Graphical abstract
Keywords: SARS-CoV-2, Covid-19, Avidity, Protective immunity, Vaccination, Neutralizing antibody
Abstract
Background
Avidity is defined as the strength of binding between immunoglobulin G (IgG) and its specific target epitope. IgG of high avidity is established during affinity maturation. Failure to achieve high avidity IgG may result in a lack of protective immunity towards infection and disease. It is known that the interaction between SARS-CoV-2 spike protein and its cellular receptor is driven by high affinity. Therefore, it is predictable that protective antibodies towards SARS-CoV-2 should show high affinity/avidity.
Avidity after SARS-CoV-2 infection
Recent findings by several groups demonstrate that the serological response towards infection with SARS-CoV-2 and seasonal coronaviruses is characterized by incomplete avidity maturation, followed by a decline of the serological response. This response might facilitate reinfection, prevent herd immunity and potentially allow repeated cycles of infection.
Consequences for vaccination towards SARS-CoV-2
Therefore, the sole focus on antibody titers reached after vaccination towards SARS-CoV-2 might not be sufficient to evaluate the degree of achieved protection. Rather, it is suggested to include avidity determination to optimize vaccination protocols and achieve high avidity IgG directed towards SARS-CoV-2 through vaccination. Avidity determination might also be useful to control for truly protective immunity towards SARS-CoV-2 in individual cases.
Protection towards viral infection or disease requires high avidity IgG
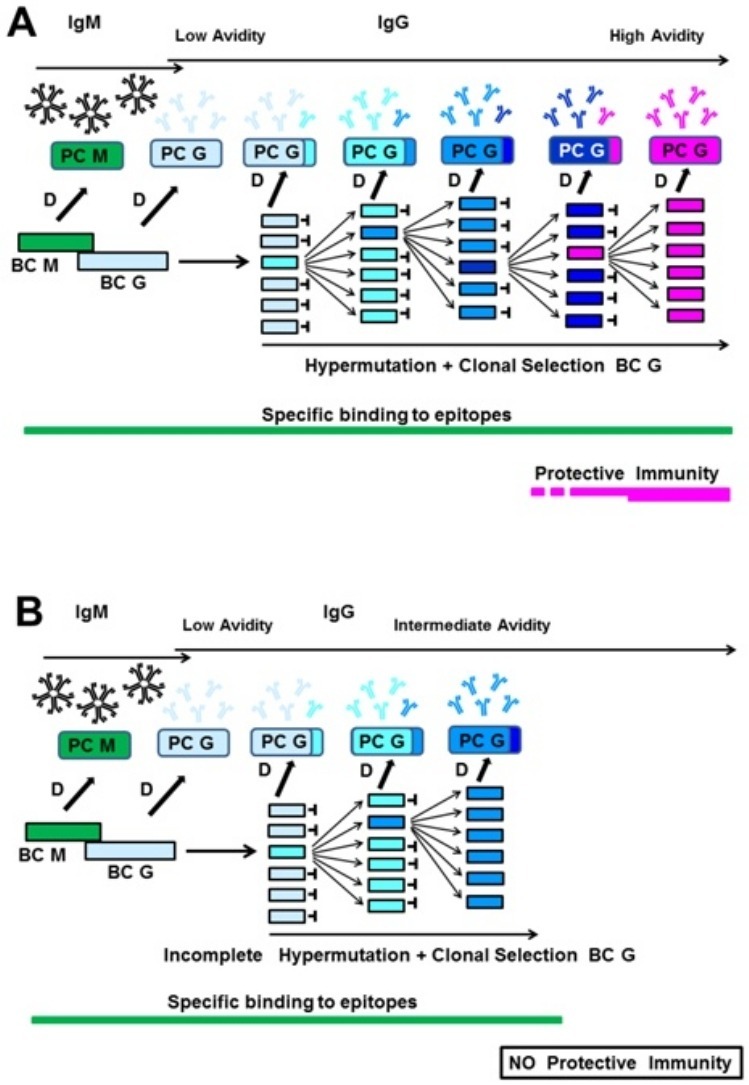
The avidity of immunoglobulin G (IgG) is determined by its affinity and denotes the strength of binding to its target epitope. High avidity is reached after affinity/avidity maturation and reflects the best fit between IgG and epitope. Avidity of IgG is low during acute infection and reaches high values several weeks or months later (Hedman et al., 1997, Bauer, 2021). Memory B cells express and utilize high avidity IgG to establish effective anamnestic responses (Eisen, 2014).
High avidity of neutralizing antibodies plays an important role in antibody-mediated protection against viral infections. Protection against viral infections and/or resultant diseases seems to fail if avidity maturation of IgG directed towards the virus is incomplete. Junker and Tilley (1994) showed that low avidity of IgG towards varicella zoster virus (VZV) was associated with the risk of repeated chickenpox, and Martin et al. (1994) reported on the risk of pregnant women with low avidity anti-VZV IgG for acquiring chickenpox. Boppana and Britt (1995) concluded that failure to generate high avidity IgG towards cytomegalovirus (CMV) plays a role in the intrauterine transmission of CMV. Boppana and Britts’ conclusions agree with the findings of Lazzarotto et al. (2009), Seo et al. (2009) and Kaneko et al. (2017). Kontio et al. (2012) reported that the concentration of IgG directed towards the measles virus correlated with the avidity inherent to this IgG, and therefore concluded that waning antibody levels and low avidity might be the cause of reinfections. The significance of IgG avidity for protective immunity towards the measles virus has also been demonstrated by Paunio et al. (2005). Puschnik et al. (2013) demonstrated that IgG avidity correlates with the neutralizing capacity of IgG directed towards the Dengue virus. Delgado et al. (2009) presented evidence that the failure of the formalin-treated vaccine against the respiratory syncytial virus (RSV) was not, as previously assumed, due to the destruction of essential epitopes by formalin. Rather, it was due to the vaccine’s failure to induce an antibody response of high avidity. Immunization studies with Simian human immunodeficiency virus (SHIDV) in macaques showed a strong correlation between the avidity of IgG directed towards the viral envelope protein and protection against infection (Lai et al. 2007). These findings have been confirmed by Pegu et al. (2013), who demonstrated that the avidity of IgG towards the envelope protein was higher in immunized animals that were protected from infection with SHIDV than in non-protected animals.
Biophysical data indicate that neutralizing antibodies towards SARS-CoV-2 should show high affinity (avidity)
The interaction between the receptor-binding domain of SARS-CoV-2 spike protein and angiotensin-converting enzyme-2 (ACE2) on target cells is the initial step in infection (Barnes et al., 2020a, Barnes et al., 2020b). The binding between the receptor-binding domain (RBD) of SARS-CoV-2 and the angiotensin-converting enzyme 2 (ACE2) is characterized by high affinity (Khatri et al., 2020). Therefore, Khatri et al. concluded that efficient neutralization of SARS-CoV-2 would require antibodies of high avidity, adding to our classical concept of neutralizing antibodies the additional requirement for high affinity/avidity.
Incomplete avidity maturation of IgG after natural infection with SARS-CoV-2 and other coronaviruses
In a review published in May 2020, the author of this perspective critically evaluated 16 publications on serological findings related to the diagnosis of SARS-CoV-2 infections (Bauer, 2021). The review outlined that the kinetic patterns of IgM and IgG responses towards SARS-CoV-2 were characterized by a high degree of variability, which did not allow acute and past SARS-CoV-2 infections to be distinguished merely by determining IgM and IgG. The review (i) summarized similar findings in many other viral systems; (ii) proposed a model to explain the observed variability; (iii) suggested introducing avidity determination for an unequivocal serological diagnosis. The latter suggestion was based on the premise that the serological response towards SARS-CoV-2 would show classical avidity maturation, as observed before for many other viruses.
The subsequent analysis of outpatients with Covid-19 and SARS-CoV-2 infection confirmed by positive polymerase chain reaction (PCR) led, however, to a rather unexpected result (Bauer et al., 2021a, Bauer et al., 2021b). In contrast to the regular interaction between the humoral immune system and viruses, the avidity of IgG towards SARS-CoV-2 antigens seemed to remain low in the majority of patients, even several months after the onset of clinical symptoms. Bauer et al. (2021a) demonstrated that (i) the avidity of IgG directed towards SARS-CoV-2 nucleocapsid protein (NP), RBD and spike glycoprotein 1 (S1) was increasing with time; but that (ii) the final level of avidity was much lower in most sera than found for other viral systems. Importantly, however, in a few sera, high avidity was reached. These essential positive controls of high avidity allowed the conclusion that the low avidity found in most sera was not due to trivial reasons such as incompatibility of SARS-CoV-2 antigens with urea treatment during avidity determination. Bauer et al. (2021a) defined a model of analysis that should allow the use of avidity determination to discriminate between acute and past SARS-CoV-2 infections despite low overall avidity. In a follow-up study with 93 sera from 70 SARS-CoV-2-infected Covid-19 outpatients, the frequent incomplete avidity maturation of IgG towards SARS-CoV-2 NP, RBD and S1 was confirmed (Bauer et al., 2021b). This study showed that the low avidity found in most cases was due to discontinuous avidity maturation after an initial increase rather than to unusually slow avidity maturation. The kinetic analysis demonstrated that the point of abrogation of avidity maturation correlated with the IgG production breakpoint and the beginning IgG concentration decline. Waning IgG titers after SARS-CoV-2 infection, including for neutralizing antibodies, have also been shown by other groups (Long et al., 2020, Beaudoin-Bussières et al., 2020, Seow et al., 2020). Compared to the Epstein–Barr virus (EBV) (a classical system for the application of avidity determination), SARS-CoV-2 infection is similar in low avidity during the first few weeks of acute infection but completely different in avidity reached in past infection. Less than 15% of SARS-CoV-2 infected patients reached avidity indices >0.6 several months after infection, in contrast to more than 90% in the case of EBV. Similar high avidity indices have been reported for past infections with hepatitis A virus (HAV) (Roque-Afonso et al., 2004), hepatitis C virus (HCV) (Gaudy-Graffin et al., 2010), West Nile virus (Levett et al., 2005, Fox et al., 2006) and other viruses (Bauer, 2021).
Our findings and conclusions are in line with reports from several other groups.
Strömer et al., 2020a, Strömer et al., 2020b also describe the predominant occurrence of low avidity IgG towards SARS-CoV-antigens while using the prototype and final version of our assay system (Bauer et al., 2021a). Liu et al. (2020) reported that IgG towards S1 and RBD of SARS-CoV-2 showed avidity indices <0.3 within the first 45 days after onset of disease, except for 1 serum with an avidity index of approximately 0.8 before day 10.
Benner et al. (2020) stated that (i) antibody avidity increased over the duration of infection and remained elevated; (ii) antibody avidity correlated with infection duration and higher neutralizing titers. However, the dissociation constant (DC)50 values for urea during avidity determination, as shown for convalescent sera in their study, had a median between 4–5 M for IgG towards the spike protein and 6 M for NP. These values indicate that at 7 M urea, the discriminative concentration used in our studies, an avidity index of <0.5 would have been determined for most sera. Therefore, despite the measurable avidity maturation, these sera remained at low or borderline avidity.
Klein et al. (2020) show (their Figure 2B) that their 126 convalescent sera were below an avidity index of 0.4 at 7 M urea, except for 3 sera. Luo et al. (2020) report on an increase over time in the avidity of IgG towards SARS-CoV-2 RBD. This kinetics is useful for the differentiation between acute and past SARS-CoV-2 infections. However, their assay was performed with 3 M urea, so the high avidity indices measured are converted to the low avidity range, where 7 M urea is used instead of 3 M. Therefore, their finding also supports the view that avidity maturation is frequently at a low level, which is best explained by incomplete avidity maturation, according to our findings.
Incomplete avidity maturation: an essential strategy of coronaviruses?
Incomplete avidity maturation was found for the serological response towards SARS-CoV-2 and the immune responses towards 4 seasonal coronaviruses (Bauer et al., 2021a, Bauer et al., 2021b). It is also known that the immune responses towards seasonal coronaviruses are characterized by a relatively rapid decline after an initial burst (Callow et al., 1990, Edridge et al., 2020). It seems that these 2 characteristics of the immune response towards seasonal coronaviruses, i.e. declining antibody concentrations and frequent low avidity, are connected with the high probability of repeated waves of reinfections with these viruses (Edridge et al., 2020, Galanti and Shaman, 2021).
Revisiting the data published for SARS CoV-1, where complete avidity maturation had been claimed (Chan et al., 2005), revealed that the analysis had been performed with 4 M urea. This low concentration of urea does not lead to sharp discrimination between low and high avidity, unlike 7 M urea which has been established as a standard concentration in most other studies. Therefore, the humoral response towards SARS CoV-1 also seems to be restricted to IgG of lower avidity.
These data allow to speculate that coronaviruses interfere with avidity maturation to ensure incomplete protection towards reinfection, allowing replication through repeated waves of infection in the host population.
Suggested goals for vaccination programs towards SARS-CoV-2
Incomplete avidity maturation after SARS-CoV-2 infection, combined with waning IgG titers, may prevent herd immunity and potentially enable repeated cycles of reinfection. Reinfections with SARS-CoV-2, despite specific humoral immune responses after primary infection, have been reported (Overbaugh, 2020, To et al., 2020, Tillett et al., 2020, Gupta et al., 2020).
The concept of a coronavirus strategy that renders immune responses non-protective, combined with the biophysical data on the high affinity between SARS-CoV-2 RBD and its cellular receptor, allow us to propose that vaccination should induce certain qualities of IgG in order to establish protective immunity. Vaccination programs should achieve:
-
i)
sufficiently high and long-lasting IgG concentrations that specifically target viral structures that are relevant for binding to cellular receptors, such as RBD;
-
ii)
completed avidity maturation of neutralizing IgG towards SARS-CoV-2; and
-
iii)
the potential of these antibodies to prevent the binding of the virus to the cells.
Therefore, the suggested goal is that immunization achieves a response that outcompetes the quality of the immune response reached after natural infection.
Monitoring the avidity of IgG may have the potential to control the success of vaccination
Can high avidity be reached after vaccination, even if natural infection fails to reach this state in most cases? Abrogated avidity maturation after natural infection with SARS-CoV-2 infection is conceivably due to (i) an insufficient supply of the immune system with viral antigen, thus preventing a sufficient number of cycles of hypermutation and clonal selection of B cells; or (ii) a suppressive effect of SARS-CoV-2 on the immune system (Zhou et al., 2020), potentially also affecting avidity maturation (Kaneko et al., 2020). Both mechanisms of suppression of avidity maturation have been demonstrated to occur in vivo in the case of HIV infections (Nair et al., 2009, Re et al., 2010). Therefore, vaccination towards SARS-CoV-2 with defined antigens might provide optimal antigen concentrations, and this would occur independently of the potential negative immunomodulatory effects associated with viral infection. Therefore, the induction of high avidity protective IgG through vaccination seems to be feasible. This conclusion has been confirmed in experiments performed after submission.
In this respect, it is encouraging to see that the mRNA-based vaccine BNT162b1 elicits antibody titers much higher than the titers reached after natural infection (Mulligan et al., 2020, Şahin et al., 2020). Avidity determination in these studies should also clarify whether high avidity has been achieved.
Quantitative measurement of avidity might also provide answers to whether protection towards Covid-19 through immunization towards SARS-CoV-2 is based on sterile immunity or whether the state of protection towards disease is paralleled by limited replication of the virus not sufficient to establish disease. Resolving these questions may be instrumental for further understanding and controlling the spread of SARS-CoV-2 in the human population.
Conflict of interest
Georg Bauer is co-inventor for a pending patent application describing a method to determine the avidity of antibodies towards several SARS-CoV-2 antigens in one assay (“Verfahren zur Bestimmung der Avidität von gegen Coronavirus gerichteten Antikörpern sowie hierzu geeignete Testkits” (EP 2019/2550)).
Funding source
Publication costs will be sponsored by the Medical Faculty of the University of Freiburg, Germany. No other funding was obtained.
Ethical approval
No ethical approval was required for this work.
Acknowledgment
The financial support for publication fees by the Medical Faculty of the University of Freiburg is acknowledged.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.01.061.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Barnes C.O., Jette C.A., Abernathy M.E., Dam K.-M.A., Esswein S.R., Gristick H.B. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.O., West A.P., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842. doi: 10.1101/2020.05.28.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G. The variability of the serological response to SARS-corona virus-2: potential resolution of ambiguity through determination of avidity (functional affinity) J Med Virol. 2021;93:311–322. doi: 10.1002/jmv.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Struck F., Schreiner P., Staschik E., Soutschek E., Motz M. The challenge of avidity determination in SARS-CoV-2 serology. J Med Virol. 2021;93:3092–3104. doi: 10.1002/jmv.26863. under revision. Preprint deposited at the server of the World Health Organization, 2021 a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Struck F., Schreiner P., Staschik E., Soutschek E., Motz M. The serological response to SARS corona virus-2 is characterized by frequent incomplete maturation of functional affinity (avidity) Sci Rep. 2021 doi: 10.21203/rs.3.rs-104847/v1. under review, Preprint available at Research Square, b. [DOI] [Google Scholar]
- Beaudoin-Bussières G., Laumaea A., Anand S.P., Prévost J., Gasser R., Goyette G. Decline of humoral responses against SARS-CoV-2 Spike in convalescent individuals. mBio. 2020;11 doi: 10.1128/mBio.02590-20. e02590–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner S., Patel E.U., Laeyendecker O., Pekosz A., Littlefield K., Eby Y. SARS-CoV-2 antibody avidity responses in covid-19 patients and convalescent plasma donors. J Infect Dis. 2020;222:1974–1984. doi: 10.1093/infdis/jiaa581. jiaa581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boppana S.B., Britt W.J. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis. 1995;171:1115–1121. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A.J. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K.S., Lim P.-L., Liu E.Y.M., Cheung J.L.K., Leung D.T.M., Sung J.J.Y. Antibody avidity maturation during severe acute respiratory syndrome—associated coronavirus infection. J Infect Dis. 2005;192:166–169. doi: 10.1086/430615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1099/jgv.0.001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K. Seasonal corona virus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- Eisen H.N. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res. 2014;2:381–392. doi: 10.1158/2326-6066.CIR-14-0029. [DOI] [PubMed] [Google Scholar]
- Fox J.L., Hazel S.L., Tobler L.H., Busch M.P. Immunoglobulin G avidity in differentiation between early and late antibody responses to West Nile virus. Clin Vaccine Immunol. 2006;13:33–36. doi: 10.1128/CVI.13.1.33-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti M., Shaman J. Direct observation of repeated infections with endemic coronaviruses. J Infect Dis. 2021;223:409–415. doi: 10.1093/infdis/jiaa392. jiaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudy-Graffin C., Lesage G., Kousignian I., Laperche S., Girault A., Dubois F. Use of an anti-hepatitis C virus (HCV) IgG avidity assay to identify recent HCV infection. J Clin Microbiol. 2010;48:3281–3287. doi: 10.1128/JCM.00303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Bhoyar R.C., Jain A., Srivastava S., Upadhayay R., Imran M. Asymptomatic reinfection in 2 healthcare workers from India with genetically distinct severe acute respiratory syndrome coronavirus 2. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman K., Lappalainen M., Söderlund M., Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1997;4:123–129. [Google Scholar]
- Junker A.K., Tilley P. Varicella-zoster virus antibody avidity and subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119–124. doi: 10.1002/jmv.1890430204. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Ohhashi M., Minematsu T., Muraoka J., Kusumoto K., Sameshima H. Maternal immunoglobulin G avidity as a diagnostic tool to identify pregnant women at risk of congenital cytomegalovirus infection. J Infect Chemother. 2017;23:173–176. doi: 10.1016/j.jiac.2016.12.001. [Epub 26 December 2016]. PMID: 28034524. [DOI] [PubMed] [Google Scholar]
- Kaneko N., Kuo H.-H., Bucau J., Farmer J.R., Mahajan V.S. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in Covid-19. Cell. 2020;183:143–157. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri I., Staal F.J.T., van Dongen J.J.M. Blocking of the high-affinity interaction-synapse between SARS CoV-2 spike and human ACE2 proteins likely requires multiple high-affinity antibodies: an immune perspective. Front Immunol. 2020;11:570018. doi: 10.3389/fimmu.2020.570018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Pekosz A., Park H.-S., Ursin R.L., Shapiro J.R., Benner S.E. Sex, age, and hospitalization drive antibody responses in 1 a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130:6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontio M., Jokinen S., Paunio M., Peltola H., Davidkin I. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. J Infect Dis. 2012;206:1542–1548. doi: 10.1093/infdis/jis568. [DOI] [PubMed] [Google Scholar]
- Lai L., Vödrös D., Kozlowski P.A., Montefiori D.C., Wilson R.L., Akerstrom V.L. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369:153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarotto T., Varani S., Spezzacatena P., Gabriell L., Pradelli P., Guerra B. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Virol Immunol. 2009;13:137–141. doi: 10.1089/vim.2000.13.137. [DOI] [PubMed] [Google Scholar]
- Levett P.N., Sonnenberg K., Sidaway F., Shead S., Niedrig M., Steinhagen K. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol. 2005;43:5873–5875. doi: 10.1128/JCM.43.12.5873-5875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Hsiung J., Zhao S., Kost J., Sreedhar D., Hanson C.V. Quantification of antibody avidities and accurate detection of SARS-CoV-2 antibodies in serum and saliva on plasmonic substrates. Nat Biomed Eng. 2020;4:1188–1196. doi: 10.1038/s41551-020-00642-4. [DOI] [PubMed] [Google Scholar]
- Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Luo Y.R., Chakraborty I., Yun C., Wu A.H.B., Lynch K.L. Kinetics of SARS-CoV-2 antibody avidity maturation and association with disease severity. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.A., Junker A.K., Thomas E.E., Van Allen M.I., Friedman J.M. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991–995. doi: 10.1093/infdis/170.4.991. [DOI] [PubMed] [Google Scholar]
- Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Nair N., Moss W.J., Scott S., Mugala N., Ndhlovu Z.M., Lilo K. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus–specific immunoglobulin G after vaccination and infection. J Infect Dis. 2009;200:1031–1038. doi: 10.1086/605648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J. Understanding protection from SARS CoV-2 by studying reinfection. Nat Med. 2020;26:1678–1685. doi: 10.1038/s41591-020-1121-z. [DOI] [PubMed] [Google Scholar]
- Paunio M., Hedman K., Davidkin I., Peltola H. IgG avidity to distinguish secondary from primary measles vaccination failures: prospects for a more effective global measles elimination strategy. Exp Opin Pharmacother. 2005;4:1215–1225. doi: 10.1517/14656566.4.8.1215. [DOI] [PubMed] [Google Scholar]
- Pegu P., Vaccari M., Gordon S., Keele B.F., Doster M., Guan Y. Antibodies with high avidity to the gp120 envelope protein in protection from Simian Immunodeficiency Virus SIVmac251. Acquisition in an immunization regimen that mimics the RV-144 Thai Trial. J Virol. 2013;87:1708–1719. [Google Scholar]
- Puschnik A., Lau L., Cromwell E.A., Balmaseda A., Zompi S., Harris E. Correlation between Dengue-specific neutralizing antibodies and serum avidity in primary and secondary Dengue virus 3 natural infections in humans. PLoS Negl Trop Dis. 2013;7:e2274. doi: 10.1371/journal.pntd.0002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re M.C., Schiavone P., Bon I., Vitone F., De Crignis E., Biagetti C. Incomplete IgG response to HIV-1 proteins and low avidity levels in recently converted HIV patients treated with early antiretroviral therapy. Int J Infect Dis. 2010;14:e1008–e1012. doi: 10.1016/j.ijid.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Roque-Afonso A.-M., Grangeot-Keros L., Roquebert B., Desbois D., Poveda J.-D., Vincent Mackiewicz V. Diagnostic relevance of immunoglobulin G avidity for hepatitis A virus. J Clin Microbiol. 2004;42:5121–5124. doi: 10.1128/JCM.42.11.5121-5124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahin U., Mulk A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 Tcell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Seo S., Cho Y., Park J. Serologic screening of pregnant Korean women for primary human cytomegalovirus infection using IgG avidity test. Korean J Lab Med. 2009;29:557–562. doi: 10.3343/kjlm.2009.29.6.557. PMID: 20046088. [DOI] [PubMed] [Google Scholar]
- Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömer A., Grobe O., Rose R., Fickenscher H., Lorentz T., Krumbholz A. Diagnostic accuracy of six commercial SARS-CoV-2 IgG/total antibody assays and identification of SARS-CoV-2 neutralizing antibodies in convalescent sera. medRxiv. 2020 doi: 10.1101/2020.06.15.20131672. [DOI] [Google Scholar]
- Strömer A., Rose R., Grobe O., Neumann F., Fickenscher H., Lorentz T. Kinetics of nucleo- and spike protein-specific immunoglobulin G and of virus-neutralizing antibodies after SARS-CoV-2 infection. Microorganisms. 2020;8:1572–1583. doi: 10.3390/microorganisms8101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2020;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.-W., Hung I.F.-N., Ip J.D., Chu A.W.-C., Chan W.-M., Tam A.R. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., To K.K.-W., Wong Y.-C., Liu L., Zhou B., Li X. SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53:864–877. doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


