Abstract
Background and aim of the work:
The reverse shoulder arthroplasty (RSA) has risen exponentially, this has entailed an increasing number of complications and reoperations. In RSA, loads are transferred directly to the glenoid component. As a result, failure of the glenoid component is one of the most common complications. CT 3D preoperative planning, patient-specific and the possibility of performing a more precise and controlled surgical gesture in the operating room are increasingly important. The use of the GPS navigation on CT 3D planning has proved to be useful above all in terms of accuracy, reliability and the possibility of reproducing the planned gesture preoperatively.
Methods:
This study analyzes the precision, safety, and reproducibility of the GPS system for the reverse shoulder prosthesis tested on 6 scapulohumeral cadaver specimens, subsequently subjected to anatomical dissection to verify the correct positioning of the glenoid components and the percentage of appropriateness in the field of planning previously virtually assumed.
Results:
Postoperative macroscopic dissection revealed no central peg perforated or screws malpositioned, no leaking from the bone or injury to the adjacent neurovascular structures. The average length of the screws was 42 mm (range 36 mm to 46 mm) for the lower screw and 40 mm for the upper one (range 36 mm to 42 mm).
Conclusions:
This cadaver study has shown that GPS navigation offers greater efficiency in baseplate and screws placement and can avoid intra- and postoperative complications. (www.actabiomedica.it)
Keywords: GPS, Shoulder arthroplasty, Navigation, RSA
Introduction
For a decade, total shoulder arthroplasty (TSA) has been the gold-standard treatment for end-stage arthritis of the glenohumeral joint. However, TSA in patient with concomitant rotator cuff pathology has been associated with early failure due to the high rate of glenoid looseing (1). Recently, however, reverse shoulder arthroplasty (RSA) has emerged as an alternative surgical option. RSA provides a mechanical advantage for shoulder elevation in patients with rotator cuff disease (2). An aging population, improved implant designs, and broader indications have all been implicated for increasing volume and utilization (3). RSA has risen exponentially, and this has entailed an increasing number of complications and reoperations (4). Zumstein et al. reported a 20% rate of postoperative complications; 105 implants required reintervention: 79 (10.1%) surgical revisions and 26 (3.3%) reoperations. The complication rate was almost 3-fold higher in cases of revision for the failure of the anatomic implant than in primary RSA: 33.3% vs. 13.4%4.
With these semi-constrained prostheses, loads applied to the humerus are transferred directly to the fixation of the glenoid component. As a result, failure of the glenoid component fixation is one of the most common complications of the reverse total shoulder (5,6). The glenoid component position must be optimized for version, inclination, and overhang to maximize the bone stock available for fixation. A review of articles regarding reverse total shoulder prostheses, especially those showing glenoid component fixation failure, reveals a wide variability in the placement of glenoid fixation screws in the limited bone available in the scapula (7). On the other side, several cadaveric studies have demonstrated considerable natural variability in anatomic parameters of the glenoid: this variability affects prosthesis design, instrumentation, and intraoperative implantation techniques. When the scapular anatomy is distorted, as is often the case in rotator cuff tear arthropathy or in revision shoulder arthroplasty where reverse total shoulder arthroplasties are often used, achieving secure purchase with each screw may be even more difficult. Inferior scapular bone resorption, or notching, associated with the reverse total shoulder prosthesis may jeopardize the security of the inferior screw (8). For primary osteoarthritis, the most common pattern is glenoid wear with varying degrees of posterior subluxation of the humeral head. Inflammatory arthritis is often associated with central glenoid erosion, which may be accompanied by the presence of cysts within the glenoid vault. Anterior glenoid erosion can also be encountered (9). Moreover, the anatomy “beyond the glenoid fossa” thus became a factor in screw fixation strength and potential injury (10). An important neurovascular structure at risk from screw position and drill bit plunge is the suprascapular nerve. The suprascapular nerve provides motor innervation to the supraspinatus and infraspinatus with some branches to the teres minor (11). The major innervation of the teres minor is provided by the axillary nerve, which also provides motor supply to the deltoid and the teres minor. On average, the axillary nerve is approximately 32 mm from the inferior glenoid, although this position may vary with arm position (12), while the suprascapular nerve is, on average, 1.8 cm from the posterior superior glenoid rim (13). Pain sensory innervation to the glenohumeral articulation is largely supplied by the suprascapular nerve, axillary nerve, and lateral pectoral nerve. The inferior, lateral, and anterior joint structures are supplied by branches from the axillary nerve. The posterior, medial, and superior joint supply come from the suprascapular nerve and lateral pectoral nerve. Injury to these nerves could lead to increased postoperative pain and suboptimal outcomes (14).
In consideration of all the elements described above, an adequate CT 3D preoperative planning, patient-specific, is increasingly important, and also the possibility of performing a more precise and controlled surgical gesture in the operating room. In recent years, in this regard, we could benefit to the GPS navigation technology, already developed and implemented in hip and knee prosthetics, and which today in shoulder prosthetic seems to offer the greatest advantages, this in consideration of the reduced bone surface on where you need to work and the proximity to all the neurovascular structures around that may be at risk. The use of the GPS navigation on CT 3D planning has proved to be useful above all in terms of accuracy, reliability and the possibility of reproducing the planned gesture preoperatively directly in the operating room. This study analyzes the precision, safety, and reproducibility of the Exactech GPS system for the reverse shoulder prosthesis tested on 6 scapulohumeral cadaver specimens, subsequently subjected to anatomical dissection to verify the correct positioning of the glenoid components and the percentage of appropriateness in the field of planning previously virtually assumed.
Materials and Methods
Six paired fresh-frozen cadaver scapulothoracic specimens were obtained from patients who had not undergone previous surgery on the shoulder or scapula. The mean age was 66 years (range, 58-72). Preoperative computed tomography (CT) scans were performed on each specimen. The scan was of the entire scapula in the axial plane, with the specimen in the supine position and the arm in an adducted position to the side. The tube current was set to at least 120 kV (peak) with image reconstruction using a convolution bone kernel with a field of view of 154 to 410 mm and a standard image matrix size of 512 _ 512 pixels, yielding between 200 and 450 images. The interslice distance was between 0.3 and 1.0 mm. The CT file was sent to the manufacturer for uploading into the surgical planning software and rendering it to a 3-dimensional model for visualization. CT scans were loaded into the RSA planning software (Exactech) and manually segmented by the manufacturer to reconstruct the shoulder in a 3-dimensional model for preoperative planning. Glenoid version, inclination, and any wear or deformity were accurately measured for all specimens, and the surgeon provides to plan the best virtual positioning of baseplate and screws on the planning software (Fig. 1).
Figure 1.
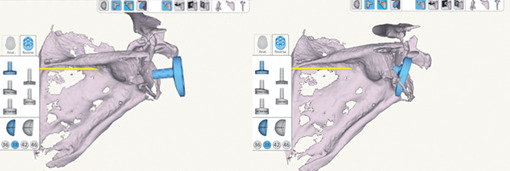
Virtual planning of baseplate positioning.
A deltopectoral approach was used for all procedures, taking care of exposing the superior surface of the coracoid to place the GPS tracker using two screws to its inferolateral base. At this point, the recognition phase of the glenoid surface and anatomic landmarks begins using a handheld tracker on the surface that recognizes and matches the specimen scapula with the virtual planning model (Fig. 2). After registration, the software provided 2- and 3-dimensional guidelines to achieve the preoperative plan. At this point, every next phase from drilling, reaming and positioning of the baseplate and screws were performed under image-guided navigation using instruments with trackers mounted and GPS guided.
Figure 2.
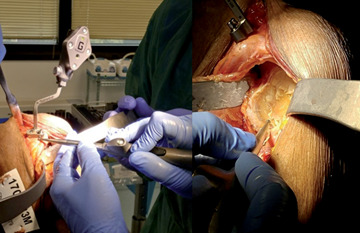
Matching phase of the glenoid surface and anatomic landmarks with the virtual planning model.
Postoperatively, all glenoids were dissected and possible perforation of the central peg of the glenoid base plate or malposition of the 2 screws concerning the glenoid vault or surrounding soft tissues was assessed (Fig. 3). In addition, the coracoid process and the position of the reference pins were verified. Then, all glenoid specimens received a multi-slice CT scan and the position of the glenoid baseplate and the locking screws were studied. Glenoid component version relative to the long axis of the scapula was measured on the first axial cut below the coracoid process. Glenoid component tilt was measured on the oblique cut relative to the frontal axis of the scapula. The positioning of the superior and inferior locking screws was assessed on the axial, coronal, and sagittal cuts.
Figure 3.
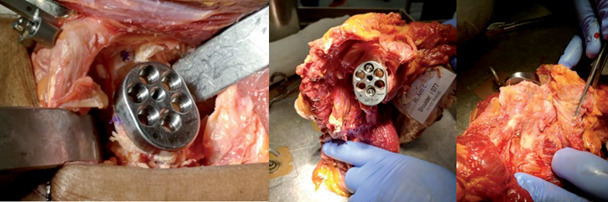
Sacpula dissection. Central peg and screws positioning verification.
Results
Preoperative CT scans did not show bone loss or deformity in any specimen. The mean native glenoid version was 5.6° (range -2° to +7°). The mean version of the glenoid was 3.1° of anteversion (range 0° to +8°). The mean tilt of the glenoid component was -5.4° of inferior tilt (range -2° to -10°). The range of error for the version was ±2° compared to the ideal position planned before. For component tilt, the range of error was ±3°. Postoperative macroscopic dissection revealed no central peg perforated or screws malpositioned, no leaking from the bone or injury to the adjacent neurovascular structures. The average length of the screws was 42 mm (range 36 mm to 46 mm) for the lower screw and 40 mm for the upper one (range 36 mm to 42 mm).
During the 6 computer-assisted navigation procedures, there were no complications reported related to the placement of the 2 fixation pins in the coracoid process. There was no need for an extended surgical approach, and the reference remained stable and visible for the navigation station throughout the complete procedure.
Discussion
The glenoid component positioning in RSA is crucial to prevent failure, loosening and biomechanical mismatch that affect the function and clinical result. Crucial is the coverage by the baseplate of the glenoid surface and the correct positioning in terms of version, inclination, and off-set, equally essential part is the positioning of the longest screws possible but which at the same time do not cause injury to adjacent structures or impingement (9,15). However, the glenoid anatomy presents an extreme variability from one subject to another, without taking into account that in the arthrosis and arthritic scapulohumeral joints this is upset especially in terms of bone quality and bone stock. Furthermore, the difficult surgical exposure of the glenoid, the limited size and difficult visualization of anatomical reference landmarks may jeopardize optimal placement and stable bone fixation of the base plate and screws. Lastly, mispositioned screws may be harmful to the surrounding soft tissues, such as the axillary (inferior screw) or suprascapular (superior screw) nerve, blood vessels, or rotator cuff muscles. This cadaver study has shown that GPS navigation offers greater efficiency in baseplate and screws placement and can avoid intra- and postoperative complications.
We believe that the possibility of implanting longer screws is directly correlated with the resistance of the glenoid implant and therefore with its duration. The navigation allows us to always implant the longest possible screw without leaking from the bone and risking injuries to adjacent noble structures. Concerning the positioning of the base-plate and gleno-sphere it is now established that any malposition leads inevitably to failure of the system, for this fundamental is the precision to the minimum degree (16).
While the specimens in the current study did not show severe glenoid bone loss, we know how arthrosis pathology can subvert the anatomy of the glenoid and how much every anatomical landmark can be distorted, it is precisely in this situation that GPS navigation takes on an increasingly important role. This is even truer in revisions, where often there is a need to tackle a lack of bone and poor quality.
This study showed how useful CT 3D programming is to identify the best positioning of each component and the usefulness of receiving directly in the operating room real-time feedback on the change in position, version, and tilt of the glenoid component and improves the accuracy of their placement. This real-time guide allows going beyond all the problems of surgical exposure of the glenoid, anatomical variability and safety in the positioning of the components. We showed how the variability in the positioning of the baseplate was 2-3 degrees in the various planes, an accuracy that is difficult to reproduce without navigation assistance.
There are, however, several main disadvantages of this image-based system. First of all the higher costs of instrumentation and software compared to the classical technique, secondly the increase in surgical times, and finally, the need often to enlarge the surgical access to discover the coracoid and the risk related to the positioning of the tracker to break the coracoid itself. Obviously, we think that if the result consists of improving the positioning and therefore both the function and longevity, these are acceptable compromises.
There are several limitations to this study. First, the limited number of specimens investigated in navigated and control specimens. Second, the absence of a control group. Third, we did not perform biomechanical tests to compare the initial stability of the glenoid component. Surely a long-term follow-up of a large number of patients is needed to confirm the hypothesis that navigation may improve long-term functional outcomes.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Westermann RW, Pugely AJ, Martin CT, Gao Y, Wolf BR, Hettrich CM. Reverse Shoulder Arthroplasty in the United States: A Comparison of National Volume, Patient Demographics, Complications, and Surgical Indications. Iowa Orthop J. 2015;35:1–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Jt Surg - Ser A. 2011;93(24):2249–2254. doi: 10.2106/JBJS.J.01994. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 3.Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: A systematic review. J Shoulder Elb Surg. 2011;20(1):146–157. doi: 10.1016/j.jse.2010.08.001. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res. 2016;102(1):S33–S43. doi: 10.1016/j.otsr.2015.06.031. doi:10.1016/j.otsr.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elb Surg. 2005;14(5):524–528. doi: 10.1016/j.jse.2004.09.010. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Chebli C, Huber P, Watling J, Bertelsen A, Bicknell RT, Matsen F. Factors affecting fixation of the glenoid component of a reverse total shoulder prothesis. J Shoulder Elb Surg. 2008;17(2):323–327. doi: 10.1016/j.jse.2007.07.015. doi: 10.1016/j.jse.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Giannotti S, Bottai V, Dell’Osso G, et al. Thirty-six consecutive reverse shoulder arthroplasties in cuff arthropathy: Our experience with the anterosuperior approach. Eur Orthop Traumatol. 2014;5(3):293–298. doi: 10.1007/s12570-014-0248-0. [Google Scholar]
- 8.Scalise JJ. Reverse total shoulder arthroplasty. Minerva Ortop e Traumatol. 2009;60(1):55–59. doi: 10.2106/00004623-200707000-00011. [Google Scholar]
- 9.Codsi MJ, Iannotti JP. The effect of screw position on the initial fixation of a reverse total shoulder prosthesis in a glenoid with a cavitary bone defect. J Shoulder Elb Surg. 2008;17(3):479–486. doi: 10.1016/j.jse.2007.09.002. doi: 10.1016/j.jse.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Hart ND, Clark JC, Wade Krause FR, Kissenberth MJ, Bragg WE, Hawkins RJ. Glenoid screw position in the Encore Reverse Shoulder Prosthesis: An anatomic dissection study of screw relationship to surrounding structures. J Shoulder Elb Surg. 2013;22(6):814–820. doi: 10.1016/j.jse.2012.08.013. doi: 10.1016/j.jse.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Plancher KD. Sports Medicine : Shoulder. 2012:113–116. [Google Scholar]
- 12.Bigliani LU, Dalsey RM, McCann PD, April EW. An anatomical study of the suprascapular nerve. Arthrosc J Arthrosc Relat Surg. 1990;6(4):301–305. doi: 10.1016/0749-8063(90)90060-q. doi: 10.1016/0749-8063(90)90060-Q. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Singh A, Higgins L, Warner J. Suprascapular neuropathy secondary to reverse shoulder arthroplasty: A case report. J Shoulder Elb Surg. 2010;19(3):e5. doi: 10.1016/j.jse.2009.10.004. doi:10.1016/j.jse.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Cofield RH. Bone grafting for glenoid bone deficiencies in shoulder arthritis: A review. J Shoulder Elb Surg. 2007;16(5 SUPPL):273–281. doi: 10.1016/j.jse.2007.03.005. doi:10.1016/j.jse.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in “reverse” total shoulder arthroplasty: A biomechanical evaluation. J Shoulder Elb Surg. 2005;14(1 SUPPL):S162–S167. doi: 10.1016/j.jse.2004.09.030. doi:10.1016/j.jse.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elb Surg. 2008;17(6):925–935. doi: 10.1016/j.jse.2008.02.010. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]


