Abstract
Since its first reported application, renal biopsy became an important part of the diagnostic algorithm, considered advantages and risks, to better manage therapeutic options. The biopsy can be performed with different techniques (open, laparoscopic, transjugular, transurethral and percutaneous). Currently, the percutaneous approach is the modality of choice. Percutaneous biopsy can be performed under CT or US guidance, but critical benefits and disadvantages have to be considered. Core needle biopsy is usually preferred to fine-needle aspiration because of the sample quality, usually obtaining multiple cores, especially in heterogeneous tumors. Principal complications are hematuria (1-10%), perinephric hematoma (10-90%), pneumothorax (0,6%), clinically significant pain (1,2%). (www.actabiomedica.it)
Keywords: percutaneous renal biopsy, small renal mass, renal cell carcinoma, US-guided biopsy, CT-guided biopsy, coaxial technique
Introduction
Interventional radiology techniques have been developed and used widely, becoming critical both for the diagnosis and therapeutic management of many diseases (1-15). The first renal biopsy approach was surgical, performed by Gwyn (16). More recently, different methods (open, laparoscopic, transjugular, transurethral, and percutaneous) (17, 18) and improvements have been made (19). Nowadays, the percutaneous approach (Percutaneous Renal Biopsy or PRB) is considered the modality of choice, but in case of failure or major contraindication, other methods could be preferred. The transjugular approach, even in consideration of its disadvantages, allows multiorgan biopsies during the same procedure, and it can be recommended in case of failure of PRB and in patients with severe coagulopathies since it gives the possibility to perform a selective embolization in case of bleeding. The transurethral biopsy may be considered in case of patients undergoing a cystoscopic examination and do not wish to undergo PRB separately, or when there is the suspected involvement of upper urinary tract.
Indications
The main indications to perform a renal biopsy, following nephrologists recommendation, are cases of idiopathic nephritic and nephrotic syndromes and the diagnosis of unknown primary lesions (20). Renal biopsy could also be useful in detecting acute or chronic renal allograft rejection (in presence of increasing serum creatinine levels) or in order to evaluate the response to antirejection therapy.
Timing and utility of the biopsy are still debated even in presence of an unknown primary lesion, though the consensus on the need of tissue sampling if the management could be conditioned. Different theories explain the poor adoption of renal tumor biopsy as a standard of care for small renal masses (SRMs, size <4 cm), but none is well-supported by studies reported over the past years (21, 22). Safety, discordance with diagnosis after surgery, low diagnostic rates, and lack of perceived impact on clinical management are reported as the leading causes for the low consensus between specialists for practicing renal tumor biopsy (RTB) (23). Despite these concerns, the number of renal biopsies is grown thanks to the fact that procedural risks, such as tumor seeding and bleeding, have been reduced through improving the experience of operators and perfecting those techniques (24) (fig 1). Moreover, an international panel has recommended RTB before any ablative treatment (25).
Figure 1.
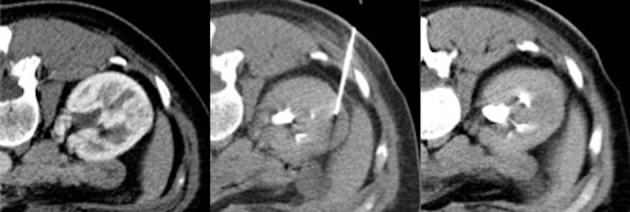
A core needle biopsy is performed on a single lesion in the para-hilar region of the right kidney. The lesion is in the proximity of important vascular and urinary system structures. The choice of a correct approach prevents post procedural complications such as bleeding and urinary tract lesions.
Imaging methods
Accurate preprocedural imaging study is crucial for a proper diagnosis, preoperative planning, and postoperative follow-up (26-28). Though the application of MRI is growing in the field of interventional radiology, most procedures are performed under fluoroscopic, ultrasound, and CT imaging guidance (19, 29-39). Percutaneous biopsy can be performed under CT or US guidance, but critical benefits and disadvantages have to be considered. Ultrasonography has the advantages to offer a real-time view during needle placement, which allows to avoid vascular structures; furthermore, US is a low-cost technique and allows multiplanar imaging (40). However, US does not allow an accurate visualization of all renal mass, thus requiring contrast media administration (41).
CT is frequently used, with a step-and-shoot approach or as CT-fluoroscopy, allowing a better and less operator-dependent target detection. CT approach is characterized by high spatial resolution and a large field of view, enabling multiplanar reconstruction (MPR) to obtain an adequate visualization of the path of the needle (19) (fig 2). The main disadvantages are related to the more difficult positioning of the needle due to the patient’s respiratory movement, as well as the side effects related to ionizing radiation.
Figure 2.
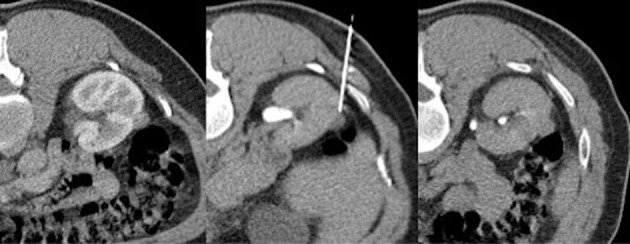
A single, small lesion at the middle third of the right kidney in the nearby of the ascending colon. Choosing the best approach, thanks to the large field of view and multiplanarity offered by CT, a fine needle aspiration biopsy is performed without any post-procedural complication.
The technique
There are two primary modalities for performing a percutaneous biopsy: fine-needle aspiration biopsy (FNAB) and core needle biopsy (CNB) (42, 43).
FNAB is a cytologic technique involving the use of a small needle (18-25G) equipped with an inner stylet. Once in the target, the stylet is removed, and a syringe is connected.
Individual cells for cytological evaluation could be obtained after creating a vacuum and moving gently and repeatedly back-and-forth the system.
CNB involves devices with larger needles (usually 16-18G) and different mechanisms to obtain the specimen (manually or automatically cutting systems) (44). CNB is usually preferred to fine-needle aspiration (FNA) due to sample quality. Coaxial needle technique is a safe and proven technique consisting in the use of a guide needle (9-19G), previously advanced towards the target, in which the biopsy needle could be inserted to retrieve multiple specimens in a single puncture. Multiple cores allow a better assessment of tissue architecture and histologic subtype (22). Moreover, CNB does not increase the recurrence of complications and could decrease the tumor cells seeding risk along the needle path (45, 46). The sensitivity (97,5% - 99,7%) and specificity (96,2% - 99,1%), as reported in two large meta-analyses, are very high, allowing to consider RTB a highly accurate test in the detection of malignancy (47). When performing a percutaneous biopsy in nephritic or nephrotic syndrome, the target is usually the lower pole of the kidney. In lesions suspected for malignancy, the location should be chosen on the basis of the tumor size: in large tumors (>4cm), necrotic phenomena can occur, especially into the center of the lesion, making that site inappropriate for the sample quality (fig 3). In such lesions, to improve sensitivity and accuracy, a multi-quadrant technique is useful: obtaining multiple cores from different areas within the tumor has proven to better detect aggressive pathologic futures as sarcomatoid dedifferentiation (48). In that cases, occurring usually in metastatic RCC with a poor life expectancy in the long-term (49-51), the biopsy has shown to have an important role in avoiding cytoreductive nephrectomy in patients who are unlikely to benefit and selecting different strategies as systemic therapy.
Fig 3.
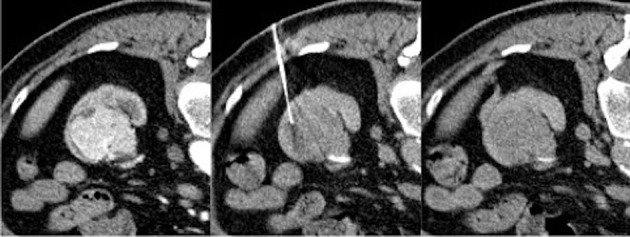
A large, hyperenhancing focal lesion at the lower third of the left kidney in the first scan (on the left). CT guided fine needle aspiration biopsy is performed with the patient in prone position. In such large lesions a peripheral approach is preferred in order to avoid the center of the lesion which could be necrotic and not useful for histologic characterization
In large lesions, despite only 6,3% of masses higher than 7cm are not RCC-tumors, the biopsy could be essential in directing the appropriate treatment. E.g., lymphoma requires chemotherapeutical treatment instead of surgery (52), as large sarcomas need presurgical radiation (53). Although the benefit of retroperitoneal lymph node dissection (RPLND) for RCC is debatable (54), several studies reported high-risk features for lymph node metastases (55-57), pointing out the utility of RTB in the selection of patients with not-clinically-evident metastases who may benefit from aggressive surgery with RPLND.
Contraindications and complications
Reported absolute contraindications are severe uncontrolled hypertension, poor patient compliance to undergo the procedure, solitary kidney, and uncontrollable bleeding diathesis. Relative contraindications are renal morphologic abnormalities, urinary tract infections, severe azotemia, and coagulation disorders (58).
Principal complications include bleeding diathesis, consisting in hematuria (1-10%), perinephric hematoma (10-90%) to major bleeding requiring transfusion (0,3% - 7,4%) and nephrectomy (0,1%-0,5%) (fig 4). The risk is higher in cases of clotting disorders, thrombocytopenia, pharmacological treatment with anticoagulants or antiplatelets. According to the SIR-guidelines, renal biopsy is considered a high bleeding risk technique and appropriate preprocedural coagulation tests should be obtained (suggested laboratory value thresholds: correct INR to within range of 1.5–1.8 or less and consider platelet transfusion if platelet count is < 50 × 109/L) (59).
Fig 4.
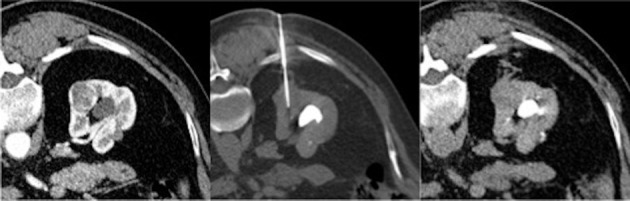
A core needle biopsy is performed on an exophytic lesion at the lower third of the right kidney; in the post-procedural scan (on the right) a minimal haematic suffusion of the pararenal tissue, along the needle path.
Other, less frequent complications are pneumothorax (0,6%), clinically significant pain (1,2%), infection and arteriovenous fistula. Tumor seeding is considered a fearsome complication but is very rare and reported only in a few cases (24,43,60-65),
Conclusions
Renal biopsy is a safe and effective technique in the majority of patients, useful in the diagnosis and management of certain medical conditions. Renal biopsy has a pivotal role in the algorithm of small renal masses (SMRs), avoiding unnecessary surgeries for benign disease and selecting appropriate candidates for focal ablation or active surveillance.
The biopsy is very useful for choosing the best management strategy, especially for SRMs, that is still based on the clinical setting of the patient and on histological characteristic of the lesion, selecting between active surveillance, focal ablation, and surgical options. In tumors greater than 4cm and metastatic disease, the biopsy has a critical role in identifying patients that are unlikely to benefit from surgical operation or who may benefit from pre-nephrectomy systemic therapy, investigating the molecular and genetic information, and lymph node involvement in order to plan a different surgical approach, as the lymph node dissection.
Conflict of interest:
Authors declare that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.D’Amico G, Di Crescenzo V, Muto M, et al. Cytological diagnosis of lymph nodes by instrumental guide: ultrasonography and CT. Recenti Prog Med. 2013;104:367–70. doi: 10.1701/1315.14577. [DOI] [PubMed] [Google Scholar]
- 2.Carrafiello G, Fontana F, Cotta E, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med. 2011;116:1059–66. doi: 10.1007/s11547-011-0697-2. [DOI] [PubMed] [Google Scholar]
- 3.Carrafiello G, Lagana D, Nosari AM, et al. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections. Study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol Med. 2006;111:33–41. doi: 10.1007/s11547-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 4.Belfiore G, Belfiore MP, Reginelli A, et al. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med Oncol. 2017;34:38. doi: 10.1007/s12032-017-0887-4. [DOI] [PubMed] [Google Scholar]
- 5.Barile A, Quarchioni S, Bruno F, et al. Interventional radiology of the thyroid gland: critical review and state of the art. Gland Surg. 2018;7:132–46. doi: 10.21037/gs.2017.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masciocchi C, Arrigoni F, La Marra A, Mariani S, Zugaro L, Barile A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol. 2016;89:20150356. doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoccali C, Rossi B, Zoccali G, et al. A new technique for biopsy of soft tissue neoplasms: a preliminary experience using MRI to evaluate bleeding. Minerva Med. 2015;106:117–20. [PubMed] [Google Scholar]
- 8.Arrigoni F, Bruno F, Zugaro L, et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed. 2018;89:166–74. doi: 10.23750/abm.v89i1-S.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrigoni F, Gregori LM, Zugaro L, Barile A, Masciocchi C. MRgFUS in the treatment of MSK lesions: a review based on the experience of the University of L’Aquila, Italy. Translational Cancer Research. 2014;3:442–48. [Google Scholar]
- 10.Campobasso D, Marchioni M, Altieri V, et al. GreenLight Photoselective Vaporization of the Prostate: One Laser for Different Prostate Sizes. J Endourol. 2020;34:54–62. doi: 10.1089/end.2019.0478. [DOI] [PubMed] [Google Scholar]
- 11.Castellani D, Cindolo L, De Nunzio C, et al. Comparison Between Thulium Laser VapoEnucleation and GreenLight Laser Photoselective Vaporization of the Prostate in Real-Life Setting: Propensity Score Analysis. Urology. 2018;121:147–52. doi: 10.1016/j.urology.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Pavan N, Autorino R, Lee H, et al. Impact of novel techniques on minimally invasive adrenal surgery: trends and outcomes from a contemporary international large series in urology. World J Urol. 2016;34:1473–9. doi: 10.1007/s00345-016-1791-9. [DOI] [PubMed] [Google Scholar]
- 13.Greco F, Pini G, Alba S, Altieri VM, Verze P, Mirone V. Minilaparoendoscopic Single-site Pyeloplasty: The Best Compromise Between Surgeon’s Ergonomy and Patient’s Cosmesis (IDEAL Phase 2a) Eur Urol Focus. 2016;2:319–26. doi: 10.1016/j.euf.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Floridi C, Radaelli A, Pesapane F, et al. Clinical impact of cone beam computed tomography on iterative treatment planning during ultrasound-guided percutaneous ablation of liver malignancies. Med Oncol. 2017;34:113. doi: 10.1007/s12032-017-0954-x. [DOI] [PubMed] [Google Scholar]
- 15.Nicolini D, Agostini A, Montalti R, et al. Radiological response and inflammation scores predict tumour recurrence in patients treated with transarterial chemoembolization before liver transplantation. World J Gastroenterol. 2017;23:3690–701. doi: 10.3748/wjg.v23.i20.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwyn NB. Biopsies and the Completion of certain Surgical Procedures. Can Med Assoc J. 1923;13:820–3. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuo L, Wang H, Chen D, Lu H, Zou G, Li W. Alternative renal biopsies: past and present. Int Urol Nephrol. 2018;50:475–79. doi: 10.1007/s11255-017-1668-x. [DOI] [PubMed] [Google Scholar]
- 18.Carrafiello G, Fontana F, Mangini M, et al. Upper urinary tract biopsy: an old device for a new approach. Radiol Med. 2012;117:1152–60. doi: 10.1007/s11547-012-0799-5. [DOI] [PubMed] [Google Scholar]
- 19.Bevilacqua A, D’Amuri FV, Pagnini F, et al. Percutaneous needle biopsy of retroperitoneal lesions: technical developments. Acta Biomed. 2019;90:62–67. doi: 10.23750/abm.v90i5-S.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandari J, Fuller TW. Turner capital I UiURM D’Agostino LA Renal biopsy for medical renal disease: indications and contraindications. Can J Urol. 2016;23:8121–6. [PubMed] [Google Scholar]
- 21.Marconi L, Dabestani S, Lam TB, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. Eur Urol. 2016;69:660–73. doi: 10.1016/j.eururo.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 22.Richard PO, Jewett MA, Bhatt JR, et al. Renal Tumor Biopsy for Small Renal Masses: A Single-center 13-year Experience. Eur Urol. 2015;68:1007–13. doi: 10.1016/j.eururo.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Richard PO, Martin L, Lavallee LT, et al. Identifying the use and barriers to the adoption of renal tumour biopsy in the management of small renal masses. Can Urol Assoc J. 2018;12:260–66. doi: 10.5489/cuaj.5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpe A, Finelli A, Gill IS, et al. Rationale for percutaneous biopsy and histologic characterisation of renal tumours. Eur Urol. 2012;62:491–504. doi: 10.1016/j.eururo.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Tsivian M, Rampersaud EN, Jr, del Pilar Laguna Pes M, et al. Small renal mass biopsy--how, what and when: report from an international consensus panel. BJU Int. 2014;113:854–63. doi: 10.1111/bju.12470. [DOI] [PubMed] [Google Scholar]
- 26.Cortellini A, Bozzetti F, Palumbo P, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Sci Rep. 2020;10:1456. doi: 10.1038/s41598-020-58498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agliata G, Schicchi N, Agostini A, et al. Radiation exposure related to cardiovascular CT examination: comparison between conventional 64-MDCT and third-generation dualsource MDCT. Radiol Med. 2019;124:753–61. doi: 10.1007/s11547-019-01036-6. [DOI] [PubMed] [Google Scholar]
- 28.Agostini A, Mari A, Lanza C, et al. Trends in radiation dose and image quality for pediatric patients with a multidetector CT and a third-generation dual-source dual-energy CT. Radiol Med. 2019;124:745–52. doi: 10.1007/s11547-019-01037-5. [DOI] [PubMed] [Google Scholar]
- 29.Barile A, Arrigoni F, Bruno F, et al. Present role and future perspectives of interventional radiology in the treatment of painful bone lesions. Future Oncol. 2018;14:2945–55. doi: 10.2217/fon-2017-0657. [DOI] [PubMed] [Google Scholar]
- 30.Carrafiello G, Lagana D, Pellegrino C, et al. Ablation of painful metastatic bone tumors: a systematic review. Int J Surg. 2008;6(Suppl 1):S47–52. doi: 10.1016/j.ijsu.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Carrafiello G, Fontana F, Pellegrino C, et al. Radiofrequency ablation of abdominal wall endometrioma. Cardiovasc Intervent Radiol. 2009;32:1300–3. doi: 10.1007/s00270-008-9500-8. [DOI] [PubMed] [Google Scholar]
- 32.Arrigoni F, Napoli A, Bazzocchi A, et al. Magnetic-resonance-guided focused ultrasound treatment of non-spinal osteoid osteoma in children: multicentre experience. Pediatr Radiol. 2019;49:1209–16. doi: 10.1007/s00247-019-04426-0. [DOI] [PubMed] [Google Scholar]
- 33.Pagnini F, D’Amuri FV, Bevilacqua A, et al. Ultrasoundguided percutaneous irrigation of calcific tendinopathy: technical developments. Acta Biomed. 2019;90:95–100. doi: 10.23750/abm.v90i5-S.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrafiello G, Mangini M, Fontana F, et al. Microwave ablation of lung tumours: single-centre preliminary experience. Radiol Med. 2014;119:75–82. doi: 10.1007/s11547-013-0301-z. [DOI] [PubMed] [Google Scholar]
- 35.Carrafiello G, Ierardi AM, Duka E, et al. Usefulness of Cone-Beam Computed Tomography and Automatic Vessel Detection Software in Emergency Transarterial Embolization. Cardiovasc Intervent Radiol. 2016;39:530–7. doi: 10.1007/s00270-015-1213-1. [DOI] [PubMed] [Google Scholar]
- 36.Lagana D, Carrafiello G, Mangini M, et al. Indications for the use of the Amplatzer vascular plug in interventional radiology. Radiol Med. 2008;113:707–18. doi: 10.1007/s11547-008-0306-1. [DOI] [PubMed] [Google Scholar]
- 37.Mangini M, Lagana D, Fontana F, et al. Use of Amplatzer Vascular Plug (AVP) in emergency embolisation: preliminary experience and review of literature. Emerg Radiol. 2008;15:153–60. doi: 10.1007/s10140-007-0696-8. [DOI] [PubMed] [Google Scholar]
- 38.Cornelis FH, Borgheresi A, Petre EN, Santos E, Solomon SB, Brown K. Hepatic Arterial Embolization Using Cone Beam CT with Tumor Feeding Vessel Detection Software: Impact on Hepatocellular Carcinoma Response. Cardiovasc Intervent Radiol. 2018;41:104–11. doi: 10.1007/s00270-017-1758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol. 2017;34:52. doi: 10.1007/s12032-017-0906-5. [DOI] [PubMed] [Google Scholar]
- 40.Uppot RN, Harisinghani MG, Gervais DA. Imaging-guided percutaneous renal biopsy: rationale and approach. AJR Am J Roentgenol. 2010;194:1443–9. doi: 10.2214/AJR.10.4427. [DOI] [PubMed] [Google Scholar]
- 41.Israel GM, Bosniak MA. Pitfalls in renal mass evaluation and how to avoid them. Radiographics. 2008;28:1325–38. doi: 10.1148/rg.285075744. [DOI] [PubMed] [Google Scholar]
- 42.Gupta P, Rajwanshi A, Nijhawan R, et al. Fine needle aspiration in retroperitoneal lesions. APMIS. 2017;125:16–23. doi: 10.1111/apm.12627. [DOI] [PubMed] [Google Scholar]
- 43.Tomozawa Y, Inaba Y, Yamaura H, et al. Clinical value of CT-guided needle biopsy for retroperitoneal lesions. Korean J Radiol. 2011;12:351–7. doi: 10.3348/kjr.2011.12.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misra RK, Mitra S, Jain RK, Vahikar S, Bundela A, Misra P. Image-guided fine needle cytology with aspiration versus non-aspiration in retroperitoneal masses: is aspiration necessary? J Pathol Transl Med. 2015;49:129–35. doi: 10.4132/jptm.2015.01.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Filippo M, Saba L, Rossi E, et al. Curved Needles in CT-Guided Fine Needle Biopsies of Abdominal and Retroperitoneal Small Lesions. Cardiovasc Intervent Radiol. 2015;38:1611–6. doi: 10.1007/s00270-015-1107-2. [DOI] [PubMed] [Google Scholar]
- 46.Pedote P, Gaudio F, Moschetta M, Cimmino A, Specchia G, Angelelli G. CT-guided needle biopsy performed with modified coaxial technique in the diagnosis of malignant lymphomas. Radiol Med. 2010;115:1292–303. doi: 10.1007/s11547-010-0559-3. [DOI] [PubMed] [Google Scholar]
- 47.Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J Urol. 2016;195:1340–47. doi: 10.1016/j.juro.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abel EJ, Heckman JE, Hinshaw L, et al. Multi-Quadrant Biopsy Technique Improves Diagnostic Ability in Large Heterogeneous Renal Masses. J Urol. 2015;194:886–91. doi: 10.1016/j.juro.2015.03.106. [DOI] [PubMed] [Google Scholar]
- 49.Kyriakopoulos CE, Chittoria N, Choueiri TK, et al. Outcome of patients with metastatic sarcomatoid renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clin Genitourin Cancer. 2015;13:e79–85. doi: 10.1016/j.clgc.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Shuch B, Said J, La Rochelle JC, et al. Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology--is up-front resection indicated and, if not, is it avoidable? J Urol. 2009;182:2164–71. doi: 10.1016/j.juro.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kassouf W, Sanchez-Ortiz R, Tamboli P, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma with nonclear cell histology. J Urol. 2007;178:1896–900. doi: 10.1016/j.juro.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 52.Kose F, Sakalli H, Mertsoylu H, et al. Primary renal lymphoma: report of four cases. Onkologie. 2009;32:200–2. doi: 10.1159/000203331. [DOI] [PubMed] [Google Scholar]
- 53.Baldini EH, Wang D, Haas RL, et al. Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys. 2015;92:602–12. doi: 10.1016/j.ijrobp.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Barrisford GW, Gershman B, Blute ML, Sr. The role of lymphadenectomy in the management of renal cell carcinoma. World J Urol. 2014;32:643–9. doi: 10.1007/s00345-014-1294-5. [DOI] [PubMed] [Google Scholar]
- 55.Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. J Urol. 2004;172:465–9. doi: 10.1097/01.ju.0000129815.91927.85. [DOI] [PubMed] [Google Scholar]
- 56.Crispen PL, Breau RH, Allmer C, et al. Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: indications and recommendations for surgical templates. Eur Urol. 2011;59:18–23. doi: 10.1016/j.eururo.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 57.Capitanio U, Suardi N, Matloob R, et al. Extent of lymph node dissection at nephrectomy affects cancer-specific survival and metastatic progression in specific sub-categories of patients with renal cell carcinoma (RCC) BJU Int. 2014;114:210–5. doi: 10.1111/bju.12508. [DOI] [PubMed] [Google Scholar]
- 58.Clinical competence in percutaneous renal biopsy. Health and Public Policy Committee. American College of Physicians. Ann Intern Med. 1988;108:301–3. [PubMed] [Google Scholar]
- 59.Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30:1168–84 e1. doi: 10.1016/j.jvir.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 60.Tomaszewski JJ, Uzzo RG, Smaldone MC. Heterogeneity and renal mass biopsy: a review of its role and reliability. Cancer Biol Med. 2014;11:162–72. doi: 10.7497/j.issn.2095-3941.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Filippo M, Bozzetti F, Martora R, et al. Radiofrequency thermal ablation of renal tumors. Radiol Med. 2014;119:499–511. doi: 10.1007/s11547-014-0412-1. [DOI] [PubMed] [Google Scholar]
- 62.De Filippo M, Saba L, Concari G, et al. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol Med. 2013;118:1071–81. doi: 10.1007/s11547-013-0965-4. [DOI] [PubMed] [Google Scholar]
- 63.De Filippo M, Saba L, Silva M, et al. CT-guided biopsy of pulmonary nodules: is pulmonary hemorrhage a complication or an advantage? Diagn Interv Radiol. 2014;20:421–5. doi: 10.5152/dir.2014.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Filippo M, Gira F, Corradi D, Sverzellati N, Zompatori M, Rossi C. Benefits of 3D technique in guiding percutaneous retroperitoneal biopsies. Radiol Med. 2011;116:407–16. doi: 10.1007/s11547-010-0604-2. [DOI] [PubMed] [Google Scholar]
- 65.Blute ML, Jr, Drewry A, Abel EJ. Percutaneous biopsy for risk stratification of renal masses. Ther Adv Urol. 2015;7:265–74. doi: 10.1177/1756287215585273. [DOI] [PMC free article] [PubMed] [Google Scholar]


