Abstract
The management of patients undergoing surgical resection for liver malignancies requires a multidisciplinary team, including a dedicated radiologist. In the preoperative workup, the radiologist has to provide precise, relevant information to the surgeon. This requires the radiologist to know the basics of surgical techniques as well as liver surgical anatomy in order to help to avoid unexpected surgical scenarios and complications. Moreover, virtual resections and volumetries on radiological images will be discussed, and basic concepts of postoperative liver failure, regeneration, and methods for hypertrophy induction will be provided. (www.actabiomedica.it)
Keywords: Imaging, liver malignancies, liver resection, surgical planning, MRI, CT, US
Introduction
Surgical resection often represents the only curative treatment in patients with liver malignancies. In the last 50 years, from a pioneering surgery, the liver resections have pushed up its limits with remarkable achievements in terms of effectiveness, clinical outcomes, and safety (1-3).
The reasons of these achievements rely in part on the evolution of surgical and anesthetic techniques; on the other side, the advances in cross-sectional imaging provided a significant contribution in the preoperative workup allowing a better treatment allocation and safety (1, 4). The management of these complex patients requires a multidisciplinary team, including dedicated radiologists with specific skills and knowledge. Cross-sectional imaging (MRI, CT, and US) techniques gained extensive application in gastrointestinal radiology; they are advised as first-line techniques in the diagnosis, staging, and follow-up in many diseases (5-11).
In this paper, we will provide an overview of the main concepts the radiologist needs to know to manage a presurgical workup of a patient with liver lesions. In particular, the present review will discuss: 1. the basics of surgical anatomy and terminology; 2. the relevant anatomical variants in liver resections; 3. The role of imaging, with a focus on virtual resections and volumetric estimations (the advanced techniques for the evaluation of diffuse disease go beyond the scope of this review); 4. The pathophysiological elements of liver regeneration, liver failure, and techniques for induction of liver hypertrophy.
Liver surgical anatomy and resections
Couinaud’s segmentation
The modern techniques for liver resections rely on Couinaud’s segmental anatomy of the liver: each segment is fed by an independent portal branch (12). The main anatomical landmarks are the hepatic veins (right, middle, and left), each of them lying in the so-called “portal scissurae” (12). The main portal scissura includes the middle hepatic vein, which corresponds to the Cantlie’s line (Fig. 1). The right portal scissura contains the right hepatic vein and divides the right hemiliver into two sectors: right postero-lateral and right antero-medial. The two right sectors are further divided into two segments each (respectively to the hilar plane): segments VII and VI laterally, and segment V and VIII medially. The left hemiliver is divided into two sectors by the left portal scissura (containing the left hepatic vein). The left anterior sector contains segments IV and III, while segment II is in the left posterior sector. The Spigel lobe (segment I) has autonomous feeding vessels (Fig. 1) (12).
Figure 1.
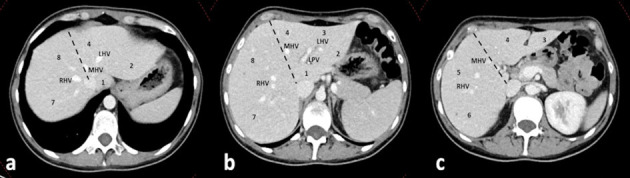
Normal segmental anatomy of the liver. a, b, c, cranio-caudal axial CT slices, portal venous phase. Dashed line: Cantlie’s line dividing the two hemilivers. RHV: right hepatic vein; MHV: middle hepatic vein; LHV: left hepatic vein; LPV: left portal vein. Arabic numbers: Couinaud’s segments.
Liver surgical resections
The modern surgical terminology considers the anatomic and non-anatomic resections (respectively AR and NAR) of the liver.
In AR, one or more Couinaud’s segments are removed together with the inside tumor and relative portal pedicles (13). The rationale is the oncological effectiveness assuming that hepatic tumors spread through portal branches. The actual definitions of AR refer to the “Brisbane 2000 Terminology of Liver anatomy and Resections” proposed by the International Hepato-Pancreato-Biliary Association (IHPBA) (14). This classification uses a modified version of Couinaud’s segmental anatomy: the term sector is replaced with “section,” and the left lateral section is composed of the Couinaud’s segments II and III (13, 14). The IHPBA define AR in relation to the order of portal divisions:
Hepatectomy (first-order): the two hemilivers are divided by the Cantlie’s line (Couinaud’s middle hepatic scissura);
Sectionectomy (second-order): the right sections correspond to Couinaud’s. The left medial section is segment IV, and the left lateral section includes segments II+III. The Counauds terminology and segmentation are retained as an alternative.
Segmentectomy (third-order): resection of one or more of Couinaud’s segments.
In NAR, also named atypical or wedge resections, a portion of hepatic parenchyma (with the tumor inside) is resected independently of vascular segmental anatomy. The rationale is the parenchymal sparing, which balances the oncological effectiveness in patients with underlying chronic liver disease (4).
Anatomical variants of surgical relevance
The normal vascular and biliary anatomy is present in up to 70% of cases, and anatomical variants are relatively commons (15). The radiologist has to recognize the relevant anatomical variants for surgery, keeping in mind two concepts: an unexpected vascular injury leads to significant bleeding, and the remnant liver needs vascular and biliary structures to work properly.
Hepatic Artery
Several variants of the hepatic artery (HA) have surgical relevance and need to be reported (16). Besides the normal anatomy (HA originating from the celiac trunk), the significant variants involve the “accessory” or “replaced” arteries from other vessels than the celiac trunk. The critical factor to be considered is the spatial relationship between the vessel and the type of resection, to prevent injuries to the aberrant branch and irrorated liver (e.g. left aberrant hepatic arteries are relevant for left hepatectomy) (Fig. 2a) (15, 16).
Figure 2.
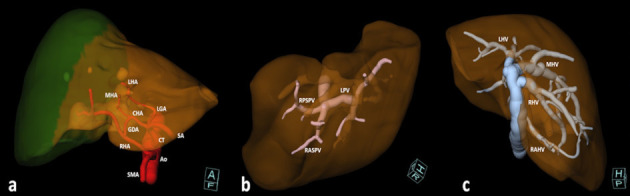
Volume Rendering (VR) reconstructions of relevant vascular variants for surgery. A: antero-inferior view of a variant of the hepatic artery (red). Replaced left hepatic artery from the left gastric artery, middle hepatic artery from common hepatic artery, and replaced right hepatic artery from the superior mesenteric artery. Variants of the left hepatic artery are relevant in left hepatectomies (brown hemiliver), while variants of the right hepatic artery are relevant for right hepatectomies (green hemiliver). Ao: aorta. CT: celiac trunk. SMA: superior mesenteric artery. CHA: common hepatic artery. SA: splenic artery. LGA: left gastric artery. GDA: gastro-duodenal artery. LHA: left hepatic artery. MHA: middle hepatic artery. RHA: right hepatic artery. B: portal trifurcation (pink), cranial-right view. RPSPV: right posterior sectorial portal vein. RASPV: right anterior sectorial portal vein. LPV: left portal vein. C: variants of hepatic veins (blue) with right accessory hepatic vein, cranio-posterior view. RAHV: right accessory hepatic vein. RHV: right hepatic vein. MHV: middle hepatic vein. LHV: left hepatic vein.
Portal Vein
Typically, the portal vein bifurcates at the hilum to feed the left and right hemi-livers in 65% of cases (17), respectively. The relevant portal variants to be recognized to avoid accidental ligation involve the bifurcation of the main portal trunk. The most frequent are the portal trifurcation and the so-called “Z-type” portal vein, where the right portal vein is absent, and right sectorial branches originate from the main trunk (Fig. 2b). Other relevant variants involve the segment VIII fed by the left portal vein (16,17).
Hepatic Veins
The importance of hepatic vein variants relies on the drained territories and the potential congestive status of the remnant liver after ligation of an aberrant vessel. The classification of hepatic veins is more complex: the right hepatic vein has four variants, while the middle and left hepatic veins are classified into three variants each (18-20). Usually, the right hepatic vein drains the segments V, VI, and VII; if a variant drains the entire right hemiliver, the middle hepatic vein can be removed during left hepatectomy (18). An accessory right hepatic vein with caval confluence of at least 20 mm caudally to the right hepatic vein may allow for resection of segment VII and VIII together with the right hepatic vein (fig. 2c) (18). The middle and left hepatic veins present a common trunk in 65-85% of cases with difficult selective clamping of the middle hepatic vein alone (19,20). The relevant variants of middle and left hepatic veins regard the drainage of segments V, VIII, and IV to be evaluated before the ligation of the left or the middle hepatic vein (18).
Biliary Tree
In the normal biliary anatomy, the confluence of the right and left hepatic duct makes the main hepatic duct at the hilum (80% of cases) (21). Even though systematic correlations between biliary and vascular variants have not been demonstrated (21), a not-recognized biliary variant may lead to postoperative leakage or biliary obstruction in a portion of the remnant liver (16). The most relevant biliary variants involve a segment or sector drained contralaterally or in an ectopic way; these frequently involve the right posterior sectorial duct draining into the left hepatic duct, in the main hepatic duct or the cystic duct (16, 21).
Imaging techniques
Ultrasound
Transabdominal liver ultrasound (US) is generally the first level for the evaluation of a focal liver lesion; elastography techniques are used for the evaluation of liver fibrosis (22, 23). It is fast, diffuse and cheap, but is not panoramic and strongly dependent on the operator. With the administration of contrast material (CEUS), it may be helpful in the characterization of liver lesions (24).
Conversely, the US plays a fundamental role in intraoperative imaging, allowing for an accurate evaluation of the lesions, vascular pedicles, and their relationships and is also helpful in the evaluation of segmental anatomy (e.g., the US finger compression) (25).
Computer Tomography
The Computer Tomography (CT) examination is fundamental in the preoperative evaluation and the administration of intravenous contrast material is mandatory. A post-contrast triphasic protocol is necessary for an accurate evaluation of the arterial vessels, the intrahepatic portal and venous vessels as well as for detection, localization and characterization of the liver lesions (16). Adequate dose of contrast material and bolus tracking techniques are recommended for optimal acquisition timing and adequate contrast resolution between the vascular pedicles, the lesions, and the liver parenchyma (26). Advanced techniques, such as Dual-Energy CT, allow for further optimization of contrast administration (27, 28). The purpose of the radiological report is to help the surgeon to decide whether the procedure is indicated and, eventually, to choose the best surgical method. The report should include the size, number, and location of the lesions, including their relationship with the vessels and biliary tree, by using the Couinaud’s landmarks. The relevant anatomical variants with potential influence on the surgical technique (see the previous section) need to be reported. Moreover, the patency of the vascular pedicles, as well as the presence of neoplastic portal thrombus, need to be reported (4).
The radiologist should highlight potential contraindications to surgery, such as radiological signs of portal hypertension (i.e., ascites, splenomegaly, patency of umbilical vein, and abdominal varices), associated with a higher rate of perioperative complications (4).
Furthermore, the radiologist should recognize and report signs of chronic liver disease (cirrhosis and steatosis) or the presence of biliary dilatation / obstructive jaundice suggesting an impairment of the function of the relative parenchyma (29).
Compared to MRI, CT provides a relatively poor representation of the biliary duct system but is faster and provides a higher spatial resolution of the vascular structures. This is relevant for 3D reconstructions of the liver parenchyma and vessels provided for the multidisciplinary evaluation and surgical planning. A further step is the virtual resections with volumetric estimations, discussed in the next sections (4).
Finally, the evaluation of diffuse liver disease with advanced techniques such as Dual-Energy is not fully standardized and validated (30).
Magnetic Resonance Imaging
Magnetic Resonance Imaging (MRI) represents the gold-standard for the evaluation of the liver focal and diffuse disease and the biliary tree. In this regard, MRI has assumed a primary role in the study of various districts and pathologies, thanks to its high contrast resolution and the absence of ionizing radiations compared to CT (31-34).
An adequate MRI protocol should include the basic sequences (axial and coronal T2-weighted turbo spin-echo, axial T1-weighted gradient-echo in- and out-of-phase with eventual Dixon technique) together with Diffusion-Weighted imaging (DWI), magnetic resonance cholangiopancreatography (MRCP) and post-contrast acquisitions (35, 36).
The DWI is helpful in the detection and characterization of focal lesions and eventual neoplastic portal vein thromboses (35, 37).
The first advantage of hepatobiliary contrast agents (HCA) is the remarkably high accuracy in detection and mapping of focal lesions, in particular the smaller ones (<1 cm); this is cost-effective by reducing the rate of inadequate surgical treatments in patients with colorectal liver metastases (38). Moreover, the evaluation of the biliary tree in the excretory phase, integrated with MRCP, may be helpful for the evaluation of eventual lesions or variants of the biliary tract and the spatial relations with hepatic nodules (35).
The advanced techniques, such as MR Elastography, Proton Density Fat Fraction (PDFF), the evaluation of iron overload, and the T1 mapping with hepatobiliary contrast agents for liver function, can provide valuable information on liver parenchyma, though not widely available (35).
Quantitative evaluation: the role of Virtual Resections
The remnant liver and the Post-Hepatectomy Liver Failure (PHLF)
The curative effectiveness of a major liver resection needs to be balanced with the amount of remnant liver parenchyma. In the postoperative period, the remnant liver has contemporarily to sustain liver regeneration (induced by the increased sinusoidal shear stress) and metabolic functions. In the case of imbalance between the metabolic and regenerating functions, the functional reserve, and the volume of the remnant liver, the post-hepatectomy liver failure (PHLF) occurs (29).
There are several definitions of PHLF; a widely accepted one describes the PHLF as the postoperative impairment of metabolic functions of the remnant liver with hyperbilirubinemia and increased INR (with or without clinical symptoms) on or after postoperative day 5 (29, 39). The PHLF may present with different grades of severity, with variable mortality up to 54% (39).
The portal hyperperfusion of the remnant liver (because of the loss of vascular space after resection), the imbalance of the metabolism of biliary salts, dysregulation of the innate hepatic immune system and the presence of underlying chronic liver disease (e.g., steatosis or cirrhosis) are the major pathophysiological factors of PHLF. Thus, the combination of patient’s and hepatic intrinsic factors (e.g., cirrhosis) and operative factors (e.g., loss of vascular) are responsible for the occurrence of PHLF (29, 40).
Virtual Resections
Cross-sectional imaging plays a pivotal role in the estimation of the postoperative liver remnant (Future Liver Remnant, FLR). The FLR is a percentage of the estimated remnant liver volume (RLV) over the estimated preoperative total liver volume (TLV) (41).
The RLV is accurately calculated on CT or MRI images following the Couinaud’s landmarks with dedicated software. A relevant technical factor is the slice thickness, responsible for partial volume artifacts: acceptable values are below 6 mm in CT and below 8 mm in MRI (easily achieved with modern scanners) (42).
The recent advances in artificial intelligence allow for segmentation of big volumes (TLV) in short time with no more needs for calculation of standard liver volumes from anthropometric data; moreover, tumoral lesions are easily segmented (Tumor volume, TuV) (43). Thus, the FLR can be easily calculated as a fraction of the RLV on the Functional Liver Volume (FLV=TLV-TuV) (Fig. 3) (41):
Figure 3.
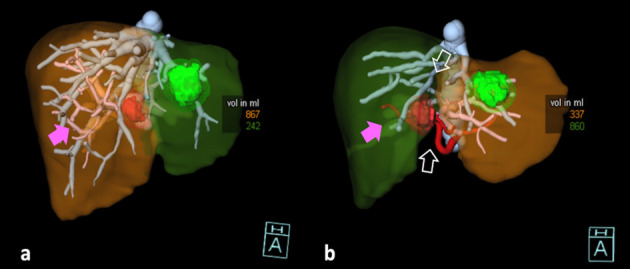
Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS), Volume rendering reconstructions (VR). Hepatic artery: red. Hepatic veins: blue. Portal vein: pink. Red lesion: tumor. Green lesion: cyst. A: Baseline CT before step 1: simulation of right hepatectomy extended to segment 4. Green liver: Remnant Liver Volume (RLV) including Couinaud’s segments 1, 2, 3. Brown Liver: resected liver (segments 4, 5, 6, 7, 8). The RLV had a volume of 242 ml and 230 ml without the cyst. The calculated future liver remnant was 21% (fraction of RLV on Functional Liver), below the safe threshold for resection (FLR>=25%). The patient was candidate to ALPPS. B: follow-up CT on 7° post-operative day after ALPPS step 1. The RLV (brown liver) increased from 242 ml to 337 ml (equal to 325 ml without the cyst). The calculated FLR was 28%, and the patient underwent to right hepatectomy extended to segment 4 on the following day. The procedures performed in step 1 can be observed: the right portal vein was ligated (not visible in b, pink arrows), and the in-situ splitting was performed (empty white arrows).
The estimation of FLR does not consider the presence of underlying liver disease that has to be estimated separately. If a cutoff of FLR=25-30% can be considered as safe in patients without chronic liver diseases, higher thresholds (more than 40%) are necessary in the presence of steatosis, cirrhosis, or obstructive jaundice (29, 41). Thus, the volumetric estimations from virtual resections need to be integrated with clinical data (e.g., MELD Score, Child-Pugh, Indocyanine test, and eventual liver biopsy) in a multidisciplinary environment (4, 29).
Induction of liver hypertrophy
It is possible to increase a not-sufficient FLR by induction of liver hypertrophy with selective occlusion of portal branches with different techniques (44).
The portal vein embolization (PVE) is an interventional technique for percutaneous occlusion of portal branches, with a reported mean increase in FLR of nearly 38% at 4-weeks CT (44). The relatively low invasiveness needs to be balanced with a relatively low increase of FLR and long times with a reported 20% drop-out rate because of tumor progression (44, 45).
The portal vein ligation (PVL) is usually associated with wedge resections in two-stage hepatectomies in colorectal liver metastases: a hemiliver is debulked, and the contralateral portal branch is ligated for subsequent hepatectomy (46). The data available do not suggest significant differences between the outcomes of PVE and PVL (44).
The ALPPS (Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy) is a two-stage hepatectomy with several variants (47, 48). The first step includes the portal ligation together with the partial or total liver transection to avoid portal shunts (47). After a median time of 7-9 days, a mean increase in FLR of 69-75% has been reported on CT studies, and the hepatectomy can be safely performed in the 2nd step (Fig. 3) (44, 47). The absence of almost null tumor progression needs to be balanced with a reported higher mortality rate (49).
Conclusions
In this overview, we provided a basic discussion on the surgical techniques and the underlying anatomical concepts the radiologist needs to be aware of. The patients with hepatobiliary malignancies require a multidisciplinary approach in which radiology plays a pivotal role in qualitative and quantitative evaluations.
Conflict of interest:
Authors declare that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Kokudo N, Takemura N, Ito K, Mihara F. The history of liver surgery: Achievements over the past 50 years. Ann Gastroenterol Surg. 2020;4:109–17. doi: 10.1002/ags3.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calise F, Giuliani A, Sodano L, et al. Segmentectomy: is minimally invasive surgery going to change a liver dogma? Updates Surg. 2015;67:111–5. doi: 10.1007/s13304-015-0318-z. [DOI] [PubMed] [Google Scholar]
- 3.Marte G, Scuderi V, Rocca A, Surfaro G, Migliaccio C, Ceriello A. Laparoscopic splenectomy: a single center experience. Unusual cases and expanded inclusion criteria for laparoscopic approach. Updates Surg. 2013;65:115–9. doi: 10.1007/s13304-013-0197-0. [DOI] [PubMed] [Google Scholar]
- 4.Shin DS, Ingraham CR, Dighe MK, et al. Surgical resection of a malignant liver lesion: what the surgeon wants the radiologist to know. AJR Am J Roentgenol. 2014;203:W21–33. doi: 10.2214/AJR.13.11701. [DOI] [PubMed] [Google Scholar]
- 5.Belfiore MP, Reginelli A, Maggialetti N, et al. Preliminary results in unresectable cholangiocarcinoma treated by CT percutaneous irreversible electroporation: feasibility, safety and efficacy. Med Oncol. 2020;37:45. doi: 10.1007/s12032-020-01360-2. [DOI] [PubMed] [Google Scholar]
- 6.Giannitto C, Campoleoni M, Maccagnoni S, et al. Unindicated multiphase CT scans in non-traumatic abdominal emergencies for women of reproductive age: a significant source of unnecessary exposure. Radiol Med. 2018;123:185–90. doi: 10.1007/s11547-017-0819-6. [DOI] [PubMed] [Google Scholar]
- 7.Petrillo M, Patella F, Pesapane F, et al. Hypoxia and tumor angiogenesis in the era of hepatocellular carcinoma transarterial loco-regional treatments. Future Oncol. 2018;14:2957–67. doi: 10.2217/fon-2017-0739. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis FH, Borgheresi A, Petre EN, Santos E, Solomon SB, Brown K. Hepatic Arterial Embolization Using Cone Beam CT with Tumor Feeding Vessel Detection Software: Impact on Hepatocellular Carcinoma Response. Cardiovasc Intervent Radiol. 2018;41:104–11. doi: 10.1007/s00270-017-1758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgheresi A, Gonzalez-Aguirre A, Brown KT, et al. Does Enhancement or Perfusion on Preprocedure CT Predict Outcomes After Embolization of Hepatocellular Carcinoma? Acad Radiol. 2018;25:1588–94. doi: 10.1016/j.acra.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belfiore G, Belfiore MP, Reginelli A, et al. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med Oncol. 2017;34:38. doi: 10.1007/s12032-017-0887-4. [DOI] [PubMed] [Google Scholar]
- 11.Carrafiello G, Fontana F, Cotta E, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med. 2011;116:1059–66. doi: 10.1007/s11547-011-0697-2. [DOI] [PubMed] [Google Scholar]
- 12.Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg. 1999;16:459–67. doi: 10.1159/000018770. [DOI] [PubMed] [Google Scholar]
- 13.Bismuth H. Revisiting liver anatomy and terminology of hepatectomies. Ann Surg. 2013;257:383–6. doi: 10.1097/SLA.0b013e31827f171f. [DOI] [PubMed] [Google Scholar]
- 14.Strasberg SM, Belghiti J, Clavien PA, et al. The Brisbane 2000 Terminology of Liver Anatomy and Resections. Hpb. 2000;2:333–39. [Google Scholar]
- 15.Lopez-Andujar R, Moya A, Montalva E, et al. Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers. Liver Transpl. 2007;13:1401–4. doi: 10.1002/lt.21254. [DOI] [PubMed] [Google Scholar]
- 16.Catalano OA, Singh AH, Uppot RN, Hahn PF, Ferrone CR, Sahani DV. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008;28:359–78. doi: 10.1148/rg.282075099. [DOI] [PubMed] [Google Scholar]
- 17.Covey AM, Brody LA, Getrajdman GI, Sofocleous CT, Brown KT. Incidence, patterns, and clinical relevance of variant portal vein anatomy. AJR Am J Roentgenol. 2004;183:1055–64. doi: 10.2214/ajr.183.4.1831055. [DOI] [PubMed] [Google Scholar]
- 18.Barbaro B, Soglia G, Alvaro G, et al. Hepatic veins in presurgical planning of hepatic resection: what a radiologist should know. Abdom Imaging. 2013;38:442–60. doi: 10.1007/s00261-012-9900-8. [DOI] [PubMed] [Google Scholar]
- 19.Reichert PR, Renz JF, D’Albuquerque LA, et al. Surgical anatomy of the left lateral segment as applied to living-donor and split-liver transplantation: a clinicopathologic study. Ann Surg. 2000;232:658–64. doi: 10.1097/00000658-200011000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masselot R, Leborgne J. Anatomical study of hepatic veins. Anat Clin. 1978;1:109–25. [Google Scholar]
- 21.Macdonald DB, Haider MA, Khalili K, et al. Relationship between vascular and biliary anatomy in living liver donors. AJR Am J Roentgenol. 2005;185:247–52. doi: 10.2214/ajr.185.1.01850247. [DOI] [PubMed] [Google Scholar]
- 22.Rodgers SK, Fetzer DT, Gabriel H, et al. Role of US LIRADS in the LI-RADS Algorithm. Radiographics. 2019;39:690–708. doi: 10.1148/rg.2019180158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy P, Wagner M, Castera L, et al. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology. 2018;286:738–63. doi: 10.1148/radiol.2018170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burrowes DP, Medellin A, Harris AC, Milot L, Wilson SR. Contrast-enhanced US Approach to the Diagnosis of Focal Liver Masses. Radiographics. 2017;37:1388–400. doi: 10.1148/rg.2017170034. [DOI] [PubMed] [Google Scholar]
- 25.Torzilli G, Makuuchi M. Ultrasound-guided finger compression in liver subsegmentectomy for hepatocellular carcinoma. Surg Endosc. 2004;18:136–9. doi: 10.1007/s00464-003-9024-x. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa T, Erturk SM, Araki T. Multiphasic contrastenhanced multidetector-row CT of liver: contrast-enhancement theory and practical scan protocol with a combination of fixed injection duration and patients’ body-weighttailored dose of contrast material. Eur J Radiol. 2006;58:165–76. doi: 10.1016/j.ejrad.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 27.Agostini A, Borgheresi A, Mari A, et al. Dual-energy CT: theoretical principles and clinical applications. Radiol Med. 2019;124:1281–95. doi: 10.1007/s11547-019-01107-8. [DOI] [PubMed] [Google Scholar]
- 28.Agostini A, Mari A, Lanza C, et al. Trends in radiation dose and image quality for pediatric patients with a multidetector CT and a third-generation dual-source dual-energy CT. Radiol Med. 2019;124:745–52. doi: 10.1007/s11547-019-01037-5. [DOI] [PubMed] [Google Scholar]
- 29.Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 30.Agostini A, Borgheresi A, Mari A, et al. Dual-energy CT: theoretical principles and clinical applications. Radiol Med. 2019 doi: 10.1007/s11547-019-01107-8. [DOI] [PubMed] [Google Scholar]
- 31.Mungai F, Pasquinelli F, Mazzoni LN, et al. Diffusionweighted magnetic resonance imaging in the prediction and assessment of chemotherapy outcome in liver metastases. Radiol Med. 2014;119:625–33. doi: 10.1007/s11547-013-0379-3. [DOI] [PubMed] [Google Scholar]
- 32.Carrafiello G, Ierardi AM, Piacentino F, Cardim LN. Percutaneous transhepatic embolization of biliary leakage with Nbutyl cyanoacrylate. Indian J Radiol Imaging. 2012;22:19–22. doi: 10.4103/0971-3026.95398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrafiello G, D’Ambrosio A, Mangini M, et al. Percutaneous cholecystostomy as the sole treatment in critically ill and elderly patients. Radiol Med. 2012;117:772–9. doi: 10.1007/s11547-012-0794-2. [DOI] [PubMed] [Google Scholar]
- 34.Calistri L, Castellani A, Matteuzzi B, Mazzoni E, Pradella S, Colagrande S. Focal Liver Lesions Classification and Characterization: What Value Do DWI and ADC Have? J Comput Assist Tomogr. 2016;40:701–8. doi: 10.1097/RCT.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 35.Agostini A, Kircher MF, Do RK, et al. Magnetic Resonanance Imaging of the Liver (Including Biliary Contrast Agents)-Part 2: Protocols for Liver Magnetic Resonanance Imaging and Characterization of Common Focal Liver Lesions. Semin Roentgenol. 2016;51:317–33. doi: 10.1053/j.ro.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agostini A, Kircher MF, Do R, et al. Magnetic Resonance Imaging of the Liver (Including Biliary Contrast Agents) Part 1: Technical Considerations and Contrast Materials. Semin Roentgenol. 2016;51:308–16. doi: 10.1053/j.ro.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liver EAftSot EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of hepatology. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Zech CJ, Grazioli L, Jonas E, et al. Health-economic evaluation of three imaging strategies in patients with suspected colorectal liver metastases: Gd-EOB-DTPA-enhanced MRI vs. extracellular contrast media-enhanced MRI and 3-phase MDCT in Germany, Italy and Sweden. Eur Radiol. 2009;19(Suppl 3):S753–63. doi: 10.1007/s00330-009-1432-4. [DOI] [PubMed] [Google Scholar]
- 39.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–24. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 40.van Mierlo KM, Schaap FG, Dejong CH, Olde Damink SW. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J Hepatol. 2016;65:1217–31. doi: 10.1016/j.jhep.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Schindl MJ, Redhead DN, Fearon KC, et al. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–96. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiner CS, Karlo C, Petrowsky H, Marincek B, Weishaupt D, Frauenfelder T. Preoperative liver volumetry: how does the slice thickness influence the multidetector computed tomography-and magnetic resonance-liver volume measurements? J Comput Assist Tomogr. 2009;33:390–7. doi: 10.1097/RCT.0b013e3181806c29. [DOI] [PubMed] [Google Scholar]
- 43.Chlebus G, Schenk A, Moltz JH, van Ginneken B, Hahn HK, Meine H. Automatic liver tumor segmentation in CT with fully convolutional neural networks and object-based postprocessing. Sci Rep. 2018;8:15497. doi: 10.1038/s41598-018-33860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandanaboyana S, Bell R, Hidalgo E, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015;157:690–8. doi: 10.1016/j.surg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 45.van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25–34. doi: 10.1007/s00270-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popescu I, Alexandrescu ST. Surgical options for initially unresectable colorectal liver metastases. HPB Surg. 2012;2012:454026. doi: 10.1155/2012/454026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–14. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 48.Vivarelli M, Vincenzi P, Montalti R, et al. ALPPS Procedure for Extended Liver Resections: A Single Centre Experience and a Systematic Review. PLoS ONE. 2015;10:e0144019. doi: 10.1371/journal.pone.0144019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olthof PB, Coelen RJS, Wiggers JK, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:381–87. doi: 10.1016/j.hpb.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


