Abstract
COVID-19 can affect the central nervous system (CNS) indirectly by inflammatory mechanisms and even directly enter the CNS. Thereby, COVID-19 can evoke a range of neurosensory conditions belonging to infectious, inflammatory, demyelinating, and degenerative classes. A broad range of non-specific options, including anti-viral agents and anti-inflammatory protocols, is available with varying therapeutic. Due to the high mortality and morbidity in COVID-19–related brain damage, some changes to these general protocols, however, are necessary for ensuring the delivery of therapeutic(s) to the specific components of the CNS to meet their specific requirements. The biomaterials approach permits crossing the blood–brain barrier (BBB) and drug delivery in a more accurate and sustained manner. Beyond the BBB, drugs can protect neural cells, stimulate endogenous stem cells, and induce plasticity more effectively. Biomaterials for cell delivery exist, providing an efficient tool to improve cell retention, survival, differentiation, and integration. This paper will review the potentials of the biomaterials approach for the damaged CNS in COVID-19. It mainly includes biomaterials for promoting synaptic plasticity and modulation of inflammation in the post-stroke brain, extracellular vesicles, exosomes, and conductive biomaterials to facilitate neural regeneration, and artificial nerve conduits for treatment of neuropathies. Also, biosensing surfaces applicable to the first sensory interface between the host and the virus that encourage the generation of accelerated anti-viral immunity theoretically offer hope in solving COVID-19.
Keywords: Artificial nose, Biomaterial, Brain, Central nervous system, CNS, COVID-19, Cytokine storm, Drug delivery, Infection, Inflammation, Intracerebral hemorrhage, Neuropathies, Nose, Olfaction, Stroke
Introduction
Today, materials science’s interests are interdisciplinary that by designing new materials and recognizing their simple properties make progress in understanding the behavior of complex systems. For the condition of COVID-19 emerged as a global pandemic in Wuhan, China, and continued to take lives with more than 2 million deaths as of writing this [1], the need is for devices that offer early prevention and diagnosis of disease with high accuracy and high precision. To this end, the year 2020 has seen strenuous efforts to develop prediction models and diagnostic methods using laboratory data, imaging features, and clinical manifestations [2–4]. Biosensing applications of advanced nanomaterials-based devices that can participate in detecting biomarkers are of particular interest to us because it depends only on the samples of bodily fluids and tissues collected from patients and can take us rapidly and precisely to the situation of making a diagnosis [5]. Also, some biosensing materials have allowed robotic systems to operate better in the detection, tracking, and disinfection of crowded public environments. Amid the COVID-19 pandemic, nano-enabled biosensing systems are an intelligent example to remarkably improve healthcare management [6]. The applications of material science and engineering are really of far greater importance when we realize the potential therapeutic effects of materials. For example, aqueous synthesized quantum dots (QDs) that exhibit both electric and optical properties are a beautiful example of the broad applicability of biomaterials to viral infections, inflammation, and autoimmunity [7]. Also, biomaterials have characteristics that serve to function as carriers for delivering a wide variety of drugs. Solubility, the small size suitable for oral administration, and being able to interact with cells and cross the blood–brain barrier are among others. These amazing abilities indicated that biomaterials, notably nanosized materials, the so-called nanoparticles (NPs), could be recognized as a unique system for delivering anti-viral drugs, tumor-directed therapies, and vaccines [8, 9]. Cell microencapsulation technologies further augment the capacity of this system and its products, which are especially important in treating diseases affecting the central nervous system (CNS) [10]. More recently, researchers have called out studies for nanomaterials-based strategies to treat coronaviruses [11–13]. Tissue engineering is another context within which smart biomaterials and scaffolds are currently under investigation for their activities in tissue regeneration. COVID-19 is an infectious disease that can cause damage to multiple organs and systems, including lungs, cardiovascular system [14–17], gastrointestinal system [18, 19], hematological system [20], nephrological system [21], and CNS [22–25]; because of this, tissue engineering can benefit patients by triggering repair and regeneration mechanisms [26–28].
This introduction will follow an updated synopsis of COVID-19 and related neurological impairments. It will outline several mechanisms COVID-19 has to cause neurosensory damage. In the meantime, this paper will provide evidence supporting biomaterial approaches, including therapeutic biomaterials and biosensing surfaces, for the CNS in COVID-19.
Neurological manifestations in COVID-19
In December 2019, unusual pneumonia made the Chinese health services investigate the origins of the disease. The data from the first cases showed a new coronavirus (CoV) that resembles the causative virus of the severe acute respiratory syndrome (SARS) and so coined the name SARS-CoV-2 [29–31]. A broad range of non-specific options, including anti-viral agents [32–35], plasma therapy [36], statins [37], dietary strategies for immunoregulation [38], and anti-inflammatory protocols [27, 39, 40], is available with varying therapeutic ability in cases with SARS-CoV-2 infection. The proof of their inability is, however, in the nearly 2% death rate related to the virus. Such a percentage of all 70 million cases affected by the disease, in addition to the fact that it may recur, are serious alarm signals [41–43] in the healthcare system, which had to deal with increasing fatigue, burn-out, and stress [44, 45] over the course of the pandemic year. It will undoubtedly call for globally integrated collaboration to respond to this complex problem [46–54] in the context of national online platforms [55].
COVID-19 first manifested itself as a respiratory infection of unknown origin. It, however, revealed to us its multifaceted picture over time. It usually involves the respiratory tract, but it also can lead to cardiovascular problems, gastrointestinal symptoms, and cutaneous manifestations and results in dysregulation of hormonal levels [15, 16, 22, 56, 57].
First reports of CNS involvement reveal that COVID-19-associated neurological diseases can belong to infectious, inflammatory, demyelinating, and degenerative classes. The first case of CNS involvement in COVID-19 was a young man who presented with neck stiffness, generalized seizures, and consciousness loss [58]. The observation of inflammatory changes in the brain, as represented in hyperintense signals in the right mesial temporal lobe and hippocampus, in addition to the presence of SARS-CoV-2 RNA in the cerebrospinal fluid (CSF), could be highly suggestive for COVID-19-associated meningitis/encephalitis [58]. Among early reports of neurological problems in COVID-19 was a middle-aged woman with interstitial pneumonia, loss of consciousness, anosmia, and ageusia who tested positive for SARS-CoV-2. On brain MRI, this patient also had demyelinating lesions involving the bulbo-medullary junction and the cervical and dorsal spinal cord [59]. Moreover, there has been a case report of COVID-19 co-occurring with Creutzfeldt–Jakob disease, speeding up the process of neurodegeneration [60]. Both the latter cases tested negative for SARS-CoV-2 in CSF, implying other mechanisms’ involvement rather than direct infection of neural cells in these cases. In contrast, stand brain tissues showing the evidence of small clusters of infection developed due to SARS-CoV-2 around blood vessels [61]. It is, therefore, possible to assume a SARS-CoV-2-associated neurotropism and neuroinvasion, but rather the virus will affect the CNS indirectly by driving inflammatory responses.
Overall, we can already understand COVID-19 is a process usually manifested itself by respiratory symptoms but also associated with neurological manifestations, namely ataxia, dizziness, fatigue, headache, myalgia, nausea, taste and smell dysfunctions, neuropathic pain, and vomiting on the first presentation or subsequently [22–24, 62, 63] and in severe to critical cases, complicated with agitation, altered consciousness, abnormal wakefulness after sedation discontinued, confusion, and seizures [64, 65]. Brain MRI shows the medial temporal lobe as being mostly involved in severe COVID-19 [64]. Brain lesions include multifocal white matter hyperintensities with associated hemorrhagic lesions and extensive and isolated white matter microhemorrhages [64]. Moreover, patients with severe COVID-19 are at significantly higher risk of developing acute cerebrovascular diseases than patients with mild disease (about 6% vs. 1%) [62]. Those who progress to critical condition, including those who develop acute respiratory distress syndrome (ARDS) and those who need intensive care unit (ICU) admission, are more likely to have an intracerebral hemorrhage [64].
Biomaterials for the CNS and COVID-19: mechanisms and implications
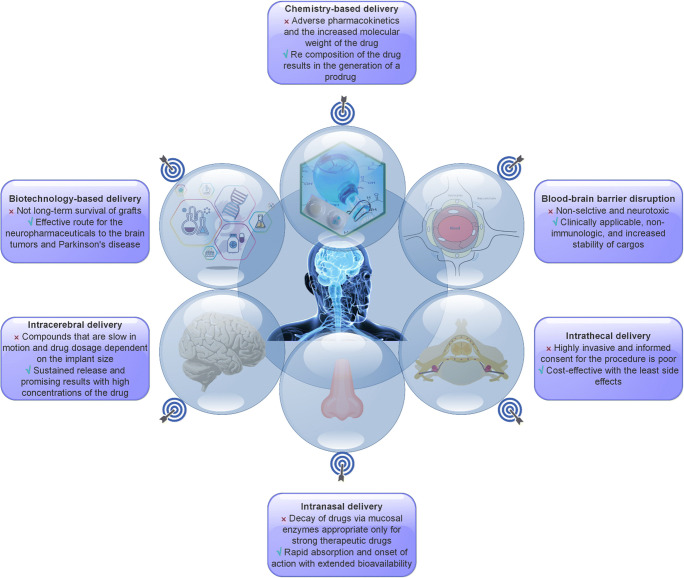
Multiple barriers, including the blood–brain barrier (BBB), blood–CSF barrier, the blood–retinal barrier, and the blood–spinal cord barrier, encircle the CNS without which neural circuits would never be able to play the flute peacefully [66]. However, at the therapeutic level, they are a primary concern for CNS drug delivery. There are common approaches to the problem of drug delivery to the brain, including BBB disruption, biotechnology-based delivery, chemistry-based delivery, intracerebral delivery, intrathecal delivery, intranasal delivery, and non-invasive delivery [66], but with the advantages and disadvantages as summarized in Fig. 1.
Fig. 1.
Approaches for drug delivery to the brain: advantages and disadvantages (Prepared with data from [66])
Biomaterials for the CNS include CNS shunts, cortical neural prosthetics, drug delivery strategies, hydrogel scaffolds for CNS repair, and neural stem cell encapsulation [67]. In particular, therapeutic biomaterials for the brain are of natural and synthetic types. Extracellular matrix (ECM) components, e.g., hyaluronic acid (HA), collagen, fibrin, laminin, heparin, peptides, proteins, and xenobiotic elements, e.g., alginate, chitosan, Matrigel, silk, and methylcellulose (MC), are essential ingredients in the backbone of naturally occurring biomaterials. Synthetic polymers are the main components of synthetically derived biomaterials, e.g., polyethylene glycol (PEG), poly(d,l-lactic acid), polyglycolic acid (PGA), poly(d,l-lactic acid co-glycolic acid) (PLGA), poly(d-lysine), poly(sebacic acid) (PSA), and polycaprolactone (PCL). Different forms of biomaterials used in brain therapy and repair are injectable hydrogels, NPs, microparticles, and electrospun fibers.
Biomaterials, stroke, and COVID-19
Stroke in COVID-19
As reported by The American Heart Association/American Stroke Association, on average, approximately 6% of patients with COVID-19 will experience stroke at 10 days after onset of the disease [62]. Meta-summary reports the pooled incidence of acute ischemic stroke (AIS) as 1.2%, of whom about 40% died [68]. These estimates, coupled with the incidence of stroke in young patients with no risk factors for cerebrovascular diseases and no source of thrombosis identified, provides partial support for the pro-coagulopathy state due to COVID-19 [69–71]. Moreover, coagulopathy is a process associated with inflammation [72, 73]. Therefore, another reason for the large rise of developing thromboembolic events of both venous and arterial and macro and microvascular types in patients with COVID-19 [74] is because an inflammatory response develops in almost all cases of COVID-19, and this inflammation will develop with varying degrees dependent on the genetic background [75–77] and pre-existing condition [78] and corresponds to immune dysregulation occurring at both the local and systemic levels [79–81]. Thrombolytic therapy using recombinant tissue plasminogen activator (rtPA) looks promising in terms of improving a neurological and respiratory condition as represented in the reduction of hypercapnia, alveolar dead space, and ventilatory ratio [82]. However, it increases the risk of hemorrhage.
From the storm of cytokines to the development of stroke in COVID-19
Cells and pattern recognition receptors (PRRs) sense invading pathogens and release cytokines as part of the development of the first line of immune defense, i.e., innate immunity, arranging a mixture of proteins, including chemokines, adhesion molecules, and transcription factors, that mediate the transition to the specialized immune responses, i.e., adaptive immunity [83, 84]. Cytokines are functionally diverse messengers. They can interact with many immune and non-immune cells, including being involved in biological activities and signaling pathways, namely inflammation and thrombopoietic activities [85, 86]. Cytokines can affect endothelial cells in the CNS with various pro-coagulant effects and contribute to the development of coagulopathies and subsequent in the cause–consequence loop.
A storm orchestrated by elevated levels of pro-inflammatory cytokines causes vital organs and systems to fall down. Such is often easy to find in patients with COVID-19 pneumonia [79, 81, 87–91]. Following this storm, a systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome MODS are what we expect to see within a few days from symptom onset [89]. More than 10 cytokines contribute to COVID-19-associated cytokine storm [90], although interleukin-6 (IL-6) is the key player. Patients with severe to critical disease have significantly higher concentrations of IL-6 [92], and the use of monoclonal antibodies targeting this cytokine could provide a better survival. Such findings explain the ideas about the elaborate implementation of multiple immunomodulatory strategies, corticosteroids, immune checkpoint inhibitors (ICIs), intravenous immunoglobulin therapy, and plasmapheresis [40].
Strokes occur due to blood flow obstruction caused by thrombosis (ischemic stroke) except for about 15% of cases associated with hemorrhage (hemorrhagic stroke). Following tissue ischemia, disruption of the BBB integrity initiates infiltration of peripheral leukocytes into the brain and activation of the glial population. Also, it will stimulate the injured cells to release intracellular molecules known as damage-associated molecular patterns (DAMPs). This will cause the activation of immune cells and promote their reaction by producing inflammatory and cytotoxic factors [93]. Accordingly, post-stroke is a condition predisposed to immune dysregulation, laying down in the peri-infarct region composed of glial cells, which then critically contribute to the processes that re-establish homeostasis of the CNS. In the CNS, glial cells are of four main types: oligodendrocytes, astrocytes, ependymal cells, and microglia. Microglia cells are brain-resident macrophages that can sense pathological changes in the CNS environment, serving as the cornerstone of immunity in the CNS. Following tissue ischemia, activated microglial cells respond to ischemic stroke by an inflammatory response, which will mount injury to neuronal tissue within the first one to two hours. In contrast, when microglia cells undergo alternatively activation, an anti-inflammatory reaction occurs corresponding to neuronal injury improvement. Macrophage/microglial activation response determines the degree of damage to the brain. Similarly, astrocytes also become activated by ischemic injury and play a dual function; the release of inflammatory markers and glial scar formation on the one hand, and on the other hand, the control of glutamate excitotoxicity and conservation of function of the brain waste clearance system.
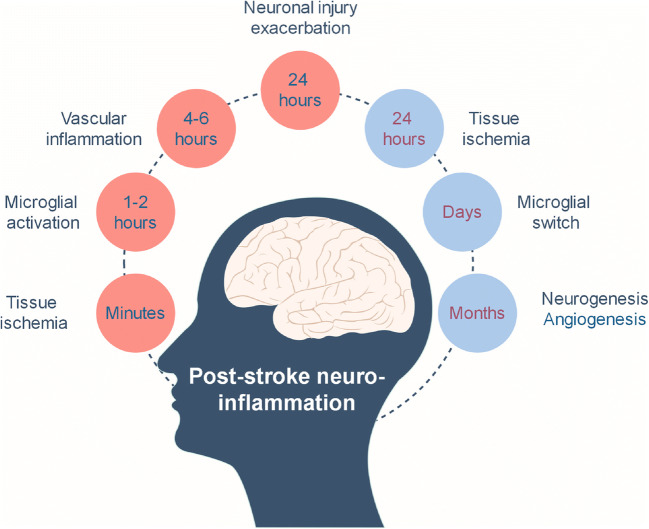
Figure 2 is a timeline indicating inflammatory mechanisms that occur early or late in patients with ischemic stroke. Six hours after the tissue ischemia, there may be several factors that can exacerbate neuronal injury avoiding the activity of recoverable cells tracking their mother, ECM, in motion. There is thus little time for us to refigure the CNS after an ischemic stroke and no time for neurons to recall their previously established functions [94]. Challenges with cell and cytokine therapies for strokes are poor viability of transplanted cells, uncontrolled cell differentiation, cell engraftment failure, and lack of controlled growth factor delivery [95]. For this, stroke remains the third leading cause of disability in adult people, while many restoration approaches have emerged [94].
Fig. 2.
Timeline of early (pink) and delayed (blue) inflammatory mechanisms in the post-stroke brain (Prepared with data from [93])
Endogenous and exogenous modulation of synaptic plasticity after stroke
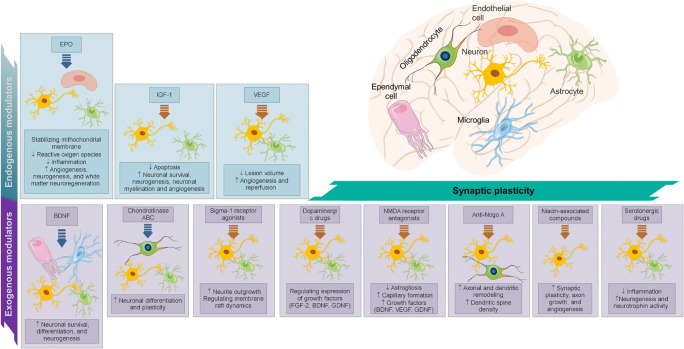
The brain inherits the habit of dynamic modeling in response to the internal and external environment, and by aggregation of this process, also known as neuroplasticity, makes changes at different levels of neural systems, neural and glial cells, and even non-cellular components, i.e., ECM, usually inhabiting a new structural and therefore functional land. The injury progression, reactive astrocytes, the glial scar, and brain-derived neurotrophic factor (BDNF) make up an endogenous response to stroke, along with other factors, mainly including EPO, VEGF, and IGF-1. Serotonergic drugs, dopaminergic drugs, NMDA receptor antagonists, Sigma-1 receptor agonist, niacin-associated compounds, chondroitinase ABC (ChABC), anti-Nogo A, and BDNF can exogenously act as plasticity-modulating biomolecules. The transplantation of cells is another method of exogenously modulating plasticity (Fig. 3).
Fig. 3.
Endogenous and exogenous modulators of synaptic plasticity after stroke (Prepared with data from [68])
Angiogenesis refers to generating new blood vessels on a pre-existing vasculature [96]. Unwanted angiogenesis is the underlying mechanism of pathological conditions, like cancer, diabetic retinopathy, and psoriasis [96], although angiogenesis critically contributes to embryonic development and the menstruation cycle in a physiologically well-regulated manner [96]. Angiogenesis results in the formation of a vascular network, providing a local environment rich in nutrients and oxygen required for wound healing and the recovery of injured tissues [96] while serving as a “supervisor” that helps neural progenitor cells navigate to their targets [97]. In this manner, the post-stroke brain under the influence of angiogenesis and its impact on the phenomena of neurogenesis as forming new neurons will achieve functional recovery in a better and more rapid way [98].
Noted that the strength of angiogenesis and its amenability to alteration in the brain is relatively low and its network’s complexity remains less understood [98], the delivery of exogenous modulators of synaptic plasticity, angiogenesis, inflammation, and neurogenesis to the brain would be the most effective of all delivery ways, and this is challenging unless biomaterials of the finest shape and highest quality bridge the distance between systemic circulation and CNS to reach the target, i.e., the site of injury. The site of the cavity of its own lacks ECM, poses a barrier for cell infiltration and tissue regeneration after stroke, and can evolve into a scar within days. However, it is highly favored for biomaterial administration because the peri-infarct area, where cavity formation occurs, supports great neurovascular plasticity, and a large volume injection can be administered.
Biomaterials: an endless source of synaptic plasticity for the post-stroke brain
Biomaterials can cross the BBB to exert synaptic plasticity-promoting effects, alone or in combination with drugs and cells [97]. The use of a biomaterial approach would allow controlling drug release in a more accurate and sustained manner, without unwanted side effects stemming from the need for higher doses, multiple injections, and invasive procedures for implant placement [97]. Beyond the BBB, it is more possible for drugs to protect neural cells, stimulate endogenous stem cells, and induce plasticity. Biomaterials for cell delivery are an efficient tool to improve cell retention, survival, differentiation, and integration. For some, biomaterials alone can support synaptic plasticity [97].
In stroke treatment, biomaterials exist, operating with both systemic and local delivery of exogenous factors and serving as scaffolding vehicles and encapsulating neural stem cells [97]. Therapeutics delivered to the brain include BDNF, CsA, EGF, VEGF, EPO, anti-Nogo-A, EGF combined with EPO, VEGF combined with Ang1 and anti-Nogo-A, BDNF combined with AMPAkine or GDNF, NT-3, and HGF or IGF1.
Systemic and local delivery of exogenous factors
NP biomaterial formulations, including liposomes and polymeric NPs, help systemic and local delivery to the CNS, especially when the integration of systems mediating receptor-mediated transcytosis (RMT) across the BBB and the methods guiding CNS molecular delivery is relevant [97, 99, 100]. The potential advantage of nanoliposomes, including dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylserine (DPPS), and ganglioside GM1, is the delivery of higher levels of citicoline to the brain so the pace and amount of functional recovery and synaptic plasticity after stroke would be higher than expected in intravenous use of citicoline. Furthermore, the formation of nano-assemblies from the lipid squalene might support the nucleoside adenosine that extends into circulation and contributes to the potential neuroprotective treatment of stroke. To achieve greater liposomal stability is the advantage offered by surface coatings available to protect liposomal bilayers from the sequestration by the reticuloendothelial system (RES). Such PEGylated liposomal formulations (PEGylated liposome 1-Palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC), didodecyl dimethyl ammonium bromide (DDAB), and polyethylene glycol) help in functional recovery after stroke by acting as carriers for plasmids related to VEGF. When conjugated with transferrin in the formulation of distearoylphosphosphatidylethanolamine (PEG2000-DSPE), maleimide-derivatized PEG2000-DSPE (Mal-PEG2000-DSP), they could be of further support by allowing the VEGF-related plasmids to be better fitted for the purpose, BBB targeting. Studies illustrate that neutral and negatively charged particles can produce a beneficial effect on such fitness with regard to simvastatin, a medication involved in lipid-lowering activities, neurogenesis, synaptic plasticity and transmission, and axon growth. Examples of these biomaterials are 1,2-didodecanoyl-sn-glycero-3-phosphocholine (DLPC), cholesteryl–polyethylene, glycol 600 sebacate (CHOL–PEG), 1,2-dioleoyl-sn-glycero-3-phosphoric acid, monosodium salt (DOPA−), cholesteryl 3β-N-(dimethylaminoethyl) carbamate, hydrochloride (CHOL+), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE). Also, polymeric NPs used for the systemic and local delivery of therapeutics to the CNS include chitosan (bFGF, z-DEVD-FMK), PLGA and PLA (camptothecin), polyethylene glycol–poly(l-glutamate) diblock copolymer (BDNF), and gelatin (osteopontin, IGF, HGF). Chitosan is a kind of semisynthetic, mucoadhesive material that can penetrate the BBB and form hydrogels [101]. Chitosan-based biomaterials serve to function as carriers for delivering drugs, cells, and genes into the CNS and provide porous scaffolds paving the way for neuroregeneration [101]. Chitosan leads to an increase in the stability of bFGF, as for camptothecin with PLGA and PLA. Both polyethylene glycol–poly(l-glutamate) diblock copolymer and gelatin improve the bioavailability of therapeutics delivered, e.g., BDNF, osteopontin, IGF, and HGF. Antibodies to transferrin and custom peptides promise to develop functionalized polymers chitosan, PLGA, and PLA, providing them with increased fitness for BBB targeting.
Scaffolding biomaterials
To provide an infarcted brain with both structural and functional support, the biomaterial approach made scaffolds baked especially for this double purpose when acting as carriers for the cells themselves and for the exogenous factors that can underlie increased cell survival. Scaffolds are 3D structures designed to develop a microenvironment favorable for endogenous neural stem cell stimulation (neurogenesis) and exogenous stem cell transplantation. An effective scaffold needs to be non-toxic, injectable, biocompatible with transplanted cells and tissues, biodegradable, biomechanical competent, work in a particular place, and maintain stemness [102]. Biomaterial scaffold structures include hydrogels, microspheres and microparticles, and polymers (natural and synthetic) and can develop with the incorporation of growth factors, namely, VEGF and FGF-2.
The ECM itself is not a cellular compartment, but the point is its relations to cell and tissue biology, by the effects like physical scaffolding to direct cellular systems and biochemical and biomechanical cues for the control of tissue morphogenesis [103]. The composition of the ECM varies across tissues and organs. For example, the brain ECM may consist of laminin, fibronectin, type IV collagen, hyaluronan, heparan sulfate proteoglycan, chondroitin sulfate proteoglycan, and tenascin R that occur in the three major sites of the basement membrane, perineuronal nets, and neural interstitial matrix [104]. Changes in the composition of the brain ECM have an important role in neuropathologies [104]. Tissue engineering and biomaterials attempt for the ECM to be in equilibrium [105].
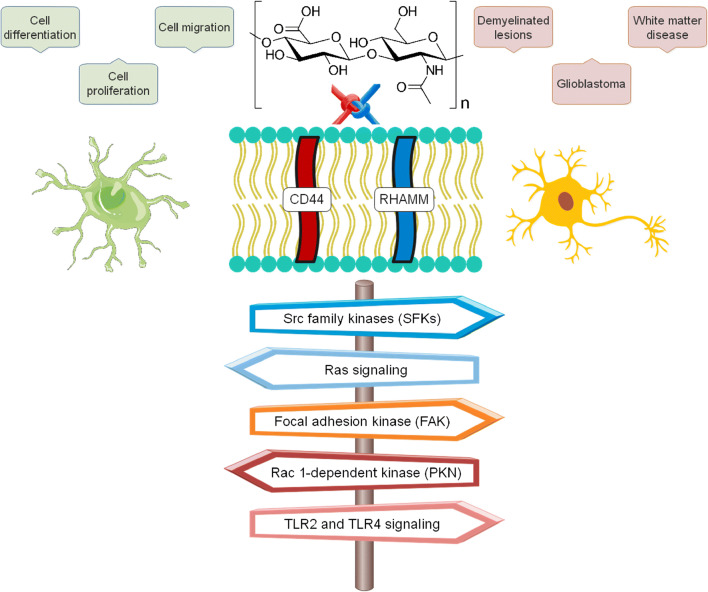
HA is a polysaccharide component of the ECM produced by hyaluronan synthases. In the CNS, while structurally localized to myelinated fibers, HA secreted from neurons and astrocytes affects tissue homeostasis by taking part in the formation of perineuronal nets, functionally controlling neuroplasticity and brain development and maturation. A biocompatible, linear structure enables HA to bind to many cell surface receptors, bringing cells into response [106]. HA mostly engage the CD44 receptor, a glycoprotein on the surface of glial and neuronal cells. This receptor then triggers Rac 1-dependent PKN (protein kinase N-) pathway, allowing astrocytes to undergo cytoskeletal activation. HA can also bind at the RHAMM (Receptor for Hyaluronan Mediated Motility) expressed on the cell surface and also floated in the cytoplasm and nucleus of cells. In this way, Src family kinases (SFKs), focal adhesion kinase (FAK), and Ras signaling come to cast cell-specific behaviors, e.g., cell migration, proliferation, and differentiation. HA regulates molecular mechanisms that contribute to cell responses in a molecular weight (Mw)–dependent manner. For instance, low Mw HA promotes the release of inflammatory mediators. In contrast, high Mw HA does seem to prevent this. It is not surprising then that the accumulation of HA and CD44 positive astrocytes is a neuropathologic hallmark of demyelinated lesions, white matter disease, and glioblastoma. They upregulate the expression of toll-like receptors (TLRs), importantly TLR2 and TLR4. Activation of these TLRs and their downstream signaling cascade would promote intracerebral injuries (Fig. 4) [107].
Fig. 4.
Hyaluronic acid and its receptors and signaling pathways in neural and glial cells. Physiologically, they contribute to cell-specific behaviors, although by upregulation of TLR2 and TL4 signaling pathways they may play role in neuropathologies (Prepared with data from [106])
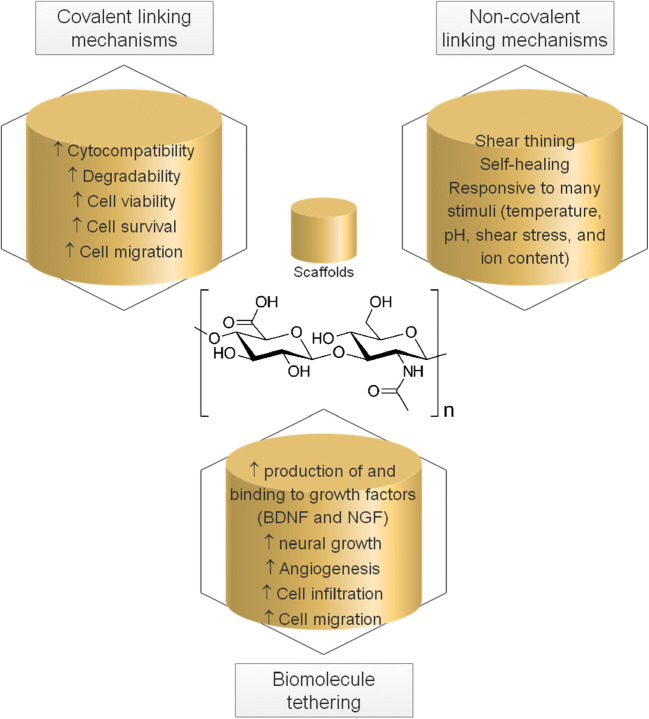
Using cross-linking mechanisms, including covalent and non-covalent mechanisms, HA-based biomaterials achieve a high degree of stability and a better biomechanical structure suited to different kinds of applications in the CNS for regeneration and repair (Fig. 5) [106]. HA-based biomaterials include hydrogels, granular hydrogels and microgels, and composite material systems [106].
Fig. 5.
The potentials of hyaluronic acid (HA) scaffolds prepared by chemical modifications (Prepared with data from [106])
When HA undergoes modifications using covalent cross-linking mechanisms such as the addition of a thiol functional group, adipic acid dihydrazide, and a protease-degradable peptide (notably matrix metalloproteinases, MMP2 and MMP9, which function in processes that contribute to the modulation of neuroinflammation [108]), the resultant biomaterials, i.e., thiol-modified HA, dihydrazide-modified HA, and 3D HA scaffolds can exhibit cytocompatibility and degradability and improve cell viability, survival, and migration even more than non-chemically modified HA. Using guest–host chemistry, thermally induced gelation, ionic and hydrophobic bonds, and interactions between proteins and their ligands, non-covalent cross-linking mechanisms, one can produce cross-links between HA polymer chains that are reversible and dynamic (unlike permanently cross-linked HA provided by covalent mechanisms) in response to many stimuli, including temperature, pH, shear stress, and ion concentration. These could be key features of an HA-based material designed to provide shear-thinning and rapidly self-healing, biochemical criteria that any material for regenerative application to the CNS must address. Cross-linking mechanisms may introduce a problem: decreased affinity binding of HA. Biomolecule tethering may help resolve this concern by simply incorporating biomolecules into the HA-based scaffolds. Proteins and peptides capable of surface bonding (adhesion), e.g., laminin and peptides derived from laminin (IKVAV and LRE) and fibronectin (RGD) and binding to growth factors, e.g., heparin, are available for this purpose. HA-based scaffolds lead to enhanced cell migration and infiltration, angiogenesis, and neurite outgrowth and promote the production of growth factors, mainly nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), by the administration of laminin and laminin-derived peptides. HA-based scaffolds incorporating fibronectin-derived peptide are more able to support viable neural stem/progenitor cells. Heparin has its advantage for binding to growth factors, a clear sign of a reduction in degradation of these proteins that are necessary for neural growth.
HAMC is an injectable hydrogel containing over 90% water and offers an effective way for local delivery of EPO to the brain [95]. EPO critically contributes to neurogenesis and post-stroke recovery [109]. In a rat model for an ischemic stroke model induced by middle cerebral artery occlusion (MCAO), alginate hydrogel combined with VEGF could confer protection in terms of the reduced ischemic lesion and functional recovery [95]. Alginate is a natural polysaccharide of high chemical stability and biocompatibility.
Hybrid biomaterials, e.g., gelatin and 3-(glycidoxypropyl) trimethoxysilane (GPSM) and polydimethysiloxane and tetraethoxysilane (PDMS-TEOS), become more purposeful. The ability to make a surface with a higher degree of hydrophilicity, which will be needed in cell adhesion and growth, favors PDMS-TEOS over GPSM. VEGF appears to increase neural regeneration, and so is a potential candidate for combination with PDMS-TEOS [110]. Synthetically derived VEGFs (low, medium, and high) bound to nH promoted endothelial cell differentiation.
Biomaterials for cell delivery
Natural biomaterials apply to the delivery of cells, including iPSC-derived neural progenitor cells (hyaluronan/methylcellulose (HAMC), HA, fibrin, and self-assembling peptides), ESC-derived progenitor cells (Matrigel and collagen), primary rodent NPCs (HA and self-assembling peptides), and adult neural stem/progenitor cells (HAMC), to the brain.
Heparin nanoparticles (nH) combined with HA hydrogel led to a reduction in macrophage/microglial activation and brain concentrations of the pro-inflammatory cytokine, TNF-α, along with increased vascularization occurring at the site of the stroke cavity in the distal middle cerebral artery occlusion (MCAo) [98]. Also, nH diminished over the region of reactive astrocytes; without any significant effect on lesion size.
When a Matrigel matrix used as scaffolding biomaterial for human ESC-derived NPCs was implanted into the infarct cavity, adult rats revealed improvements in terms of cavity size reduction, increased survival and differentiation of transplanted cells, electrophysiological properties presented by some of these transplanted cells, and better behavioral outcomes. The effect on cell survival when NPCs were used alone was not as good as that for cells cultured on Matrigel [111]. Moreover, no effect on cavity size occurred when each of Matrigel and NPCs used alone. Aged rats developed the infarcts of larger sizes, but they could reduce infarct size comparable to that for young adult rats, even when delivery of NPCs cultured on Matrigel was delayed for 3 weeks. This observation seems to be due to immunosenescence, corresponding to a decreased risk of acute rejection in aged rats [111]. Such would be a clear advantage to the current condition of COVID-19, in which older people are the most vulnerable population [80].
In a mouse model of photochemically induced cerebral ischemia, a hyaluronan–heparin–collagen hydrogel seeded with stem cells could diminish macrophage/microglia infiltration and increase survival of NPCs. Collagen is present in the ECM appreciated for the role in cell adhesion and migration. Using collagen type I combined with NSCs, the survival and differentiation of NPCs were improved along with synaptogenesis and functional recovery in a rat model of cerebral ischemic injury induced by MCAO.
Biomaterials for modulation of inflammation in the post-stroke brain
Pre-clinical studies have found that injection of PEG-MeNPs, PEG-HCCs, MAP hydrogels, HA–PLGA that incorporates VEGF and Ang1, HA gel plus heparin nanoparticles (nH) bound to VEGF, and amine-modified single-walled carbon nanotubes (a-SWNTs) and transplantation of HP carbon nanotubes impregnated with subventricular zone neural progenitor cells (SVZ NPCs) yielded a satisfactory reduction in post-stroke brain inflammation induced by MCAO in animals (rats and mice). They could accomplish such a task by inhibiting the expression of pro-inflammatory markers and the activation of microglia and astrocytes. Also, the safety and efficacy of perflutren lipid microspheres are subjected to study for use as an ultrasound enhancer for acute ischemic stroke. However, it could get approval from the U.S. Food and Drug Association (FDA) to be used as an ultrasound contrast agent. These NPs and hydrogels are a few of the strategies regenerative medicine uses to minimize neuroinflammation in the post-stroke brain. Regenerative medicine has uncovered a range of therapeutics suing cell therapy and gene therapy with anti-inflammatory effects for ischemic strokes [93] and COVID-19 [27].
Biomaterials, olfactory dysfunction, and COVID-19
Olfactory dysfunction in COVID-19
People with COVID-19 frequently report smell loss. Occurrences range from 5% to 85% [112]. Interestingly, having anosmia would predict a milder course of the disease. It can be attributed to earlier immune responses induced by olfactory epithelia.
Olfactory sensory neurons: an immune-sensory interface between the SARS-CoV-2 and host
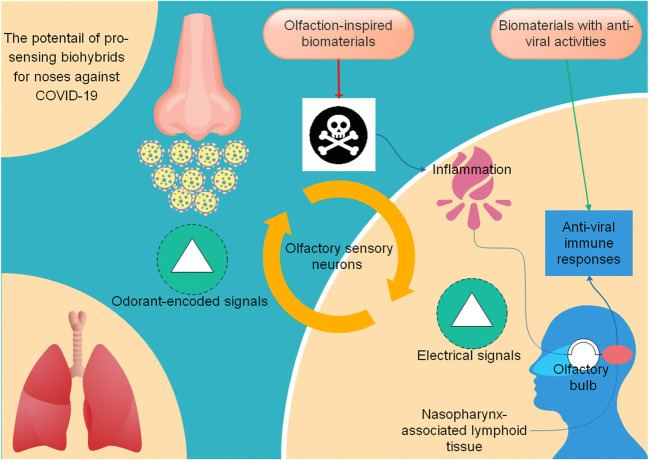
Olfactory sensory neurons (OSNs) constitute a tiny population of neurons in the nasal cavity that work on the inside and outside of the body simultaneously. In humans, air enters the respiratory system via the nose, travels through the pharynx, larynx, and trachea, and eventually terminates into bronchi and air sacs, the so-called alveoli. OSNs are in close contact with the external environment and therefore can provide detection of environmental pathogens. These neurons can convert odorant-encoded signals into electrical ones. The olfactory nerve carries electrical signals and conducts them to the olfactory bulb (OB), from where they can hit the central nervous system (CNS) and other nerves in the body. Moreover, OSNs stand nearly enough to enable the nasopharynx-associated lymphoid tissue (NALT) to bring about anti-viral immune responses upon stimulation. In the study of crypt OSNs in rainbow trout, neuronal apoptosis occurred following intranasal administration of rhabdoviruses. OSN apoptosis, in turn, gives rise to inflammatory responses while paving the way for infiltration of CD8+ T cells, which vitally play a role in anti-viral immunity, in the olfactory organ (OO). It results from the viral glycoprotein interacting with the host OSN tropomyosin-related kinase A receptor (TrkA), as reflected in the loss of TrkA reactivity (and therefore, its internalization increases). In contrast, olfactory bulb neurons (OBN) displayed decreased inflammatory responses. NALT-mediated immunity would be ultra-rapid in a TrkA-dependent manner and possible to avoid subsequent infection stages [113]. TrkA conservation with high values suggests anti-viral immunity in OO by a similar mechanism in mice, humans, and rainbow trout.
Biosensing surfaces for the generation of accelerated anti-viral immunity
With the recent emergence of olfaction-inspired biomaterials, each bioelectronic nose can detect an odor in the environment wherein odorous substances exist at the nanomole or lower levels. Biological elements used in chemical sensors to create biosensors include whole animals, insect antennae, olfactory-related proteins, olfactory tissues, olfactory cells, odorant-binding proteins, synthetic polypeptides, and olfactory sensory neurons, each with advantages and disadvantages described in [114]. In addition to these elements that can stimulate the biological olfactory system and perceive a wide range of odors, some biomaterials exhibit cell–cell interactions directing immune responses’ activation. Elastomeric surfaces are solid materials utilized in microstructured and nanostructured forms with reversible cell adhesion [115]. With the use of antibody-coated arrays of microscale elastomer pillars, mouse CD4+ T cells respond by infiltration into the pillars and interaction with antigen-presenting cells (APCs) [116].
Moreover, poly (ethylene-co-vinyl alcohol) (EVAL) is a biocompatible polymer that, when added to human olfactory neuroepithelial cells (HONCs), mature olfactory sensory neurons (OSNs) were able to grow further, possibly via neuropeptide Y (NPY)-mediated signaling upregulation [117]. CD4+ T cells are a director of anti-viral immunity [118], while NPY and receptors help modulate inflammation and autoimmunity [119]. Designing biohybrids that enhance anti-viral immunity and reduce inflammation and are applicable at the first sensory interface between the pathogen and host may be useful in combating COVID-19 (Fig. 6).
Fig. 6.
Pro-sensing biohybrids for the generation of accelerated anti-viral immunity applicable to the first sensory interface between the host and SARS-CoV-2
Biomaterials, neural degeneration, and COVID-19
Neurodegeneration in COVID-19
The relation of COVID-19 to neurodegeneration is an important relation. Despite heterogeneous data avoiding us to arrive at a definite conclusion [120], there is evidence of worsening the severity of neurological diseases such as multiple sclerosis and myopathies with COVID-19. Moreover, COVID-19 has been shown to cause symptoms mainly comprising cognitive, motor, and non-motor symptoms and conditions, including ischemic stroke, Guillain-Barré syndrome, polyneuropathy, encephalitis, meningitis, and parkinsonism [121], suggestive of inflammatory, degenerative, and demyelinating processes taking place during the disease in the CNS, along with systemic inflammation and ischemia. Accumulation of higher concentrations of ferritin frequently occurs in COVID-19. Therefore, ferrosenescence, which refers to the more replication of the virus in cells containing a high amount of iron, is a mechanism proposed to underlie dysregulation of immunity and subsequent neurodegeneration in COVID-19 [122]. Also, neurotropism is a direct path for the virus to cause neurodegeneration [123]. Neurons and glial cells express ACE2 [124], known to mediate virus entry into the cells [38, 125–128]. Activation of ACE2 by the virus leads to inflammation in the CNS. Aberrant protein folding is another mechanism proposed for neuroinflammation in COVID-19 [129]. Potential therapies include immunotherapy with natural killer cells, targeting angiotensin II receptor (sartans), iron chelators, and dipeptidyl peptidase 4 inhibitors (gliptins) [122].
Extracellular vesicles-based biomaterials for neural regeneration
Biomaterials commonly used in neurodegenerative diseases mainly comprise metal elemental (gold nanoparticles, silver nanoparticles), metal oxide (iron oxide nanoparticles, cerium oxide nanoparticles, zinc oxide nanoparticles), inorganic compound (quantum dots), metalloid elemental (silica nanoparticles), lipids (liposomes, micelles, solid lipid nanoparticles, extracellular vesicles (EVs)), and polymers (polylactic acid, PLGA, PEG, hydrogel). Especially, biomaterials based on EVs could assist drug loading with several promising characteristics, e.g., BBB penetration, biocompatibility, stability, low toxicity, low immunogenicity, and a non-invasive procedure of administration [130]. Moreover, EVs on their own hold biological activities, notably immunoregulatory and pathogen suppressive activities and regenerative capacity. For this, the use of EV-based drug delivery seems very interesting to Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and traumatic brain injury. In the case of COVID-19-associated brain damage, it is of particular importance for anti-inflammatory agents and small RNA to deliver their therapeutic effects. Also, there is pre-clinical and experimental evidence for the effectiveness of mesenchymal stem cells (MSC)-EVs in stimulating endogenous neural stem cells, neurite remodeling, and synaptic plasticity following ischemia and hypoxia. Clinical studies provide evidence for the role of MSC-EVs in attenuating autoimmune features in patients with GvHD, a condition to which COVID-19 resembles analogically. EL-4-derived exosomes allow curcumin, a natural polyphenol, to have a higher degree of solubility and exert protection against tumorigenesis, inflammation, oxidative stress, and more interestingly, against neurodegeneration and motor problems as demonstrated in models of PD. Exosomes can also effectively contribute as carriers of microRNAs (miRNAs), small non-coding RNAs helping with the post-transcriptional gene regulation [131]. The computational approach has depicted several pathways that serve as targets for miRNAs in COVID-19 [132]. Exosomes carrying relevant miRNAs are a promising therapeutic for damage associated with COVID-19.
Conductive biomaterials for neural regeneration
The electrical conductivity is the force allowing ion channels at the surface of neurons to engage an array of signaling pathways (ERK-dependent pathways, cAMP pathway, PTEN pathway, MAPK pathway, STAT3 pathway) and growth factors (NGF, BDNF) in the neuronal growth, axonal elongation, and axonal regeneration in the mammalian brain.
The electrical conductivity of biomaterials, such as polypyrrole, polyaniline, poly(3,4-ethylene dioxythiophene), multi-walled carbon nanotubes, single-wall carbon nanotubes, graphene, and graphite oxide, reflects the intrinsic surface properties [133] stepping over obstacles in the path of tissue regeneration in the CNS, which mainly consist of myelin-associated proteins, myelin-associated glycoprotein, neurite outgrowth inhibitor (Nogo-A), oligodendrocyte/myelin glycoprotein, ephrins, chondroitin sulfate proteoglycans, and semaphorin 3A (Sema3A) along with activated microglia and macrophages and glial scar, and providing guidance cues to neural stem cells (NSC) by different mechanisms that contribute to the regulation of NSC differentiation [133].
Biomaterials, neuropathies, and COVID-19
Neuropathies in COVID-19
COVID-19-associated nerve injuries mostly rooted in the ulnar nerve and the cords of the brachial plexus are presented by neuropathic pain and muscle wasting and affect the upper limb [134]. In addition to the effect of prolonged prone positioning [135] and related nerve compression injuries and vascular suppression and hematoma and related entrapment of the nerve(s) [136], neuroinflammation plays a role in nerve damages due to COVID-19. Using autografts and allografts deals with several challenges due to lack of appropriate efficiency, cost-effectiveness, and the need for a second operation and related increases in death.
Biomaterials for neuropathies
Engineering artificial nerve conduits permit the improvement of either motor or sensory (or both) function. However, tuning the conduit microenvironment is of primary significance achievable by optimizing cues and vehicles, e.g., channels, cells, drugs, fibers, growth factors, and hydrogels, applied intraluminally [137].
Biomaterials, intracerebral hemorrhage, and COVID-19
Intracerebral hemorrhage in COVID-19
Studies report a broad range of incidence for ICH in patients with COVID-19 from less than 1% to about 8% [138], with being more common in older and non-Caucasian people and in those with respiratory failure who need mechanical ventilation and anticoagulation therapy [139].
Biomaterials for intracerebral hemorrhage
Extensive cell death in the hematoma area is the main characteristic of a hemorrhagic stroke. When applied intranasally, RADA16-I self-assembling peptide (SAP) could reduce brain edema, necrotic cell death within the hematoma, and the perihematomal area, and cavity volume along with functional recovery improvement in a rat model of ICH induced by intrastriatal collagenase injection [140].
Biomaterials, spinal cord injury, and COVID-19
Spinal cord injury in COVID-19
It does occur very rarely, but recent reports of neuromyelitis optica, in which the immune system attacks the spinal cord and optic nerves, paraplegia due to an epidural abscess, and cervicothoracic myelopathy, in patients with COVID-19 from that we can confirm that COVID-19 affects the spinal cord are available in the literature [141–144].
Biomaterials for spinal cord injury
There is a sequence of events following spinal cord injury similar to that in stroke, i.e., neuroinflammation, damage to and death of different kinds of glial cells, including neurons, microglia, astrocytes, and oligodendrocytes, cavity formation, disruption of spinal cord circuitry, and neuronal activity. The biomaterials approach can act within the previously discussed context of delivering drugs to the CNS that serve a protective function [145]. Also, the ECM-like scaffolding biomaterials, such as hydrogel-based scaffolds, can support stem cell transplants within the context of a planned neural regeneration in the spinal cord, thanks to which they contain immunomodulatory and regenerative factors allowing the cells within the spinal cord to communicate with each other successfully [146, 147]. In addition to biomaterials, stem cells, growth factors, drugs, and exosomes can regenerate the injured spine [148]. However, neurorehabilitation through exercise training, electrical stimulation (ES), and brain–computer interfaces (BCIs) are necessary to re-establish functionally appropriate neural connections [149].
Biomaterials for modeling COVID-19-associated brain damage
Since February 2020, a surge of animal models for COVID-19 has spread internationally. As reviewed in [150], these models fall into six main aspects of investigating virus replication in upper and lower respiratory tracts and other organs, clinical signs (fever, nasal discharge, and difficult breathing), pneumonia involving both lungs, and leading to ground-glass opacities, focal edema and inflammation, and ARDS, transmission, host responses (T-cell immunity, seroconversion, neutralizing antibody titers, and pro-inflammatory cytokines), and demographics of SARS-CoV-2 infection. Mice, hamsters, ferrets, non-human primates, mink, cats, dogs, pigs, chickens and ducks, and fruit bats are the animals used for modeling COVID-19. However, the need for modeling the immunopathogenic mechanisms of COVID-19-associated local CNS disease as well as COVID-19-associated systemic disease is still great [151, 152]. Decellularized tissues, used for modeling diseases of the brain, heart, kidney, large intestine, liver, lung, skeletal muscle, skin, and tongue [153], can help us as biomaterial platforms to meet this need. Engineering in vitro tissue models, for example, using hPSC-derived neural organoids (hNOs), is necessary to realize the effect of small molecules, drugs, and vaccines on the neuropathogenesis of COVID-19 [154]. Also, glial interfaces might enlighten us on precisely how COVID-19 behaves in the brain for the purpose of predicting its behavior in reality [155, 156].
Biomaterials to surpass COVID-19-related immunological disorders at all games
The non-linear behavior of the immune system calls for the solution of non-linear materials. On the one side, infectious diseases and cancer rise due to immune responses of not adequate quality and quantity, while autoimmune diseases rooted in the heightened immune system are on the other side. Biomaterials science can approach poor immunity by immunogenic or antigen-loaded or coated microneedles and bring us better immune responses, as by tolerogenic or self-antigen-loaded nanoparticles to modulate inflammatory responses in the case of autoimmunity [157].
The COVID-19’s face is strange; on the one hand, it manifests itself as a multi-system disease characterized by auto-inflammatory and autoimmune features [88, 91, 158–162], and on the other hand, are people with immunodeficiency who also appear susceptible to infection and lethal complications [163–166]. Therefore, engineering biomaterials to selectively target the immune system components has the potential of inducing tolerance; for instance, toll-like receptors (TLRs) and APCs, which respectively mediate innate immune responses [167] and antigen-specific adaptive immune responses [166, 168–171]. Also, biomaterials offer tools to help cell-mediated immune responses against viral vaccines and gene delivery for vaccines against viral infections, such as HIV.
Biomaterials for modulation of inflammation
Biomaterials with intrinsically anti-inflammatory effects mainly include polysaccharides naturally derived from shells of crustaceans (chitosan) and plants (such as mushrooms and seaweed) and synthetic polymers (PEG) [172]. When studying chitosan through the chitosan–gelatin hybrid materials and chitosan-based materials, there could be an inhibitory effect on the NO/iNOS pathway and mRNA expression of COX-2 and IL-6 along with reactive oxygen species (ROS) scavenging properties responsible for such anti-inflammatory effects [172]. Moreover, it was possible to achieve the needed anti-inflammatory properties by hybridizing PEG and peptides, such as GRGDSPG and the interleukin-1 receptor inhibitory peptide (IL-1RIP) FEWTPGWYQPY-NH2, in the mouse islet cells [172]. Besides, some biomaterials simply integrate anti-inflammatory properties over their characteristic function. For our example are 2-methacryloyloxyethyl phosphorylcholine (MPC) and HA hydrogels that tend to reduce inflammation in the gingival epithelium and myocardial tissue, respectively.
Biomaterial developments resting upon the idea of controlled inflammation include agents and reservoirs for delivering anti-inflammatory therapeutics, such as the chemokine stromal-cell-derived factor-1 (SDF-1 aka CXCL12), ibuprofen, dexamethasone, and triptolide-micelles and bone morphogenic protein-2 (BMP-2). At this level of research, the emphasis is on the interaction of anti-inflammatory agents and incorporating materials, leaving these materials as the one ideal for protection against inflammation while serving to promote repair and regeneration mechanisms [172].
Biomaterials for potential anti-viral activity
Some nanomaterials exhibit antimicrobial and anti-viral activities. Of particular interest are silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) for their activities against viral infections, including HIV (AgNPs, glucose-coated AuNPs, AuNPs conjugated with peptide triazoles, sulfated ligands-coated AuNPs, fluorescein-labeled oligomannoside AuNPs, mercaptobenzoic acid-coated AuNPs, and quantum rods), HBV (solid lipid nanoparticles), HSV-1 (PVP-stabilized AgNPs, mercaptoethanesulfonate-capped AgNPs, and mercaptoethane sulfonate-capped AuNPs), HPV (PVP-stabilized AgNPs), H1N1 influenza (AgNPs/chitosan composites), and Tacaribe virus (polysaccharide-coated AgNPs). In addition to these nanomaterials, tannic acid is a naturally driven phenolic acid that provides defense against influenza A virus, papillomaviruses, noroviruses, HSV, and HIV [173].
Biomaterials for delivery of anti-viral agents to the brain
Polymeric NPs possess a tremendous brain-targeting ability because of retention in brain capillaries [174]. For this, some anti-viral agents are available in the formulas of biomaterials. An example is acyclovir-coated solid lipid nanoparticles (ACV-SLNs) used for the brain in rabbits with herpes encephalitis. With the application of chitosan, particle changes in terms of size (increased) and charge (positive), residence time (increased), sustained-release, and stability and adhesive properties (better) provided ACV to have the higher ability to target the brain cells [175]. Also, poly l-lysine is a naturally derived biomaterial used for scaffolding in tissue engineering. The antimicrobial effects of poly l-lysine enable it to provide hydrogels for the delivery of drugs. Poly-l-lysine-coated nanoparticles help improve the efficacy of DNA vaccines against viral infections [176]. Some biomaterials, in particular, supramolecular self-assembled peptides, can be a part of vaccine development, and biomolecular engineering, microfabrication techniques, and nanotechnology support this idea [177, 178].
Precautions
Non-degradable biomaterials, e.g., silicone, PVA, P(AN/VC), PSU, PES, PET, and PP, can maintain an acceptable level of stability. However, due to limited regenerative capacity, their CNS applications are less than those of degradable biomaterials, mainly including guidance channels and macroencapsulation devices. Biodegradable biomaterials used for the nervous system include PGA, PLLA, PDLLA, PLGA, and PLGA-PLLA [179]. PLGA is a degradable biomaterial with the capacity of structural support for cells. In a mouse model of ischemic stroke due to MCAO, tissue infarction and brain edema were decreased with PLGA-PEG encapsulating T3 (thyroid hormone).
Bioincompatability reactions are the main problem with biomaterials, mainly including inflammation, infections, and downregulation or loss of biomaterials’ function [180]. For modulation of inflammation, anti-inflammatory drugs can be loaded, biomaterials can be coated with biocompatible surfaces, and designs need to adopt finer and more flexible shapes. Dexamethasone retrodialysis and systemic minocycline could reduce neuroinflammation. However, caution should be paid in the administration of resveratrol, an anti-oxidant drug that caused a hemorrhage in chronic use [181]. Dissociation of a chemical element from the implant is another source of concern. For example, gallium phosphide (GaP) nanowires offer implantable devices with the capability to induce the growth of neurons that express synaptophysin, a protein contributing to activity-dependent synapse development and remodeling in the CNS. At week 12, after implantation, increased levels of Ga in the brain are proof of dissociation of Ga from the implant, paving the way for implant-induced immune responses in the brain [182].
Conclusion
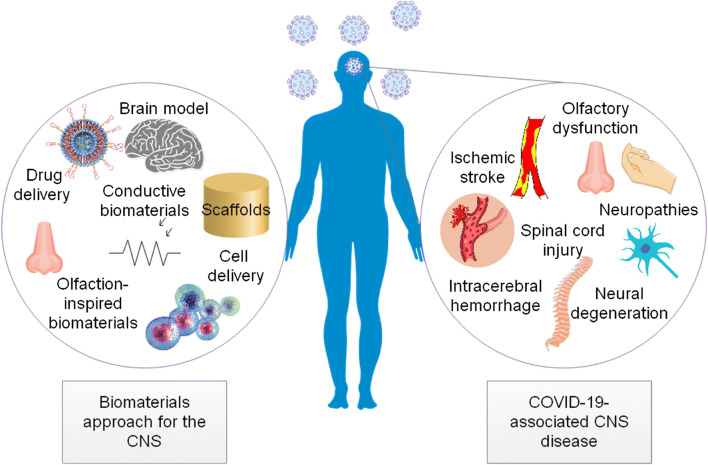
COVID-19 can involve the CNS and cause a range of neurosensory manifestations, including olfactory dysfunction, neuropathies, ischemic stroke, intracerebral hemorrhage, and spinal cord injury. Here, the potentials of the biomaterials approach for the CNS in COVID-19 were discussed. It included, but not limited to, promotion of synaptic plasticity, modulation of inflammation, and enhancement of functional recovery after stroke, biosensing surfaces for the generation of accelerated anti-viral immunity applicable to the first sensory interface between the host and the virus, biomaterials for delivery of anti-viral drugs to the brain, biomaterials for the facilitation of neural regeneration, and biomaterials for treatment of neuropathies and intracerebral hemorrhage. Biomaterials can also provide effective tools for modeling COVID-19-associated brain damage (Fig. 7).
Fig. 7.
Biomaterials: potential tools for the CNS in COVID-19
References
- 1.COVID-19 CORONAVIRUS PANDEMIC (2021) https://www.worldometers.info/coronavirus/. Accessed February 08, 2021
- 2.A. Basiri, A. Heidari, M.F. Nadi, M.T.P. Fallahy, S.S. Nezamabadi, M. Sedighi, A. Saghazadeh, N. Rezaei, Microfluidic devices for detection of RNA viruses. Reviews in medical virology:e2154. (2020). 10.1002/rmv.2154 [DOI] [PMC free article] [PubMed]
- 3.Rabiee N, Rabiee M, Bagherzadeh M, Rezaei N. COVID-19 and picotechnology: potential opportunities. Med. Hypotheses. 2020;144:109917. doi: 10.1016/j.mehy.2020.109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M.M. Tantuoyir, N. Rezaei, Serological tests for COVID-19: potential opportunities. Cell Biol. Int. (2020). 10.1002/cbin.11516
- 5.AlMaadeed MA (2020) Emergent materials and industry 4.0 contribution toward pandemic diseases such as COVID-19. Emergent Mater:1-2. 10.1007/s42247-020-00102-4 [DOI] [PMC free article] [PubMed]
- 6.Mujawar MA, Gohel H, Bhardwaj SK, Srinivasan S, Hickman N, Kaushik A. Nano-enabled biosensing systems for intelligent healthcare: towards COVID-19 management. Materials Today Chemistry. 2020;17:100306. doi: 10.1016/j.mtchem.2020.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Z, Song B, Xu L, Zhong Y, Peng F, Ji X, Zhu F, Yang C, Zhou J, Su Y. Aqueous synthesized quantum dots interfere with the NF-κB pathway and confer anti-tumor, anti-viral and anti-inflammatory effects. Biomaterials. 2016;108:187–196. doi: 10.1016/j.biomaterials.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 8.Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86(3):215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood–brain barrier. Drug Dev. Ind. Pharm. 2002;28(1):1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 10.T.B. Lopez-Mendez, E. Santos-Vizcaino, J.L. Pedraz, R.M. Hernandez, G. Orive, Cell microencapsulation technologies for sustained drug delivery: clinical trials and companies. Drug Discov. Today (2020). 10.1016/j.drudis.2020.11.019 [DOI] [PubMed]
- 11.Medhi R, Srinoi P, Ngo N, Tran H-V, Lee TR. Nanoparticle-based strategies to combat COVID-19. ACS Applied Nano Materials. 2020;3(9):8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 12.Mehta M, Prasher P, Sharma M, Shastri MD, Khurana N, Vyas M, Dureja H, Gupta G, Anand K, Satija S. Advanced drug delivery systems can assist in targeting coronavirus disease (COVID-19): a hypothesis. Med. Hypotheses. 2020;144:110254. doi: 10.1016/j.mehy.2020.110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoieb SM, El-Ghiaty MA, El-Kadi AOS (2020) Targeting arachidonic acid–related metabolites in COVID-19 patients: potential use of drug-loaded nanoparticles. Emergent materials:1-13 [DOI] [PMC free article] [PubMed]
- 14.A. Hessami, A. Shamshirian, K. Heydari, F. Pourali, R. Alizadeh-Navaei, M. Moosazadeh, S. Abrotan, L. Shojaie, S. Sedighi, D. Shamshirian, N. Rezaei, Cardiovascular diseases burden in COVID-19: systematic review and meta-analysis. Am. J. Emerg. Med. (2020). 10.1016/j.ajem.2020.10.022 [DOI] [PMC free article] [PubMed]
- 15.Nejadghaderi SA, Heidari A, Shakerian N, Saghazadeh A, Rezaei N. Cardiovascular system is at higher risk of affecting by COVID-19. Acta bio-medica. Atenei Parmensis. 2020;91(3):e2020018. doi: 10.23750/abm.v91i3.9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vakhshoori M, Heidarpour M, Shafie D, Taheri M, Rezaei N, Sarrafzadegan N. Acute cardiac injury in COVID-19: a systematic review and meta-analysis. Archives of Iranian Medicine. 2020;23(11):801–812. doi: 10.34172/aim.2020.107. [DOI] [PubMed] [Google Scholar]
- 17.Jenab Y, Rezaei N, Hedayat B, Naderian M, Shirani S, Hosseini K. Occurrence of acute coronary syndrome, pulmonary thromboembolism, and cerebrovascular event in COVID-19. Clin Case Rep. 2020;8(12):2414–2417. doi: 10.1002/ccr3.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aleebrahim-Dehkordi E, Soveyzi F, Deravi N, Rabbani Z, Saghazadeh A, Rezaei N. Human coronaviruses SARS-CoV, MERS-CoV, and SARS-CoV-2 in children. J. Pediatr. Nurs. 2020;56:70–79. doi: 10.1016/j.pedn.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotfi M, Rezaei N. SARS-CoV-2: a comprehensive review from pathogenicity of the virus to clinical consequences. J. Med. Virol. 2020;92(10):1864–1874. doi: 10.1002/jmv.26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.K.K. Sahu, A.D. Siddiqui, From hematologistʼs desk: the effect of COVID-19 on the blood system. Am. J. Hematol. (2020) [DOI] [PMC free article] [PubMed]
- 21.Ertuğlu LA, Kanbay A, Afşar B, Afşar RE, Kanbay M. COVID-19 and acute kidney injury. Tuberkuloz ve toraks. 2020;68(4):407–418. doi: 10.5578/tt.70010. [DOI] [PubMed] [Google Scholar]
- 22.Jahanshahlu L, Rezaei N. Central nervous system involvement in COVID-19. Arch. Med. Res. 2020;51(7):721–722. doi: 10.1016/j.arcmed.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleki K, Banazadeh M, Saghazadeh A, Rezaei N. The involvement of the central nervous system in patients with COVID-19. Rev. Neurosci. 2020;31(4):453–456. doi: 10.1515/revneuro-2020-0026. [DOI] [PubMed] [Google Scholar]
- 24.Yazdanpanah N, Saghazadeh A, Rezaei N. Anosmia: a missing link in the neuroimmunology of coronavirus disease 2019 (COVID-19) Rev. Neurosci. 2020;31(7):691–701. doi: 10.1515/revneuro-2020-0039. [DOI] [PubMed] [Google Scholar]
- 25.Amanat M, Rezaei N, Roozbeh M, Shojaei M, Tafakhori A, Zoghi A, Darazam IA, Salehi M, Karimialavijeh E, Lima BS (2021) Neurological manifestations as the predictors of severity and mortality in hospitalized individuals with COVID-19: a multicenter prospective clinical study. [DOI] [PMC free article] [PubMed]
- 26.A. Aydin, G. Cebi, Z.E. Demirtas, H. Erkus, A. Kucukay, M. Ok, L. Sakalli, S. Alpdagtas, O. Gunduz, C.B. Ustundag, Combating COVID-19 with tissue engineering: a review. Emergent Mater, 1–21 (2020). 10.1007/s42247-020-00138-6 [DOI] [PMC free article] [PubMed]
- 27.A. Basiri, Z. Pazhouhnia, N. Beheshtizadeh, M. Hoseinpour, A. Saghazadeh, N. Rezaei, Regenerative medicine in COVID-19 treatment: real opportunities and range of promises. Stem Cell Rev. Rep., 1–13 (2020). 10.1007/s12015-020-09994-5 [DOI] [PMC free article] [PubMed]
- 28.Sharma D, Ferguson M, Kamp TJ, Zhao F. Constructing biomimetic cardiac tissues: a review of scaffold materials for engineering cardiac patches. Emergent Mater. 2019;2:181–191. doi: 10.1007/s42247-019-00046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K. Lundstrom, M. Seyran, D. Pizzol, P. Adadi, T. Mohamed Abd El-Aziz, S.S. Hassan, A. Soares, R. Kandimalla, M.M. Tambuwala, A.A.A. Aljabali, G. Kumar Azad, P. Pal Choudhury, V.N. Uversky, S.P. Sherchan, B.D. Uhal, N. Rezaei, A.M. Brufsky, Viewpoint: origin of SARS-CoV-2. Viruses 12(11) (2020). 10.3390/v12111203 [DOI] [PMC free article] [PubMed]
- 30.M. Seyran, D. Pizzol, P. Adadi, T.M.A. El-Aziz, S.S. Hassan, A. Soares, R. Kandimalla, K. Lundstrom, M. Tambuwala, A.A.A. Aljabali, A. Lal, G.K. Azad, P.P. Choudhury, V.N. Uversky, S.P. Sherchan, B.D. Uhal, N. Rezaei, A.M. Brufsky, Questions concerning the proximal origin of SARS-CoV-2. J. Med. Virol. (2020). 10.1002/jmv.26478 [DOI] [PMC free article] [PubMed]
- 31.Sharifkashani S, Bafrani MA, Khaboushan AS, Pirzadeh M, Kheirandish A, Yavarpour Bali H, Hessami A, Saghazadeh A, Rezaei N. Angiotensin-converting enzyme 2 (ACE2) receptor and SARS-CoV-2: potential therapeutic targeting. Eur. J. Pharmacol. 2020;884:173455. doi: 10.1016/j.ejphar.2020.173455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotfi M, Hamblin MR, Rezaei N (2020) COVID-19: transmission, prevention, and potential therapeutic opportunities. Clinica chimica acta; international journal of clinical chemistry 508:254-266. 10.1016/j.cca.2020.05.044 [DOI] [PMC free article] [PubMed]
- 33.K. Mohamed, N. Yazdanpanah, A. Saghazadeh, N. Rezaei, Computational drug discovery and repurposing for the treatment of COVID-19: a systematic review. Bioorganic chemistry:104490. (2020). 10.1016/j.bioorg.2020.104490 [DOI] [PMC free article] [PubMed]
- 34.Daoud S, Alabed SJ, Dahabiyeh LA. Identification of potential COVID-19 main protease inhibitors using structure-based pharmacophore approach, molecular docking and repurposing studies. Acta Pharma. 2021;71(2):163–174. doi: 10.2478/acph-2021-0016. [DOI] [PubMed] [Google Scholar]
- 35.Zarandi PK, Zinatizadeh MR, Zinatizadeh M, Yousefi MH, Rezaei N. SARS-CoV-2: from the pathogenesis to potential anti-viral treatments. Biomed. Pharmacother. 2021;137:111352. doi: 10.1016/j.biopha.2021.111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R. Pourahmad, B. Moazzami, N. Rezaei, Efficacy of plasmapheresis and immunoglobulin replacement therapy (IVIG) on patients with COVID-19. SN Comprehensive Clinical Medicine, 1–5 (2020). 10.1007/s42399-020-00438-2 [DOI] [PMC free article] [PubMed]
- 37.Peymani P, Dehesh T, Aligolighasemabadi F, Sadeghdoust M, Kotfis K, Ahmadi M, Mehrbod P, Iranpour P, Dastghaib S, Nasimian A, Ravandi A, Kidane B, Ahmed N, Sharma P, Shojaei S, Bagheri Lankarani K, Madej A, Rezaei N, Madrakian T, Los MJ, Labouta HI, Mokarram P, Ghavami S. Statins in patients with COVID-19: a retrospective cohort study in Iranian COVID-19 patients. Translational Medicine Communications. 2021;6(1):3. doi: 10.1186/s41231-021-00082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.E. Shojaeefar, N. Malih, N. Rezaei, The possible double-edged sword effects of vitamin D on COVID-19: a hypothesis. Cell Biol. Int. (2020). 10.1002/cbin.11469 [DOI] [PubMed]
- 39.Fathi N, Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol. Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saghazadeh A, Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84:106560. doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanaei S, Rezaei N. COVID-19: developing from an outbreak to a pandemic. Arch. Med. Res. 2020;51(6):582–584. doi: 10.1016/j.arcmed.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jabbari P, Jabbari F, Ebrahimi S, Rezaei N. COVID-19: A chimera of two pandemics. Disaster medicine and public health preparedness. 2020;14(3):e38–e39. doi: 10.1017/dmp.2020.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jabbari P, Rezaei N. With risk of reinfection, is COVID-19 here to stay? Disaster Med Public Health Prep. 2020;14(4):e33. doi: 10.1017/dmp.2020.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezaei N. COVID-19 affects healthy pediatricians more than pediatric patients. Infect. Control Hosp. Epidemiol. 2020;41(9):1106–1107. doi: 10.1017/ice.2020.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salehi M, Amanat M, Mohammadi M, Salmanian M, Rezaei N, Saghazadeh A, Garakani A. The prevalence of post-traumatic stress disorder related symptoms in Coronavirus outbreaks: a systematic-review and meta-analysis. J. Affect. Disord. 2021;282:527–538. doi: 10.1016/j.jad.2020.12.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.N. Samieefar, R. Yari Boroujeni, M. Jamee, M. Lotfi, M.R. Golabchi, A. Afshar, H. Miri, M.A. Khazeei Tabari, P. Darzi, M. Abdullatif Khafaie, B. Amirheidari, A. Tamadon, N. Rambod Rad, N. Samimi, M. Farjam, F. Shiravi, N. Farshidi, M. Hedayati Ch, D. Doostkamel, R. Alikhani, M. Razmkhah, S. Abdollahifard, R. Nasiri Kalmarzi, R. Kelishadi, H. Khazaei, A. Aghamohammadi, F.S. Jafari Mousavi, M. Shamsizadeh, A. Khojasteh, N. Rezaei, Country quarantine during COVID-19: critical or not? Disaster medicine and Public Health Preparedness, 1–2 (2020). 10.1017/dmp.2020.384
- 47.S. Hanaei, A. Takian, R. Majdzadeh, C.R. Maboloc, I. Grossmann, O. Gomes, M. Milosevic, M. Gupta, A.A. Shamshirsaz, A. Harbi, A.M. Burhan, L.Q. Uddin, A. Kulasinghe, C.M. Lam, S. Ramakrishna, A. Alavi, J.L. Nouwen, T. Dorigo, M. Schreiber, A. Abraham, N. Shelkovaya, W. Krysztofiak, M. Ebrahimi Warkiani, F. Sellke, S. Ogino, F.J. Barba, S. Brand, C. Vasconcelos, D.B. Salunke, N. Rezaei, Emerging standards and the hybrid model for organizing scientific events during and after the COVID-19 pandemic. Disaster medicine and public health preparedness, 1–17 (2020). 10.1017/dmp.2020.406 [DOI] [PMC free article] [PubMed]
- 48.Mohamed K, Rezaei N, Rodríguez-Román E, Rahmani F, Zhang H, Ivanovska M, S AM. Joya M, Makuku R, Md Shahidul I, Nesrine R, Laila R, Goda R, Sunny OA, Mujtaba S, Zoghi S, Irtsyan S, Ling I, Orsolya C, Attig-Bahar F, Hazar Sayar E, Soloukey C, Giulia G. International efforts to save healthcare personnel during COVID-19. Acta bio-medica. Atenei Parmensis. 2020;91(3):e2020044. doi: 10.23750/abm.v91i3.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Momtazmanesh S, Ochs HD, Uddin LQ, Perc M, Routes JM, Vieira DN, Al-Herz W, Baris S, Prando C, Rosivall L, Abdul Latiff AH, Ulrichs T, Roudenok V, Aldave Becerra JC, Salunke DB, Goudouris E, Condino-Neto A, Stashchak A, Kryvenko O, Stashchak M, Bondarenko A, Rezaei N. All together to fight COVID-19. The American Journal of Tropical Medicine and Hygiene. 2020;102(6):1181–1183. doi: 10.4269/ajtmh.20-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moradian N, Ochs HD, Sedikies C, Hamblin MR, Camargo CA, Jr, Martinez JA, Biamonte JD, Abdollahi M, Torres PJ, Nieto JJ, Ogino S, Seymour JF, Abraham A, Cauda V, Gupta S, Ramakrishna S, Sellke FW, Sorooshian A, Wallace Hayes A, Martinez-Urbistondo M, Gupta M, Azadbakht L, Esmaillzadeh A, Kelishadi R, Esteghamati A, Emam-Djomeh Z, Majdzadeh R, Palit P, Badali H, Rao I, Saboury AA, Jagan Mohan Rao L, Ahmadieh H, Montazeri A, Fadini GP, Pauly D, Thomas S, Moosavi-Movahed AA, Aghamohammadi A, Behmanesh M, Rahimi-Movaghar V, Ghavami S, Mehran R, Uddin LQ, Von Herrath M, Mobasher B, Rezaei N. The urgent need for integrated science to fight COVID-19 pandemic and beyond. J. Transl. Med. 2020;18(1):205. doi: 10.1186/s12967-020-02364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.K. Mohamed, N. Rezaei, COVID-19 pandemic is not the time of trial and error. Am. J. Emerg. Med. (2020). 10.1016/j.ajem.2020.09.020 [DOI] [PMC free article] [PubMed]
- 52.Mohamed K, Rodríguez-Román E, Rahmani F, Zhang H, Ivanovska M, Makka SA, Joya M, Makuku R, Islam MS, Radwan N, Rahmah L, Goda R, Abarikwu SO, Shaw M, Zoghi S, Irtsyan S, Ling I, Cseprekal O, Faten AB, Hazar Sayar E, Soloukey C, Grancini G, Rezaei N. Borderless collaboration is needed for COVID-19—a disease that knows no borders. Infect. Control Hosp. Epidemiol. 2020;41(10):1245–1246. doi: 10.1017/ice.2020.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moazzami B, Razavi-Khorasani N, Dooghaie Moghadam A, Farokhi E, Rezaei N. COVID-19 and telemedicine: Immediate action required for maintaining healthcare providers well-being. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2020;126:104345. doi: 10.1016/j.jcv.2020.104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.P. Jabbari, N. Taraghikhah, F. Jabbari, S. Ebrahimi, N. Rezaei, Adherence of the general public to self-protection guidelines during the COVID-19 pandemic. Disaster medicine and public health preparedness, 1–12 (2020). 10.1017/dmp.2020.445 [DOI] [PMC free article] [PubMed]
- 55.Azadnajafabad S, Saeedi Moghaddam S, Rezaei N, Ghasemi E, Naderimagham S, Azmin M, Mohammadi E, Jamshidi K, Fattahi N, Zokaei H (2021) A report on statistics of an online self-screening platform for COVID-19 and its effectiveness in Iran. Int. J. Health Policy Manag. [DOI] [PMC free article] [PubMed]
- 56.Heidarpour M, Vakhshoori M, Abbasi S, Shafie D, Rezaei N. Adrenal insufficiency in coronavirus disease 2019: a case report. J. Med. Case Rep. 2020;14(1):134. doi: 10.1186/s13256-020-02461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.S. Goudarzi, F. Dehghani Firouzabadi, M. Dehghani Firouzabadi, N. Rezaei, Cutaneous lesions and COVID-19: cystic painful lesion in a case with positive SARS-CoV-2. Dermatologic therapy:e14266. (2020). 10.1111/dth.14266 [DOI] [PubMed]
- 58.T. Moriguchi, N. Harii, J. Goto, D. Harada, H. Sugawara, J. Takamino, M. Ueno, H. Sakata, K. Kondo, N. Myose, A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. (2020) [DOI] [PMC free article] [PubMed]
- 59.L. Zanin, G. Saraceno, P.P. Panciani, G. Renisi, L. Signorini, K. Migliorati, M.M. Fontanella, SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir., 1–4 (2020) [DOI] [PMC free article] [PubMed]
- 60.Young MJ, O'Hare M, Matiello M, Schmahmann JD. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav. Immun. 2020;89:601–603. doi: 10.1016/j.bbi.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, Al Rasheed M (2020) Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv
- 62.Zhou YL, Lu J, Cheng YB, Xin N. Nervous system complications of COVID-19 with a focus on stroke. Eur. Rev. Med. Pharmacol. Sci. 2020;24:13044–13048. doi: 10.26355/eurrev_202012_24210. [DOI] [PubMed] [Google Scholar]
- 63.S. Sadeghmousavi, N. Rezaei, COVID-19 and multiple sclerosis: predisposition and precautions in treatment. SN Comprehensive Clinical Medicine, 1–6 (2020). 10.1007/s42399-020-00504-9 [DOI] [PMC free article] [PubMed]
- 64.Kremer S, Lersy F, de Sèze J, Ferré J-C, Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G, Martin-Blondel G. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.X. Chen, S. Laurent, O.A. Onur, N.N. Kleineberg, G.R. Fink, F. Schweitzer, C. Warnke, A systematic review of neurological symptoms and complications of COVID-19. J. Neurol., 1–11 (2020) [DOI] [PMC free article] [PubMed]
- 66.A. Akhtar, A. Andleeb, T.S. Waris, M. Bazzar, A.R. Moradi, N.R. Awan, M. Yar, Neurodegenerative diseases and effective drug delivery: a review of challenges and novel therapeutics. J. Control. Release (2020). 10.1016/j.jconrel.2020.11.021 [DOI] [PubMed]
- 67.Zhong Y, Bellamkonda RV. Biomaterials for the central nervous system. J. R. Soc. Interface. 2008;5(26):957–975. doi: 10.1098/rsif.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan Y-K, Goh C, Leow AST, Tambyah PA, Ang A, Yap E-S, Tu T-M, Sharma VK, Yeo LLL, Chan BPL, Tan BYQ. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J. Thromb. Thrombolysis. 2020;50(3):587–595. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Immovilli P, Terracciano C, Zaino D, Marchesi E, Morelli N, Terlizzi E, De Mitri P, Vollaro S, Magnifico F, Colombi D. Stroke in COVID-19 patients—a case series from Italy. Int. J. Stroke. 2020;15(6):701–702. doi: 10.1177/1747493020938294. [DOI] [PubMed] [Google Scholar]
- 71.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D (2020) COVID-19 presenting as stroke. Brain, behavior, and immunity [DOI] [PMC free article] [PubMed]
- 72.Saghazadeh A, Hafizi S, Rezaei N. Inflammation in venous thromboembolism: cause or consequence? Int. Immunopharmacol. 2015;28(1):655–665. doi: 10.1016/j.intimp.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 73.Saghazadeh A, Rezaei N. Inflammation as a cause of venous thromboembolism. Crit. Rev. Oncol. Hematol. 2016;99:272–285. doi: 10.1016/j.critrevonc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 74.J. Goswami, T.A. MacArthur, M. Sridharan, R.K. Pruthi, R.D. McBane 2nd, T.E. Witzig, M.S. Park, A review of pathophysiology, clinical features, and management options of COVID-19 associated coagulopathy. Shock Publish Ahead of Print. (2020). 10.1097/shk.0000000000001680 [DOI] [PMC free article] [PubMed]
- 75.Darbeheshti F, Rezaei N. Genetic predisposition models to COVID-19 infection. Med. Hypotheses. 2020;142:109818. doi: 10.1016/j.mehy.2020.109818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yousefzadegan S, Rezaei N. Case report: death due to COVID-19 in three brothers. The American journal of tropical medicine and hygiene. 2020;102(6):1203–1204. doi: 10.4269/ajtmh.20-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.N. Jabalameli, F. Rajabi, A. Firooz, N. Rezaei, The overlap between genetic susceptibility to COVID-19 and skin diseases. Immunol. Investig., 1–8 (2021) [DOI] [PubMed]
- 78.A. Razavi, M.R. Hamblin, N. Rezaei, COVID-19 in patients with cancer: risks and precautions. Am. J. Emerg. Med. (2021). 10.1016/j.ajem.2021.01.067 [DOI] [PMC free article] [PubMed]
- 79.L.M. Khosroshahi, N. Rezaei, Dysregulation of the immune response in COVID-19. Cell Biol. Int. (2020). 10.1002/cbin.11517
- 80.Saghazadeh A, Rezaei N. Immune-epidemiological parameters of the novel coronavirus – a perspective. Expert. Rev. Clin. Immunol. 2020;16(5):465–470. doi: 10.1080/1744666x.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.M. Torabi-Rahvar, N. Rezaei, Storm at the time of Corona: a glimpse at the current understanding and therapeutic opportunities of the SARS-CoV-2 cytokine storm. Curr. Pharm. Des. (2020). 10.2174/1381612826666201125102649 [DOI] [PubMed]
- 82.D. Kosanovic, A.I. Yaroshetskiy, N.A. Tsareva, Z.M. Merzhoeva, N.V. Trushenko, G.V. Nekludova, R.T. Schermuly, S.N. Avdeev, Recombinant tissue plasminogen activator treatment for COVID-19 associated ARDS and acute cor pulmonale. Int. J. Infect. Dis. (2020) [DOI] [PMC free article] [PubMed]
- 83.Striz I, Brabcova E, Kolesar L, Sekerkova A. Cytokine networking of innate immunity cells: a potential target of therapy. Clin. Sci. 2014;126(9):593–612. doi: 10.1042/CS20130497. [DOI] [PubMed] [Google Scholar]
- 84.Strieter RM, Belperio JA, Keane MP. Host innate defenses in the lung: the role of cytokines. Curr. Opin. Infect. Dis. 2003;16(3):193–198. doi: 10.1097/00001432-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Nakashima K, Taga T gp130 and the IL-6 family of cytokines: signaling mechanisms and thrombopoietic activities. In, 1998. WB Saunders Ltd, pp 210-221 [PubMed]
- 86.Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit. Care Med. 2002;30(5):S294–S301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 87.Nasab MG, Saghazadeh A, Rezaei N. SARS-CoV-2—a tough opponent for the immune system. Arch. Med. Res. 2020;51(6):589–592. doi: 10.1016/j.arcmed.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rokni M, Hamblin MR, Rezaei N. Cytokines and COVID-19: friends or foes? Human vaccines & immunotherapeutics. 2020;16(10):2363–2365. doi: 10.1080/21645515.2020.1799669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun X, Wang T, Cai D, Hu Z, Ja C, Liao H, Zhi L, Wei H, Zhang Z, Qiu Y, Wang J, Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaninov N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020;20(5):277–277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256:117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur. Cytokine Netw. 2020;31(2):44–49. doi: 10.1684/ecn.2020.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajkovic O, Potjewyd G, Pinteaux E. Regenerative medicine therapies for targeting neuroinflammation after stroke. Front. Neurol. 2018;9:734. doi: 10.3389/fneur.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esteban-Garcia N, Nombela C, Garrosa J, Rascón-Ramirez FJ, Barcia JA, Sánchez-Sánchez-Rojas L. Neurorestoration approach by biomaterials in ischemic stroke. Front. Neurosci. 2020;14:431. doi: 10.3389/fnins.2020.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang J, Yang W, Xie H, Song Y, Li Y, Wang L. Ischemic stroke and repair: current trends in research and tissue engineering treatments. Regenerative medicine research. 2014;2(1):3. doi: 10.1186/2050-490X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22(42):6549–6556. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- 97.Obermeyer JM, Ho E, Gracias A, Shoichet MS. Influencing neuroplasticity in stroke treatment with advanced biomaterials-based approaches. Adv. Drug Deliv. Rev. 2019;148:204–218. doi: 10.1016/j.addr.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 98.Nih LR, Gojgini S, Carmichael ST, Segura T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 2018;17(7):642–651. doi: 10.1038/s41563-018-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.F. Gosselet, R.A. Loiola, A. Roig, A. Rosell, M. Culot, Central nervous system delivery of molecules across the blood–brain barrier. Neurochem Int:104952. (2021). 10.1016/j.neuint.2020.104952 [DOI] [PubMed]
- 100.Chai W-Y, Chu P-C, Tsai C-H, Lin C-Y, Yang H-W, Lai H-Y, Liu H-L. Image-guided focused-ultrasound CNS molecular delivery: an implementation via dynamic contrast-enhanced magnetic-resonance imaging. Sci. Rep. 2018;8(1):4151. doi: 10.1038/s41598-018-22571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D.D. Ojeda-Hernández, A.A. Canales-Aguirre, J. Matias-Guiu, U. Gomez-Pinedo, J.C. Mateos-Díaz, Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Frontiers in Bioengineering and Biotechnology 8 (2020) [DOI] [PMC free article] [PubMed]
- 102.Skop NB, Calderon F, Cho CH, Gandhi CD, Levison SW. Improvements in biomaterial matrices for neural precursor cell transplantation. Molecular and cellular therapies. 2014;2(1):19. doi: 10.1186/2052-8426-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J. Cell Sci. 2010;123(24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat. Rev. Neurosci. 2013;14(10):722–729. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 105.Purvis EM, O'Donnell JC, Chen HI, Cullen DK. Tissue engineering and biomaterial strategies to elicit endogenous neuronal replacement in the brain. Front. Neurol. 2020;11:344. doi: 10.3389/fneur.2020.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jensen G, Holloway JL, Stabenfeldt SE. Hyaluronic acid biomaterials for central nervous system regenerative medicine. Cells. 2020;9(9):2113. doi: 10.3390/cells9092113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Y. Wang, P. Ge, Zhu Y (2013) TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediat. Inflamm. (2013) [DOI] [PMC free article] [PubMed]
- 108.Hannocks MJ, Zhang X, Gerwien H, Chashchina A, Burmeister M, Korpos E, Song J, Sorokin L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019;75-76:102–113. doi: 10.1016/j.matbio.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J. Neurosci. 2006;26(4):1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamashita T, Deguchi K, Nagotani S, Abe K. Vascular protection and restorative therapy in ischemic stroke. Cell Transplant. 2011;20(1):95–97. doi: 10.3727/096368910X532800. [DOI] [PubMed] [Google Scholar]
- 111.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, Gorostiza O, Wang X, Greenberg DA. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J. Cereb. Blood Flow Metab. 2010;30(3):534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Izquierdo-Dominguez A, Rojas-Lechuga MJ, Mullol J, Alobid I. Olfactory dysfunction in the COVID-19 outbreak. J Investig Allergol Clin Immunol. 2020;30(5):317–326. doi: 10.18176/jiaci.0567. [DOI] [PubMed] [Google Scholar]
- 113.Sepahi A, Kraus A, Casadei E, Johnston CA, Galindo-Villegas J, Kelly C, García-Moreno D, Muñoz P, Mulero V, Huertas M. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrkA-dependent manner. Proc. Natl. Acad. Sci. 2019;116(25):12428–12436. doi: 10.1073/pnas.1900083116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wasilewski T, Gębicki J, Kamysz W. Advances in olfaction-inspired biomaterials applied to bioelectronic noses. Sensors Actuators B Chem. 2018;257:511–537. [Google Scholar]
- 115.Kim S, Wu J, Carlson A, Jin SH, Kovalsky A, Glass P, Liu Z, Ahmed N, Elgan SL, Chen W, Ferreira PM, Sitti M, Huang Y, Rogers JA. Microstructured elastomeric surfaces with reversible adhesion and examples of their use in deterministic assembly by transfer printing. Proc. Natl. Acad. Sci. 2010;107(40):17095. doi: 10.1073/pnas.1005828107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jin W, Tamzalit F, Chaudhuri PK, Black CT, Huse M, Kam LC. T cell activation and immune synapse organization respond to the microscale mechanics of structured surfaces. Proc. Natl. Acad. Sci. 2019;116(40):19835–19840. doi: 10.1073/pnas.1906986116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li S-T, Young T-H, Huang T-W. Poly (ethylene-co-vinyl alcohol) is a suitable substrate for human olfactory neuroepithelial cell differentiation in vitro through a defined regulatory pathway. Acta Biomater. 2018;68:204–213. doi: 10.1016/j.actbio.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 118.Sant AJ, McMichael A. Revealing the role of CD4(+) T cells in viral immunity. J. Exp. Med. 2012;209(8):1391–1395. doi: 10.1084/jem.20121517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wheway J, Herzog H, Mackay F. NPY and receptors in immune and inflammatory diseases. Curr. Top. Med. Chem. 2007;7(17):1743–1752. doi: 10.2174/156802607782341046. [DOI] [PubMed] [Google Scholar]
- 120.Study finds no link between COVID-19, Guillain-Barré syndrome. (2020). https://www.cidrap.umn.edu/news-perspective/2020/12/study-finds-no-link-between-covid-19-guillain-barr-syndrome.
- 121.Gromova OA, Torshin IY, Semenov VA, Putilina MV, Chuchalin AG. Direct and indirect neurological manifestations of COVID-19. Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova. 2020;120(11):11–21. doi: 10.17116/jnevro202012011111. [DOI] [PubMed] [Google Scholar]
- 122.Sfera A, Osorio C, Maguire G, Rahman L, Azaal J, Cummings M, Maldonado JC (2020) COVID-19, ferrosenescence and neurodegeneration, a mini-review. Progress in Neuro-Psychopharmacology and Biological Psychiatry:110230 [DOI] [PMC free article] [PubMed]
- 123.Vavougios GD (2020) Potentially irreversible olfactory and gustatory impairments in COVID-19: indolent vs. fulminant SARS-CoV-2 neuroinfection. Brain, behavior, and immunity [DOI] [PMC free article] [PubMed]
- 124.Mahalakshmi AM, Ray B, Tuladhar S, Bhat A, Paneyala S, Patteswari D, Sakharkar MK, Hamdan H, Ojcius DM, Bolla SR. Does COVID-19 contribute to development of neurological disease? Inflammation and Disease: Immunity; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.S.S. Hassan, S. Ghosh, D. Attrish, P.P. Choudhury, A.A.A. Aljabali, B.D. Uhal, K. Lundstrom, N. Rezaei, V.N. Uversky, M. Seyran, D. Pizzol, P. Adadi, A. Soares, T.M.A. El-Aziz, R. Kandimalla, M.M. Tambuwala, G.K. Azad, S.P. Sherchan, W. Baetas-da-Cruz, K. Takayama, Á. Serrano-Aroca, G. Chauhan, G. Palu, A.M. Brufsky, Possible transmission flow of SARS-CoV-2 based on ACE2 features. Molecules (Basel, Switzerland) 25 (24). (2020). 10.3390/molecules25245906 [DOI] [PMC free article] [PubMed]
- 126.M. Seyran, K. Takayama, V.N. Uversky, K. Lundstrom, G. Palù, S.P. Sherchan, D. Attrish, N. Rezaei, A.A.A. Aljabali, S. Ghosh, D. Pizzol, G. Chauhan, P. Adadi, T. Mohamed Abd El-Aziz, A.G. Soares, R. Kandimalla, M. Tambuwala, S.S. Hassan, G.K. Azad, P. Pal Choudhury, W. Baetas-da-Cruz, Á. Serrano-Aroca, A.M. Brufsky, B.D. Uhal, The structural basis of accelerated host cell entry by SARS-CoV-2†. FEBS J. (2020). 10.1111/febs.15651
- 127.Rezaei N. COVID-19 and medical biotechnology. Avicenna journal of medical biotechnology. 2020;12(3):139. [PMC free article] [PubMed] [Google Scholar]
- 128.P. Palit, D. Chattopadhyay, S. Thomas, A. Kundu, H.S. Kim, N. Rezaei, Phytopharmaceuticals mediated Furin and TMPRSS2 receptor blocking: can it be a potential therapeutic option for Covid-19? Phytomedicine:153396. (2020). 10.1016/j.phymed.2020.153396 [DOI] [PMC free article] [PubMed]
- 129.G. Pedrioli, R. Patani, P. Paganetti, Chloroquine, the coronavirus crisis, and neurodegeneration: a perspective. Front. Neurol. 11 (2020) [DOI] [PMC free article] [PubMed]
- 130.Kumar A, Zhou L, Zhi K, Raji B, Pernell S, Tadrous E, Kodidela S, Nookala A, Kochat H, Kumar S. Challenges in biomaterial-based drug delivery approach for the treatment of neurodegenerative diseases: opportunities for extracellular vesicles. Int. J. Mol. Sci. 2021;22(1):138. doi: 10.3390/ijms22010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang X, Sun C (2020) The roles of MicroRNAs in neural regenerative medicine. Experimental Neurology:113394 [DOI] [PubMed]
- 132.Aydemir MN, Aydemir HB, Korkmaz EM, Budak M, Cekin N, Pinarbasi E. Computationally predicted SARS-COV-2 encoded microRNAs target NFKB, JAK/STAT and TGFB signaling pathways. Gene Rep. 2021;22:101012. doi: 10.1016/j.genrep.2020.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.M. Farokhi, F. Mottaghitalab, M.R. Saeb, S. Shojaei, N.K. Zarrin, S. Thomas, S. Ramakrishna, Conductive biomaterials as substrates for neural stem cells differentiation towards neuronal lineage cells. Macromol Biosci:e2000123. (2020). 10.1002/mabi.202000123 [DOI] [PubMed]
- 134.C. Miller, J. O'Sullivan, J. Jeffrey, D. Power, Brachial plexus neuropathies during the COVID-19 pandemic: a retrospective case series of 15 patients in critical care. Phys. Ther. 101(1) (2021). 10.1093/ptj/pzaa191 [DOI] [PMC free article] [PubMed]
- 135.L. Brugliera, M. Filippi, U. Del Carro, C. Butera, F. Bianchi, P. Castellazzi, P. Cimino, P. Capodaglio, G. Monti, P. Mortini, L.G. Pradotto, L. Priano, A. Spina, S. Iannaccone, Nerve compression injuries after prolonged prone position ventilation in SARS-CoV-2 patient: a case series. Arch. Phys. Med. Rehabil. (2020). 10.1016/j.apmr.2020.10.131 [DOI] [PMC free article] [PubMed]
- 136.C.E. Fernandez, C.K. Franz, J.H. Ko, J.M. Walter, I.J. Koralnik, S. Ahlawat, S. Deshmukh, Imaging review of peripheral nerve injuries in patients with COVID-19. Radiology:203116. (2020). 10.1148/radiol.2020203116 [DOI] [PMC free article] [PubMed]
- 137.Dalamagkas K, Tsintou M, Seifalian A. Advances in peripheral nervous system regenerative therapeutic strategies: a biomaterials approach. Mater. Sci. Eng. C. 2016;65:425–432. doi: 10.1016/j.msec.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 138.Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, Lewis A, Czeisler B, Kahn DE, Zhou T, Ishida K, Torres J, Riina HA, Shapiro M, Nossek E, Nelson PK, Tanweer O, Gordon D, Jain R, Dehkharghani S, Henninger N, de Havenon A, Grory BM, Lord A, Melmed K (2020) Anticoagulation use and hemorrhagic stroke in SARS-CoV-2 patients treated at a New York Healthcare System. Neurocrit Care:1-12. 10.1007/s12028-020-01077-0 [DOI] [PMC free article] [PubMed]
- 139.K.R. Melmed, M. Cao, S. Dogra, R. Zhang, S. Yaghi, A. Lewis, R. Jain, S. Bilaloglu, J. Chen, B.M. Czeisler, E. Raz, A. Lord, J.S. Berger, J.A. Frontera, Risk factors for intracerebral hemorrhage in patients with COVID-19. J. Thromb. Thrombolysis, 1–8 (2020). 10.1007/s11239-020-02288-0 [DOI] [PMC free article] [PubMed]
- 140.Sang LY, Liang YX, Li Y, Wong WM, Tay DK, So KF, Ellis-Behnke RG, Wu W, Cheung RT. A self-assembling nanomaterial reduces acute brain injury and enhances functional recovery in a rat model of intracerebral hemorrhage. Nanomedicine. 2015;11(3):611–620. doi: 10.1016/j.nano.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 141.Batum M, Kisabay Ak A, Mavioğlu H (2020) Covid-19 infection-induced neuromyelitis optica: a case report. Int J Neurosci:1-7. 10.1080/00207454.2020.1860036 [DOI] [PubMed]
- 142.Ghosh R, De K, Roy D, Mandal A, Biswas S, Biswas S, Sengupta S, Naga D, Ghosh M, Benito-León J. A case of area postrema variant of neuromyelitis optica spectrum disorder following SARS-CoV-2 infection. J. Neuroimmunol. 2020;350:577439. doi: 10.1016/j.jneuroim.2020.577439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khedr EM, Karim AA, Soliman RK. Case report: acute spinal cord myelopathy in patients with COVID-19. Front. Neurol. 2020;11:610648. doi: 10.3389/fneur.2020.610648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soh P, Doan N, Manning B, Doan H. Spinal cord injury from an epidural abscess as a serious complication of COVID-19 infection. Cureus. 2020;12(11):e11327. doi: 10.7759/cureus.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Abbas WA, Ibrahim ME, El-Naggar M, Abass WA, Abdullah IH, Awad BI, Allam NK. Recent advances in the regenerative approaches for traumatic spinal cord injury: materials perspective. ACS Biomater Sci Eng. 2020;6(12):6490–6509. doi: 10.1021/acsbiomaterials.0c01074. [DOI] [PubMed] [Google Scholar]
- 146.Lv B, Zhang X, Yuan J, Chen Y, Ding H, Cao X, Huang A. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res Ther. 2021;12(1):36. doi: 10.1186/s13287-020-02090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ghane N, Beigi MH, Labbaf S, Nasr-Esfahani MH, Kiani A. Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. J. Mater. Chem. B. 2020;8(47):10712–10738. doi: 10.1039/d0tb01842b. [DOI] [PubMed] [Google Scholar]
- 148.Papa S, Pizzetti F, Perale G, Veglianese P, Rossi F. Regenerative medicine for spinal cord injury: focus on stem cells and biomaterials. Expert. Opin. Biol. Ther. 2020;20(10):1203–1213. doi: 10.1080/14712598.2020.1770725. [DOI] [PubMed] [Google Scholar]
- 149.Yang B, Zhang F, Cheng F, Ying L, Wang C, Shi K, Wang J, Xia K, Gong Z, Huang X, Yu C, Li F, Liang C, Chen Q. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis. 2020;11(6):439. doi: 10.1038/s41419-020-2620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M, Qin C, Crozier I, Dallmeier K, de Waal L, de Wit E, Delang L, Dohm E, Duprex WP, Falzarano D, Finch CL, Frieman MB, Graham BS, Gralinski LE, Guilfoyle K, Haagmans BL, Hamilton GA, Hartman AL, Herfst S, Kaptein SJF, Klimstra WB, Knezevic I, Krause PR, Kuhn JH, Le Grand R, Lewis MG, Liu WC, Maisonnasse P, McElroy AK, Munster V, Oreshkova N, Rasmussen AL, Rocha-Pereira J, Rockx B, Rodríguez E, Rogers TF, Salguero FJ, Schotsaert M, Stittelaar KJ, Thibaut HJ, Tseng CT, Vergara-Alert J, Beer M, Brasel T, Chan JFW, García-Sastre A, Neyts J, Perlman S, Reed DS, Richt JA, Roy CJ, Segalés J, Vasan SS, Henao-Restrepo AM, Barouch DH. Animal models for COVID-19. Nature. 2020;586(7830):509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cleary SJ, Magnen M, Looney MR, Page CP. Update on animal models for COVID-19 research. Br. J. Pharmacol. 2020;177(24):5679–5681. doi: 10.1111/bph.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cleary SJ, Pitchford SC, Amison RT, Carrington R, Robaina Cabrera CL, Magnen M, Looney MR, Gray E, Page CP. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br. J. Pharmacol. 2020;177(21):4851–4865. doi: 10.1111/bph.15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCrary MW, Bousalis D, Mobini S, Song YH, Schmidt CE (2020) Decellularized tissues as platforms for in vitro modeling of healthy and diseased tissues. Acta Biomater. 111:1-19. doi:10.1016/j.actbio.2020.05.031 [DOI] [PubMed]
- 154.N.J. Fedorchak, N. Iyer, R.S. Ashton, Bioengineering tissue morphogenesis and function in human neural organoids. Semin. Cell Dev. Biol. (2020). 10.1016/j.semcdb.2020.05.025 [DOI] [PMC free article] [PubMed]
- 155.Chakraborty J, Banerjee I, Vaishya R, Ghosh S. Bioengineered in vitro tissue models to study SARS-CoV-2 pathogenesis and therapeutic validation. ACS Biomater Sci Eng. 2020;6(12):6540–6555. doi: 10.1021/acsbiomaterials.0c01226. [DOI] [PubMed] [Google Scholar]
- 156.Maiolo L, Guarino V, Saracino E, Convertino A, Melucci M, Muccini M, Ambrosio L, Zamboni R, Benfenati V. Glial interfaces: advanced materials and devices to uncover the role of astroglial cells in brain function and dysfunction. Adv Healthc Mater. 2021;10(1):e2001268. doi: 10.1002/adhm.202001268. [DOI] [PubMed] [Google Scholar]
- 157.Oakes RS, Froimchuk E, Jewell CM. Engineering biomaterials to direct innate immunity. Advanced Therapeutics. 2019;2(6):1800157. doi: 10.1002/adtp.201800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.A. Bahrami, M. Vafapour, B. Moazzami, N. Rezaei, Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J. Paediatr. Child Health (2020). 10.1111/jpc.15048 [DOI] [PMC free article] [PubMed]
- 159.M. Sarzaeim, N. Rezaei, Kawasaki disease and multisystem inflammatory syndrome in children with COVID-19. SN comprehensive clinical medicine, 1–6 (2020). 10.1007/s42399-020-00558-9 [DOI] [PMC free article] [PubMed]
- 160.Sahu KK, Siddiqui AD, Rezaei N, Cerny J. Challenges for management of immune thrombocytopenia during COVID-19 pandemic. J. Med. Virol. 2020;92(11):2277–2282. doi: 10.1002/jmv.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moradian N, Gouravani M, Salehi MA, Heidari A, Shafeghat M, Hamblin MR, Rezaei N. Cytokine release syndrome: inhibition of pro-inflammatory cytokines as a solution for reducing COVID-19 mortality. Eur. Cytokine Netw. 2020;31(3):81–93. doi: 10.1684/ecn.2020.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.A.R. Safdarian, K. Momenzadeh, F. Kahe, P. Farhangnia, N. Rezaei, Death due to COVID-19 in a patient with diabetes, epilepsy, and gout comorbidities. Clin Case Rep. (2020). 10.1002/ccr3.3557 [DOI] [PMC free article] [PubMed]
- 163.H. Ahanchian, N. Moazzen, S.H. Joghatayi, A. Saeidinia, M. Khoshkhui, M.H. Aelami, N. Rezaei, N.S.M. Haghi, Death due to COVID-19 in an infant with combined immunodeficiencies. Endocr Metab Immune Disord Drug Targets (2020). 10.2174/1871530320666201021142313 [DOI] [PubMed]
- 164.Babaha F, Rezaei N. Primary immunodeficiency diseases in COVID-19 pandemic: a predisposing or protective factor? Am J Med Sci. 2020;360(6):740–741. doi: 10.1016/j.amjms.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.S. Delavari, H. Abolhassani, F. Abolnezhadian, F. Babaha, S. Iranparast, H. Ahanchian, N. Moazzen, M. Nabavi, S. Arshi, M. Fallahpour, M.H. Bemanian, S. Shokri, T. Momen, M. Sadeghi-Shabestari, R. Molatefi, A. Shirkani, A. Vosughimotlagh, M. Safarirad, M. Sharifzadeh, S. Pashangzadeh, F. Salami, P. Shirmast, A. Rezaei, T. Moeini Shad, M. Mohraz, N. Rezaei, L. Hammarström, R. Yazdani, A. Aghamohamamdi, Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J. Clin. Immunol., 1–11 (2020). 10.1007/s10875-020-00928-x [DOI] [PMC free article] [PubMed]
- 166.Mansourabadi AH, Sadeghalvad M, Mohammadi-Motlagh HR, Rezaei N. The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life Sci. 2020;258:118185. doi: 10.1016/j.lfs.2020.118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.S. Khanmohammadi, N. Rezaei, Role of Toll-like receptors in the pathogenesis of COVID-19. J. Med. Virol. (2021) [DOI] [PMC free article] [PubMed]
- 168.S. Seyedpour, B. Khodaei, A.H. Loghman, N. Seyedpour, M.F. Kisomi, M. Balibegloo, S.S. Nezamabadi, B. Gholami, A. Saghazadeh, N. Rezaei, Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: a systematic review of in vitro and in vivo studies. J. Cell. Physiol. (2020). 10.1002/jcp.30032 [DOI] [PubMed]
- 169.L. Jahanshahlu, N. Rezaei, Monoclonal antibody as a potential anti-COVID-19. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 129:110337. (2020). 10.1016/j.biopha.2020.110337 [DOI] [PMC free article] [PubMed]
- 170.M. Lotfi, N. Rezaei, CRISPR/Cas13: a potential therapeutic option of COVID-19. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 131:110738. (2020). 10.1016/j.biopha.2020.110738 [DOI] [PMC free article] [PubMed]
- 171.Pashaei M, Rezaei N. Immunotherapy for SARS-CoV-2: potential opportunities. Expert. Opin. Biol. Ther. 2020;20(10):1111–1116. doi: 10.1080/14712598.2020.1807933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Griffith M, Islam MM, Edin J, Papapavlou G, Buznyk O, Patra HK. The quest for anti-inflammatory and anti-infective biomaterials in clinical translation. Frontiers in bioengineering and biotechnology. 2016;4:71. doi: 10.3389/fbioe.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.B. Kaczmarek, Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—a minireview. Materials (Basel) 13(14) (2020). 10.3390/ma13143224 [DOI] [PMC free article] [PubMed]
- 174.Muthu MS, Singh S (2009) Targeted nanomedicines: effective treatment modalities for cancer, AIDS and brain disorders. [DOI] [PubMed]
- 175.El-Gizawy SA, El-Maghraby GM, Hedaya AA. Formulation of acyclovir-loaded solid lipid nanoparticles: 2. Brain targeting and pharmacokinetic study. Pharm. Dev. Technol. 2019;24(10):1299–1307. doi: 10.1080/10837450.2019.1667386. [DOI] [PubMed] [Google Scholar]
- 176.Minigo G, Scholzen A, Tang CK, Hanley JC, Kalkanidis M, Pietersz GA, Apostolopoulos V, Plebanski M. Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy. Vaccine. 2007;25(7):1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 177.Abudula T, Bhatt K, Eggermont LJ, O'Hare N, Memic A, Bencherif SA. Supramolecular self-assembled peptide-based vaccines: current state and future perspectives. Front Chem. 2020;8:598160. doi: 10.3389/fchem.2020.598160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou X, Jiang X, Qu M, Aninwene GE, 2nd, Jucaud V, Moon JJ, Gu Z, Sun W, Khademhosseini A. Engineering antiviral vaccines. ACS Nano. 2020;14(10):12370–12389. doi: 10.1021/acsnano.0c06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wong FSY, Chan BP, Lo ACY. Carriers in cell-based therapies for neurological disorders. Int. J. Mol. Sci. 2014;15(6):10669–10723. doi: 10.3390/ijms150610669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007;44(1-3):82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 181.Tsui C, Koss K, Churchward MA, Todd KG. Biomaterials and glia: progress on designs to modulate neuroinflammation. Acta Biomater. 2019;83:13–28. doi: 10.1016/j.actbio.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 182.Linsmeier CE, Wallman L, Faxius L, Schouenborg J, Bjursten LM, Danielsen N. Soft tissue reactions evoked by implanted gallium phosphide. Biomaterials. 2008;29(35):4598–4604. doi: 10.1016/j.biomaterials.2008.08.028. [DOI] [PubMed] [Google Scholar]