Abstract
Degenerative tendon pathology represents one of the most frequent and disabling musculoskeletal disorders. Diagnostic radiology plays a fundamental role in the clinical evaluation of tendon pathologies. Moreover, several minimally invasive treatments can be performed under imaging guidance to treat tendon disorders, maximizing the efficacy and reducing procedural complications. In this review article we describe the most relevant diagnostic features of conventional and advanced US and MRI imaging in tendon disorders, along with the main options for image-guided intervention. (www.actabiomedica.it)
Keywords: tendon, tendinosis, MRI, T2 mapping, ultrasound elastography
Introduction
Together with osteochondral (1-4) and synovial pathology (5-11), tendon pathology represents one of the main musculoskeletal disorders, with traumatic and - above all - degenerative pathology (12). Imaging plays a fundamental role in the clinical evaluation of tendon pathologies, as an accurate and timely diagnosis is crucial to allow the establishment of conservative measures, to reduce or delay the need for surgery (13-20). Moreover, several minimally invasive MSK interventional treatments can be performed under imaging guidance to maximize efficacy and reduce procedural complications (21,22).
This article aims to review the relevant diagnostic features of conventional and advanced imaging in tendon disorders – namely the degenerative pathology - and the main options for image-guided intervention.
Histology of tendon diseases and imaging correlates
Tendons are made up of dense fibrous connective tissue, mainly represented by multiple subunits of collagen fibers. This three-dimensional structure allows adequate transmission of mechanical force preventing damage and disruption of the fibers under stress. Tendons can be affected by different types of diseases that differ both macroscopically and microscopically (23).
Tendinosis, the main degenerative tendon alteration, is characterized histologically by collagen disorientation, fiber disorganization, and separation due to increased mucoid ground substance, increased cellularity and vascular spaces with or without neovascularization, focal necrosis, and calcifications (24). In more advanced stages, partial tendon rupture may show the superimposed presence of tears, including fibroblastic and myofibroblastic proliferation, hemorrhage, and organizing granulation tissue. Together with degenerative changes, reactive inflammatory changes in the tendon sheaths and paratenonium (tendonitis/paratenonitis) are often associated (25).
MRI and ultrasound are potent tools for the assessment of tendon anatomy. The imaging appearance correlates with the histological tendon structure and the changes that occur during pathologic processes (26).
Thanks to its availability (27-29), low cost (30, 31), absence of ionizing radiation (32-35), and the ability to study superficial anatomical structures (36-39), ultrasound is often the first imaging method of approach to the evaluation of tendons. The ultrasound appearance of tendinopathy generally shows tendon thickening with loss of the normal fibrillar structure, and increased spacing of the hyperechoic fibrillar lines with reduced tendon echogenicity; calcifications may also be detected, usually near tendon insertion. Doppler study may also reveal the proliferation of neovessels and increased vascularization in case of inflammation. In severe tendinosis/partial rupture, anechoic fluid may be visible inside the tear in acute/subacute stages; in more chronic phases, the echogenicity may increase, making the differentiation with the tendon less evident. Dynamic imaging during muscle contraction or passive movement is often useful to unveil small tendon gaps. Doppler imaging may also be useful to distinguish small intrasubstance tears from vessels that have developed in the tendinopathic tendon. Regarding the localization, degenerative changes are more frequent in the midportion and areas of critical vascularization, while tendon anomalies in spondyloarthritis tend to be more closely related to fibrocartilaginous enthesis (40-42).
Being more panoramic (43, 44) and multiparametric (45-50), magnetic resonance imaging (MRI) is the other imaging modality of choice for the evaluation of tendon diseases, including tendinopathies. The tendon structure plays a critical role in determining the appearance in magnetic resonance imaging. The water and collagen in the tendon are aligned, reducing the T2 and T1 relaxation time. Along with tendon thickening, an increase in signal intensity in T2-weighted imaging sequences is often the first sign of tendon anomaly at MRI. Although the appearance of a complete or partial tear is variable, the main finding is the T2-hyperintense fluid signal at the tear site and in surrounding tissues; however, in late stages, intermediate signal scar tissue can obscure this finding. The fibroadipose involution of the myotendinous junction is another typical finding of chronic lesions (26, 34).
Advanced diagnostic imaging in tendon diseases
T2 mapping
As mentioned above, conventional MRI sequences can detect morphologic changes occurring in tendinopathy. However, the sensitivity and specificity of MRI to determine disease grading have proven to be variable with the evaluation of the signal intensity alone (35). Furthermore, the diagnosis and monitoring of post-surgical tendon healing remain primarily subjective. In recent years, advanced MRI sequences capable of identifying and showing structural and biochemical tissue changes have been implemented. Among them, T2 mapping was developed to exploit the sensitivity of magnetic resonance imaging to the biophysical properties of numerous tissues. In musculoskeletal pathology applications, T2 mapping demonstrated to be sensitive to structural and biochemical composition in the cartilage matrix (2). In particular, T2 relaxation time mapping correlates with the changes in collagen matrix integrity and cartilage water content that occur during the pathophysiology of degenerative osteoarthritis. Paralleling cartilage histology, biochemical and degenerative changes in tendons are related to proteoglycan loss and disorganization of the collagen matrix, which becomes less elastic, allowing increased mobility of water and consequentially increased levels of H2O proton content; this leads to an increase of T2 relaxation values compared to normal levels. Both T2 and T2* mapping sequences can detect biochemical changes that occur in the early stage of tendinopathy, namely the change of collagen orientation, and the collagen and water content (35).
Although the experiences in literature are limited, focused mainly on Achilles and supraspinatus tendon, early evidence shows that T2 mapping values are significantly higher in tendons tears, compared to tendinosis and healthy tendons (36). Some works also confirm the validity of this sequence in identifying and monitoring tendon healing after surgery (37). From our unpublished experience, T2 mapping sequences may also be valid for identifying changes in tendon structure after percutaneous minimally invasive tendon treatment, such as tendon needling and Platelet-rich-plasma injections (fig. 1).
Figure 1.
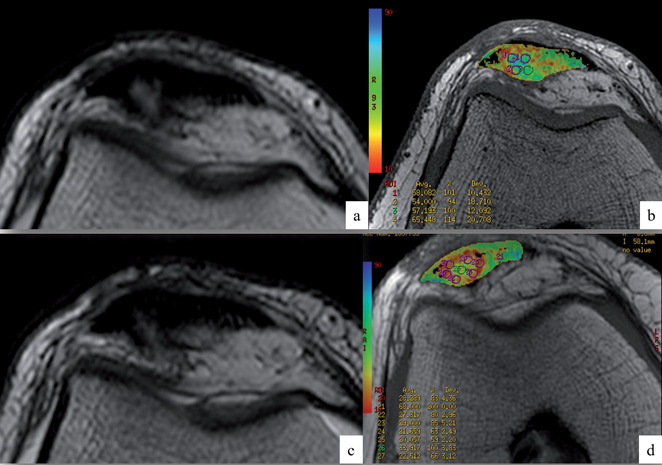
Axial images of standard T2 (a, c) and T2 mapping (b, d) sequences in a patellar tendon, before (a, b) and six months after (c, d) percutaneous US-guided intratendinous PRP injection treatment. Note the reduction of mean T2 relaxation values of circular ROIs within the most tendinopathic area (visible as a hyperintense intrasubstance alteration on standard imaging) after treatment, consistent with signs of tendon healing
Sonoelastography
Elastography is an US method for quantitative imaging of the distribution of biological tissue strains and elasticity, that has been evaluated in various settings in clinical radiology as a reliable and useful complementary modality to conventional ultrasound in the evaluation of lesions in the liver, spleen, breast, thyroid, and prostate (38).
Over the past years, the number of studies on elastography in tendon pathologies has risen considerably, and several studies in vitro and in vivo have tried to provide answers regarding the normal and pathologic biomechanical and structural properties of tendons (39). However, they have also aimed to assess the reliability of elastography and the prospects offered by this technique in daily clinical practice. There are several techniques of elastosonography, the most used being:
Quasi-static elastography (QSE). The operator exerts, through the US probe handheld compression, slow mechanical stress (strain) on tissues that induce tissue displacement. Comparing the position of the structures at rest and under compression, the displacement generated by the stress can be estimated. The degree of stiffness is represented in a color scale that identifies the “softest” and “hardest” tissue areas (38, 40).
Shear wave elastography (SWE). By generating several near-simultaneous pushes moved through the medium at supersonic speed, the device creates high-intensity shear waves that move through the medium transversely relative to the initial radiation force. Shear waves cause particulate moves recorded with high-frequency imaging (5000 to 30,000 Hz), from which the system calculates color elastograms in real-time (quantitative analysis) (41).
The literature on the application of elastosonography to the study of tendons has been focused mainly on the Achilles tendon in most studies (fig. 2). In QSE, the strain ratio (tendon/Kager’s fat) was found to be higher and the tendon softer in case of tendinopathy, when compared to healthy subjects. QSE could also be a reliable tool in monitoring Achilles tendon after surgical repair: one research revealed that after percutaneous tenorrhaphy, tendons tend to stiffen progressively for at least a year, reflecting the abnormal collagen composition during scar formation. QSE was also used to detect small partial tears of the supraspinatus tendon (fig. 3) and to evaluate structural and biomechanical changes of the Achilles tendon in metabolic diseases such as diabetes, acromegaly, severe renal insufficiency (38, 42, 43).
Figure 2.
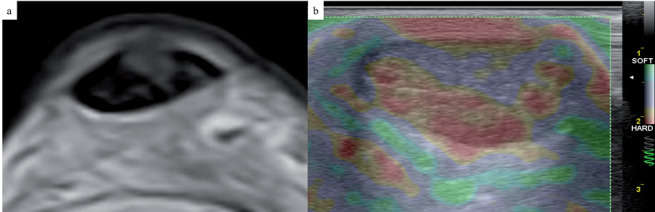
Axial T2-weighted image (a) and short-axis compression US elastography image (b) of an Achilles tendon. Note the intrasubstance hyperintense area of tendinosis within the tendon, evident at a “softer” area (in purple) at US compression elastogram
Figure 3.
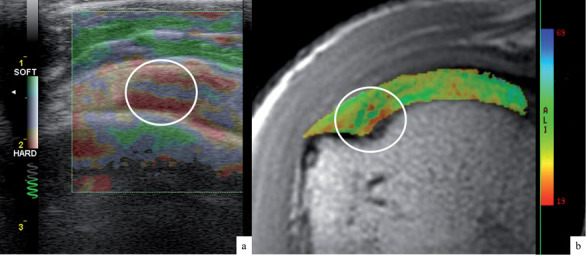
Concordance of US compression elastogram (a) and T2 mapping colorimetric scale (b) in a supraspinatus tendon tendinosis at the insertion area (white circle)
Interventional radiology procedures in tendon diseases
Several intervention have been introduced for the conservative treatment of tendinosis, including rest, oral and topical analgesics, and physical therapy (cryotherapy, stretching, eccentric strengthening, taping, bracing, extracorporeal shock-wave therapy) (44). Few of these treatments have been shown to be effective, and corticosteroid injections, once a mainstay of tendinopathy treatment, have been found to be harmful to tendons. Consequently, clinicians have sought safer and more effective interventions for the treatment of this condition.
In recent years, the indications for interventional radiology procedures have expanded thanks to the use of all diagnostic methods (45-50). With the use of US, the specific location of a tendon pathology can be accurately delineated, and this also allows to target many percutaneous minimally invasive treatments (51, 52). Among them, the most popular ones include percutaneous needle tenotomy (PNT), high-volume injection (HVI), and orthobiologic interventions, such as intratendinous injection of Platelet-rich Plasma (PRP) (53).
Percutaneous needle tenotomy (PNT), also termed needling, consists in the repeated passing of a needle (16-22 gauge) through the tendon, to disrupt the chronic degenerative process (including scar tissue), induce localized bleeding and fibroblast proliferation, which can lead to growth factor release, collagen formation, and ultimately healing. The number of needle passes (usually from 20 to 40) may vary based on numerous factors, such as patient characteristics, severity, and size of the tendinopathic area, presence or absence of tears, operator experience, and comfort level. This technique can be used alone or in combination with the injection of orthobiologic products. There is considerable variability in postprocedural care, such as the use of anti-inflammatory medications, the use of bracing, rehabilitation protocols, and return-to-activity guidelines. Most publications in literature are limited series on gluteus tendons, hamstring tendons, tensor fascia lata tendon, common extensor tendon of the elbow, rotator cuff tendons, Achilles and patellar tendon. Almost all studies showed improvement in clinical symptoms and reported rare complications (54-56).
High-volume injection (HVI) treatment focuses on using high volumes of injectate (about 10 mL of 0.5% bupivacaine, 25mg of hydrocortisone, and between 12-40 mL of normal saline) to disrupt neovessels and neonerves, which may reduce neurogenic inflammation and pain, and promote healing. This technique has mainly been studied in patellar and Achilles tendinopathy, where most neovessels and neonerves are sonographically observed between the affected tendon and an adjacent fat pad. US-guided HVI for the treatment of patellar and midportion Achilles tendinopathy has shown promise in improvement of both pain and functional scores, with positive effects persisting for up to 15 months. No significant complications have been reported. Another potential advantage of this procedure is that, because the tendon is not mechanically debrided, rehabilitation and return to activity may progress at a faster rate than with PNT (57, 58).
In the last decade, the use of biological therapies for the treatment of musculoskeletal diseases has increased significantly. Among them, platelet-rich plasma (PRP) is one of the most used compounds in regenerative musculoskeletal medicine, in particular for tendinopathies and degenerative joint pathologies, namely osteoarthritis. PRP is the blood-derived plasma fraction and contains high concentrations of platelets that release several growth factors with a critical role in tissue healing, such as platelet-derived growth factor, transforming growth factor-beta, and vascular endothelial growth factor. There are many studies and authors who report an intensification of the healing of tendinopathies treated with PRP. Several trials showed that PRP is an effective treatment for lateral epicondylitis, with short-term and long-term (up to 24 months) efficacy compared to steroids. Best available evidence suggests explicitly that LR-PRP (leucocyte-rich PRP) should be the treatment of choice. Only two trials of high-quality evidence – though with limited follow-up of 6 months - support the use of PRP in chronic refractory patellar tendinopathy, and recommended LR-PRP over LP-PRP (leucocyte-poor PRP). Concerning Achilles tendinopathy, one clinical trial reported efficacy of 4 LP-PRP injections, but also found similar results for high-volume injection of anesthetic, corticosteroid, and saline, perhaps suggesting the benefit may be due to mechanical volume effects (59, 60). Although there remains a paucity of evidence to recommend PRP injections for rotator cuff tendinopathy routinely, PRP may be a safe and effective alternative to corticosteroid injections in conservative treatment of rotator cuff tendinopathy. PRP injections are an effective treatment for improving pain and function in chronic plantar fasciitis and may be superior to corticosteroids, especially considering the complications of multiple corticosteroid injections that are not associated with PRP. Nevertheless, there is substantial evidence that corticosteroids can be useful in chronic tendinopathy at relieving pain, reducing swelling, and improving function in the short term (61).
Recently, the anti-inflammatory and lubrication properties of hyaluronic acid have drawn the scientific community’s interest to treat tendinopathies. Some in vitro and animal in vivo studies demonstrate encouraging results in terms of the ability of hyaluronic acid to facilitate tendon gliding, as well as creating better tendon structural organization. Moreover, the injection of hyaluronic acid may limit the proinflammatory effect by restoring viscoelasticity and by stimulating the endogenous synthesis of hyaluronic acid (62, 63).
Conclusions
In conclusion, diagnostic and interventional radiology are nowadays a matter of fact in all clinical settings (64-70). Tendon evaluation has improved with advances in ultrasound technology and MRI sequences. Beyond the anatomical changes detected with conventional imaging, MRI T2 mapping sequences and ultrasound elastography can provide information about the biochemical changes in tendon tissue and the consequent mechanical alteration induced by tendinopathy, giving reliable quantitative data for the diagnosis, staging, and follow-up.
The significant advancements in the use of musculoskeletal US for interventional purposes have played a critical role in the development of minimally invasive interventions to treat tendon diseases.
Conflict of interest:
Authors declare that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Bruno F, Arrigoni F, Palumbo P, et al. The Acutely Injured Wrist. Radiol Clin North Am. 2019;57:943–55. doi: 10.1016/j.rcl.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bruno F, Arrigoni F, Palumbo P, et al. New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints. Radiol Med. 2019;124:1121–27. doi: 10.1007/s11547-019-01003-1. [DOI] [PubMed] [Google Scholar]
- 3.Bruno F, Barile A, Arrigoni F, et al. Weight-bearing MRI of the knee: a review of advantages and limits. Acta Biomed. 2018;89:78–88. doi: 10.23750/abm.v89i1-S.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoccali C, Arrigoni F, Mariani S, Bruno F, Barile A, Masciocchi C. An unusual localization of chondroblastoma: The triradiate cartilage; from a case report a reconstructive technique proposal with imaging evolution. J Clin Orthop Trauma. 2017;8:S48–s52. doi: 10.1016/j.jcot.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacomelli R, Gorla R, Trotta F, et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology (Oxford) 2015;54:792–7. doi: 10.1093/rheumatology/keu398. [DOI] [PubMed] [Google Scholar]
- 6.Giacomelli R, Afeltra A, Alunno A, et al. Guidelines for biomarkers in autoimmune rheumatic diseases - evidence based analysis. Autoimmun Rev. 2019;18:93–106. doi: 10.1016/j.autrev.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still’s disease. J Autoimmun. 2018;93:24–36. doi: 10.1016/j.jaut.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Cipriani P, Di Benedetto P, Ruscitti P, et al. Perivascular Cells in Diffuse Cutaneous Systemic Sclerosis Overexpress Activated ADAM12 and Are Involved in Myofibroblast Transdifferentiation and Development of Fibrosis. J Rheumatol. 2016;43:1340–9. doi: 10.3899/jrheum.150996. [DOI] [PubMed] [Google Scholar]
- 9.Giacomelli R, Liakouli V, Berardicurti O, et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int. 2017;37:853–63. doi: 10.1007/s00296-016-3636-7. [DOI] [PubMed] [Google Scholar]
- 10.Di Geso L, Zardi EM, Afeltra A, et al. Comparison between conventional and automated software-guided ultrasound assessment of bilateral common carotids intimamedia thickness in patients with rheumatic diseases. Clin Rheumatol. 2012;31:881–4. doi: 10.1007/s10067-011-1915-y. [DOI] [PubMed] [Google Scholar]
- 11.Salaffi F, Carotti M, Di Carlo M, Farah S, Gutierrez M. Adherence to Anti-Tumor Necrosis Factor Therapy Administered Subcutaneously and Associated Factors in Patients With Rheumatoid Arthritis. J Clin Rheumatol. 2015;21:419–25. doi: 10.1097/RHU.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 12.Carotti M, Salaffi F, Di Carlo M, Giovagnoni A. Relationship between magnetic resonance imaging findings, radiological grading, psychological distress and pain in patients with symptomatic knee osteoarthritis. Radiol Med. 2017;122:934–43. doi: 10.1007/s11547-017-0799-6. [DOI] [PubMed] [Google Scholar]
- 13.Pogliacomi F, De Filippo M, Paraskevopoulos A, Alesci M, Marenghi P, Ceccarelli F. Mini-incision direct lateral approach versus anterior mini-invasive approach in total hip replacement: results 1 year after surgery. Acta Biomed. 2012;83:114–21. [PubMed] [Google Scholar]
- 14.De Filippo M, Pesce A, Barile A, et al. Imaging of postoperative shoulder instability. Musculoskelet Surg. 2017;101:15–22. doi: 10.1007/s12306-017-0461-4. [DOI] [PubMed] [Google Scholar]
- 15.Barile A, Bruno F, Arrigoni F, et al. Emergency and Trauma of the Ankle. Semin Musculoskelet Radiol. 2017;21:282–89. doi: 10.1055/s-0037-1602408. [DOI] [PubMed] [Google Scholar]
- 16.Barile A, Bruno F, Mariani S, et al. Follow-up of surgical and minimally invasive treatment of Achilles tendon pathology: a brief diagnostic imaging review. Musculoskelet Surg. 2017;101:51–61. doi: 10.1007/s12306-017-0456-1. [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Rossi B, Zoccali G, et al. A new technique for biopsy of soft tissue neoplasms: a preliminary experience using MRI to evaluate bleeding. Minerva Med. 2015;106:117–20. [PubMed] [Google Scholar]
- 18.Di Pietto F, Chianca V, de Ritis R, et al. Postoperative imaging in arthroscopic hip surgery. Musculoskelet Surg. 2017;101:43–49. doi: 10.1007/s12306-017-0459-y. [DOI] [PubMed] [Google Scholar]
- 19.Barile A, Bruno F, Mariani S, et al. What can be seen after rotator cuff repair: a brief review of diagnostic imaging findings. Musculoskelet Surg. 2017;101(Suppl 1):3–14. doi: 10.1007/s12306-017-0455-2. [DOI] [PubMed] [Google Scholar]
- 20.Patriarca L, Letteriello M, Di Cesare E, Barile A, Gallucci M, Splendiani A. Does evaluator experience have an im pact on the diagnosis of lumbar spine instability in dynamic MRI? Interobserver agreement study. Neuroradiol J. 2015;28:341–6. doi: 10.1177/1971400915594508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrigoni F, Napoli A, Bazzocchi A, et al. Magnetic-resonance-guided focused ultrasound treatment of non-spinal osteoid osteoma in children: multicentre experience. Pediatr Radiol. 2019;49:1209–16. doi: 10.1007/s00247-019-04426-0. [DOI] [PubMed] [Google Scholar]
- 22.Cazzato RL, Arrigoni F, Boatta E, et al. Percutaneous management of bone metastases: state of the art, interventional strategies and joint position statement of the Italian College of MSK Radiology (ICoMSKR) and the Italian College of Interventional Radiology (ICIR) Radiol Med. 2019;124:34–49. doi: 10.1007/s11547-018-0938-8. [DOI] [PubMed] [Google Scholar]
- 23.Putz R, Muller-Gerbl M. Anatomy and pathology of tendons. Orthopade. 1995;24:180–6. [PubMed] [Google Scholar]
- 24.Nakagaki K, Ozaki J, Tomita Y, Tamai S. Magnetic resonance imaging of rotator cuff tearing and degenerative tendon changes: Correlation with histologic pathology. J Shoulder Elbow Surg. 1993;2:156–64. doi: 10.1016/S1058-2746(09)80052-9. [DOI] [PubMed] [Google Scholar]
- 25.Backman C, Boquist L, Friden J, Lorentzon R, Toolanen G. Chronic achilles paratenonitis with tendinosis: an experimental model in the rabbit. J Orthop Res. 1990;8:541–7. doi: 10.1002/jor.1100080410. [DOI] [PubMed] [Google Scholar]
- 26.Weinreb JH, Sheth C, Apostolakos J, et al. Tendon structure, disease, and imaging. Muscles Ligaments Tendons J. 2014;4:66–73. [PMC free article] [PubMed] [Google Scholar]
- 27.Piccolo CL, Galluzzo M, Ianniello S, et al. Pediatric musculoskeletal injuries: role of ultrasound and magnetic resonance imaging. Musculoskelet Surg. 2017;101:85–102. doi: 10.1007/s12306-017-0452-5. [DOI] [PubMed] [Google Scholar]
- 28.Perrotta FM, Astorri D, Zappia M, Reginelli A, Brunese L, Lubrano E. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol Int. 2016;36:1579–83. doi: 10.1007/s00296-016-3562-8. [DOI] [PubMed] [Google Scholar]
- 29.Zappia M, Reginelli A, Russo A, et al. Long head of the biceps tendon and rotator interval. Musculoskelet Surg. 2013;97(Suppl 2):S99–108. doi: 10.1007/s12306-013-0290-z. [DOI] [PubMed] [Google Scholar]
- 30.Zytoon AA, Eid H, Sakr A, El Abbass HA, Kamel M. Ultrasound assessment of elbow enthesitis in patients with seronegative arthropathies. J Ultrasound. 2014;17:33–40. doi: 10.1007/s40477-013-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syha R, Springer F, Ketelsen D, et al. Achillodynia - radiological imaging of acute and chronic overuse injuries of the achilles tendon. Rofo. 2013;185:1041–55. doi: 10.1055/s-0033-1335170. [DOI] [PubMed] [Google Scholar]
- 32.Gurgenidze T, Mizandari MG, Gadeliia GT. Normal sonoanatomy and ultrasound diagnosis of achilles tendon pathology. Georgian Med News. 2009:119–25. [PubMed] [Google Scholar]
- 33.Cortellini A, Bozzetti F, Palumbo P, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Sci Rep. 2020;10:1456. doi: 10.1038/s41598-020-58498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber J, Buchhorn T. Midportion Achilles tendinopathy. Unfallchirurg. 2017;120:1038–43. doi: 10.1007/s00113-017-0411-5. [DOI] [PubMed] [Google Scholar]
- 35.Anz AW, Lucas EP, Fitzcharles EK, Surowiec RK, Millett PJ, Ho CP. MRI T2 mapping of the asymptomatic supraspinatus tendon by age and imaging plane using clinically relevant subregions. Eur J Radiol. 2014;83:801–5. doi: 10.1016/j.ejrad.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Bachmann E, Rosskopf AB, Gotschi T, et al. T1-and T2*-Mapping for Assessment of Tendon Tissue Biophysical Properties: A Phantom MRI Study. Invest Radiol. 2019;54:212–20. doi: 10.1097/RLI.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 37.Ganal E, Ho CP, Wilson KJ, et al. Quantitative MRI characterization of arthroscopically verified supraspinatus pathology: comparison of tendon tears, tendinosis and asymptomatic supraspinatus tendons with T2 mapping. Knee Surg Sports Traumatol Arthrosc. 2016;24:2216–24. doi: 10.1007/s00167-015-3547-2. [DOI] [PubMed] [Google Scholar]
- 38.Fusini F, Langella F, Busilacchi A, et al. Real-time sonoelastography: principles and clinical applications in tendon disorders. A systematic review. Muscles Ligaments Tendons J. 2017;7:467–77. doi: 10.11138/mltj/2017.7.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klauser AS, Miyamoto H, Tamegger M, et al. Achilles tendon assessed with sonoelastography: histologic agreement. Radiology. 2013;267:837–42. doi: 10.1148/radiol.13121936. [DOI] [PubMed] [Google Scholar]
- 40.Tan S, Kudas S, Ozcan AS, et al. Real-time sonoelastography of the Achilles tendon: pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skeletal Radiol. 2012;41:1067–72. doi: 10.1007/s00256-011-1339-4. [DOI] [PubMed] [Google Scholar]
- 41.Ooi CC, Malliaras P, Schneider ME, Connell DA. “Soft, hard, or just right?” Applications and limitations of axialstrain sonoelastography and shear-wave elastography in the assessment of tendon injuries. Skeletal Radiol. 2014;43:1–12. doi: 10.1007/s00256-013-1695-3. [DOI] [PubMed] [Google Scholar]
- 42.Busilacchi A, Olivieri M, Ulisse S, et al. Real-time sonoelastography as novel follow-up method in Achilles tendon surgery. Knee Surg Sports Traumatol Arthrosc. 2016;24:2124–32. doi: 10.1007/s00167-014-3484-5. [DOI] [PubMed] [Google Scholar]
- 43.Tudisco C, Bisicchia S, Stefanini M, Antonicoli M, Masala S, Simonetti G. Tendon quality in small unilateral supraspinatus tendon tears. Real-time sonoelastography correlates with clinical findings. Knee Surg Sports Traumatol Arthrosc. 2015;23:393–8. doi: 10.1007/s00167-013-2551-7. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Royo MP, Gomez-Trullen EM, Ortiz-Lucas M, et al. Comparative study of treatment interventions for patellar tendinopathy: a protocol for a randomised controlled trial. BMJ Open. 2020;10:e034304. doi: 10.1136/bmjopen-2019-034304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrafiello G, Dionigi G, Ierardi AM, et al. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renal lesions Bosniak III or IV after 24 months follow up. Int J Surg. 2013;11(Suppl 1):S30–5. doi: 10.1016/S1743-9191(13)60010-2. [DOI] [PubMed] [Google Scholar]
- 46.Ierardi AM, Piacentino F, Fontana F, et al. The role of endovascular treatment of pelvic fracture bleeding in emergency settings. Eur Radiol. 2015;25:1854–64. doi: 10.1007/s00330-015-3589-3. [DOI] [PubMed] [Google Scholar]
- 47.Ierardi AM, Tsetis D, Ioannou C, et al. Ultra-low profile polymer-filled stent graft for abdominal aortic aneurysm treatment: a two-year follow-up. Radiol Med. 2015;120:542–8. doi: 10.1007/s11547-015-0499-z. [DOI] [PubMed] [Google Scholar]
- 48.Carrafiello G, Ierardi AM, Duka E, et al. Usefulness of Cone-Beam Computed Tomography and Automatic Vessel Detection Software in Emergency Transarterial Embolization. Cardiovasc Intervent Radiol. 2016;39:530–7. doi: 10.1007/s00270-015-1213-1. [DOI] [PubMed] [Google Scholar]
- 49.Arrigoni F, Bruno F, Zugaro L, et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed. 2018;89:166–74. doi: 10.23750/abm.v89i1-S.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Floridi C, Pesapane F, Angileri SA, et al. Yttrium-90 radioembolization treatment for unresectable hepatocellular carcinoma: a single-centre prognostic factors analysis. Med Oncol. 2017;34:174. doi: 10.1007/s12032-017-1021-3. [DOI] [PubMed] [Google Scholar]
- 51.Peck E, Jelsing E, Onishi K. Advanced Ultrasound-Guided Interventions for Tendinopathy. Phys Med Rehabil Clin N Am. 2016;27:733–48. doi: 10.1016/j.pmr.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Adler RS, Sofka CM. Percutaneous ultrasound-guided injections in the musculoskeletal system. Ultrasound Q. 2003;19:3–12. doi: 10.1097/00013644-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89:20150355. doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hung CY, Chang KV. Ultrasound-Guided Percutaneous Needle Tenotomy with Platelet-Rich Plasma Injection for an Uncommon Case of Proximal Gluteus Medius Tendinopathy. J Med Ultrasound. 2019;27:111–12. doi: 10.4103/JMU.JMU_86_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattie R, Wong J, McCormick Z, Yu S, Saltychev M, Laimi K. Percutaneous Needle Tenotomy for the Treatment of Lateral Epicondylitis: A Systematic Review of the Literature. PM R. 2017;9:603–11. doi: 10.1016/j.pmrj.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Hopkins J, Sampson M. Percutaneous tenotomy: Development of a novel, percutaneous, ultrasound-guided needlecutting technique for division of tendons and other connective tissue structures. J Med Imaging Radiat Oncol. 2014;58:327–30. doi: 10.1111/1754-9485.12113. [DOI] [PubMed] [Google Scholar]
- 57.Maffulli N, Spiezia F, Longo UG, Denaro V, Maffulli GD. High volume image guided injections for the management of chronic tendinopathy of the main body of the Achilles tendon. Phys Ther Sport. 2013;14:163–7. doi: 10.1016/j.ptsp.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Crisp T, Khan F, Padhiar N, et al. High volume ultrasound guided injections at the interface between the patellar tendon and Hoffa’s body are effective in chronic patellar tendinopathy: A pilot study. Disabil Rehabil. 2008;30:1625–34. doi: 10.1080/09638280701830936. [DOI] [PubMed] [Google Scholar]
- 59.Kaux JF, Libertiaux V, Dupont L, et al. Platelet-rich plasma (PRP) and tendon healing: comparison between fresh and frozen-thawed PRP. Platelets. 2020;31:221–25. doi: 10.1080/09537104.2019.1595563. [DOI] [PubMed] [Google Scholar]
- 60.Yuan T, Zhang CQ, Wang JH. Augmenting tendon and ligament repair with platelet-rich plasma (PRP) Muscles Ligaments Tendons J. 2013;3:139–49. [PMC free article] [PubMed] [Google Scholar]
- 61.Rees JD, Stride M, Scott A. Tendons--time to revisit inflammation. Br J Sports Med. 2014;48:1553–7. doi: 10.1136/bjsports-2012-091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaux JF, Samson A, Crielaard JM. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2015;5:264–9. doi: 10.11138/mltj/2015.5.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abate M, Schiavone C, Salini V. The use of hyaluronic acid after tendon surgery and in tendinopathies. Biomed Res Int. 2014;2014:783632. doi: 10.1155/2014/783632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruno F, Smaldone F, Varrassi M, et al. MRI findings in lumbar spine following O2-O3 chemiodiscolysis: A long term follow-up. Interventional neuroradiology: journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences. 2017;23:444–450. doi: 10.1177/1591019917703784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruno F, Arrigoni F, Palumbo P, et al. New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints. Radiol Med. 2019;124:1121–27. doi: 10.1007/s11547-019-01003-1. [DOI] [PubMed] [Google Scholar]
- 66.Mariani S, La Marra A, Arrigoni F, et al. Dynamic measurement of patello-femoral joint alignment using weight-bearing magnetic resonance imaging (WB-MRI) European journal of radiology. 2015;84:2571–8. doi: 10.1016/j.ejrad.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 67.Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol. 2017;34:52. doi: 10.1007/s12032-017-0906-5. [DOI] [PubMed] [Google Scholar]
- 68.Masciocchi C, Arrigoni F, La Marra A, Mariani S, Zugaro L, Barile A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. The British journal of radiology. 2016;89:20150356–20150356. doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salvati F, Rossi F, Limbucci N, Pistoia ML, Barile A, Masciocchi C. Mucoid metaplastic degeneration of anterior cruciate ligament. The. Journal of sports medicine and physical fitness. 2008;48:483–487. [PubMed] [Google Scholar]
- 70.Zappia M, Castagna A, Barile A, Chianca V, Brunese L, Pouliart N. Imaging of the coracoglenoid ligament: a third ligament in the rotator interval of the shoulder. Skeletal Radiol. 2017;46:1101–11. doi: 10.1007/s00256-017-2667-9. [DOI] [PubMed] [Google Scholar]


