Abstract
Good knowledge of the various approaches of embolization of peripheral bleedings and different embolic materials available is of paramount importance for successful and safe embolization. We review and illustrate the main endovascular and percutaneous techniques used for embolization, along with the characteristics of the different embolic materials, and the potential complications. (www.actabiomedica.it)
Keywords: embolization, interventional radiology, sac packing, sandwich technique
Introduction
In recent years, radiology has been one of the medical branches that has experienced the most important technological revolution and expansion, both in diagnostic (1-3) and interventional applications. In the interventional setting, numerous and different techniques have been introduced (4-7), allowing the treatment of several medical (8) and surgical (9-11) conditions in practically all body areas (12-19). In this context, embolization has become a major arm of modern interventional radiology practice, with growing scope and complexity in diverse clinical scenarios. The goal of embolization is to obtain the occlusion or decrease of the blood/ lymph flow through the endovascular application of different agents or materials. Over the past decades, endovascular embolization has witnessed an increasing use because of a combination of the trend towards conservative treatment protocols, advances in catheter technology, the introduction of new embolic agents, and improvements in digital imaging (20, 21).
Different endovascular procedures have been applied for previously unsolvable problems, replacing open surgery in many settings thanks to their low invasiveness, reduced morbidity and mortality rates, shortened hospital stay and recovery time, and an overall positive impact on the health system economics (20, 21).
After the first intuition in 1953 by Seldinger, of a simple technique allowing the percutaneous catheter replacement of a needle or trocar, Dotter described, in the early 1960s, the possibility of using catheters to perform intravascular surgery; however, only in the mid-1970s, transcatheter therapeutic procedures started to become popular (22). Since then, embolization has become a fundamental procedure and has been included in the treatment protocols for a wide variety of conditions: trauma, emergency bleeding; non-traumatic hemorrhage including hemoptysis or gastro-intestinal bleeding; cancer care, through direct therapy delivery and solid tumor chemoembolization, preoperative devascularization, hepatic growth stimulation before surgery; congenital or acquired vascular malformations and aneurysms; treatment of uterine myomas and other benign conditions (23-32).
A comprehensive understanding and practical knowledge of these techniques are essential for the optimal and safe use in different scenarios. In this work, we describe different approaches of embolization with a focus on “tips and tricks” of each modality, providing interventional strategies for avoiding and managing procedure-related complications.
Endovascular Approach
The endovascular approach is the most used, with different available techniques.
Proximal Embolization
Proximal embolization (PE) is recommended in cases of multifocal injury or in cases of contrast blush detected at CT-angiography, without clear evidence of bleeding with catheter angiography. This approach requires a proper planning in the different vascular areas, to assess the presence of “good” collaterals to the target tissue, and thus preventing ischemic lesions (33).
Spleen
PE plays a crucial role in the management of traumatic splenic lesions, decreasing the perfusion pressure within the splenic parenchyma, and maintaining the collateral flow to preserve long-term splenic function. PE of splenic artery reduces the risk of acute rupture, infarction, and abscess formation. Splenic PE is performed mainly using endovascular plugs and coils, to spare the origin of the dorsal pancreatic artery and great pancreatic artery, and to maintain the collateral flow (34, 35).
Proximal splenic embolization, if permed in selected patients, is also an efficient treatment for hypersplenism, increasing white blood cells (WBC) and platelets count in these patients (36).
Liver
Blunt liver trauma are more frequently associated with venous injuries, commonly treated conservatively. In case of arterial lesions in hemodynamically stable patients, the endovascular approach is recommended; this can be performed through permanent and distal embolization (e.g., coils) if the bleeding site is easily evidenced by angiogram; otherwise temporary embolization (usually with gelfoam) should be chosen for “shower” embolization, distally to the origin of the gastro-duodenal artery. Procedural complications include liver necrosis and abscess formations (37, 38).
Renal artery embolization
Renal artery embolization (RAE) is recommended in various pathologic conditions, including traumatic or iatrogenic vascular injuries, and reports have described good preservation of renal function after embolization. RAE should always be performed as selective as possible in order to reduce the risk of loss of nephrons and renal function (39). Some authors stated that detachable coils are safer than liquid embolizing agents (40), because they allow a more reliable controlled release (fig. 1).
Figure 1.
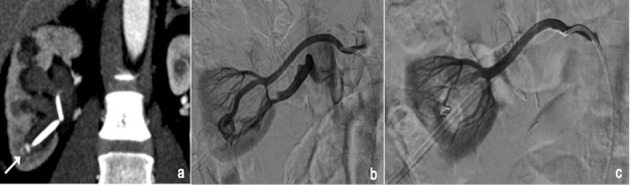
CT coronal view shows pseudoaneurysm (PSA) after nephrostomy (a); arteriogram confirms PSA and reveals an artero-venous fistula also (b); final angiogram after embolization with coil (c).
Recently, Jardinet et al. (41) proposed the use of microvascular plugs as embolic agents in case of renal bleeding; they reported a superselective embolization with a minimal resulting renal infarction volume.
Pelvic Trauma
In case of active arterial bleeding from pelvic fractures or organ injuries (muscle laceration), endovascular embolization is nowadays considered a safe option in order to stabilize a patient with polytrauma (33, 42, 43). Internal iliac artery (IIA) branches are the most common source of pelvic bleeding followed by branches of the external iliac and common femoral arteries. In pelvic trauma, both proximal and distal embolization were described (44, 45).
Proximal embolization is usually adopted in patients with multiple lesions and/or unstable patients. As hemodynamic stabilization should be considered the main purpose of TAE, the operator may also use temporary embolic materials to save valuable time in hemodynamically unstable patients.
Selective embolization is preferred in stable patients and when one or more bleeding point are clearly visualized at preliminary angiogram (33, 45).
In literature, both mechanical and liquid embolic agents were described as effective and safe (46, 47). Gelfoam and coils seem to be the most used materials, often used in combination. Gelfoam is a biodegradable gelatine sponge, lasts for 7–21 days, and it is relatively manageable and economical. Furthermore, no long or short-term complications were associated with its use (46). Coils are commonly used for more specific and selective embolization, allowing a fast mechanical occlusion. However, multiple coils are often necessary to obtain the complete bleeding control. Liquid agents, including adhesive (glue) or non-adhesive (Onyx), represent interesting tools for very distal vessels, and can also be used in cases of rebleeding. The main limitations of liquid embolising agents are the costs, the lack of controlling the amount used and the risk of back-flow (45).
Lower gastrointestinal bleeding
Embolization is currently proposed as the first step in the treatment of acute, life-threatening LGIB, when an endoscopic approach is not possible or unsuccessful (48). Despite the high success rate, some complications like rebleeding and bowel infarction are reported in literature. Due to the terminal bowel vascular branches with no collateral circulation, the ideal area to perform LGIB embolization is at the level of vasa recta. The ideal embolic agent should also be characterized by controlled release to reduce the risk of extravasation in the marginal vessels. Some Authors (49) suggested the use of selective flow-directed particles; however, these cannot be visualized or precisely deposited and may reflux into non-target arteries. Better results, in terms of rebleeding rates, were achieved with cyanoacrylates, despite their use require experienced operator, due to the increased risk of non-targeted embolization and microcatheter entrapment (50). To overcome this limitation, detachable coils were introduced, allowing a safe and selective embolization also in cases in which vessels are thin and/or tortuous. Liquid embolic (LE) materials like non-adhesive agents, such as ethylene-vinyl alcohol co-polymer (EVOH), are characterized by controlled release, non-adherence, progressive solidification, cohesiveness, high vascular penetration, and a weak inflammatory effect on the endothelium.
Moreover, they polymerize gradually from the periphery out towards the center. This represents a great difference from cyanoacrylates, which polymerize instantaneously. Furthermore, another advantage is that LE is displaced by pressure applied by the operator on the syringe and is not blood-flow dependent. Consequently, the procedure is safer and allows to leave the distal tip of the catheter in situ, thus permitting selective angiographic control after some minutes.
Bronchial artery hypertrophy embolization
Bronchial artery embolization is the recommended treatment for hemoptysis in case of airways bleeding. Both mechanical and liquid agents could be used. Among the mechanical ones, the most used are particles and coils (fig. 2). The particles are highly effective on the distal microcirculation (51). Coils represent a safer alternative, with high success rates, however, in case of large-caliber bronchial artery with tortuous course (V-shaped), the use of coils can be problematic. Liquid embolic agents overcame those limitations, the most used being n-Butyl-2-cyanoacrylate (NBCA). Nevertheless, liquid embolizing agents have some limitations such as the need of compatible microcatheters, the cost and the mandatory use of dimethyl sulfoxide DMSO, which can cause vasospasm, endothelial wall damage and pain (52).
Figure 2.
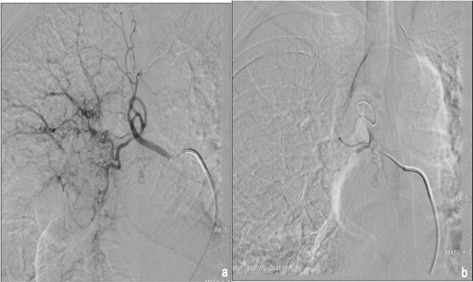
selective arteriogram of the right hypertrophic bronchial artery (a); final angiogram after embolization performed with particles (b).
Sac packing
In case of aneurysm or pseudoaneurysm, a sac packing (SP) technique can be used for embolization (53). This procedure implies coils placement in a vascular sac until it is obliterated or excluded from the circulation. This technique is well suited for saccular aneurysms with a narrow neck, allowing retention of the coils in the sac and preserving the parent vessel flow to the visceral end-organ (54-56) (fig. 3). Balloon assisted SP, bare metal stent-assisted SP and percutaneous SP cast forming may also be considered techniques to fill the sac with one or more embolic agents. This technique consists in the coaxial positioning of an inflated balloon, in order to reduce the risk of spillage of the embolic agent; moreover, in the event of intra-procedural acute aneurysm rupture, the balloon could be used as a fast hemostatic agent (57).
Figure 3.
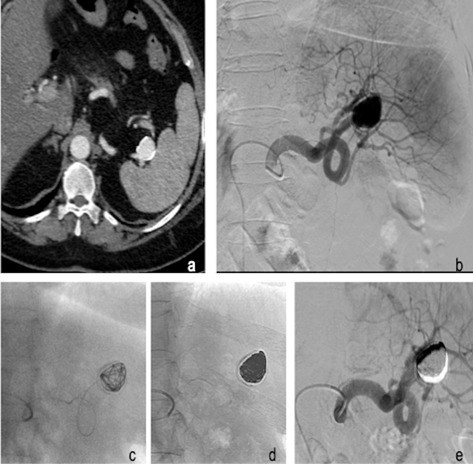
52 years old female with incidental finding of distal splenic artery aneurysm. Axial CT acquisition shows a distal splenic artery aneurysm with a diameter of cm 3 (a); Digital Substruction Angiography (DSA) confirms the arterial dilation of the distal part of the splenic artery (b). Super-selective arteriogram performed with microcatheter with the distal end inside the aneurysmal sac (c). Single shot acquisition demonstrates coils compacted in the aneurysm (d). Post procedural DSA corfims regular patency of the splenic artery with the complete exclusion of the distal splenic artery aneurysm (e).
Bare metal stent-assisted SP, always used in combination with coils, aims to avoid the coils migration and, at the same time, maintain the patency of the treated vessel (58, 59).
Percutaneous direct puncture of the sac may be considered when the endovascular approach is not feasible or unsuccessful. Of course, the sac must be accessible with the percutaneous approach; imaging guidance must be usable (ultrasound, CT, Cone Beam CT, fluoroscopic). The sac may be filled with one or more embolic agents using more than one imaging technique as guidance.
Sandwich Technique
The sandwich technique implicates embolization of both the afferent and efferent vessels to completely remove all portions of a target lesion from the circulation. Such a method is usually preferred in patients in whom collateral flow could “flow back” into the lesion if only one segment of the vessel is occluded (60). Sandwich technique is indicated in peripheral embolization, especially in districts in which backflow from collaterals should be fill the bleeding site (fig. 4). A clear understanding of the target vessel is critical, especially if it contains extensive collateral supply (e.g., via muscular branches) as these can provide distal flow and supply to the bleeding vessel and therefore result in continued bleeding if they are not also embolized. Accordingly, both proximal and distal segments to the site of injury of the artery, are routinely embolized to prevent this complication (61). The intraprocedural impossibility to reach the distal branch during an endovascular exclusion of a PSA represents an example of a condition that is a common challenge. An example of sandwich technique application is that of embolization of splenic artery aneurysm/pseudoaneurysm that could be evidenced in the setting of pancreatitis, splenic trauma, or mycotic infection of the arterial wall. As with most pseudoaneurysms, there is a high risk of rupture without appropriate management, and all splenic lesions should be treated regardless of size or clinical manifestation (62). Such proper management is usually a coil embolization or a stent-graft placement across the lesion when the anatomy allows (for instance, more proximal lesions and non-tortuous arterial segments to facilitate device passage).
Figure 4.
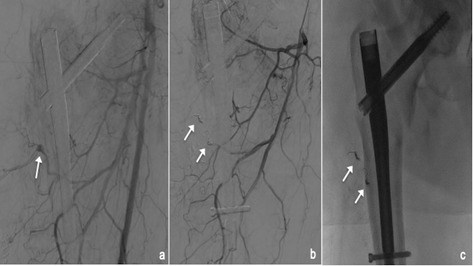
small pseudoaneurysm (PSA) of the profunda femoral artery (white arrow,a); angiogram at the end of the embolization with sandwich technique performed with 2 microcoils (white arrows, b); single shot confirms the presence of 2 coils (white arrows, c).
Distal and proximal embolization (sandwich technique) across the aneurysm neck is typically required to prevent collateral circulation resulting in continued sac pressurization. Since the aneurysm sac could continue to be pressurized through short gastric or distal pancreatic arteries acting as retrograde-filling collateral vessel, embolization of only the feeding-afferent artery would be unsatisfactory indeed. The “back door”, also named the efferent artery, is usually closed first, followed by the “front door”, namely the afferent artery. Alternatively, a sac-packing technique with coils or parent artery glue embolization has also been used. Intentional embolization of the entire splenic artery may be required in complex, high-risk splenic pseudoaneurysms (63).
The sandwich technique may also be applied in more complex settings of multiple afferent or efferent vessels.
Stent placement
Aneurysm and PSA with wide neck have an increased risk of migration of embolic material. To avoid this complication, stent-graft (covered stent) placement, stent-assisted coiling and balloon remodeling techniques are useful (53). These techniques also help in preserving the patency of parent artery. Recently, flow diverting multi-layered bare stents are available, which facilitate slowing the blood flow within the visceral artery aneurysms and maintaining patency of the parent artery as well as any branches arising from or proximal to the aneurysm. Although it is used in the treatment of true aneurysms, its use in pseudoaneurysms is limited, as thrombosis occurs slowly and there is a possibility of rupture in the interim (53).
Percutaneous approach
It is usually used for cases of failed endovascular approach or pseudoaneurysms or bleeding not accessible endovascularly, PSA with narrow neck or localized in solid organs. Bleeding sites must be visible with imaging guides. More often, ultrasound or CT guidance is used as imaging guidance; cone-beam CT may be used as well. More rarely, operator may take advantage from the stasis of the contrast media into the pseudoaneurysm after angiogram acquisition; after realizing the impossibility to reach the bleeding site by endovascular route, fluoroscopic guidance may be successfully used. Multiple projections are usually necessary to guide the needle. Its correct position may be confirmed with the injection of contrast media; once in the desired location, the embolic agent is injected under fluoroscopic guidance until the embolization in completed
The pseudoaneurysm is usually punctured using a 22 G Chiba needle. Care should be taken to keep the tip of the needle away from the neck, to avoid non-target embolization. Once within the pseudoaneurysm, embolization is performed as described above, until thrombosis of the pseudoaneurysm occurs (fig. 5). Thrombin, glue and occasionally coils are used as embolic materials. Complications include rupture of the pseudoaneurysm, non- target embolization, and recurrence (64, 65).
Figure 5.
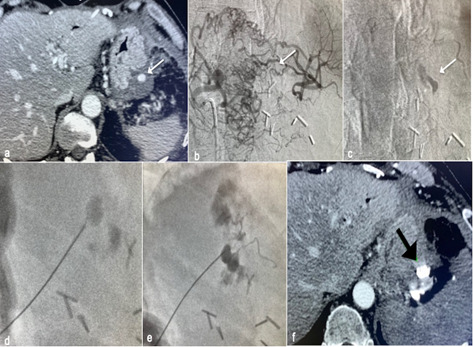
CT scan reveals gastric pseudoaneurysm (PSA) (a); arteriogram of the celiac trunk shows occlusion of the splenic artery and the PSA filled by small vessels, impossible to catheterize (b); contrast medium remained in the PSA (white arrow,c); percutaneous puncture was performed under fluoroscopic guidance (d); glue was used as embolic agent (e); CT was performed to check the complete embolization of the PSA (black arrow, f).
Complications
Complications may be divided into complications of the puncture site, of the embolization site and post-embolization (64). Complications of the puncture site occur in approximately 10-20% of cases and they are usually classified as minor complications (pain, bleeding and hematoma) that may be easily managed intraprocedurally (66). Infection or, rarely, pseudoaneurysm at the puncture site can be managed with supportive care, percutaneous drainage, or other non-invasive management.
Complications related to the embolization are essentially the inadvertent distal infarction of nonexpendable vessels and damages in the adjacent organs (67).
For this reason, a clear understanding of the target vessel is critical, especially if it contains extensive collateral supply, as these can provide flow to the bleeding vessel. Therefore, routinely both segments of the artery proximal and distal to the site of injury, are embolised.
A technically successful embolization requires the catheter tip to be placed so that embolic material is deposited only in blood vessels that serve the abnormal area, preventing injury to healthy tissue. This can be technically impossible in a small percentage of cases (68).
Post-embolization complications include the post-embolization syndrome, which is one of the most common side effects of such procedure and it is more often associated with large fibroids or large tumors or solid organ embolization. This includes fever, nausea and/or vomiting, and pain. It is often a self-limiting phenomenon and usually occurs within the first 72 hours after the procedure and generally starting to subside after 72 hours. Although the etiology of the post-embolization syndrome is partially unknown, it was hypothesized that tissue hypoxia and cell death lead to the release of tissue breakdown products, inflammatory mediators, and vasoactive substances from the tumor and or adjacent healthy tissues. Early imaging following embolization, either by ultrasound or CT, may reveal intralesional gas. This is not to be mistaken for abscess without additional factors (69).
Post-embolization syndrome usually is self-limiting, and treatment is symptomatic with analgesics and intravenous fluids. Prophylactic use of antipyretic and antiemetic therapy may be considered prior to the embolization of large tumors/fibroids (70).
Another complication that may follow embolization procedures is the continued bleeding distal to the point of embolization secondary to collateral flow.
In general, up to 85% of complications of embolization can be managed with only supportive care, percutaneous drainage, or other non-invasive management (64, 66, 67).
Conflict of interest:
Authors declare that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.D’Orazio F, Splendiani A, Gallucci M. 320-Row Detector Dynamic 4D-CTA for the Assessment of Brain and Spinal Cord Vascular Shunting Malformations. A Technical Note. Neuroradiol J. 2014;27:710–7. doi: 10.15274/NRJ-2014-10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzei MA, Gentili F, Mazzei FG, et al. High-resolution MR lymphangiography for planning lymphaticovenous anastomosis treatment: a single-centre experience. Radiol Med. 2017;122:918–27. doi: 10.1007/s11547-017-0795-x. [DOI] [PubMed] [Google Scholar]
- 3.Carrafiello G, Lagana D, Recaldini C, et al. Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience. Cardiovasc Intervent Radiol. 2006;29:969–74. doi: 10.1007/s00270-005-0267-x. [DOI] [PubMed] [Google Scholar]
- 4.Gatta G, Parlato V, Di Grezia G, et al. Ultrasound-guided aspiration and ethanol sclerotherapy for treating endometrial cysts. Radiol Med. 2010;115:1330–9. doi: 10.1007/s11547-010-0586-0. [DOI] [PubMed] [Google Scholar]
- 5.Carrafiello G, Fontana F, Mangini M, et al. Initial experience with percutaneous biopsies of bone lesions using Xper-Guide cone-beam CT (CBCT): technical note. Radiol Med. 2012;117:1386–97. doi: 10.1007/s11547-012-0788-1. [DOI] [PubMed] [Google Scholar]
- 6.Perri M, Grattacaso G, di Tunno V, et al. T2 shine-through phenomena in diffusion-weighted MR imaging of lumbar discs after oxygen-ozone discolysis: a randomized, double blind trial with steroid and O2-O3 discolysis versus steroid only. Radiol Med. 2015;120:941–50. doi: 10.1007/s11547-015-0519-z. [DOI] [PubMed] [Google Scholar]
- 7.Belfiore G, Belfiore MP, Reginelli A, et al. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med Oncol. 2017;34:38. doi: 10.1007/s12032-017-0887-4. [DOI] [PubMed] [Google Scholar]
- 8.Carrafiello G, Mangini M, Fontana F, et al. Suprarenal inferior vena cava filter implantation. Radiol Med. 2012;117:1190–8. doi: 10.1007/s11547-012-0851-5. [DOI] [PubMed] [Google Scholar]
- 9.Carrafiello G, Piffaretti G, Lagana D, et al. Endovascular treatment of ruptured abdominal aortic aneurysms: aorto-uniiliac or bifurcated endograft? Radiol Med. 2012;117:410–25. doi: 10.1007/s11547-011-0717-2. [DOI] [PubMed] [Google Scholar]
- 10.Carrafiello G, D’Ambrosio A, Mangini M, et al. Percutaneous cholecystostomy as the sole treatment in critically ill and elderly patients. Radiol Med. 2012;117:772–9. doi: 10.1007/s11547-012-0794-2. [DOI] [PubMed] [Google Scholar]
- 11.Carrafiello G, Dionigi G, Ierardi AM, et al. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renal lesions Bosniak III or IV after 24 months follow up. Int J Surg. 2013;11(Suppl 1):S30–5. doi: 10.1016/S1743-9191(13)60010-2. [DOI] [PubMed] [Google Scholar]
- 12.Barile A, Arrigoni F, Bruno F, et al. Present role and future perspectives of interventional radiology in the treatment of painful bone lesions. Future Oncol. 2018;14:2945–55. doi: 10.2217/fon-2017-0657. [DOI] [PubMed] [Google Scholar]
- 13.Belfiore MP, Reginelli A, Maggialetti N, et al. Preliminary results in unresectable cholangiocarcinoma treated by CT percutaneous irreversible electroporation: feasibility, safety and efficacy. Med Oncol. 2020;37:45. doi: 10.1007/s12032-020-01360-2. [DOI] [PubMed] [Google Scholar]
- 14.Detti B, Baki M, Becherini C, et al. High-dose intensitymodulated radiation therapy as primary treatment of prostate cancer: genitourinary/gastrointestinal toxicity and outcomes, a single-institution experience. Radiol Med. 2019;124:422–31. doi: 10.1007/s11547-018-0977-1. [DOI] [PubMed] [Google Scholar]
- 15.Consoli A, Grazzini G, Renieri L, et al. Effects of hyperearly (<12 hours) endovascular treatment of ruptured intracranial aneurysms on clinical outcome. Interv Neuroradiol. 2013;19:195–202. doi: 10.1177/159101991301900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barile A, Quarchioni S, Bruno F, et al. Interventional radiology of the thyroid gland: critical review and state of the art. Gland Surg. 2018;7:132–46. doi: 10.21037/gs.2017.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masciocchi C, Arrigoni F, La Marra A, Mariani S, Zugaro L, Barile A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol. 2016;89:20150356. doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoccali C, Rossi B, Zoccali G, et al. A new technique for biopsy of soft tissue neoplasms: a preliminary experience using MRI to evaluate bleeding. Minerva Med. 2015;106:117–20. [PubMed] [Google Scholar]
- 19.Arrigoni F, Gregori LM, Zugaro L, Barile A, Masciocchi C. MRgFUS in the treatment of MSK lesions: a review based on the experience of the University of L’Aquila, Italy. Translational Cancer Research. 2014;3:442–48. [Google Scholar]
- 20.Tsetis D, Uberoi R, Fanelli F, et al. The Provision of Interventional Radiology Services in Europe: CIRSE Recommendations. Cardiovasc Intervent Radiol. 2016;39:500–6. doi: 10.1007/s00270-016-1299-0. [DOI] [PubMed] [Google Scholar]
- 21.Carnevale A, Pellegrino F, Cossu A, et al. Current concepts in ablative procedures for primary benign liver lesions: a step forward to minimize the invasiveness of treatment when deemed necessary. Med Oncol. 2020;37:31. doi: 10.1007/s12032-020-01355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy TP, Soares GM. The evolution of interventional radiology. Semin Intervent Radiol. 2005;22:6–9. doi: 10.1055/s-2005-869570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraverty S, Flood K, Kessel D, et al. CIRSE guidelines: quality improvement guidelines for endovascular treatment of traumatic hemorrhage. Cardiovasc Intervent Radiol. 2012;35:472–82. doi: 10.1007/s00270-012-0339-7. [DOI] [PubMed] [Google Scholar]
- 24.Borgheresi A, Gonzalez-Aguirre A, Brown KT, et al. Does Enhancement or Perfusion on Preprocedure CT Predict Outcomes After Embolization of Hepatocellular Carcinoma? Acad Radiol. 2018;25:1588–94. doi: 10.1016/j.acra.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornelis FH, Borgheresi A, Petre EN, Santos E, Solomon SB, Brown K. Hepatic Arterial Embolization Using Cone Beam CT with Tumor Feeding Vessel Detection Software: Impact on Hepatocellular Carcinoma Response. Cardiovasc Intervent Radiol. 2018;41:104–11. doi: 10.1007/s00270-017-1758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol. 2017;34:52. doi: 10.1007/s12032-017-0906-5. [DOI] [PubMed] [Google Scholar]
- 27.Arrigoni F, Bruno F, Zugaro L, et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed. 2018;89:166–74. doi: 10.23750/abm.v89i1-S.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angileri SA, Mailli L, Raspanti C, Ierardi AM, Carrafiello G, Belli AM. Prophylactic occlusion balloon placement in internal iliac arteries for the prevention of postpartum haemorrhage due to morbidly adherent placenta: short term outcomes. Radiol Med. 2017;122:798–806. doi: 10.1007/s11547-017-0777-z. [DOI] [PubMed] [Google Scholar]
- 29.Campobasso D, Marchioni M, Altieri V, et al. GreenLight Photoselective Vaporization of the Prostate: One Laser for Different Prostate Sizes. J Endourol. 2020;34:54–62. doi: 10.1089/end.2019.0478. [DOI] [PubMed] [Google Scholar]
- 30.Castellani D, Cindolo L, De Nunzio C, et al. Comparison Between Thulium Laser VapoEnucleation and GreenLight Laser Photoselective Vaporization of the Prostate in Real-Life Setting: Propensity Score Analysis. Urology. 2018;121:147–52. doi: 10.1016/j.urology.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Floridi C, Radaelli A, Pesapane F, et al. Clinical impact of cone beam computed tomography on iterative treatment planning during ultrasound-guided percutaneous ablation of liver malignancies. Med Oncol. 2017;34:113. doi: 10.1007/s12032-017-0954-x. [DOI] [PubMed] [Google Scholar]
- 32.Nicolini D, Agostini A, Montalti R, et al. Radiological response and inflammation scores predict tumour recurrence in patients treated with transarterial chemoembolization before liver transplantation. World J Gastroenterol. 2017;23:3690–701. doi: 10.3748/wjg.v23.i20.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ierardi AM, Duka E, Lucchina N, et al. The role of interventional radiology in abdominopelvic trauma. Br J Radiol. 2016;89:20150866. doi: 10.1259/bjr.20150866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangini M, Lagana D, Fontana F, et al. Use of Amplatzer Vascular Plug (AVP) in emergency embolisation: preliminary experience and review of literature. Emerg Radiol. 2008;15:153–60. doi: 10.1007/s10140-007-0696-8. [DOI] [PubMed] [Google Scholar]
- 35.Lagana D, Carrafiello G, Mangini M, et al. Indications for the use of the Amplatzer vascular plug in interventional radiology. Radiol Med. 2008;113:707–18. doi: 10.1007/s11547-008-0306-1. [DOI] [PubMed] [Google Scholar]
- 36.Quencer KB, Smith TA. Review of proximal splenic artery embolization in blunt abdominal trauma. CVIR Endovasc. 2019;2:11. doi: 10.1186/s42155-019-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin JG, Shah J, Robinson C, Dariushnia S. Evaluation and Management of Blunt Solid Organ Trauma. Tech Vasc Interv Radiol. 2017;20:230–36. doi: 10.1053/j.tvir.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Carrafiello G, Ierardi AM, Piacentino F, Cardim LN. Percutaneous transhepatic embolization of biliary leakage with N-butyl cyanoacrylate. Indian J Radiol Imaging. 2012;22:19–22. doi: 10.4103/0971-3026.95398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginat DT, Saad WE, Turba UC. Transcatheter renal artery embolization: clinical applications and techniques. Tech Vasc Interv Radiol. 2009;12:224–39. doi: 10.1053/j.tvir.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Ierardi AM, Floridi C, Fontana F, et al. Transcatheter embolisation of iatrogenic renal vascular injuries. Radiol Med. 2014;119:261–8. doi: 10.1007/s11547-013-0343-2. [DOI] [PubMed] [Google Scholar]
- 41.Jardinet T, Bonne L, Oyen R, Maleux G. Initial Experience With the Microvascular Plug in Selective Renal Artery Embolization. Vasc Endovascular Surg. 2020;54:240–46. doi: 10.1177/1538574419897500. [DOI] [PubMed] [Google Scholar]
- 42.Carrafiello G, Ierardi AM, Duka E, et al. Usefulness of Cone-Beam Computed Tomography and Automatic Vessel Detection Software in Emergency Transarterial Embolization. Cardiovasc Intervent Radiol. 2016;39:530–7. doi: 10.1007/s00270-015-1213-1. [DOI] [PubMed] [Google Scholar]
- 43.Ierardi AM, Piacentino F, Fontana F, et al. The role of endovascular treatment of pelvic fracture bleeding in emergency settings. Eur Radiol. 2015;25:1854–64. doi: 10.1007/s00330-015-3589-3. [DOI] [PubMed] [Google Scholar]
- 44.Barentsz MW, Vonken EP, van Herwaarden JA, Leenen LP, Mali WP, van den Bosch MA. Clinical outcome of intraarterial embolization for treatment of patients with pelvic trauma. Radiol Res Pract. 2011;2011:935484. doi: 10.1155/2011/935484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awwad A, Dhillon PS, Ramjas G, Habib SB, Al-Obaydi W. Trans-arterial embolisation (TAE) in haemorrhagic pelvic injury: review of management and mid-term outcome of a major trauma centre. CVIR Endovasc. 2018;1:32. doi: 10.1186/s42155-018-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frevert S, Dahl B, Lonn L. Update on the roles of angiography and embolisation in pelvic fracture. Injury. 2008;39:1290–4. doi: 10.1016/j.injury.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Travis T, Monsky WL, London J, et al. Evaluation of shortterm and long-term complications after emergent internal iliac artery embolization in patients with pelvic trauma. J Vasc Interv Radiol. 2008;19:840–7. doi: 10.1016/j.jvir.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Ierardi AM, Urbano J, De Marchi G, et al. New advances in lower gastrointestinal bleeding management with embolotherapy. Br J Radiol. 2016;89:20150934. doi: 10.1259/bjr.20150934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urbano J, Manuel Cabrera J, Franco A, Alonso-Burgos A. Selective arterial embolization with ethylene-vinyl alcohol copolymer for control of massive lower gastrointestinal bleeding: feasibility and initial experience. J Vasc Interv Radiol. 2014;25:839–46. doi: 10.1016/j.jvir.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Venturini M, Lanza C, Marra P, et al. Transcatheter embolization with Squid, combined with other embolic agents or alone, in different abdominal diseases: a single-center experience in 30 patients. CVIR Endovasc. 2019;2:8. doi: 10.1186/s42155-019-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo S, Yoon CJ, Chung JW, et al. Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269:594–602. doi: 10.1148/radiol.13130046. [DOI] [PubMed] [Google Scholar]
- 52.Izaaryene J, Vidal V, Bartoli JM, Gaubert JY. Multiple bronchial artery aneurysms: Successful treatment with ethylenevinyl alcohol copolymer (Onyx(R)) Diagn Interv Imaging. 2016;97:125–7. doi: 10.1016/j.diii.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Madhusudhan KS, Venkatesh HA, Gamanagatti S, Garg P, Srivastava DN. Interventional Radiology in the Management of Visceral Artery Pseudoaneurysms: A Review of Techniques and Embolic Materials. Korean J Radiol. 2016;17:351–63. doi: 10.3348/kjr.2016.17.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loffroy R, Rao P, Ota S, et al. Packing technique for endovascular coil embolisation of peripheral arterial pseudoaneurysms with preservation of the parent artery: safety, efficacy and outcomes. Eur J Vasc Endovasc Surg. 2010;40:209–15. doi: 10.1016/j.ejvs.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Briganti F, Leone G, Ugga L, et al. Mid-term and longterm follow-up of intracranial aneurysms treated by the p64 Flow Modulation Device: a multicenter experience. J Neurointerv Surg. 2017;9:70–76. doi: 10.1136/neurintsurg-2016-012502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briganti F, Leone G, Ugga L, et al. Safety and efficacy of flow re-direction endoluminal device (FRED) in the treatment of cerebral aneurysms: a single center experience. Acta Neurochir (Wien) 2016;158:1745–55. doi: 10.1007/s00701-016-2875-4. [DOI] [PubMed] [Google Scholar]
- 57.Hobo R, Sybrandy JE, Harris PL, Buth J, Collaborators E. Endovascular repair of abdominal aortic aneurysms with concomitant common iliac artery aneurysm: outcome analysis of the EUROSTAR Experience. J Endovasc Ther. 2008;15:12–22. doi: 10.1583/07-2217.1. [DOI] [PubMed] [Google Scholar]
- 58.Oderich GS, Ricotta JJ, 2nd. Novel surgeon-modified hypogastric branch stent graft to preserve pelvic perfusion. Ann Vasc Surg. 2010;24:278–86. doi: 10.1016/j.avsg.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Tang H, Tang X, Fu W, et al. Coil embolization of renal artery bifurcation and branch aneurysms with flow preservation. J Vasc Surg. 2018;68:451–58 e2. doi: 10.1016/j.jvs.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Jesinger RA, Thoreson AA, Lamba R. Abdominal and pelvic aneurysms and pseudoaneurysms: imaging review with clinical, radiologic, and treatment correlation. Radiographics. 2013;33:E71–96. doi: 10.1148/rg.333115036. [DOI] [PubMed] [Google Scholar]
- 61.Ierardi AM, Petrillo M, Bacuzzi A, et al. Endovascular retreatment of a splenic artery aneurysm refilled by collateral branches of the left gastric artery: a case report. J Med Case Rep. 2014;8:436. doi: 10.1186/1752-1947-8-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandel SR, Jaques PF, Sanofsky S, Mauro MA. Nonoperative management of peripancreatic arterial aneurysms. A 10-year experience. Ann Surg. 1987;205:126–8. doi: 10.1097/00000658-198702000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loffroy R, Guiu B, Cercueil JP, et al. Transcatheter arterial embolization of splenic artery aneurysms and pseudoaneurysms: short-and long-term results. Ann Vasc Surg. 2008;22:618–26. doi: 10.1016/j.avsg.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 64.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol. 2017;40:1141–46. doi: 10.1007/s00270-017-1703-4. [DOI] [PubMed] [Google Scholar]
- 65.Ierardi AM, Pesapane F, Rivolta N, et al. Type 2 endoleaks in endovascular aortic repair: cone beam CT and automatic vessel detection to guide the embolization. Acta Radiol. 2018;59:681–87. doi: 10.1177/0284185117729184. [DOI] [PubMed] [Google Scholar]
- 66.Ptohis ND, Charalampopoulos G, Abou Ali AN, et al. Contemporary Role of Embolization of Solid Organ and Pelvic Injuries in Polytrauma Patients. Front Surg. 2017;4:43. doi: 10.3389/fsurg.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ekeh AP, Khalaf S, Ilyas S, Kauffman S, Walusimbi M, McCarthy MC. Complications arising from splenic artery embolization: a review of an 11-year experience. Am J Surg. 2013;205:250–4. doi: 10.1016/j.amjsurg.2013.01.003. discussion 54. [DOI] [PubMed] [Google Scholar]
- 68.Pesapane F, Nezami N, Patella F, Geschwind JF. New concepts in embolotherapy of HCC. Med Oncol. 2017;34:58. doi: 10.1007/s12032-017-0917-2. [DOI] [PubMed] [Google Scholar]
- 69.Ganguli S, Faintuch S, Salazar GM, Rabkin DJ. Postembolization syndrome: changes in white blood cell counts immediately after uterine artery embolization. J Vasc Interv Radiol. 2008;19:443–5. doi: 10.1016/j.jvir.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 70.Pesapane F, Leenknegt B, Ammar T, Panella S, Garzillo G, Huang DY. Intraoperative microvascular assessment with contrast-enhanced ultrasound (CEUS) during uterine artery embolisation (UAE): a case report and literature review. J Ultrasound. 2020 doi: 10.1007/s40477-020-00441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


