Abstract
Parkinson’s disease (PD) is a common neurological disorder characterized by gait impairment. PD has no cure, and an impediment to developing a treatment is the lack of any accepted method to predict disease progression rate. The primary aim of this study was to develop a model using clinical measures and biomechanical measures of gait and postural stability to predict an individual’s PD progression over two years. Data from 160 PD subjects were utilized. Machine learning models, including XGBoost and Feed Forward Neural Networks, were developed using extensive model optimization and cross-validation. The highest performing model was a neural network that used a group of clinical measures, achieved a PPV of 71% in identifying fast progressors, and explained a large portion (37%) of the variance in an individual’s progression rate on held-out test data. This demonstrates the potential to predict individual PD progression rate and enrich trials by analyzing clinical and biomechanical measures with machine learning.
Keywords: Parkinson’s Disease, Prognosis, Machine Learning, Biomechanical Measures, Progression Rate
1. INTRODUCTION
Parkinson’s Disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease, with 60,000 new PD diagnoses made annually resulting in a prevalence estimate by 2020 in the USA of 930,000 people [1]. PD is characterized by a progressive loss of dopaminergic neurons, resulting in resting tremor, limb stiffness, and bradykinesia, which often manifests early on with a reduction in arm swing amplitude when walking. The primary aim of this study was to develop a model using clinical and biomechanical gait and postural stability measures capable of identifying fast PD progressors with a high Positive Predictive Value (PPV). Achieving this goal will allow enrichment of future disease-modifying drug trials with fast progressors who are most likely to show detectable changes during a trial. Gait and postural stability measures were chosen as independent variables for our models because they have been previously found to be predictive of PD risk, disease severity, Freezing of Gait detection, and PD diagnosis [2–6]. While their potential for indexing PD progression has been suggested before [7–10], to our knowledge no study has used machine learning to predict future PD progression using these measures.
Baseline clinical measures were also investigated because of the potential they have to be predictive of PD progression rate. The most closely related study is by Latourelle et al. [11], where the predictive power of a composite biomarker set consisting of genetic, CSF, DaTscan, clinical and demographic features was examined. In contrast, our work examines the predictive power of gait, postural stability, clinical and demographic features. The main contributions of this study are: (1) the development of a predictive model of an individual’s PD progression rate that achieves a high PPV in identifying fast progressors suitable for enrichment of clinical trials to help expedite the development of a cure, and (2) the first machine learning models that demonstrate prediction of PD progression rate using gait and postural stability measures.
2. MATERIALS
Data were analyzed from 160 subjects with idiopathic PD followed longitudinally for 2 years. The subjects were part of the multi-year NIH-NINDS funded Parkinson’s Disease Biomarkers Program (PDBP) [12]. Patient demographics are shown in Table 1. Disease severity was measured using the Movement Disorder Society revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). The MDS-UPDRS is a four-part assessment of PD severity as measured by a trained examiner. Part III of the assessment corresponds to the motor examination and involves 18 sections which are each scored on a scale from 0 (normal) to 4 (severe). Examples of sections include speech, facial expression, and gait examinations. Some sections have subsections for each hand (LH, RH) or for each upper and lower extremity (RUE, LUE, RLE, LLE). The total part III score has a range of 0 to 132. For this dataset, a trained and MDS certified examiner with eight years of prior experience conducted the assessments.
Table 1.
Demographics for 160 PD patients in the dataset
| Demographic | Value |
|---|---|
| Age | 64.5 ± 9.5 |
| Men | 54% |
| Baseline MDS-UPDRS part III score | 16.0 ± 7.9 |
| 2 year MDS-UPDRS part III score | 18.2 ± 7.6 |
| On any PD medication | 84% |
| On levodopa | 63% |
3. METHODS
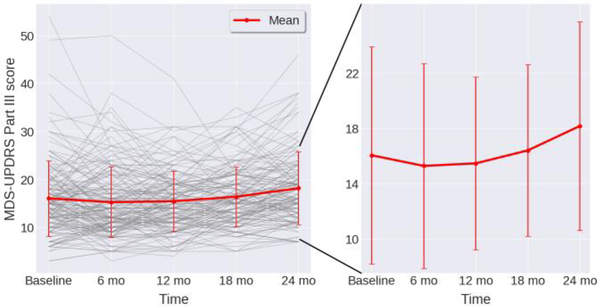
Three targets for regression were tested individually in the following experiments: (1) total part III score at 24 months, (2) 24 months-baseline change in part III, and (3) percent change in part III measured as (24 months-baseline)/baseline. These targets were chosen as it was not known a priori whether absolute severity or a change in severity is a more predictable target. The progression of the total part III score over two years is shown in Fig. 1. A paired t-test revealed that the mean score at 24 months was statistically different from that at baseline (p < 0.01) while the mean scores at previous visits were not, indicating that 24 months is the first point at which significant progression is observed. The variability of scores across subjects exemplifies PD heterogeneity and indicates the highly challenging nature of the task of predicting individual progression rate.
Figure 1.
Progression of MDS-UPDRS part III score across 24 months. Individual subjects plotted as grey lines. Mean and standard deviation across subjects plotted in red. Zoomed view on right shows increasing severity longitudinally.
For the gait and posture measures, six movement sensors called Opals® consisting of a 3-axis accelerometer, gyroscope and magnetometer (Mobility Lab, APDM Inc., Portland, OR) were attached to each subject: one on each ankle and wrist, the lower back, and the upper chest, as we described in Dewey et al. [6].
The tasks were:
The instrumented Timed-up-and-go (iTUG) test: subjects stand up from a chair, walk 6 meters, turn, walk back and sit down. Note that this extends the traditional 3 meter TUG task in order to capture more gait cycles [13]. This test gave measures such as duration of subtasks (sit-to-stand, steady-state gait, turn, and turn-to-sit), gait speed, and arm-swing velocity.
The instrumented Sway (iSway) test: subjects stand still with their feet a set distance apart and their hands across their chests for 30 seconds. This gave measures such as jerk, sway area, and mean velocity.
Three runs of iTUG and iSway were conducted at each visit and the median values of 148 summary statistics computed by the APDM software were utilized. Clinical measures were used including: age, gender, baseline MDS-UPDRS part III subscores, Levodopa Equivalent Daily Dose (LEDD), and MOntreal Cognitive Assessment (MOCA) score.
3.1. Feature set construction and feature selection:
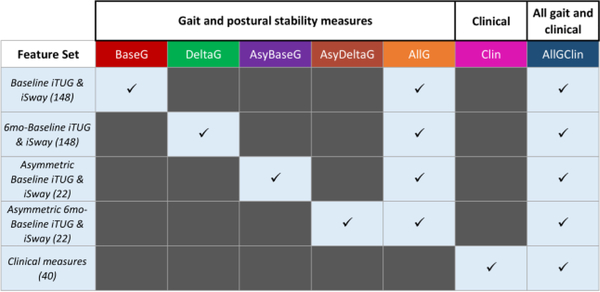
Seven sets of features were compared for predictive power, encompassing different combinations of iTUG and iSway summary statistics, clinical measures, and additional derived features (Fig. 2). These included a set with the baseline iTUG and iSway measures (which we refer to as BaseG, short for baseline gait) and a set of derived features with the difference between iTUG and iSway measures at 6 months and at baseline (DeltaG, i.e. delta gait), which capture progression of motor symptoms. Asymmetric presentation of motor dysfunctions in PD has shown to be an important marker of PD severity [14], so asymmetry measures on the 22 lateralized variables in baseline (AsyBaseG) and 6 months-baseline iTUG and iSway (AsyDeltaG) were computed with the formula . All of these iTUG and iSway measures were combined in feature set AllG, i.e. all gait measures. Clinical measures were considered by themselves (Clin) and in combination with all iTUG and iSway measures (AllGClin, i.e. all gait and clinical measures). Feature selection was conducted on the training partitions by dropping one member of each pair of highly intercorrelated features (Pearson’s r > 0.8) to minimize feature redundancy.
Figure 2.
Feature set combinations explored. Number of features in each set indicated in parentheses. Abbreviations for feature sets in column headers.
3.2. Data partitioning and model training:
XGBoost and Feed Forward Neural Network (NN) models were chosen as they are two of the most powerful models that consistently win machine-learning competitions for structured and unstructured data and have shown high performance in a wide range of tasks [15]. Mean-squared-error loss was used to train the NNs. Model performance was evaluated using the R2 score, i.e., the coefficient of determination. The dataset was partitioned using nested K-fold cross validation with 3 inner and 3 outer folds. In each outer fold, mean R2 across the held-out partitions of the inner folds was used to rank model performance and the model with the highest mean R2 was selected for evaluation on the held-out partition of the outer fold. The mean test performance over the held-out partitions in the 3 outer folds represents final model performance. Stratified k-fold partitioning was used to ensure representative target distributions across splits and appropriate model training and evaluation.
3.3. Hyperparameter optimization and model selection:
To identify optimal model hyperparameters in an unbiased manner, a random search of 1000 hyperparameter configurations for XGBoost and 300 configurations for NNs was conducted. Fewer configurations were searched for NNs due to computational requirements. The hyperparameter dimensions and ranges searched are shown in Table 2. The best-performing hyperparameter configurations were selected based on mean R2 across the inner cross-validation folds, and the model’s performance is evaluated as the mean R2 evaluated on the held-out test splits. Hyperparameter vs. performance plots [not shown] confirmed that sufficient ranges of the hyperparameters was searched such that the local maxima of performance were found.
Table 2.
Hyperparameter ranges explored for each model.
|
XGBoost |
| Number of estimators: [10, 1000] |
| Maximum depth: [5, 50] |
| L1 regularization term: (0, 1) |
| L2 regularization term: (0, 1) |
| Learning rate: [0.0001, 0.4] |
|
Feed Forward Neural Network |
| Layers: [1, 5] |
| Chance to taper: 50% |
| Taper size: {0.2, 0.5} |
| Dropout: [0.1, 1.0] |
| Activations: {ReLU, ELU, LeakyReLU, PReLU, tanh, sigmoid} |
| Number of neurons: {16, 32, 48, 64, 80, 96, 112, 128} |
| Learning rate: [0.0001, 0.005] |
| Optimizer: Nadam |
3.4. Feature importance:
To reveal what the NNs learned, feature permutation importance was used to compute feature importance. Each feature in the held-out test set was randomly permuted 100 times and the decrease in R2 was measured, with a greater mean decrease reflecting greater importance.
4. RESULTS
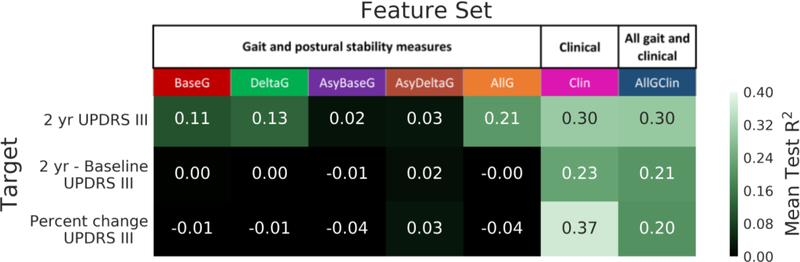
The mean test R2 performances of the best models on each feature set × target combination are shown in Fig. 3. NNs outperformed XGBoost models in every case. The feature set Clin, which used only the clinical measures, achieved the highest R2 across all prediction targets and model categories. Using this feature set, 37% of the variance was explained in the percentage change MDS-UPDRS part III score.
Figure 3.
Mean test R2 performances of best models on each feature-target combination. Feature sets are shown on the x-axis and targets are shown on the y-axis. The color bar indicates R2, with brighter green indicating better performance and black indicating 0 or negative R2 performance.
Given that predicting progression rate is a difficult problem as indicated by the large standard deviation of ± 8 points in the progression rate across subjects, explaining nearly 40% of the variance in progression rate using our model is a significant finding. This result is comparable to the 41% validation performance achieved by Latourelle et al. [11], and has the added strength that our evaluation is on held-out test data while theirs was on validation data which typically inflates the result. Our model achieved a PPV of 71% in identifying fast progressors, defined as having a 20% or more increase in MDS-UPDRS part III score from baseline (top 50% of the cohort).
This is also the first study to use machine learning to show gait and postural stability measures to be predictive of PD progression. Three of the gait and postural stability feature sets explained 10% or more of the variance in the 2 year MDS-UPDRS part III score. Feature set AllG, which included all the derived gait and postural stability measures, explained 21% of the variance.
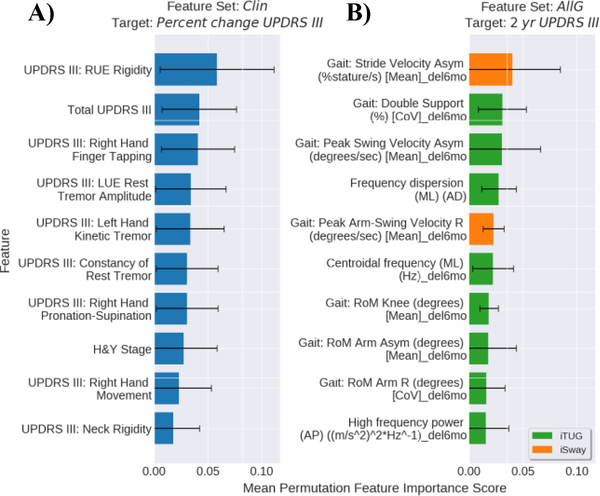
The 10 most important features learned by NNs on sets Clin and AllG are shown in Fig. 4. The MDS-UPDRS part III RUE rigidity subscore, total score, and Right Hand Finger Tapping subscore were the three most important features for predicting percent change (Fig. 4A). In the AllG feature set, the (6 months-baseline) iTUG asymmetry values ranked high in feature importance, with the iTUG gait stride velocity asymmetry change as the most important for predicting the 2 year score (Fig. 4B).
Figure 4.
Mean feature importance of the top 10 features learned by the best performing NNs. A) Top features using feature set Clin to predict the percent change in MDS-UPDRS part III and B) Top features using feature set AllG to predict 2 year MDS-UPDRS part III score. Error bars show the standard deviation across the outer splits. The “_del6mo” suffix indicates a 6 months minus baseline value.
4. DISCUSSION
The best model performance was obtained using clinical measures to predict the 2 year percent change MDS-UPDRS part III score with an NN. The model explained 37% of the variance in the target, with a PPV of 71% in identifying fast progressors. Thus, the model may be useful in enriching disease-modifying drug trials with fast progressors. Similar to Latourelle et al. [11], baseline movement scores from Clin are found to be among the most important features for predicting future progression rate.
For the gait and posture measures, the (6 months-baseline) iTUG asymmetry values ranked high in feature importance, indicating that the progression of asymmetric aspects of gait impairments are especially important for predicting PD progression. This demonstrates the prognostic value that can be provided by improved measurements of motor disability in PD subjects. While the gait and postural stability measures alone in AllG performed modestly (R2=0.21), their performance was bolstered by the inclusion of clinical measures in AllGClin (R2=0.30). However, this performance boost was not additive, suggesting that the feature sets have collinearities and measure similar aspects of PD progression.
The primary limitation of this study is that a single dataset was used. Though the dataset was fairly large (N=160 subjects) and rigorous cross-validation was performed including a held-out test set not used for training or model selection, a replication study on an independent dataset would further confirm our findings. Fortunately, other datasets are becoming available and replication of our model’s performance on these datasets is the subject of our ongoing research. Additional future studies are planned to increase predictive power using other methods to derive features from the iTUG and iSway sensor data. Such deeper analysis may enable even more prognostic applications.
5. CONCLUSION
The main contributions of this study include the development of a predictive model of an individual’s PD progression rate that achieves a 71% PPV in identifying fast progressors, which is suitable to enrich clinical trials to help expedite the development of a cure for PD. This work reaffirms the importance of clinical measures in predicting PD progression and suggests the potential for gait and postural stability measures as a predictive tool.
ACKNOWLEDGEMENTS
Data and biospecimens used in preparation of this manuscript were obtained from the Parkinson’s Disease Biomarkers Program (PDBP) Consortium, part of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health. Investigators include: Roger Albin, Roy Alcalay, Alberto Ascherio, Brad Boeve, DuBois Bowman, Alice Chen-Plotkin, Ted Dawson, Richard Dewey, Ray Dorsey, Kirk Frey, Dwight German, Lawrence Honig, Xuemei Huang, Kejal Kantarci, Jim Leverenz, Lara Mangravite, Karen Marder, Rachel Saunders-Pullman, Liana Rosenthal, Clemens Scherzer, Michael Schwarzschild, Tanya Simuni, David Vaillancourt, David Walt, Andrew West and Jing Zhang.
References
- 1.Parkinson’s Prevalence Project. Parkinson’s Foundation. http://www.parkinson.org/Understanding-Parkinsons/Causes-and-Statistics/Statistics. Accessed 1 May 2019.
- 2.Ashour AS, El-Attar A, Dey N, El-Kader HA, Abd El-Naby MM. Long short term memory based patient-dependent model for FOG detection in Parkinson’s disease. Pattern Recogn. Lett 2020;131:23–9. doi: 10.1016/j.patrec.2019.11.036. [DOI] [Google Scholar]
- 3.Buckley C, Alcock L, McArdle R, Rehman RZU, Del Din S, Mazzà C, et al. The Role of Movement Analysis in Diagnosing and Monitoring Neurodegenerative Conditions: Insights from Gait and Postural Control. Brain Sci 2019. doi: 10.3390/brainsci9020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci M, Di Lazzaro G, Pisani A, Mercuri NB, Giannini F, Saggio G. Assessment of Motor Impairments in Early Untreated Parkinson’s Disease Patients: The Wearable Electronics Impact. IEEE J. Biomedical Health Informat 2020;24:120–30. doi: 10.1109/JBHI.2019.2903627. [DOI] [PubMed] [Google Scholar]
- 5.Ur Rehman RZ, Del Din S, Shi JQ, Galna B, Lord S, Yarnall AJ, et al. Comparison of walking protocols and gait assessment systems for machine learningbased classification of parkinson’s disease. Sensors 2019. doi: 10.3390/s19245363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewey DC, Miocinovic S, Bernstein I, Khemani P, Dewey RB, Querry R, Chitnis S. Automated gait and balance parameters diagnose and correlate with severity in Parkinson disease. J Neurol Sci 2014;345:131–8. doi: 10.1016/j.jns.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toosizadeh N, Mohler J, Lei H, Parvaneh S, Sherman S, Najafi B. Motor Performance Assessment in Parkinson’s Disease: Association between Objective In-Clinic, Objective In-Home, and Subjective/Semi-Objective Measures. PLoS ONE. 2015;10:e0124763. doi: 10.1371/journal.pone.0124763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture. 2012;36:471–6. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:171–6. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricciardi C, Amboni M, Santis C de, Ricciardelli G, Improta G, Iuppariello L, et al. Classifying Different Stages of Parkinson’s Disease Through Random Forests. In: Henriques J, de Carvalho P, Neves N, editors: Springer; 2020. p. 1155–1162. doi: 10.1007/978-3-030-31635-8_140. [DOI] [Google Scholar]
- 11.Latourelle JC, Beste MT, Hadzi TC, Miller RE, Oppenheim JN, Valko MP, et al. Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson’s disease: a longitudinal cohort study and validation. The Lancet Neurology. 2017;16:908–16. doi: 10.1016/S1474-4422(17)30328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal LS, Drake D, Alcalay RN, Babcock D, Bowman FD, Chen-Plotkin A, et al. The NINDS Parkinson’s disease biomarkers program. Mov Disord 2016;31:915–23. doi: 10.1002/mds.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng 2010;18:303–10. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewek MD, Poole R, Johnson J, Halawa O, Huang X. Arm swing magnitude and asymmetry during gait in the early stages of Parkinson’s disease. Gait Posture. 2010;31:256–60. doi: 10.1016/j.gaitpost.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T, Guestrin C. XGBoost. In: Krishnapuram B, Shah M, Smola A, Aggarwal C, Shen D, Rastogi R, editors. the 22nd ACM SIGKDD International Conference; 8/12/2016 – 8/16/2016; San Francisco, California, USA. New York, New York, USA: ACM Press; 2016. p. 785–794. doi:10.1145/2939672.2939785. [Google Scholar]