Abstract
The synovial membrane is a specialized mesenchymal tissue that lines the diarthrodial joints surfaces, bursae, and tendon sheaths of the body. This article aims to provide an overview of the fundamentals of synovial tissue, with particular regard to the imaging findings of the main pathologic processes that can affect the synovia and the role of image-guided interventions. (www.actabiomedica.it)
Keywords: synovia, knee, MRI, ultrasound, CEUS, joint injection
Synovial anatomy, physiology, and pathophysiology
Normal synovium is made up of two layers, the synovial intima, and subsynovial tissue. The synovial intima is formed by a layer of loosely connected synovial cells, while the subsynovial tissue widely varies in the structure based on its location, and it may be mainly fibrous, areolar, or adipose. The subsynovium is characterized by the presence of a vascular and lymphatic network; through this capillary network, fluid enters into the joint cavity as an ultrafiltrate of blood plasma. Synovial membrane roles are the production of synovial fluid, the removal of articular debris, and the facilitation of the sliding between the articular surfaces (1).
Synovial tissue reacts to the numerous kinds of stresses it may be subjected in a fast but stereotypical manner, and the pathogenesis of the majority of the synovial disorders routes into a final and common anatomopathological pathway. The initial fast response is characterized by the increase of blood flow that causes edema, alteration of the synovial matrix, and intra-articular effusion. The process proceeds inducing synovial proliferation accompanied by mononuclear infiltration; this leads to synovial hyperplasia that encroaches upon the articular surface. In the chronic phases, fibrotic changes may also occur (2, 3).
As in most anatomical districts, although the first step of the approach to synovial pathology is clinical evaluation, this can often have low sensitivity and specificity. Diagnostic imaging modalities, uniquely if integrated, are reliable support to diagnosis by demonstrating the type of anatomic alteration, approaching - in most cases - the final pathologic diagnosis, and being a noninvasive follow-up tool (4-20).
Synovial effusion
Synovial effusion is defined as the increase of the normal quantity of fluid on the articular cavity: its distribution is specific for each joint and follows the capsule anatomy. The normal synovium is barely perceptible at MR imaging. Visualization of the synovium, in facts, suggests the presence of underlying pathologic changes. However, this is a nonspecific finding as it can be associated with traumatic, degenerative, overload, inflammatory and neoplastic pathology (21). So, effusion can be considered a sensitive indicator of joint pathology, but it may occur in either the presence or absence of proliferative synovitis. Due to the release of pluripotent cells (mainly fibroblasts), the synovial membrane undergoes to hyperplasia and/or hypertrophy that can be less or more fibrous, depending on the kind and the time lasting of the stress. Synovial thickening or proliferation can be challenging to detect in the presence of a joint effusion since both reveal themselves as an increased signal intensity on fluid-sensitive (T2-weighted) images. During the subacute stage of the pathology, in case of repeated stress, synovial thickening becomes more prominent and so more detectable from the synovial fluid. Additional morphologic findings may help in the differential diagnosis between synovial effusion and synovial thickening, such as the presence of scalloping or truncation of the prefemoral fat pad, defects of Hoffa’s fat pad and the non-visualization or irregular margins of the quadriceps fat pad, that have been described as signs of synovial proliferation. The administration of intravenous gadolinium can be considered highly accurate in differentiating proliferative synovium from joint effusion (fig. 1). Besides being highly sensitive in the detection of the presence of joint effusions and synovitis, MR imaging is a reliable tool in quantifying synovial and effusion volumes. Its ability to quantify these synovial processes has important clinical implications: assessment of disease severity and response to therapy. Several studies have shown how MR imaging with the use of advanced quantitative evaluation of synovial hypertrophy and synovial fluid is an essential tool in the diagnostic and follow-up stages of many degenerative and inflammatory conditions (22, 23). Even if MR imaging remains the gold standard in the evaluation of synovial disorders of the knee, ultrasounds technique has been considered to find correlative accuracy in measurement of synovial thickness, vascularization, and effusion, most of all for its simple, inexpensive, and beneficial properties. At US, synovial hyperplasia and hypertrophy are evident as a thickening of the synovial lining hyperechoic compared to the intra-articular effusion. Depending on the degree and chronicity of inflammation, it can have a smooth, irregular, villous/villonodular profile. Power-doppler and Color-doppler sampling are useful for documenting the degree of proliferation of neovessels within the hypertrophic synovial tissue (24). In several studies, the evaluation with contrast-enhanced ultrasound (CEUS) showed higher sensitivity (95%) for synovitis detection than CE-MRI (82%), power Doppler US (64%), or grayscale US (58%) (24, 25).
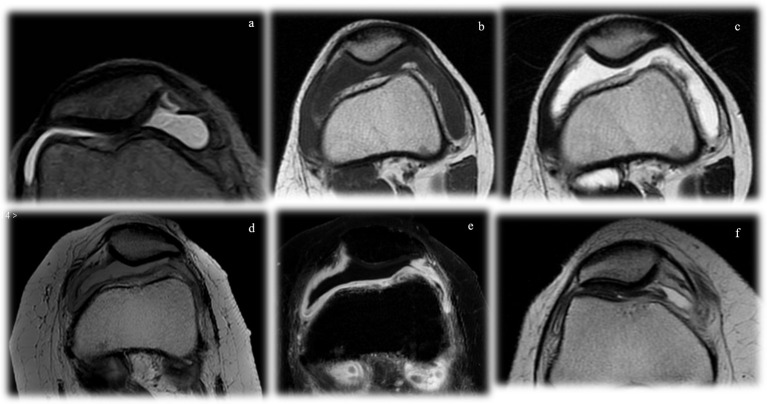
Figure 1.
Imaging features of synovial effusion. In the acute phases (a), there is an increase of joint fluid, with the typical T2-hyperintensity and T1-hypointensity. In the subacute stages (b), the thickened synovial lining can be appreciated. Progressively, synovial hyperplasia and hypertrophy can be differentiated by the synovial effusion as it is clearly thickened, with villous appearance (c, d). The distinction is even more evident with the administration of gadolinium, where only the active synovial thickening enhances (e). In the chronic stages (f), the joint fluid can be less prominent and fibrotic aspects may occur
Inflammatory arthritis
The synovia is typically involved, as a prime pathophysiological process, in a wide range of inflammatory arthritis. Among them, the most frequent are represented by rheumatoid arthritis (RA) and psoriatic arthritis (PsA) (26-36). Though bone involvement has typical different pathologic and imaging patterns, all inflammatory arthritis are progressive inflammatory disorders, primarily affecting the synovium (37-40). Articular tumefaction reflects the synovial inflammation that is the primary target of the pathological process. The earliest anatomopathological alteration is the exudative synovitis characterized by increased vascularization and permeability, synovial effusion, and inflammatory infiltration (41, 42). Synovitis is an early phase of the process, and together with the bone edema is an important predictive factor of bone lesions. Synovitis can be exudative or proliferative, and MR imaging is crucial for its differentiation. MR imaging has become a central tool in the evaluation of inflammatory arthritis because of its superior soft-tissue contrast, its ability to detect and quantify synovial thickening/volume, and the fact that these measurements correlate highly with synovial inflammatory activity. Fat-suppressed T1-weighted 3D gradient-echo images offer an excellent differentiation of cartilage and joint fluid in patients with RA (43, 44). However, it is recognized that gadolinium-enhanced MR images offer superior differentiation of enlarged or hyperplastic synovium from the adjacent joint fluid. Synovitis is described as an area in the synovial compartment that shows an increased contrast enhancement after intravenous administration of gadolinium (45). For this reason, the administration of gadolinium turns out to be essential to obtain information about vascularization and – consequently- the activity stage of the disease; this, furthermore, allows both qualitative and quantitative evaluations (45, 46). For quantitative evaluation, dynamic imaging is used in order to appreciate the synovial enhancement and to obtain values of signal intensity according to acquisition time (47). The rate of early enhancement (30 to 60 seconds following injection) correlates highly with microscopic evidence of active inflammation (vessel proliferation and mononuclear leukocyte infiltration). The distinction between synovium and joint fluid is most reliable in the first 10 minutes following injection of gadolinium contrast, because the gadolinium diffuses into the joint, thereby obscuring the border between synovium and effusion. Synovitis can be evaluated by US as well and, as MR imaging, can be considered highly reliable and accurate when used to follow disease progression and monitor response to therapy (48). The thickened intra-articular synovial pannus can show Doppler signal, according to its increased vascularization. The Doppler signal intensity relates to the quantity of neo-formed vessels and thus with the severity of the inflammation (49, 50).
Osteoarthritis
Osteoarthritis (OA) is the most common form of arthritis and a major cause of joint pain and disability (51, 52). Traditionally, OA has been considered primarily a disease of hyaline cartilage with associated bone involvement, caused by overload or overuse; hover, the pathophysiology of OA development is now appreciated to be more complex. The newest evidence suggests that synovitis and the resultant pro-inflammatory mediators have a major and early role in the pathogenesis of OA, with secondary effects on articular cartilage. In light of this, MRI imaging and US have been used to assess the presence of “macroscopic” inflammation and have supported the role of synovitis as an active component of the OA process, associated with both pain and structural progression (53-55). The histological pattern of synovium involvement in OA patients is characterized by synovial lining hyperplasia, sublining fibrosis, and stromal vascularization. Synoviocytes react by producing pro-inflammatory mediators, which in turn attract immune cells, increase angiogenesis, and induce a phenotypic shift in chondrocytes. A vicious cycle follows as chondrocytes produce additional cytokines and proteolytic enzymes that eventually increase cartilage degradation and induce further synovial inflammation (55). At the “macroscopic” level, MRI provides valuable insights into synovitis and can visualize synovial hypertrophy, synovial fluid volume, and level of synovial enhancement after intravenous injection of contrast agent. Several studies also demonstrated how MRI inflammation measures correlate well with histological inflammation and clinical symptoms.
Interventional radiology
Interventional radiology can propose a wide range of therapeutic procedures also in musculoskeletal pathology through ultrasound, CT, and MRI guidance (56-64). Based on the evidence reported above, the synovia – and namely synovial inflammation – has become one of the main therapeutic targets not only for inflammatory arthropathies but also in degenerative osteoarthritis. Corticosteroids are undoubtedly the most used anti-inflammatory drugs. The possibility, through image guidance, of direct intraarticular drug injection, is the key to maximize the therapeutic effects while minimizing the known systemic side effects (65). In addition to intra-articular administration, ultrasound imaging guidance is beneficial for intrabursal and peritendinous administration, where corticosteroids can have anti-inflammatory action on synovial tissue. The imaging guide also allows minimizing other risks of unguided infiltration of corticosteroids, such as tendon rupture. Hyaluronic acid (HA) injection is another interventional radiology procedure aimed at degenerative joint pathology (namely osteoarthritis) but primarily aimed at the synovium. Hyaluronic acid is a glycosaminoglycan consisting of highly hydrophilic chains of D-glucuronic acid and N-acetylglucosamine. There are numerous types of hyaluronic acid on the market, which mainly distinguish themselves by their molecular weight. Those with low molecular weight, able to bind to binding proteins (hyaladerine) and CD44 receptor, act mainly with a biological effect of viscoinduction (i.e., stimulating the endogenous production of HA) (66-68). Those with high molecular weight, on the other hand, have a lower biological effect, while performing a powerful viscosupplementation action, thanks to their rheological properties. Even if metanalysis highlight the heterogeneity of the available studies, intraarticular HA injections appear effective in the treatment of arthritic pain (mild-moderate OA) both at the level of the knee and the hip. The size of the effect on pain varies according to the studies, with a peak at 8 weeks (higher than corticosteroids). Cross-linked products (high molecular weight) have greater pain efficacy than linear HA, and there are evidences to support HA efficacy also concerning functional improvement (level 1B). In all guidelines, the use is recommended for the management of osteoarthritis as a second-line treatment in symptomatic patients after conservative therapy (NSAID) (69). Platelet-rich plasma injection is another therapeutic tool we can consider. This product, consisting of a platelet ultrafiltrate, performs its action through the release of several growth factors (PDGF, TGF-B, EGF, CTGF) with anti-inflammatory activity and trophic action on different joint tissues. There are several in vitro and clinical evidences that intraarticular PRP injection may exert a positive influence in patients affected by knee cartilage degeneration and OA and that it may have higher and longer efficacy than HA in improving pain and articular function (70, 71).
Synovial tumors and pseudotumors
Synovial cysts and ganglia
Synovial cysts are fluid-filled masses lined with synovium located within or about joints. They can be intraneural, extraneural, or between or within muscles. The distinction between a synovial cyst and ganglion cyst is made primarily based on the histological nature of the lining of the cyst, the contents of the cyst, and the presence/absence of communication with the joint (72, 73). Ganglion cysts are lined by spindle-shaped cells and are characterized by myxoid contents. Because true synovial cysts are, by definition, lined by synovial cells, they manifest disease and are affected by the same disease processes as other synovial structures. They are most commonly encountered about the knee and occur with synovial inflammatory disorders, rheumatologic and otherwise, as a result of trauma, and in conjunction with osteoarthritis. Synovial cysts can arise from different causes (degenerative articular processes, chronic microtrauma soft tissue/bursae, or reflect anatomical remnants), and knowledge of the most relevant localizations, pathological and radiological picture is helpful for a correct diagnosis (74).
Pigmented villonodular synovitis (PVNS)
Pigmented villonodular synovitis (PVNS) is a rare, benign synovial proliferative process whose distribution is usually monoarticular. The disorder is idiopathic with two primary forms, localized and diffuse, either intraarticular or extra-articular (75-77). Although any synovial joint may be involved, the knee is the most frequently affected. Patients usually present with nonspecific symptoms such as monoarticular pain and decreased range of motion. The disease is characterized by the presence of abundant hemosiderin-laden macrophage deposition into a bulky, mass-like synovium. MR imaging is the imaging method of choice for the diagnosis, surgical planning, and evaluation of recurrence (75). Clumps of hemosiderin-laden macrophage deposits demonstrate low signal intensity on T1- and T2-weighted images due to the paramagnetic effects of the iron in the ferric state, and this is the most reliable diagnostic feature (fig. 2). A large amount of joint effusion is usually present, but not specific. The pathological equivalent at the level of the tendon sheaths is the giant cell tumor of the tendon sheaths (GCTTS) (77).
Figure 2.
Imaging features of pigmented villonodular synovitis (PVNS) in solitary (a), diffuse (b), and extraarticular form. Note the low signal intensity on both T1- and T2-weighted sequences
Lipoma arborescens
Lipoma arborescens, or villous lipomatous proliferation of the synovial membrane, is a rare intra-articular lesion that usually involves the knee. It is characterized by villous proliferation of the synovium and extensive replacement of the subsynovial tissue by mature adipose cells. It is usually a monoarticular condition. MR imaging shows a frond-like morphology with high signal intensity (isointense to subcutaneous fat) on T1-weighted images (78, 79). Lack of enhancement after injection of intravenous contrast material excludes the diagnosis of other synovial inflammatory or neoplastic processes (fig. 3).
Figure 3.

Imaging features of synovial chondromatosis. In a and b, note the right hip joint effusion with intraarticular osteocartilaginous bodies with signs of ossification. In c, a plain film of the knee with secondary synovial chondromatosis showing osteocartilaginous bodies distributed in the joint recesses
Synovial chondromatosis
Idiopathic synovial osteochondromatosis is a non-neoplastic, proliferative, and metaplastic disorder of the synovium characterized by the presence of multiple cartilaginous or osteocartilaginous bodies within an articulation or adjacent synovial lined structure (80). Two forms of the condition occur, primary and secondary osteochondromatosis. The secondary one is more common and characterized by the presence of intraarticular osteocartilaginous bodies against a backdrop of degenerative joint disease. The second type, primary synovial osteochondromatosis, is relatively uncommon. The condition is usually progressive, leading to early osteoarthritis. The MR imaging characteristics depend on the presence and extent of calcification and or ossification in the bodies. In the case of intra-articular bodies composed primarily of cartilage, the bodies are lobulated in appearance with intermediate to low signal intensity on T1-weighted sequences with high signal intensity on fluid-sensitive sequences. In the case of calcified intraarticular bodies, the signal characteristics are described as foci of low signal intensity on both T1-weighted and fluid-sensitive sequences (81). Marrow-containing bodies will show the high signal intensity of the marrow within the bodies on T1-weighted images (fig. 4). Heterogeneous enhancement of cartilaginous bodies can be seen after the administration of intravenous contrast material.
Figure 4.

MRI imaging features of a lipoma arborescens of the knee. Note the typical frond-like thickening of the synovium in the supra-patellar recess showing signal intensity of fat (T1- and T2-hyperintense in a-b and hypointense in the fs-T2 sequence in c). After gadolinium administration (d) there is enhancement only of the superficial synovial lining
Conclusions
Imaging is a central piece of the complex puzzle of synovial diseases; the role of multimodal imaging is crucial in many aspects regarding the management of these disorders from the very beginning of the diagnosis, going through the follow-up and the treatment as well.
Conflict of interest:
Authors declare that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Broeren MGA, Waterborg CEJ, Wiegertjes R, et al. A threedimensional model to study human synovial pathology. ALTEX. 2019;36:18–28. doi: 10.14573/altex.1804161. [DOI] [PubMed] [Google Scholar]
- 2.Frick MA, Wenger DE, Adkins M. MR imaging of synovial disorders of the knee: an update. Radiol Clin North Am. 2007;45:1017–31, vii. doi: 10.1016/j.rcl.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Chung CB, Boucher R, Resnick D. MR imaging of synovial disorders of the knee. Semin Musculoskelet Radiol. 2009;13:303–25. doi: 10.1055/s-0029-1242186. [DOI] [PubMed] [Google Scholar]
- 4.Reginelli A, Silvestro G, Fontanella G, et al. Validation of DWI in assessment of radiotreated bone metastases in elderly patients. Int J Surg. 2016;33(Suppl 1):S148–53. doi: 10.1016/j.ijsu.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Reginelli A, Zappia M, Barile A, Brunese L. Strategies of imaging after orthopedic surgery. Musculoskelet Surg. 2017;101:1. doi: 10.1007/s12306-017-0458-z. [DOI] [PubMed] [Google Scholar]
- 6.Barile A, Bruno F, Mariani S, et al. What can be seen after rotator cuff repair: a brief review of diagnostic imaging findings. Musculoskelet Surg. 2017;101(Suppl 1):3–14. doi: 10.1007/s12306-017-0455-2. [DOI] [PubMed] [Google Scholar]
- 7.Cortellini A, Bozzetti F, Palumbo P, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Sci Rep. 2020;10:1456. doi: 10.1038/s41598-020-58498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccolo CL, Galluzzo M, Ianniello S, et al. Pediatric musculoskeletal injuries: role of ultrasound and magnetic resonance imaging. Musculoskelet Surg. 2017;101:85–102. doi: 10.1007/s12306-017-0452-5. [DOI] [PubMed] [Google Scholar]
- 9.Zappia M, Capasso R, Berritto D, et al. Anterior cruciate ligament reconstruction: MR imaging findings. Musculoskelet Surg. 2017;101:23–35. doi: 10.1007/s12306-017-0460-5. [DOI] [PubMed] [Google Scholar]
- 10.Bruno F, Arrigoni F, Palumbo P, et al. The Acutely Injured Wrist. Radiol Clin North Am. 2019;57:943–55. doi: 10.1016/j.rcl.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Salvati F, Rossi F, Limbucci N, et al. Mucoid metaplastic-degeneration of anterior cruciate ligament. The. Journal of sports medicine and physical fitness. 2008;48:483–487. [PubMed] [Google Scholar]
- 12.Zappia M, Castagna A, Barile A, et al. Imaging of the coracoglenoid ligament: a third ligament in the rotator interval of the shoulder. Skeletal Radiology. 2017;46:1101–1111. doi: 10.1007/s00256-017-2667-9. [DOI] [PubMed] [Google Scholar]
- 13.Mariani S, La Marra A, Arrigoni F, et al. Dynamic measurement of patello-femoral joint alignment using weight-bearing magnetic resonance imaging (WB-MRI) European journal of radiology. 2015;84:2571–8. doi: 10.1016/j.ejrad.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 14.De Filippo M, Pesce A, Barile A, et al. Imaging of postoperative shoulder instability. Musculoskelet Surg. 2017;101:15–22. doi: 10.1007/s12306-017-0461-4. [DOI] [PubMed] [Google Scholar]
- 15.Pogliacomi F, De Filippo M, Paraskevopoulos A, Alesci M, Marenghi P, Ceccarelli F. Mini-incision direct lateral approach versus anterior mini-invasive approach in total hip replacement: results 1 year after surgery. Acta Biomed. 2012;83:114–21. [PubMed] [Google Scholar]
- 16.Barile A, Bruno F, Mariani S, et al. Follow-up of surgical and minimally invasive treatment of Achilles tendon pathology: a brief diagnostic imaging review. Musculoskelet Surg. 2017;101:51–61. doi: 10.1007/s12306-017-0456-1. [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Arrigoni F, Mariani S, Bruno F, Barile A, Masciocchi C. An unusual localization of chondroblastoma: The triradiate cartilage; from a case report a reconstructive technique proposal with imaging evolution. J Clin Orthop Trauma. 2017;8:S48–s52. doi: 10.1016/j.jcot.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno F, Barile A, Arrigoni F, et al. Weight-bearing MRI of the knee: a review of advantages and limits. Acta Biomed. 2018;89:78–88. doi: 10.23750/abm.v89i1-S.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zappia M, Reginelli A, Russo A, et al. Long head of the biceps tendon and rotator interval. Musculoskelet Surg. 2013;97(Suppl 2):S99–108. doi: 10.1007/s12306-013-0290-z. [DOI] [PubMed] [Google Scholar]
- 20.Di Pietto F, Chianca V, de Ritis R, et al. Postoperative imaging in arthroscopic hip surgery. Musculoskelet Surg. 2017;101:43–49. doi: 10.1007/s12306-017-0459-y. [DOI] [PubMed] [Google Scholar]
- 21.Bredella MA, Tirman PF, Wischer TK, Belzer J, Taylor A, Genant HK. Reactive synovitis of the knee joint: MR imaging appearance with arthroscopic correlation. Skeletal Radiol. 2000;29:577–82. doi: 10.1007/s002560000259. [DOI] [PubMed] [Google Scholar]
- 22.Perry TA, Gait A, O’Neill TW, et al. Measurement of synovial tissue volume in knee osteoarthritis using a semiautomated MRI-based quantitative approach. Magn Reson Med. 2019;81:3056–64. doi: 10.1002/mrm.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Teichtahl AJ, Pelletier JP, et al. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology (Oxford) 2019;58:246–53. doi: 10.1093/rheumatology/key274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Huang C, Chen S, et al. Value of contrast-enhanced ultrasound for detection of synovial vascularity in experimental rheumatoid arthritis: an exploratory study. J Int Med Res. 2019;47:5740–51. doi: 10.1177/0300060519874159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiocco U, Stramare R, Coran A, et al. Vascular perfusion kinetics by contrast-enhanced ultrasound are related to synovial microvascularity in the joints of psoriatic arthritis. Clin Rheumatol. 2015;34:1903–12. doi: 10.1007/s10067-015-2894-1. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli R. Methotrexate in rheumatoid arthritis: optimizing therapy among different formulations. Current and emerging paradigms. Clin Ther. 2014;36:427–35. doi: 10.1016/j.clinthera.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Giacomelli R, Gorla R, Trotta F, et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology (Oxford) 2015;54:792–7. doi: 10.1093/rheumatology/keu398. [DOI] [PubMed] [Google Scholar]
- 28.Ruscitti P, Ursini F, Cipriani P, et al. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: A 1-year, single-centre, longitudinal study. PLoS One. 2017;12:e0181203. doi: 10.1371/journal.pone.0181203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruscitti P, Cipriani P, Masedu F, et al. Increased Cardiovascular Events and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients: 1 Year Prospective Single Centre Study. PLoS One. 2017;12:e0170108. doi: 10.1371/journal.pone.0170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruscitti P, Cipriani P, Liakouli V, et al. Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res Ther. 2019;21:204. doi: 10.1186/s13075-019-1975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still’s disease. J Autoimmun. 2018;93:24–36. doi: 10.1016/j.jaut.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Ruscitti P, Iacono D, Ciccia F, et al. Macrophage Activation Syndrome in Patients Affected by Adult-onset Still Disease: Analysis of Survival Rates and Predictive Factors in the Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale Cohort. J Rheumatol. 2018;45:864–72. doi: 10.3899/jrheum.170955. [DOI] [PubMed] [Google Scholar]
- 33.Ruscitti P, Cipriani P, Ciccia F, et al. Prognostic factors of macrophage activation syndrome, at the time of diagnosis, in adult patients affected by autoimmune disease: Analysis of 41 cases collected in 2 rheumatologic centers. Autoimmun Rev. 2017;16:16–21. doi: 10.1016/j.autrev.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Cipriani P, Di Benedetto P, Ruscitti P, et al. Perivascular Cells in Diffuse Cutaneous Systemic Sclerosis Overexpress Activated ADAM12 and Are Involved in Myofibroblast Transdifferentiation and Development of Fibrosis. J Rheumatol. 2016;43:1340–9. doi: 10.3899/jrheum.150996. [DOI] [PubMed] [Google Scholar]
- 35.Giacomelli R, Liakouli V, Berardicurti O, et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int. 2017;37:853–63. doi: 10.1007/s00296-016-3636-7. [DOI] [PubMed] [Google Scholar]
- 36.Di Cesare E, Battisti S, Di Sibio A, et al. Early assessment of sub-clinical cardiac involvement in systemic sclerosis (SSc) using delayed enhancement cardiac magnetic resonance (CEMRI) Eur J Radiol. 2013;82:e268–73. doi: 10.1016/j.ejrad.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Salaffi F, Carotti M, Di Carlo M, Farah S, Gutierrez M. Adherence to Anti-Tumor Necrosis Factor Therapy Administered Subcutaneously and Associated Factors in Patients With Rheumatoid Arthritis. J Clin Rheumatol. 2015;21:419–25. doi: 10.1097/RHU.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 38.Di Geso L, Zardi EM, Afeltra A, et al. Comparison between conventional and automated software-guided ultrasound assessment of bilateral common carotids intima-media thickness in patients with rheumatic diseases. Clin Rheumatol. 2012;31:881–4. doi: 10.1007/s10067-011-1915-y. [DOI] [PubMed] [Google Scholar]
- 39.Salaffi F, Carotti M, Bosello S, et al. Computer-aided quantification of interstitial lung disease from high resolution computed tomography images in systemic sclerosis: correlation with visual reader-based score and physiologic tests. Biomed Res Int. 2015;2015:834262. doi: 10.1155/2015/834262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitali C, Carotti M, Salaffi F. Is it the time to adopt salivary gland ultrasonography as an alternative diagnostic tool for the classification of patients with Sjogren’s syndrome? Comment on the article by Cornec et al. Arthritis Rheum. 2013;65:1950. doi: 10.1002/art.37945. [DOI] [PubMed] [Google Scholar]
- 41.Giacomelli R, Afeltra A, Alunno A, et al. Guidelines for biomarkers in autoimmune rheumatic diseases - evidence based analysis. Autoimmun Rev. 2019;18:93–106. doi: 10.1016/j.autrev.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Cipriani P, Berardicurti O, Masedu F, et al. Biologic therapies and infections in the daily practice of three Italian rheumatologic units: a prospective, observational study. Clin Rheumatol. 2017;36:251–60. doi: 10.1007/s10067-016-3444-1. [DOI] [PubMed] [Google Scholar]
- 43.Dakkak YJ, Boer AC, Boeters DM, Niemantsverdriet E, Reijnierse M, van der Helm-van Mil AHM. The relation between physical joint examination and MRI-depicted inflammation of metatarsophalangeal joints in early arthritis. Arthritis Res Ther. 2020;22:67. doi: 10.1186/s13075-020-02162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barendregt AM, Bray TJP, Hall-Craggs MA, Maas M. Emerging quantitative MR imaging biomarkers in inflammatory arthritides. Eur J Radiol. 2019;121:108707. doi: 10.1016/j.ejrad.2019.108707. [DOI] [PubMed] [Google Scholar]
- 45.Cimmino MA, Parodi M, Barbieri F, et al. Dynamic Contrast-Enhanced MRI Confirms Rapid And Sustained Improvement Of Rheumatoid Arthritis Induced By Tocilizumab Treatment: An Italian Multicentre Study. Biologics. 2020;14:13–21. doi: 10.2147/BTT.S209873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cimmino MA, Innocenti S, Livrone F, Magnaguagno F, Silvestri E, Garlaschi G. Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum. 2003;48:1207–13. doi: 10.1002/art.10962. [DOI] [PubMed] [Google Scholar]
- 47.Yi J, Lee YH, Song HT, Suh JS. Double-inversion recovery with synthetic magnetic resonance: a pilot study for assessing synovitis of the knee joint compared to contrast-enhanced magnetic resonance imaging. Eur Radiol. 2019;29:2573–80. doi: 10.1007/s00330-018-5800-9. [DOI] [PubMed] [Google Scholar]
- 48.Perrotta FM, Astorri D, Zappia M, Reginelli A, Brunese L, Lubrano E. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol Int. 2016;36:1579–83. doi: 10.1007/s00296-016-3562-8. [DOI] [PubMed] [Google Scholar]
- 49.Ostergaard M, Court-Payen M, Gideon P, et al. Ultrasonography in arthritis of the knee. A comparison with MR imaging. Acta Radiol. 1995;36:19–26. [PubMed] [Google Scholar]
- 50.El-Miedany YM, Housny IH, Mansour HM, Mourad HG, Mehanna AM, Megeed MA. Ultrasound versus MRI in the evaluation of juvenile idiopathic arthritis of the knee. Joint Bone Spine. 2001;68:222–30. doi: 10.1016/s1297-319x(01)00269-x. [DOI] [PubMed] [Google Scholar]
- 51.Bruno F, Arrigoni F, Palumbo P, et al. New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints. Radiol Med. 2019;124:1121–27. doi: 10.1007/s11547-019-01003-1. [DOI] [PubMed] [Google Scholar]
- 52.Carotti M, Salaffi F, Di Carlo M, Giovagnoni A. Relationship between magnetic resonance imaging findings, radiological grading, psychological distress and pain in patients with symptomatic knee osteoarthritis. Radiol Med. 2017;122:934–43. doi: 10.1007/s11547-017-0799-6. [DOI] [PubMed] [Google Scholar]
- 53.de Vries BA, van der Heijden RA, Poot DHJ, et al. Quantitative DCE-MRI demonstrates increased blood perfusion in Hoffa’s fat pad signal abnormalities in knee osteoarthritis, but not in patellofemoral pain. Eur Radiol. 2020;30:3401–08. doi: 10.1007/s00330-020-06671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oo WM, Linklater JM, Bennell KL, et al. Are OMERACT knee osteoarthritis ultrasound scores associated with pain severity, other symptoms, radiographic and MRI findings? J Rheumatol. 2020 doi: 10.3899/jrheum.191291. [DOI] [PubMed] [Google Scholar]
- 55.Shakoor D, Demehri S, Roemer FW, Loeuille D, Felson DT, Guermazi A. Are contrast-enhanced and non-contrast MRI findings reflecting synovial inflammation in knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2020;28:126–36. doi: 10.1016/j.joca.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Barile A, Arrigoni F, Bruno F, et al. Present role and future perspectives of interventional radiology in the treatment of painful bone lesions. Future Oncol. 2018;14:2945–55. doi: 10.2217/fon-2017-0657. [DOI] [PubMed] [Google Scholar]
- 57.Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol. 2017;34:52–69. doi: 10.1007/s12032-017-0906-5. [DOI] [PubMed] [Google Scholar]
- 58.Ierardi AM, Piacentino F, Fontana F, et al. The role of endovascular treatment of pelvic fracture bleeding in emergency settings. Eur Radiol. 2015;25:1854–64. doi: 10.1007/s00330-015-3589-3. [DOI] [PubMed] [Google Scholar]
- 59.Masciocchi C, Arrigoni F, La Marra A, et al. Treatment of focal benign lesions of the bone: MRgFUS and RFA. The British journal of radiology. 2016;89:20150356-56-71. doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrafiello G, Ierardi AM, Duka E, et al. Usefulness of Cone-Beam Computed Tomography and Automatic Vessel Detection Software in Emergency Transarterial Embolization. Cardiovasc Intervent Radiol. 2016;39:530–7. doi: 10.1007/s00270-015-1213-1. [DOI] [PubMed] [Google Scholar]
- 61.Perri M, Grattacaso G, di Tunno V, et al. T2 shine-through phenomena in diffusion-weighted MR imaging of lumbar discs after oxygen-ozone discolysis: a randomized, double-blind trial with steroid and O2-O3 discolysis versus steroid only. Radiol Med. 2015;120:941–50. doi: 10.1007/s11547-015-0519-z. [DOI] [PubMed] [Google Scholar]
- 62.Cazzato RL, Arrigoni F, Boatta E, et al. Percutaneous management of bone metastases: state of the art, interventional strategies and joint position statement of the Italian College of MSK Radiology (ICoMSKR) and the Italian College of Interventional Radiology (ICIR) Radiol Med. 2019;124:34–49. doi: 10.1007/s11547-018-0938-8. [DOI] [PubMed] [Google Scholar]
- 63.Arrigoni F, Barile A, Zugaro L, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34:55. doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- 64.Zoccali C, Rossi B, Zoccali G, et al. A new technique for biopsy of soft tissue neoplasms: a preliminary experience using MRI to evaluate bleeding. Minerva Med. 2015;106:117–120. [PubMed] [Google Scholar]
- 65.Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89:20150355. doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Lucia O, Murgo A, Pregnolato F, et al. Hyaluronic Acid Injections in the Treatment of Osteoarthritis Secondary to Primary Inflammatory Rheumatic Diseases: A Systematic Review and Qualitative Synthesis. Adv Ther. 2020;37:1347–59. doi: 10.1007/s12325-020-01256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henrotin Y, Lambert C, Richette P. Importance of synovitis in osteoarthritis: evidence for the use of glycosaminoglycans against synovial inflammation. Semin. Arthritis Rheum. 2014;43:579–87. doi: 10.1016/j.semarthrit.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5:351–61. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deseyne N, Conrozier T, Lellouche H, et al. Hip Inflammation MRI Scoring System (HIMRISS) to predict response to hyaluronic acid injection in hip osteoarthritis. Joint Bone Spine. 2018;85:475–80. doi: 10.1016/j.jbspin.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Paterson KL, Hunter DJ, Metcalf BR, et al. Efficacy of intraarticular injections of platelet-rich plasma as a symptom-and disease-modifying treatment for knee osteoarthritis - the RESTORE trial protocol. BMC Musculoskelet Disord. 2018;19:272. doi: 10.1186/s12891-018-2205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raeissadat SA, Ghorbani E, Sanei Taheri M, et al. MRI Changes After Platelet Rich Plasma Injection in Knee Osteoarthritis (Randomized Clinical Trial) J Pain Res. 2020;13:65–73. doi: 10.2147/JPR.S204788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Telischak NA, Wu JS, Eisenberg RL. Cysts and cystic-appearing lesions of the knee: A pictorial essay. Indian J Radiol Imaging. 2014;24:182–91. doi: 10.4103/0971-3026.134413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33:833–55. doi: 10.1148/rg.333115062. [DOI] [PubMed] [Google Scholar]
- 74.Beaman FD, Peterson JJ. MR imaging of cysts, ganglia, and bursae about the knee. Magn Reson Imaging Clin N Am. 2007;15:39–52. doi: 10.1016/j.mric.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Falek A, Niemunis-Sawicka J, Wrona K, et al. Pigmented villonodular synovitis. Folia Med Cracov. 2018;58:93–104. [PubMed] [Google Scholar]
- 76.Willimon SC, Busch MT, Perkins CA. Pigmented Villonodular Synovitis of the Knee: An Underappreciated Source of Pain in Children and Adolescents. J Pediatr Orthop. 2018;38:e482–e85. doi: 10.1097/BPO.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 77.Gouin F, Noailles T. Localized and diffuse forms of tenosynovial giant cell tumor (formerly giant cell tumor of the tendon sheath and pigmented villonodular synovitis) Orthop Traumatol Surg Res. 2017;103:S91–S97. doi: 10.1016/j.otsr.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Nambiar M, Onggo JR, Jacobson A. Lipoma arborescens: a rare cause of clicking in the knee. BMJ Case Rep. 2019:12. doi: 10.1136/bcr-2018-227949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minami S, Miyake Y, Kinoshita H. Lipoma arborescens arising in the extra-articular bursa of the knee joint. SICOT J. 2016;2:28. doi: 10.1051/sicotj/2016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Padhan P, Ahmed S. Synovial Chondromatosis. N Engl J Med. 2019;381:1364. doi: 10.1056/NEJMicm1813672. [DOI] [PubMed] [Google Scholar]
- 81.Derek Stensby J, Fox MG, Kwon MS, Caycedo FJ, Rahimi A. Primary synovial chondromatosis of the subtalar joint: case report and review of the literature. Skeletal Radiol. 2018;47:391–96. doi: 10.1007/s00256-017-2775-6. [DOI] [PubMed] [Google Scholar]