittent pairs were separated), and between current condition and experimental group (i.e., CI or IC) as test predictors in the inactivity, SDB, and abnormal behavior models. Age (years) and weight (kg) at last health check were included as control variables in the inactivity model, whereas time spent in indoor housing (months) and if the animal was the dominant pair-mate (i.e., yes or no) were controlled for in the SDB and abnormal behavior models. Animal identity (N = 22) and pair identity (N = 12) were included as random effects for the inactivity, SDB, and abnormal behavior models. Random slopes were included if the predictors varied within focal animal and pair identity. The random slopes of current pair-housing condition and observation period were included for both random effects in the inactivity and SDB models but excluded from the abnormal behavior model to facilitate model convergence. Affiliation behavior models included the interaction of current pair-housing condition and experimental group as a test predictor, with the pairing duration (years) and if the pair had a previous relationship (yes or no) as control variables and pair identity as a random effect with the corresponding random slopes of the model variables. All models included the random slopes of current pair-housing condition and the number of intervals observed per observation period as an offset term to account for differences in observation time.
Analyses were conducted in R (version 3.6.0; R core Team, 2018), and GLMMs were fit using the ‘glmmTMB’ package with the family argument set to “nbinom1” (version 0.2.3; (Brooks et al., 2017). Prior to fitting the models, we checked the distributions of control covariates and log transformed age, duration paired, and inverse transformed time spent indoors to obtain more normal distributions. Covariates were z-transformed to a mean of zero and a standard deviation of one to provide more comparable estimates and facilitate easier interpretation regarding interactions when scaled (Aiken, West, & Reno, 1991; Schielzeth, 2010).
Model stability was assessed by excluding subjects one at a time and comparing the subset model estimates with those from the full data set. Using the ‘vif’ function within the ‘car’ package (version 3.0–2; (Fox & Weisberg, 2018), we derived Variance Inflation Factors (VIF; Field, 2005; Quinn & Keough, 2002; Tabachnick & Fidell, 2001; Zuur et al., 2010) from a standard linear model lacking random effects and including all predictor and control variables separately (no interaction term) to rule out collinearity. We found no issues with collinearity (maximum VIF for all models: 2.08) or overdispersion (maximum for all models: 0.98) in any model.
To test the significance of our predictor variables, each model was compared to its null counterpart, lacking predictor variables, with a likelihood ratio test (LRT) using the ‘anova’ function with the argument test set to “Chisq” (Dobson & Barnett, 2018; Forstmeier & Schielzeth, 2011). We determined if an interaction contributed significantly to the model by using the ‘drop1’ function which compares the full model to respective reduced models in a series of likelihood ratio tests (Barr, Levy, Scheepers, & Tily, 2013); we removed those interactions (but keeping the main effects) that did not have an effect. Significance for each model term was also determined using the drop1 function once the final model for each behavior was deduced.
RESULTS
Overall, subjects were observed on average for 47.83 intervals or 15.94 minutes per observation period. Social behaviors (e.g., affiliation, aggression) with their pair-mate occurred on average in 26.73 percent of these intervals. Intervals that subjects were involved in other activities (e.g., exploring or manipulating the environment, foraging or feeding) occurred in 29.19 percent of intervals. Locomotion occurred in 20.45 percent of intervals that the subjects were observed. Interactions directed towards neighbors, the observer, or self (via mirror) occurred in 9.83 percent of intervals on average. Descriptive information for the behaviors of interest is described at the beginning of the corresponding subsection.
Inactivity
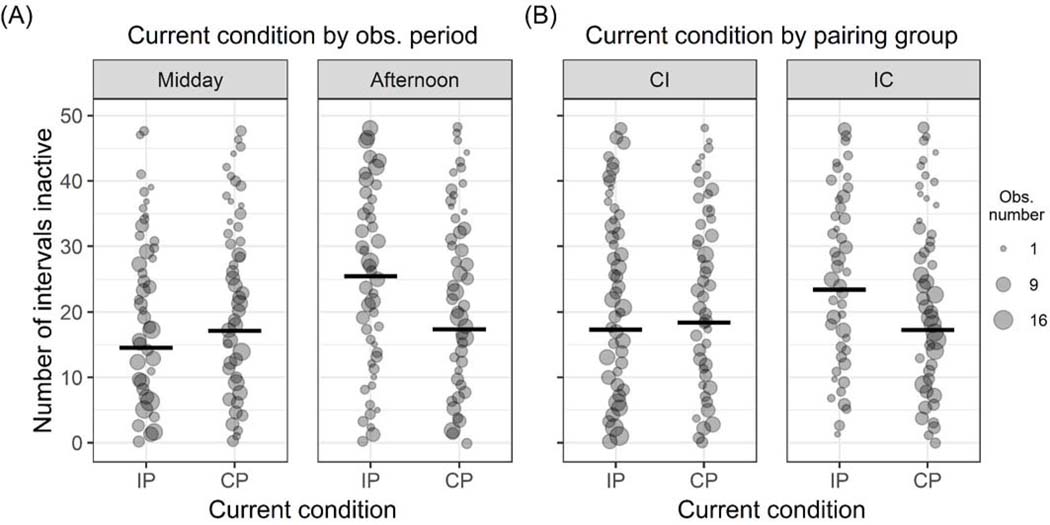
Inactivity occurred on average in 43.83 percent of intervals subjects were observed for. The model comparison between our full and null models for inactivity behavior was significant (LRT: Χ2 = 27.36, df = 5, p < 0.001). The model included significant interactions between current pair-housing condition and experimental group (LRT: Χ2 = 6.00, df = 1, p = 0.014), and current pairing housing condition and observation period (LRT: Χ2 = 11.27, df = 1, p = 0.001; Table 2). Model estimates indicate that inactivity levels were relatively stable across both observation periods when animals were continuously paired, whereas intermittently paired subjects were inactive more often in the afternoon when they were separated from their pair-mate than at midday when they were paired (Figure 2A). A comparison of model estimates across pair-housing conditions within each observation period indicate that inactivity was marginally lower for intermittent pairs than continuous pairs at midday, but substantially higher for the former over the latter during the afternoon observation period when intermittently paired subjects were separated from their pair-mate (Figure 2A). Additionally, model estimates for animals in the CI experimental group weakly indicate that inactivity was lower when they were intermittently paired than when they were continuously paired; the IC experimental group exhibited comparatively higher levels of inactivity when they were intermittently paired than the CI experimental group and when they were continuously paired (Figure 2B).
Table 2:
Results of the final GLMM examining the effect of the test and control predictors on the number of intervals that inactivity was observed.
| Estimate | SE | Z | df | χ2 | p–value | |
|---|---|---|---|---|---|---|
| Intercept | −1.31 | 0.15 | −8.82 | |||
| Test predictors | ||||||
| Current condition x Observation period † | 1 | 11.27 | 0.001 | |||
| Current condition (Continuous)a | 0.34 | 0.11 | 3.00 | |||
| Observation period (Afternoon)b | 0.55 | 0.11 | 5.22 | |||
| Current condition x Experimental group † | 1 | 6.01 | 0.014 | |||
| Current condition (Continuous)a | 0.34 | 0.11 | 3.01 | |||
| Experimental Group (IC)c | 0.28 | 0.20 | 1.39 | |||
| Control predictors | ||||||
| Age (Years)d | 0.19 | 0.09 | 2.21 | 1 | 4.37 | 0.037 |
| Weight (kg)e | −0.12 | 0.08 | −1.46 | 1 | 1.90 | 0.168 |
Interaction of specified predictors.
Current condition was dummy coded with the ‘intermittent’ pair housing condition being the reference category.
Observation period was dummy coded with ‘midday’ being the reference category.
Experimental group was dummy coded with the ‘CI’ experimental group being the reference category.
Age (years) was log and z-transformed, original values ranged from 4.84 to 10.77 years, mean ± standard deviation: 6.63 ± 1.72 years.
Weight (kg) was z-transformed, original values ranged from 4.74 to 8.72 kg, mean ± standard deviation: 6.55 ± 1.18 kg.
Figure 2:
Effects of the interactions within the final inactivity model. Point size scales with number of observations. Thick horizontal lines indicate the fitted model estimates for each condition when all other predictors and number of observation intervals are at their mean. (A) Interaction between the current pair-housing condition (intermittent: “IP”; continuous: “CP”) and observation period (midday, afternoon) on inactivity levels. Point size ranges from 1 to 16 observations. (B) Interaction between the current pairing condition and experimental group (CI, IC) on inactivity levels. Point size ranges from 1 to 17 observations.
Self-directed behavior (SDB)
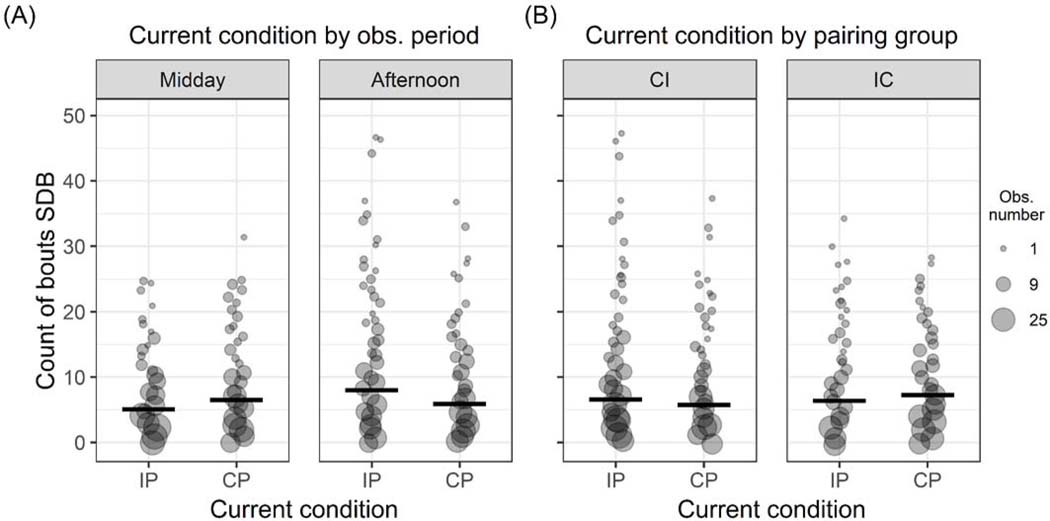
Bouts of SDB occurred on average 4.5 times per minute of observation. The full model for total bouts of SDB was significantly different than its null model (LRT: Χ2 = 16.93, df = 5, p = 0.013). The interaction of current pair-housing condition and observation period was significant (LRT: Χ2 = 7.45, df = 5, p = 0.006; Table 3). Model estimates indicate that bouts of SDB exhibited by continuously paired study subjects stayed relatively stable over observation periods, whereas bouts of SDBs exhibited when subjects were intermittently paired were lower at midday (when subjects had physical access to one another) than in the afternoon when subjects were separated from one another (Figure 3A). In comparison to continuous pair-housing levels, self-directed behavior was lower during the midday observation period, but higher in the afternoon when pair-mate separation occurred (Figure 3A). Additionally, the interaction of current pair-housing condition and experimental group was significant (LRT: Χ2 = 4.26, df = 5, p = 0.039; Table 3). Animals in the CI experimental group exhibited more bouts of SDB when intermittently paired than when continuously paired, whereas animals in the IC experimental group exhibited less SDB when intermittently paired than when continuously paired (Figure 3B).
Table 3:
Results of the final GLMM examining the effect of the test and control predictors on the number of bouts self-directed behavior was observed.
| Estimate | SE | Z | df | χ2 | p–value | |
|---|---|---|---|---|---|---|
| Intercept | −2.28 | 0.23 | −9.58 | |||
| Test predictors | ||||||
| Current condition x Observation period † | 1 | 7.45 | 0.006 | |||
| Current condition (Continuous)a | 0.14 | 0.12 | 1.19 | |||
| Observation period (Afternoon)b | 0.46 | 0.12 | 3.98 | |||
| Current condition x Experimental group † | 1 | 4.26 | 0.039 | |||
| Current condition (Continuous)a | 0.14 | 0.12 | 1.19 | |||
| Experimental group (IC)c | −0.06 | 0.27 | −0.24 | |||
| Control predictors | ||||||
| Dominant (Yes)d | 0.11 | 0.19 | 0.58 | 1 | 0.38 | 0.540 |
| Time spent indoors (Months)e | 0.11 | 0.13 | 0.84 | 1 | 0.65 | 0.419 |
Interaction of specified predictors.
Current condition was dummy coded with the ‘intermittent’ pair housing condition being the reference category.
Observation period was dummy coded with ‘midday’ being the reference category.
Experimental group was dummy coded with the ‘CI’ experimental group being the reference category.
Dominant was dummy coded with ‘no’ being the reference category.
Time spent indoors (months) was inverse and z-transformed, original values ranged from 4.50 to 81.60 months, mean ± standard deviation: 19.23 ± 18.53 months.
Figure 3:
Effects of the interactions within the final self-directed behavior (SDB) model. Point size scales with number of observations. Thick horizontal lines indicate the fitted model estimates for each condition when all other predictors and number of observation intervals are at their mean. (A) Interaction between the current pair-housing condition (intermittent: “IP”; continuous: “CP”) and observation period (midday, afternoon) on SDB. Point size ranges from 1 to 35 observations. (B) Interaction between the current pairing condition and experimental group (CI, IC) on SDB. Point size ranges from 1 to 32 observations.
Abnormal behavior
Abnormal behavior occurred on average in 3.47 percent of intervals subjects were observed for. The full-null model comparison for abnormal behavior was not significant (LRT: Χ2 = 5.11, df = 5, p = 0.402), indicating that the interactions or main effects of current pair-housing condition with experimental group, and current condition with observation period did not explain the occurrence of abnormal behavior in our study population.
Affiliative behavior
Affiliative behavior between pair-mates was assessed for 47.74 intervals of observation on average or 15.91 minutes during the midday observation period. On average, proximity occurred in 43.83 percent of intervals, grooming occurred in 29.11 percent of intervals, coalition behavior occurred in 37.63 percent of intervals, and other types of affiliation occurred on average in 8.61 percent of intervals. There was no significant difference between the full-null model comparisons for proximity behavior (LRT: Χ2 = 4.90, df = 3, p = 0.179) and coalition behavior (LRT: Χ2 = 6.58, df = 3, p = 0.087), indicating that the interaction or main effects of current pair-housing condition and experimental group did not influence the occurrence of these behaviors. The full-null model comparison for grooming behavior was significant (LRT: Χ2 = 8.01, df = 3, p = 0.046) as was that for other types of affiliation (LRT: Χ2 = 9.09, df = 3, p = 0.028). The final model for both of these behaviors included significant main effect of current pair-housing condition (grooming LRT: Χ2 = 5.27, df = 1, p = 0.022; other affiliation LRT: Χ2 = 4.59, df = 1, p = 0.032; Tables 4 and 5 respectively; Figure 4), but not experimental group (grooming LRT: Χ2 = 0.84, df = 1, p = 0.433; other affiliation LRT: Χ2 = 3.04, df = 1, p = 0.081; Tables 4 and 5 respectively). Model estimates indicate that grooming and other types of affiliation were higher during the midday observation period (when pair-mates had physical access to one another in all pair-housing conditions) when study subjects were intermittently paired than when they were continuously paired.
Table 4:
Results of the final GLMM examining the effect of the test and control predictors on the number of intervals grooming was observed.
| Estimate | SE | Z | df | χ2 | p–value | |
|---|---|---|---|---|---|---|
| Intercept | −1.15 | 0.42 | −2.75 | |||
| Test predictors | ||||||
| Current condition (Continuous)a | −0.40 | 0.15 | −2.63 | 1 | 5.27 | 0.022 |
| Experimental group (IC)b | −0.11 | 0.54 | −0.21 | 1 | 0.04 | 0.836 |
| Control predictors | ||||||
| Previous relationship (Yes)c | −0.13 | 0.48 | −0.27 | 1 | 0.08 | 0.784 |
| Pairing duration (Years)d | 0.23 | 0.28 | 0.80 | 1 | 0.61 | 0.433 |
Current condition was dummy coded with the ‘intermittent’ pair housing condition being the reference category.
Experimental group was dummy coded with the ‘CI’ experimental group being the reference category.
Previous relationship was dummy coded with ‘no’ previous relationship being the reference category.
Pairing duration (years) was log and z-transformed, original values ranged from 0.30 to 2.07 years, mean ± standard deviation: 0.90 ± 0.53 years.
Table 5:
Results of the final GLMM examining the effect of the test and control predictors on the number of intervals other types of affiliation was observed.
| Estimate | SE | Z | df | χ2 | p–value | |
|---|---|---|---|---|---|---|
| Intercept | −2.65 | 0.39 | −6.74 | |||
| Test predictors | ||||||
| Current condition (Continuous)a | −0.39 | 0.17 | −2.36 | 1 | 4.59 | 0.032 |
| Experimental group (IC)b | 0.95 | 0.50 | 1.89 | 1 | 3.04 | 0.081 |
| Control predictors | ||||||
| Previous relationship (Yes)c | −0.62 | 0.45 | −1.40 | 1 | 1.77 | 0.184 |
| Pairing duration (Years)d | −0.67 | 0.26 | −2.56 | 1 | 5.21 | 0.022 |
Current condition was dummy coded with the ‘intermittent’ pair housing condition being the reference category.
Experimental group was dummy coded with the ‘CI’ experimental group being the reference category.
Previous relationship was dummy coded with ‘no’ previous relationship being the reference category.
Pairing duration (years) was log and z-transformed, original values ranged from 0.30 to 2.07 years, mean ± standard deviation: 0.90 ± 0.53 years.
Figure 4:
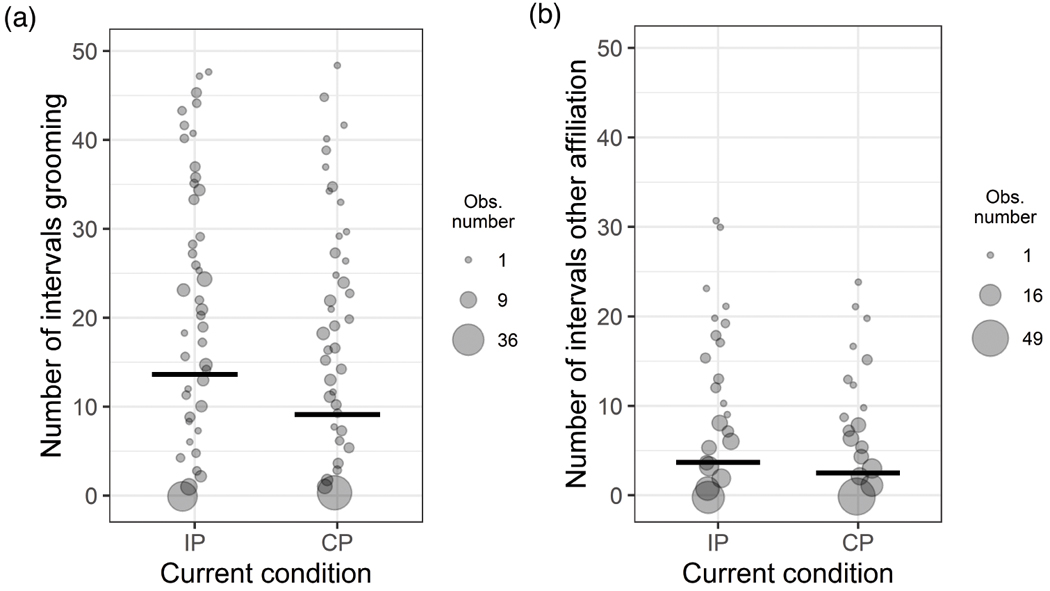
Effects of current pair-housing condition (intermittent: “IP”; continuous: “CP”) on (A) grooming and (B) other types of affiliation. Point size scales with number of observations (grooming: 1 to 44 observations; other affiliation: 1 to 51 observations). Thick horizontal lines indicate the fitted model estimates for each condition when all other predictors and number of observation intervals are at their mean.
DISCUSSION
Our study provides the first evidence that continuous pair-housing confers behavioral benefits over intermittent pair-housing. Levels of afternoon inactivity were reduced when females had access to their pair-mate (continuous pair-housing condition) compared to when pair-mates were separated (intermittent pair-housing condition). Furthermore, females exhibited self-directed behavior (SDB) less frequently during afternoon observations when they were continuously paired than when they were intermittently paired. Decreases in these measures are widely accepted as indicators of an improved behavioral repertoire in captive NHPs (Fureix et al., 2015; Leeds & Lukas, 2019; Maestripieri et al., 1992).
Other pair-housing studies of female rhesus macaques found that levels of inactivity and bouts of SDB decreased when subjects in single-housing and protected contact switched to either intermittent or continuous pair-housing (Baker et al., 2014, 2012a). However, they found no differences in levels of inactivity and SDB between intermittent and continuous housing conditions, leading to the conclusion that temporary separations due to intermittent pair-housing did not impede the overall benefits reaped from limited contact with a pair-mate (Baker et al., 2014). It is important to note that Baker et al. (2014) did not assess behavioral measures of well-being while pair-mates were separated. The presence of conspecifics in captive environments allows captive NHPs to exhibit a wide range of species-typical patterns of behavior (Novak & Suomi, 1988). Physical separation, even if only temporary, completely removes the opportunity for tactile, species-specific behaviors (Baker et al., 2012a; Novak et al., 1988), particularly affiliation and co-sleep which are key adaptive behaviors for group-living NHPs (Anderson, 2000; Dunbar, 1991). Preventing such behaviors from occurring may consequently result in elevated levels of inactivity or SDB as seen in this study. Furthermore, increased levels of inactivity may have detrimental physiological (e.g., weight gain; Katzmarzyk, 2010) and psychological outcomes (e.g., boredom, lack of cognitive stimulation; Meagher, 2019). A pair-housing option that consistently minimizes abnormal behavior, inactivity, and SDB, and that promotes positive adaptive behaviors throughout the entirety of the day, would therefore provide superior well-being for laboratory NHPs.
Interestingly, the levels of grooming and other types of affiliative behaviors measured in this study were elevated when females were intermittently paired, whereas proximity and coalition behaviors were not related to current pair-housing condition or experimental group. This result is similar to the trend Baker et al. (2014) found in their study, where adult female rhesus macaques tended to socialize more often in intermittent contact than when in continuous contact with their pair-mate. There could be several reasons why our females elevated their affiliative behavior when in intermittent contact. First, females in intermittent contact may increase rates of affiliative behavior within the window of time that they are paired, to mitigate daily stressors induced by a captive environment. Furthermore, it seems that behaviors involving physiological touch (i.e., grooming, other types of affiliation) – and not those such as proximity and coalitionary behaviors - may be the salient means for adult female rhesus to cope with the loss of physical access to their pair-mate. The social buffering hypothesis proposes that social partners temper behavioral and physiological stress responses (Balasubramaniam, Beisner, Vandeleest, Atwill, & McCowan, 2016; Cohen & Wills, 1985; Hennessy, Kaiser, & Sachser, 2009; Kikusui, Winslow, & Mori, 2006). In fact, NHPs have been known to affiliate with each other to mitigate various kinds of stress (social: Judge & Mullen, 2005; novel environment: French & Schaffner, 2000; relocation: Gust et al., 1994; sedation of neighbors: Gilbert & Baker, 2011; husbandry: Boccia et al., 1995). The ‘tend-and-befriend’ hypothesis further proposes that when levels of affiliation are inadequate, biological signaling systems (i.e., release of oxytocin) stimulate purposeful social behavior with conspecifics (Taylor et al., 2000). If social partners are supportive and comforting, these stress responses decline (Taylor, 2006). The higher rates of affiliative behaviors exhibited by females in intermittent pair-housing suggests that these females are actively trying to cope with the daily temporary separation; because continuous housing conditions provide the constant benefit of social companionship, elevated levels of these behaviors during the middle of the day may be unnecessary. This interpretation is also supported by the results found for individual levels of inactivity and SDB during the midday observation period, where these behaviors were slightly lower than levels exhibited at midday when they were continuously housed.
A second explanation for this difference in affiliative behavior between pair-housing conditions is that females may have redistributed the time they spent engaging in affiliative behavior throughout the day when in continuous pair-housing. This redistribution could occur due to the reduced pressure to affiliate during a tight time window. Furthermore, continuous pair-housing allows pair-mates to huddle overnight, during which they can receive the benefits of physical contact without needing to make up a deficit the following day.
Alternatively, lower levels of active affiliative behavior during the continuous pair-housing phase may occur to minimize the length of physical contact bouts with a pair-mate who may not be very compatible. Although we did not explore measures of pair compatibility in this study (e.g., Hannibal et al., 2018), this alternative explanation is unlikely, due to the thorough pairing process conducted by trained staff at the CNPRC at the time of our study. Despite having met CNPRC’s compatibility criteria, however, one IC pair did inflict minor wounds (scratches to face and arms) to each other during their continuous pair-housing phase, were consequently temporarily separated, and therefore not included in analyses. The change in pair-housing condition from intermittent to continuous may have triggered this incident to occur between these two pair-mates due to a sudden increase in access time. The implications of changes to pair-housing condition are not very well understood and require longer term studies (Hannibal et al., 2017).
Although we did not find differences between experimental groups for affiliative behavior, we did find opposing differences in inactivity and SDB between the groups. Over both observation periods, females in the CI experimental group had slightly higher levels of SDB and lower inactivity when intermittently paired versus than when they were continuously paired, whereas IC females exhibited lower SDB and higher inactivity in the former pair-housing condition versus the latter. One distinct group difference is that most females assigned to the CI experimental group had pair-mates they previously knew from a social group (including kin and non-kin; five of seven pairs previously knew each other), whereas most IC females did not previously know each other (one of five pairs previously knew each other). We did account for previous relationship status as a control variable in our affiliation models and these analyses were explored at the pair level, finding no effect. However, this variable may partially explain these experimental group effects seen for inactivity and SDB. At the time of the study, CNPRC pair-housing staff used this knowledge of animals’ previous relationships to match pairs as these individuals likely had previously established dominance relationships that would likely continue and therefore expedite the pair-housing process. Consequently, CI paired females may be more compatibly matched overall and may have been more agitated with the change in pair-housing condition (and reduced access to their pair-mate) as they went from being continuous pair-housed to intermittently pair-housed. Conversely, the IC experimental group experienced the reverse pair-housing change, which improved their overall activity but elevated SDB behavior, suggesting these females needed to boost these behaviors to cope with the new pair-housing arrangement.
Another explanation for experimental group effects could be that our results may have captured short-term adjustments and potential transient effects to changes in pair-housing (Baker et al., 2014). Although these effects appear to be moderate, researchers should consider incorporating a period of habituation into research plans as the impacts of pair-housing changes are relatively unknown (Capitanio, Kyes, & Fairbanks, 2006; Hannibal et al., 2017). Therefore, further long-term exploration of behavioral, as well as physiological, responses to intermittent and continuous pair-housing is necessary to understand if intermittent contact is a less favorable housing arrangement. Factors intrinsic to the individual (e.g., temperament, developmental history, early experience), the non-social (e.g., feeding regime), and social environment (e.g., who they are paired with) may also shape an individual’s response (Boccia et al., 1995; Coleman, 2012), but exploring these was not within the scope our study. These numerous factors highlight the importance of assessing welfare in NHPs at the individual level. Nonetheless, understanding which captive NHP management practices, such as type of pair-housing, enhance welfare overall, is a vital first step forward.
The occurrence of abnormal behavior was not related to current pair-housing condition, observation period, or experimental group; however, this may be because these behaviors occurred infrequently. Likely the low prevalence of abnormal behavior in our study can be attributed to our animal selection criteria (mother-reared in outdoor social groups). Previous studies have found that one of the main predictors of abnormal behavior development is restricted rearing background, such as nursery-rearing (Gottlieb, Capitanio, & McCowan, 2013; Novak et al., 2006; Rommeck, Anderson, Heagerty, Cameron, & McCowan, 2009; Vandeleest, McCowan, & Capitanio, 2011). None of our study subjects were nursery-reared, thus plausibly accounting for the low frequency of abnormal behavior observed. Recording these behaviors in the context of housing management practices, however, is critical to evaluate, particularly for vulnerable NHP populations, as these behaviors are known to occur due to experimental procedures (e.g., blood draws) and other housing management practices (i.e., cage location), and could develop into deleterious self-injurious behaviors in some cases (Gottlieb et al., 2013; Gottlieb, Maier, & Coleman, 2015).
Differences of the model estimates of SDB and other types of affiliative behavior were seemingly small (Figures 3 and 4 respectively), but statistically meaningful. Average SDB bouts increased by 69% from midday to afternoon observations for intermittently paired females and the average number of intervals other affiliation occurred increased by 44% during intermittent pair-housing in comparison to continuous pair-housing. From a practical standpoint, subtle shifts of these behaviors alone may not provide strong evidence that one pair-housing condition is superior. However, we assessed these behaviors alongside other behavioral indices of welfare and found the results largely in agreement that continuous housing confers greater behavioral welfare benefits over intermittent housing.
In the laboratory setting, intermittent pair-housing remains an alternative to single-housing when some separation is required as it offers NHPs some social contact with conspecifics while facilitating laboratory research procedures. However, modifications for carrying out common experimental procedures should be strongly considered before defaulting to intermittent pair-housing. For example, health monitoring for diarrhea or fecal sample collection can be conducted by offering pair-mates food items with differently colored dyes to distinguish feces. Macaques with a more limited social history, particularly while young, may have different outcomes under intermittent pairing. Overall, social-housing practices should prioritize the biological and psychological needs of the animals and not merely be determined by the ease with which they can be implemented by husbandry staff such as scheduling activities for convenience (Brando & Buchanan-Smith, 2018). The feasibility of using integrated social housing systems to simultaneously maximize welfare and facilitate research should be assessed in future pair-housing studies. Both welfare and research objectives may be optimized by utilizing protected pair-housing in place of complete temporary separation. This method would, for example, prevent feeding aggression and facilitate the collection of separate biological specimens, such as urine, from individuals, while maximizing the social benefits of protected pair-housing during these times.
Conclusions
Monitoring changes in a captive animal’s behavioral repertoire provides a valuable means for assessing welfare non-invasively (Honess, Johnson, & Wolfensohn, 2004). One of the main goals of captive animal welfare research is to continually refine the management practices implemented in animal care, especially in the biomedical research environment. Refining husbandry practices not only improves animal welfare, but also improves the scientific integrity of the research being carried out (Jennings & Prescott, 2009; Poole, 1997). The present study provides evidence that continuous pair-housing confers welfare benefits over intermittent pair-housing for adult female rhesus macaques. These results enhance the current literature on pair-housing and will help guide further efforts to improve NHP welfare in the biomedical research setting.
ACKNOWLEDGEMENTS
This research was supported by an NIH base grant awarded to the California National Primate Research Center (CNPRC; P51-OD011107-53). The authors would like to thank the CNPRC Primate Services for providing study subjects. Additional thanks go to the CNPRC Population Behavioral Health Services and Animal Care staff for taking care of the monkeys and assisting with the implementation of our project. Special thanks go to the two anonymous reviewers for their constructive feedback.
Footnotes
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. London, UK: Sage Publications. [Google Scholar]
- Altmann J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49(3), 227–266. DOI: 10.1163/156853974X00534 [DOI] [PubMed] [Google Scholar]
- Anderson JR (2000). Sleep-related behavioural adaptations in free-ranging anthropoid primates. Sleep Medicine Reviews, 4(4), 355–373. DOI: 10.1053/smrv.2000.0105 [DOI] [PubMed] [Google Scholar]
- Aureli F. (1992). Post-conflict behaviour among wild long-tailed macaques (Macaca fascicularis). Behavioral Ecology and Sociobiology, 31(5), 329–337. DOI: 10.1007/BF00177773 [DOI] [Google Scholar]
- Aureli F, Preston SD, & de Waal F. (1999). Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. Journal of Comparative Psychology, 113(1), 59. DOI: 10.1037/0735-7036.113.1.59 [DOI] [PubMed] [Google Scholar]
- Aureli F, & Yates K. (2010). Distress prevention by grooming others in crested black macaques. Biology Letters, 6(1), 27–29. DOI: 10.1098/rsbl.2009.0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, & Schoof VAM (2014). Comparing options for pair housing rhesus macaques using behavioral welfare measures. American Journal of Primatology, 76(1), 30–42. DOI: 10.1002/ajp.22190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof V, & Maloney M. (2012a). Benefits of pair housing are consistent across a diverse population of rhesus macaques. Applied Animal Behaviour Science, 137(3), 148–156. DOI: 10.1016/j.applanim.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Crockett CM, Lee GH, Oettinger BC, Schoof V, & Thom JP (2012b). Pair housing for female longtailed and rhesus macaques in the laboratory: Behavior in protected contact versus full contact. Journal of Applied Animal Welfare Science, 15(2), 126–143. DOI: 10.1080/10888705.2012.658330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam K, Beisner B, Vandeleest J, Atwill E, & McCowan B. (2016). Social buffering and contact transmission: network connections have beneficial and detrimental effects on Shigella infection risk among captive rhesus macaques. PeerJ, 4, e2630. DOI: 10.7717/peerj.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, & Tily HJ (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. DOI: 10.1016/j.jml.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois-Heulin C, & Jubin R. (2004). Influence of the presence of seeds and litter on the behaviour of captive red-capped mangabeys Cercocebus torquatus torquatus. Applied Animal Behaviour Science, 85(3–4), 349–362. DOI: 10.1016/j.applanim.2003.10.005 [DOI] [Google Scholar]
- Boccia ML, Laudenslager ML, & Reite ML (1995). Individual differences in macaques’ responses to stressors based on social and physiological factors: Implications for primate welfare and research outcomes. Laboratory Animals, 29(3), 250–257. DOI: 10.1258/002367795781088315 [DOI] [PubMed] [Google Scholar]
- Boccia ML, Reite M, & Laudenslager M. (1989). On the physiology of grooming in a pigtail macaque. Physiology & Behavior, 45(3), 667–670. DOI: 10.1016/0031-9384(89)90089-9 [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, & White J-SS (2009). Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution, 24(3), 127–135. DOI: 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Brando S, & Buchanan-Smith HM (2018). The 24/7 approach to promoting optimal welfare for captive wild animals. Behavioural Processes, 156, 83–95. DOI: 10.1016/j.beproc.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Brooks M,E, Kristensen K, Benthem K,J, van Magnusson A, Berg C,W, Nielsen A, … Bolker B,M (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal, 9(2), 378–400. DOI: 10.32614/RJ-2017-066 [DOI] [Google Scholar]
- Broom DM (1986). Indicators of poor welfare. British Veterinary Journal, 142(6), 524–526. DOI: 10.1016/0007-1935(86)90109-0 [DOI] [PubMed] [Google Scholar]
- Capellini I, Barton RA, McNamara P, Preston BT, & Nunn CL (2008a). Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution, 62(7), 1764–1776. DOI: 10.1111/j.1558-5646.2008.00392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellini I, Nunn CL, McNamara P, Preston BT, & Barton RA (2008b). Energetic constraints, not predation, influence the evolution of sleep patterning in mammals. Functional Ecology, 22(5), 847–853. DOI: 10.1111/j.1365-2435.2008.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, & Fairbanks LA (2006). Considerations in the selection and conditioning of Old World monkeys for laboratory research: Animals from domestic sources. ILAR Journal, 47(4), 294–306. DOI: 10.1093/ilar.47.4.294 [DOI] [PubMed] [Google Scholar]
- Carder G, & Semple S. (2008). Visitor effects on anxiety in two captive groups of western lowland gorillas. Applied Animal Behaviour Science, 115(3–4), 211–220. DOI: 10.1016/j.applanim.2008.06.001 [DOI] [Google Scholar]
- Cohen S, & Wills TA (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98(2), 310. DOI: 10.1037/0033-2909.98.2.310 [DOI] [PubMed] [Google Scholar]
- Coleman K. (2012). Individual differences in temperament and behavioral management practices for nonhuman primates. Applied Animal Behaviour Science, 137(3–4), 106–113. DOI: 10.1016/j.applanim.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Directive of the European Parliament. (2010). Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes with EEA relevance. Official Journal of the European Union 20102010 L276/33-L276/79, 47. [Google Scholar]
- Dobson AJ, & Barnett AG (2018). An Introduction to Generalized Linear Models (4th ed.). Boca Raton, FL: CRC Press. DOI: 10.1201/9781315182780 [DOI] [Google Scholar]
- Dunbar RI (1991). Functional significance of social grooming in primates. Folia Primatologica, 57(3), 121–131. DOI: 10.1159/000156574 [DOI] [Google Scholar]
- Eaton GG, Kelley ST, Axthelm MK, Iliff‐Sizemore SA, & Shiigi SM (1994). Psychological well‐being in paired adult female rhesus (Macaca mulatta). American Journal of Primatology, 33(2), 89–99. DOI: 10.1002/ajp.1350330204 [DOI] [PubMed] [Google Scholar]
- Field AP (2005). Discovering Statistics Using SPSS (2nd ed.). London, UK: Sage Publications. [Google Scholar]
- Forstmeier W, & Schielzeth H. (2011). Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behavioral Ecology and Sociobiology, 65(1), 47–55. DOI: 10.1007/s00265-010-1038-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, & Weisberg S. (2018). An R Companion to Applied Regression (3rd ed.). London, UK: Sage Publications. https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [Google Scholar]
- French J, & Schaffner C. (2000). Contextual influences on sociosexual behavior in monogamous primates. Reproduction in Context, 325–353. [Google Scholar]
- Fureix C, & Meagher RK (2015). What can inactivity (in its various forms) reveal about affective states in non-human animals? A review. Applied Animal Behaviour Science, 171, 8–24. DOI: 10.1016/j.applanim.2015.08.036 [DOI] [Google Scholar]
- Gerber P, Anzenberger G, & Schnell CR (2002). Behavioral and cardiophysiological responses of common marmosets (Callithrix jacchus) to social and environmental changes. Primates, 43(3), 201–216. DOI: 10.1007/BF02629648 [DOI] [PubMed] [Google Scholar]
- Gilbert C, McCafferty D, Le Maho Y, Martrette J-M, Giroud S, Blanc S, & Ancel A. (2009). One for all and all for one: the energetic benefits of huddling in endotherms. Biological Reviews, 85(3), 545–569. DOI: 10.1111/j.1469-185X.2009.00115.x [DOI] [PubMed] [Google Scholar]
- Gilbert MH, & Baker KC (2011). Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. Journal of Medical Primatology, 40(2), 71–78. DOI: 10.1111/j.1600-0684.2010.00447.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, & McCowan B. (2013). Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): Animal’s history, current environment, and personality. American Journal of Primatology, 75(10), 995–1008. DOI: 10.1002/ajp.22161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Maier A, & Coleman K. (2015). Evaluation of environmental and intrinsic factors that contribute to stereotypic behavior in captive rhesus macaques (Macaca mulatta). Applied Animal Behaviour Science, 171, 184–191. DOI: 10.1016/j.applanim.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, & McClure HM (1994). Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiology & Behavior, 55(4), 681–684. DOI: 10.1016/0031-9384(94)90044-2 [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright KM, & Wilson ME (1993). Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta). Hormones and Behavior, 27(3), 318–331. DOI: 10.1006/hbeh.1993.1024 [DOI] [PubMed] [Google Scholar]
- Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, & Capitanio J. (2017). Laboratory rhesus macaque social housing and social changes: Implications for research. American Journal of Primatology, 79(1), e22528. DOI: 10.1002/ajp.22528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal DL, Cassidy LC, Vandeleest J, Semple S, Barnard A, Chun K, … McCowan B. (2018). Intermittent pair-housing, pair relationship qualities, and HPA activity in adult female rhesus macaques. American Journal of Primatology, e22762. DOI: 10.1002/ajp.22762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, & Sachser N. (2009). Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology, 30(4), 470–482. DOI: 10.1016/j.yfrne.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Honess PE, Johnson P, & Wolfensohn S. (2004). A study of behavioural responses of non-human primates to air transport and re-housing. Laboratory Animals, 38(2), 119–132. DOI: 10.1258/002367704322968795 [DOI] [PubMed] [Google Scholar]
- Jennings M, & Prescott MJ (2009). Refinements in husbandry, care and common procedures for non-human primates: Ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Laboratory Animals, 43(1_suppl), 1–47. [DOI] [PubMed] [Google Scholar]
- Judge PG, & Mullen SH (2005). Quadratic postconflict affiliation among bystanders in a hamadryas baboon group. Animal Behaviour, 69(6), 1345–1355. DOI: 10.1016/j.anbehav.2004.08.016 [DOI] [Google Scholar]
- Katzmarzyk PT (2010). Physical activity, sedentary behavior, and health: Paradigm paralysis or paradigm shift? Diabetes, 59(11), 2717–2725. DOI: 10.2337/db10-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y. (2006). Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1476), 2215–2228. DOI: 10.1098/rstb.2006.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen AM, & Martin AA (1996). The effects of cage size and complexity on the behaviour of captive common marmosets, Callithrix jacchus jacchus. Laboratory Animals, 30(4), 317–326. DOI: 10.1258/002367796780739853 [DOI] [PubMed] [Google Scholar]
- Leeds A, & Lukas KE (2019). Monitoring the social behavior of a bachelor mandrill (Mandrillus sphinx) dyad participating in touchscreen-mediated cognitive testing. Zoo Biology, 38(4), 397–402. DOI: 10.1002/zoo.21490 [DOI] [PubMed] [Google Scholar]
- Lutz CK, & Novak MA (2005). Environmental enrichment for nonhuman primates: Theory and application. ILAR Journal, 46(2), 178–191. DOI: 10.1093/ilar.46.2.178 [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Schino G, Aureli F, & Troisi A. (1992). A modest proposal: Displacement activities as an indicator of emotions in primates. Animal Behaviour, 44(5), 967–979. DOI: 10.1016/S0003-3472(05)80592-5 [DOI] [Google Scholar]
- Majolo B, & Huang P. (2018). Group Living. In Vonk J & Shackelford T (Eds.), Encyclopedia of Animal Cognition and Behavior (pp. 1–12) Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Mallapur A, Sinha A, & Waran N. (2005). Influence of visitor presence on the behaviour of captive lion-tailed macaques (Macaca silenus) housed in Indian zoos. Applied Animal Behaviour Science, 94(3–4), 341–352. DOI: 10.1016/j.applanim.2005.02.012 [DOI] [PubMed] [Google Scholar]
- Mason GJ (1991). Stereotypies: A critical review. Animal Behaviour, 41(6), 1015–1037. DOI: 10.1016/S0003-3472(05)80640-2 [DOI] [Google Scholar]
- Meagher R. (2019). Is boredom an animal welfare concern? Animal Welfare, 28(1), 21–32. DOI: 10.7120/09627286.28.1.021 [DOI] [Google Scholar]
- Moberg GP, & Mench JA (2000). The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. Oxfordshire, UK: CABI. [Google Scholar]
- Mochida K, & Nishikawa M. (2014). Sleep duration is affected by social relationships among sleeping partners in wild Japanese macaques. Behavioural Processes, 103, 102–104. DOI: 10.1016/j.beproc.2013.10.010 [DOI] [PubMed] [Google Scholar]
- National Research Council. (2011). Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, D.C.: National Academies Press. DOI: 10.17226/12910 [DOI] [Google Scholar]
- Novak MA, Meyer J, Lutz C, & Tiefenbacher S. (2006). Deprived environments: Developmental insights from primatology. In Mason GJ & Rushen J (Eds.), Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare (2nd ed., pp. 153–189) Wallingford, UK: CABI. [Google Scholar]
- Novak MA, & Suomi SJ (1988). Psychological well-being of primates in captivity. American Psychologist, 43(10), 765–773. DOI: 10.1037/0003-066X.43.10.765 [DOI] [PubMed] [Google Scholar]
- Office of Laboratory Animal Welfare. (2019). National Institutes of Health. https://olaw.nih.gov/ [Google Scholar]
- Olsson IAS, & Westlund K. (2007). More than numbers matter: The effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Applied Animal Behaviour Science, 103(3–4), 229–254. DOI: 10.1016/j.applanim.2006.05.022 [DOI] [Google Scholar]
- Ostner J, & Schülke O. (2018). Linking sociality to fitness in primates: A call for mechanisms. In Naguib M, Barrett L, Healey SD, Podos J, Simmons LW, & Zuk M (Eds.), Advances in the Study of Behavior (Vol. 50, pp. 127–175) San Diego, CA: Academic Press. [Google Scholar]
- Poole T. (1997). Happy animals make good science. Laboratory Animals, 31(2), 116–124. DOI: 10.1258/002367797780600198 [DOI] [PubMed] [Google Scholar]
- Quinn G, & Keough M. (2002). Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press. [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/ [Google Scholar]
- Reamer L, Tooze Z, Coulson C, & Semple S. (2010). Correlates of self-directed and stereotypic behaviours in captive red-capped mangabeys (Cercocebus torquatus torquatus). Applied Animal Behaviour Science, 124(1–2), 68–74. DOI: 10.1016/j.applanim.2010.01.012 [DOI] [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, & McCowan B. (2009). Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. Journal of Applied Animal Welfare Science, 12(1), 61–72. DOI: 10.1080/10888700802536798 [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Suarez SA, & Porter LM (1996). Effects of social and inanimate enrichment on the behavior of yearling rhesus monkeys. American Journal of Primatology, 40(3), 247–260. DOI: [DOI] [PubMed] [Google Scholar]
- Schielzeth H. (2010). Simple Means to Improve the Interpretability of Regression Coefficients. Methods in Ecology and Evolution, 1(2), 103–113. DOI: 10.1111/j.2041-210X.2010.00012.x [DOI] [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, & Troisi A. (1996). Primate displacement activities as an ethopharmacological model of anxiety. Anxiety, 2(4), 186–191. DOI: [DOI] [PubMed] [Google Scholar]
- Schino G, Scucchi S, Maestripieri D, & Turillazzi PG (1988). Allogrooming as a tension‐reduction mechanism: A behavioral approach. American Journal of Primatology, 16(1), 43–50. DOI: 10.1002/ajp.1350160106 [DOI] [PubMed] [Google Scholar]
- Schino G, Troisi A, Perretta G, & Monaco V. (1991). Measuring anxiety in nonhuman primates: Effect of lorazepam on macaque scratching. Pharmacology Biochemistry and Behavior, 38(4), 889–891. DOI: 10.1016/0091-3057(91)90258-4 [DOI] [PubMed] [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, & Semple S. (2007). Grooming in Barbary macaques: Better to give than to receive? Biology Letters, 3(3), 231–3. DOI: 10.1098/rsbl.2007.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB (2007). Social components of fitness in primate groups. Science, 317(5843), 1347–1351. DOI: 10.1126/science.1140734 [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, … Tung J. (2020). Social determinants of health and survival in humans and other animals. Science, 368(6493). DOI: 10.1126/science.aax9553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B, & Fidell L. (2001). Using Multivariate Statistics. Boston, MA: Allyn and Bacon. [Google Scholar]
- Taylor SE (2006). Tend and befriend biobehavioral bases of affiliation under stress. Current Directions in Psychological Science, 15(6), 273–277. DOI: 10.1111/j.1467-8721.2006.00451.x [DOI] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, & Updegraff JA (2000). Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411. DOI: 10.1037/0033-295x.107.3.411 [DOI] [PubMed] [Google Scholar]
- Terry RL (1970). Primate grooming as a tension reduction mechanism. The Journal of Psychology, 76(1), 129–136. DOI: 10.1080/00223980.1970.9916830 [DOI] [PubMed] [Google Scholar]
- Troisi A. (2002). Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress, 5(1), 47–54. DOI: 10.1080/102538902900012378 [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture. (2013). Animal Welfare Act and Animal Welfare Regulations. http://awic.nal.usda.gov/government-and-professional-resources/federal-laws/animal-welfare-act [Google Scholar]
- Vandeleest JJ, McCowan B, & Capitanio JP (2011). Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta). Applied Animal Behaviour Science, 132(1–2), 81–89. DOI: 10.1016/j.applanim.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, & Elphick CS (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1(1), 3–14. DOI: 10.1111/j.2041-210X.2009.00001.x [DOI] [Google Scholar]